Abstract
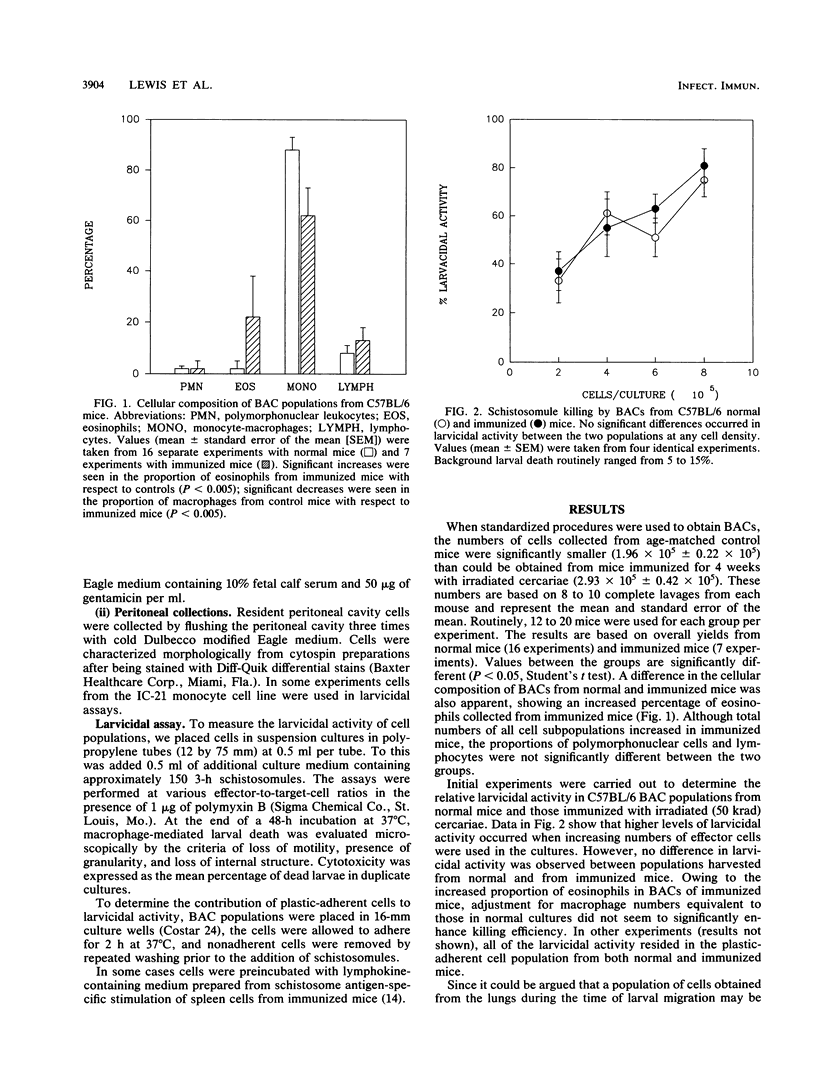
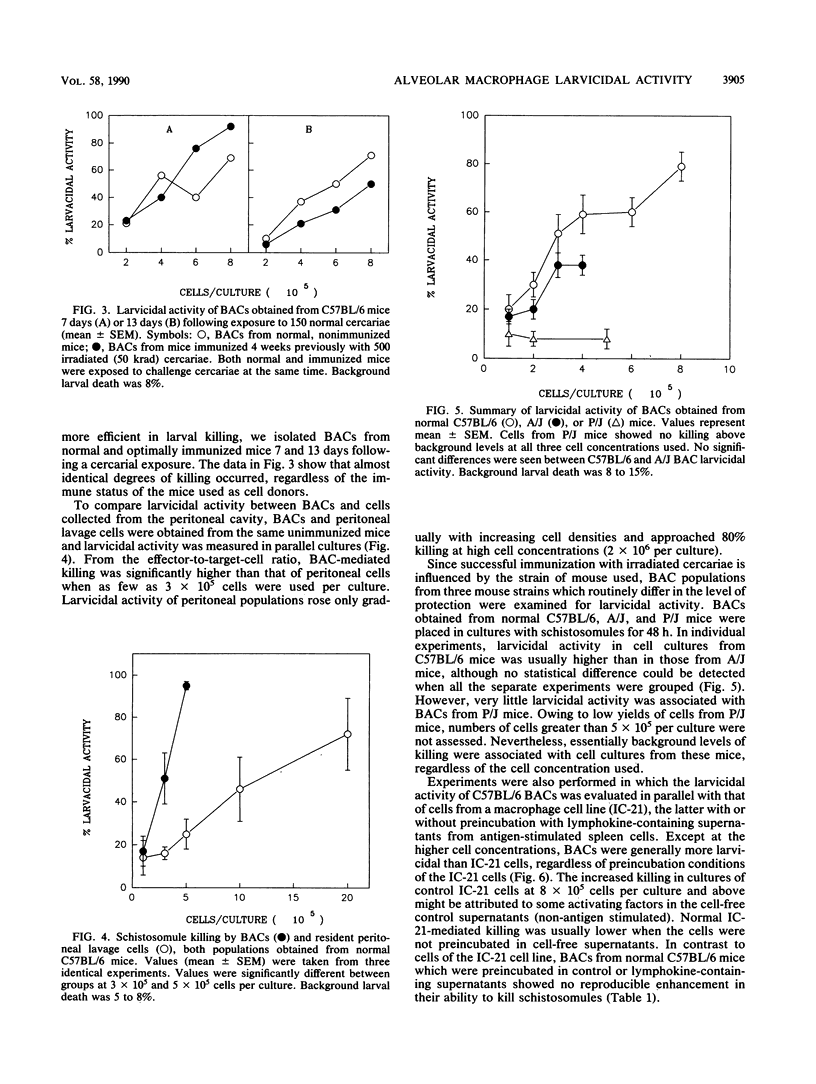
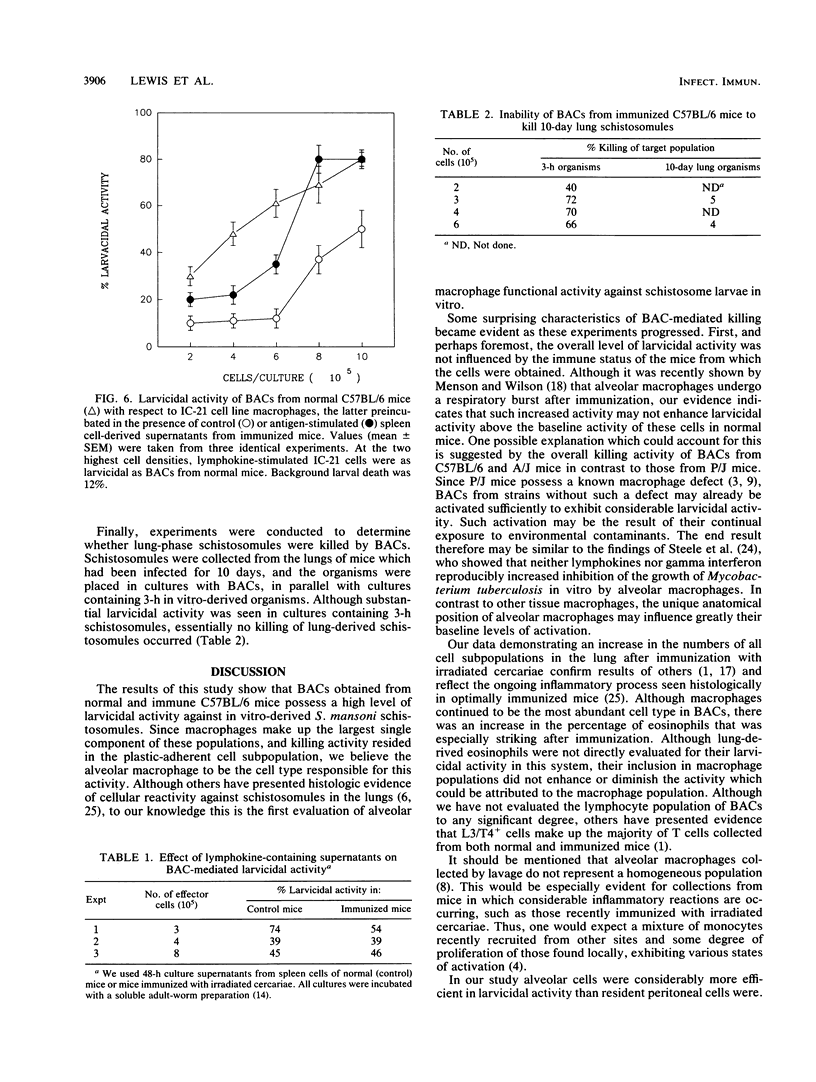
We have investigated the ability of cells obtained from both normal and immune mice by bronchoalveolar lavage (BACs) to kill Schistosoma mansoni larvae in vitro. In cultures with mechanically derived schistosomules, high levels of larvicidal activity were displayed by BACs from both normal and irradiated cercaria-immunized C57BL/6 mice. Based on effector-to-target-cell ratios, BAC-mediated killing was two- to threefold more efficient than killing mediated by macrophage-rich cell populations obtained from the peritoneal cavity. BACs from normal A/J mice were essentially as larvicidal as normal C57BL/6 cells. However, BACs from a strain of mouse (P/J) with a known macrophage defect possessed negligible larvicidal activity. Macrophages made up 85 to 95% of BACs from all three strains tested. In contrast to cells of the IC-21 macrophage cell line, B6 BACs did not show enhanced killing activity when preincubated with lymphokine-containing supernatants. Lung schistosomules harvested 10 days after cercarial penetration were refractory to BAC-mediated killing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken R., Coulson P. S., Wilson R. A. Pulmonary leukocytic responses are linked to the acquired immunity of mice vaccinated with irradiated cercariae of Schistosoma mansoni. J Immunol. 1988 May 15;140(10):3573–3579. [PubMed] [Google Scholar]
- Black C. M., Beaman B. L., Donovan R. M., Goldstein E. Intracellular acid phosphatase content and ability of different macrophage populations to kill Nocardia asteroides. Infect Immun. 1985 Feb;47(2):375–383. doi: 10.1128/iai.47.2.375-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from P/J mice: characterization of the macrophage cytotoxic defect after in vivo and in vitro activation stimuli. J Immunol. 1980 Aug;125(2):771–776. [PubMed] [Google Scholar]
