Abstract
Elevated soluble tumor necrosis factor-α receptor (sTNFR) levels in bronchoalveolar lavage fluid (BALF) are associated with poor patient outcome in acute lung injury (ALI). The mechanisms underlying these increases are unknown, but it is possible that pulmonary inflammation and increased alveolar epithelial permeability may individually contribute. We investigated mechanisms of elevated BALF sTNFRs in two in vivo mouse models of ALI. Anesthetized mice were challenged with intratracheal lipopolysaccharide or subjected to injurious mechanical ventilation. Lipopolysaccharide instillation produced acute intra-alveolar inflammation, but minimal alveolar epithelial permeability changes, with increased BALF sTNFR p75, but not p55. Increased p75 levels were markedly attenuated by alveolar macrophage depletion. In contrast, injurious ventilation induced substantial alveolar epithelial permeability, with increased BALF p75 and p55, which strongly correlated with total protein. BALF sTNFRs were not increased in isolated buffer-perfused lungs (devoid of circulating sTNFRs) subjected to injurious ventilation. These results suggest that lipopolysaccharide-induced intra-alveolar inflammation upregulates alveolar macrophage-mediated production of sTNFR p75, whereas enhanced alveolar epithelial permeability following mechanical ventilation leads to increased BALF p75 and p55 via plasma leakage. These data provide new insights into differential regulation of intra-alveolar sTNFR levels during ALI and may suggest sTNFRs as potential markers for evaluating the pathophysiology of ALI.
Keywords: lipopolysaccharide, pulmonary edema, pulmonary inflammation, ventilator-induced lung injury
acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are common causes of intensive care mortality (35). An important feature of ALI is dysregulated pulmonary inflammation, and the roles of various inflammatory mediators have been studied in depth. In particular, tumor necrosis factor-α (TNF) has been consistently implicated, but its precise role is uncertain (2, 19, 27). TNF signaling is primarily mediated by two cell-surface receptors, TNF receptor (TNFR) p55 and p75 (33). Emerging evidence suggests that each receptor can mediate differential, and even opposing roles during acute pathological conditions (9, 13, 38), and hence a proper understanding of the involvement of TNF in ALI/ARDS requires an appreciation of TNFR biology.
TNFRs also exist as cognate soluble forms produced by proteolytic cleavage (shedding) of receptor ectodomains, termed soluble TNFRs (sTNFRs) (33). These soluble receptors are still able to bind to TNF, resulting in neutralization of its bioactivity (33), or potentially prolongation of TNF bioactivity due to its stabilization (1) or reverse signaling through cell-surface TNF (10). The precise role of sTNFRs during ALI is not well defined, but they are generally considered anti-inflammatory because of their TNF neutralizing ability (33), and the fact that release of sTNFRs from cells would decrease surface receptor expression, attenuating TNFR-dependent signaling. Clinical and laboratory studies implicate sTNFRs as markers in ALI, such that elevated bronchoalveolar lavage fluid (BALF) and plasma levels are associated with morbidity and mortality in ventilated ARDS patients (24, 25, 27), and BALF levels are elevated following injurious mechanical ventilation in mice (38). However, the sources of these soluble receptors are not known. In terms of intra-alveolar sTNFRs, it has previously been reported that pulmonary epithelial cells are capable of shedding sTNFR p55 in response to inflammatory stimuli (17, 20, 25), although these in vitro data are mainly derived from cell lines (A549) and their physiological relevance may be questionable. Conversely, it has been proposed that alveolar epithelial permeability changes during ALI may cause leakage of plasma sTNFRs into the airspaces (24). Increases in BALF sTNFR levels by passive leakage or active production by shedding from the surface of cells could have very diverse effects on intra-alveolar TNF signaling. Understanding which processes actually occur during ALI is therefore required to properly interpret changes in BALF sTNFR levels in patients.
In this study we investigated mechanisms and sources of increased BALF sTNFRs during ALI. This is a vital first step toward understanding of the biological relevance of elevated sTNFR levels in patients. We studied two in vivo mouse models of ALI that exhibit distinct acute pathophysiologies: intratracheal lipopolysaccharide (LPS) administration and ventilator-induced lung injury (VILI). Our data show that these different insults, which were associated with divergent levels of intra-alveolar inflammation and alveolar epithelial permeability, induce changes in BALF sTNFR levels through entirely different mechanisms.
MATERIALS AND METHODS
All protocols were approved by the Ethical Review Board of Imperial College London and carried out under the authority of the UK Home Office in accordance with the Animals (Scientific Procedures) Act 1986, UK. Male C57Bl/6 mice (Charles River, Margate, UK) aged 10–13 wk were used throughout.