- Chen B. D., Mueller M., Olencki T. Interleukin-3 (IL-3) stimulates the clonal growth of pulmonary alveolar macrophage of the mouse: role of IL-3 in the regulation of macrophage production outside the bone marrow. Blood. 1988 Aug;72(2):685–690. [PubMed] [Google Scholar]
- Colley D. G., Wikel S. K. Schistosoma mansoni: simplified method for the production of schistosomules. Exp Parasitol. 1974 Feb;35(1):44–51. doi: 10.1016/0014-4894(74)90005-8. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Wilson R. A. The role of pulmonary cellular reactions in the resistance of vaccinated mice to Schistosoma mansoni. Parasite Immunol. 1986 May;8(3):265–285. doi: 10.1111/j.1365-3024.1986.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Dean D. A., Mangold B. L., Georgi J. R., Jacobson R. H. Comparison of Schistosoma mansoni migration patterns in normal and irradiated cercaria-immunized mice by means of autoradiographic analysis. Evidence that worm elimination occurs after the skin phase in immunized mice. Am J Trop Med Hyg. 1984 Jan;33(1):89–96. doi: 10.4269/ajtmh.1984.33.89. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Cohn Z. A. The alveolar macrophage. J Appl Physiol (1985) 1986 Feb;60(2):353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- James S. L., Correa-Oliveira R., Leonard E. J. Defective vaccine-induced immunity to Schistosoma mansoni in P strain mice. II. Analysis of cellular responses. J Immunol. 1984 Sep;133(3):1587–1593. [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- James S. L., Natovitz P. C., Farrar W. L., Leonard E. J. Macrophages as effector cells of protective immunity in murine schistosomiasis: macrophage activation in mice vaccinated with radiation-attenuated cercariae. Infect Immun. 