In vivo LPS-induced pulmonary inflammation.
Mice were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and xylazine (6 mg/kg). Animals were suspended in an upright position by their front teeth and a fine oral catheter was passed into the trachea, by direct visualization of the vocal cords under a microscope, as previously described (39). Mice were challenged intratracheally with 50 μl saline or LPS (20 ng: Ultrapure E. coli O111:B4; InVivoGen, Toulouse, France), allowed to recover from anesthesia, and euthanized 2 or 6 h after challenge. Some animals were pretreated intratracheally with 50 μl clodronate (Roche Diagnostics, Mannheim, Germany) encapsulated into liposomes (30), 48 h prior to LPS challenge to deplete alveolar macrophages.
In vivo injurious mechanical ventilation.
Our in vivo mouse model of VILI has been described in detail previously (36–38, 40). Briefly, anesthetized mice were tracheostomized and ventilated with either noninjurious low-tidal volume (VT) ventilation [VT 8–9 ml/kg, positive end-expiratory pressure (PEEP) 2.5 cmH2O, 120 breaths/min with sustained inflations (35 cmH2O, 5 s) every 30 min to prevent atelectasis, using air] or injurious high-VT ventilation (VT 36–41 ml/kg, zero PEEP, 90 breaths/min, using air +4% CO2). Animals were ventilated for up to 2 h, or until peak inspiratory pressure (PIP) increased by ∼30% (a sign of imminent cardiorespiratory collapse). A carotid cannula was placed for blood gas and blood pressure (BP) monitoring, and respiratory system compliance (Crs) and resistance (Rrs) were determined by end-inflation occlusion (12).
Ex vivo injurious mechanical ventilation using isolated perfused lungs.
Isolated buffer-perfused lungs (IPL) were used to produce VILI in the absence of systemic influences, adapted from previously described methods (22, 34). Briefly, anesthetized mice were tracheostomized, ventilated, and after exsanguination the pulmonary artery and left atrium cannulated. Lungs were perfused (37°C, flow 25 ml·kg−1·min−1, left atrial pressure 3 cmH2O) in a nonrecirculating manner using a Hugo Sachs IPL system (March-Hugstetten, Germany). Perfusate consisted of RPMI 1640 without phenol red (Invitrogen, Paisley, UK) supplemented with 4% bovine serum albumin. Lungs were ventilated using air +5% CO2 with either noninjurious low-VT (VT 8–9 ml/kg, 2.5 cmH2O PEEP, 120 breaths/min) or injurious high-VT (VT 20–25 ml/kg, zero PEEP, 80 breaths/min) ventilation for 2 h or until PIP increased by ∼30%.
BALF analyses.
On termination of experiments, saline bronchoalveolar lavage was performed as described previously (36). Total protein was quantified by Bradford assay, and sTNFR p55, p75, and macrophage inflammatory protein-2 (MIP-2) quantified using ELISA (R&D Systems, Abingdon, UK). Cells were analyzed by differential cytology using Diff-Quik (Medion Diagnostics AG, Duedingen, Switzerland) stained Cytospin slides (Shandon, Runcorn, UK).
Flow cytometry.
Macrophage depletion was assessed in BALF/lung single cell suspensions by flow cytometry (22, 23, 39, 40). Single cell suspensions, prepared from excised lungs using a GentleMACS tissue dissociator (Miltenyi Biotec, Surrey, UK), were passed through a 40-μm cell strainer (Becton Dickinson, Oxford, UK), stained with fluorescent dye-conjugated antibodies for CD11b, CD11c and F4/80, and analyzed using a FACSCalibur cytometer (Becton Dickinson). Macrophages were quantified using microsphere beads (Caltag Medsystems, Buckingham, UK), as previously described (22, 23, 40).
Data analysis.
Data are expressed as means ± SD. Statistical comparisons were made by t-tests or ANOVA with Bonferroni tests and correlations assessed by Pearson's correlation, using Prism software (GraphPad, La Jolla, CA). Significance was defined as P < 0.05.
RESULTS
Intratracheal LPS increases BALF levels of sTNFR p75, but not p55.
We first investigated intra-alveolar sTNFR production in a model of pulmonary inflammation induced by intratracheal LPS. Mice received intratracheal instillation of 20 ng of LPS or saline as a control, and were euthanized after 2 or 6 h. Mice challenged with this dose of LPS showed no sign of morbidity, i.e., the dose used was subclinical.
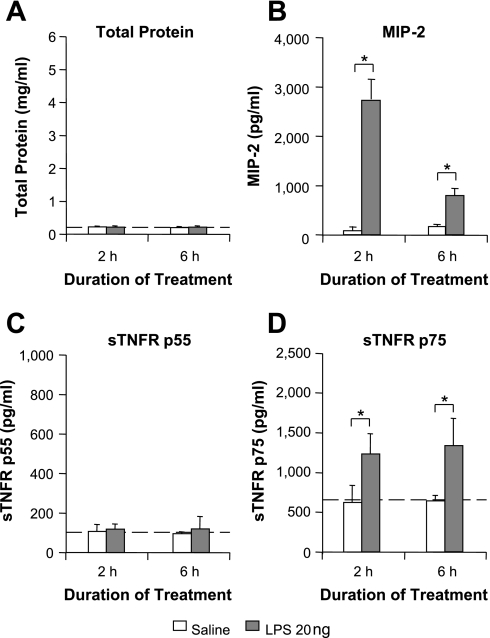
LPS challenge did not induce significant alveolar epithelial permeability changes, as measured by BALF total protein at 2 and 6 h (Fig. 1A). Similarly, lung wet:dry weight ratios were not increased 6 h post-LPS (4.53 ± 0.08 for LPS vs. 4.62 ± 0.07 for saline, n = 4). Intratracheal LPS did produce a substantial increase in BALF levels of MIP-2, indicating considerable intra-alveolar inflammation (P < 0.05; Fig. 1B). We found no increase in BALF sTNFR p55 at either 2 or 6 h following LPS challenge (Fig. 1C). In stark contrast, sTNFR p75 was rapidly upregulated after LPS compared with controls (Fig. 1D, P < 0.05). The absence of p55 upregulation was evident even with the use of a 1,000-fold higher dose (20 μg) of LPS (2 h sTNFR p55: 91.6 ± 18.5 pg/ml, n = 4; 6 h sTNFR p55: 133 ± 37.0 pg/ml, n = 4). The use of this higher dose did not further enhance the upregulation of sTNFR p75 levels (data not shown), suggesting that the subclinical 20 ng LPS dose induced a near-maximal upregulation of sTNFR p75 within 2 h. These data indicate that intratracheal LPS induces intra-alveolar production of sTNFR p75, but not p55, independently of increased alveolar epithelial permeability.
Fig. 1.
Intratracheal lipopolysaccharide (LPS) increases bronchoalveolar lavage fluid (BALF) levels of sTNFR p75, but not p55. Mice were challenged intratracheally with 20 ng of LPS in a 50-μl saline bolus. Saline alone was used as a control. After 2 or 6 h, BALF was analyzed for sTNFRs, macrophage inflammatory protein-2 (MIP-2), and total protein. A: LPS-challenged animals exhibited negligible increases in BALF total protein, indicating minimal changes in alveolar epithelial permeability. B: BALF levels of MIP-2 were significantly increased at 2 and 6 h following LPS challenge (P < 0.05), with substantially higher levels at 2 h. C: BALF sTNFR p55 levels were not increased at either time point. D: in contrast, BALF sTNFR p75 was substantially increased at both 2 and 6 h post-LPS, suggesting intra-alveolar production of this receptor. Dashed lines indicate baseline values from untreated mice. Baseline BALF levels of MIP-2 were undetectable. *P < 0.05; n = 4–7/each group.
We also found negligible neutrophil infiltration into the alveolar space 2 h after intratracheal 20 ng LPS (neutrophils as a percentage of total BALF cells: 1.1 ± 1.1% for LPS vs. 3.4 ± 4.7% for saline, n = 4–7). Neutrophil infiltration increased greatly after 6 h (31.3 ± 2.8%, n = 4), but as the increase in p75 occurred prior to this, neutrophils seem unlikely to be directly involved in intra-alveolar sTNFR p75 production.
Alveolar macrophage depletion attenuates LPS-induced sTNFR p75 increases in BALF.
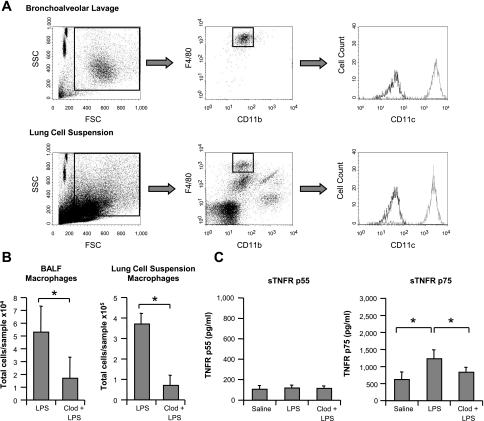
Alveolar macrophages are potential producers of intra-alveolar sTNFR p75, as they release considerable quantities of sTNFR p75 in response to LPS in vitro (7). To evaluate the contribution of alveolar macrophages to intra-alveolar sTNFR p75 production, we depleted them by pretreatment with intratracheal clodronate-liposomes 48 h prior to 20 ng LPS challenge. Consistent with the literature (14), flow cytometry of BALF and lung tissue demonstrated 70–80% depletion of alveolar macrophages (Fig. 2, A and B). Clodronate-liposome pretreatment significantly attenuated LPS-induced sTNFR p75 production by ∼65% (P < 0.05; Fig. 2C) but had no effect on p55, implicating an important role for alveolar macrophages in sTNFR p75 production in this model.