1984 Jun;44(3):569–575. doi: 10.1128/iai.44.3.569-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis F. A., Stirewalt M. A., Souza C. P., Gazzinelli G. Large-scale laboratory maintenance of Schistosoma mansoni, with observations on three schistosome/snail host combinations. J Parasitol. 1986 Dec;72(6):813–829. [PubMed] [Google Scholar]
- Lewis F. A., Wilson E. M. Regional and splenic lymphocyte proliferative responses of mice exposed to normal or irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):505–513. doi: 10.4269/ajtmh.1982.31.505. [DOI] [PubMed] [Google Scholar]
- Lewis F. A., Winestock J., James S. L. Macrophage activation as an immune correlate to protective immunity against schistosomiasis in mice immunized with an irradiated, cryopreserved live vaccine. Infect Immun. 1987 Jun;55(6):1339–1345. doi: 10.1128/iai.55.6.1339-1345.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastin A. J., Bickle Q. D., Wilson R. A. Schistosoma mansoni: migration and attrition of irradiated and challenge schistosomula in the mouse. Parasitology. 1983 Aug;87(Pt 1):87–102. doi: 10.1017/s0031182000052446. [DOI] [PubMed] [Google Scholar]
- McLaren D. J. Will the real target of immunity to schistosomiasis please stand up. Parasitol Today. 1989 Sep;5(9):279–282. doi: 10.1016/0169-4758(89)90018-5. [DOI] [PubMed] [Google Scholar]
- Menson E. N., Coulson P. S., Wilson R. A. Schistosoma mansoni: circulating and pulmonary leucocyte responses related to the induction of protective immunity in mice by irradiated parasites. Parasitology. 1989 Feb;98(Pt 1):43–55. doi: 10.1017/s0031182000059679. [DOI] [PubMed] [Google Scholar]
- Menson E. N., Wilson R. A. Lung-phase immunity to Schistosoma mansoni. Flow cytometric analysis of macrophage activation states in vaccinated mice. J Immunol. 1989 Oct 1;143(7):2342–2348. [PubMed] [Google Scholar]
- Pearce E. J., James S. L. Post lung-stage schistosomula of Schistosoma mansoni exhibit transient susceptibility to macrophage-mediated cytotoxicity in vitro that may relate to late phase killing in vivo. Parasite Immunol. 1986 Sep;8(5):513–527. doi: 10.1111/j.1365-3024.1986.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Perez H., Clegg J. A., Smithers S. R. Acquired immunity to Schistosoma mansoni in the rat: measurement of immunity by the lung recovery technique. Parasitology. 1974 Dec;69(3):349–359. doi: 10.1017/s0031182000063046. [DOI] [PubMed] [Google Scholar]
- Ryning F. W., Remington J. S. Effect of alveolar macrophages on Toxoplasma gondii. Infect Immun. 1977 Dec;18(3):746–753. doi: 10.1128/iai.18.3.746-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. I., Davis C. E. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983 Dec;42(3):1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Hieny S., James S. L., Asofsky R. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. II. Analysis of immunity in hosts deficient in T lymphocytes, B lymphocytes, or complement. J Immunol. 1982 Apr;128(4):1880–1884. [PubMed] [Google Scholar]
- Steele J., Flint K. C., Pozniak A. L., Hudspith B., Johnson M. M., Rook G. A. Inhibition of virulent Mycobacterium tuberculosis by murine peritoneal macrophages and human alveolar lavage cells: the effects of lymphokines and recombinant gamma interferon. Tubercle. 1986 Dec;67(4):289–294. doi: 10.1016/0041-3879(86)90018-8. [DOI] [PubMed] [Google Scholar]
- Von Lichtenberg F., Correa-Oliveira R., Sher A. The fate of challenge schistosomula in the murine anti-schistosome vaccine model. Am J Trop Med Hyg. 1985 Jan;34(1):96–106. doi: 10.4269/ajtmh.1985.34.96. [DOI] [PubMed] [Google Scholar]