Fig. 2.
Alveolar macrophage depletion attenuates LPS-induced sTNFR p75 increases in BALF. A: mice were treated intratracheally with a 50-μl bolus of clodronate-liposomes, and after 48 h their BALF/lungs were analyzed by flow cytometry to assess the degree of macrophage depletion. Alveolar macrophages were identified in BALF as CD11blow F4/80high events, confirmed with CD11chigh staining (dark line: isotype control). For lung cell suspension samples, the same gating strategy was employed, using the BALF population for reference. B: at 48 h after intratracheal clodronate-liposomes, mice were challenged intratracheally with 20 ng LPS, and after 2 h their BALF/lungs analyzed by flow cytometry. Mice pretreated with clodronate-liposomes (Clod + LPS) exhibited ∼70% depletion of macrophage numbers in BALF and ∼80% depletion in lung suspensions, compared with mice without the pretreatment (LPS). *P < 0.05, Clod + LPS vs. LPS; n = 5–7/each group. C: alveolar macrophage depletion significantly attenuated sTNFR p75 production in BALF 2 h after LPS by ∼65%, with no effect on p55. *P < 0.05 vs. LPS; saline and LPS data same as Fig. 1; n = 4–6/each group.
Injurious mechanical ventilation increases BALF levels of sTNFR p55 and p75.
We next investigated intra-alveolar sTNFR levels in a previously described in vivo mouse model of VILI, produced by injurious high-VT ventilation (36–38, 40). Consistent with our previous studies, high-VT ventilation produced progressive increases in PIP and Rrs as well as decreases in Crs, indicating significant induction of alveolar epithelial permeability (Table 1). Low-VT ventilation did not induce such changes. Mean BP and arterial blood gases were well maintained during both ventilation protocols, but Po2 decreased in the high-VT group at 2 h (P < 0.05). Neither ventilation protocol induced infiltration of neutrophils into the alveolar space within 2 h (data not shown).
Table 1.
Physiological parameters in the in vivo VILI model
| 0 h | 1 h | 2 h | |
|---|---|---|---|
| Low VT | |||
| PIP, cmH2O | 10.2 ± 0.5 | 10.3 ± 1.5 | 10.8 ± 0.8 |
| Rrs, cmH2O · ml−1 s | 1.76 ± 0.12 | 1.68 ± 0.10 | 1.68 ± 0.23 |
| Crs, ml/cmH2O | 0.033 ± 0.004 | 0.033 ± 0.004 | 0.032 ± 0.004 |
| BP, mmHg | 86 ± 10 | 96 ± 6* | 88 ± 9 |
| Po2, mmHg | 103 ± 11 | 101 ± 14 | |
| Pco2, mmHg | 42 ± 5 | 41 ± 5 | |
| High VT | |||
| PIP, cmH2O | 35.4 ± 0.3 | 37.2 ± 1.4 | 45.4 ± 3.0* |
| Rrs, cmH2O · ml−1 s | 1.69 ± 0.29 | 1.59 ± 0.21 | 2.41 ± 0.32* |
| Crs, ml/cmH2O | 0.035 ± 0.005 | 0.033 ± 0.005 | 0.028 ± 0.002* |
| BP, mmHg | 88 ± 14 | 96 ± 8 | 79 ± 10* |
| Po2, mmHg | 147 ± 3 | 108 ± 27† | |
| Pco2, mmHg | 38 ± 1 | 40 ± 1 |
Values are means ± SD; n = 5–7/each group. VILI, ventilator-induced lung injury; PIP, peak inspiratory pressure; Rrs, respiratory system resistance; Crs, respiratory system compliance; BP, mean arterial blood pressure. Mice were ventilated with low tidal volume (VT; 8–9 ml/kg) or high VT (36–41 ml/kg) for 2 h. High-VT ventilation induced increased PIP and Rrs and decreased Crs, consistent with increased alveolar epithelial permeability. These changes were not observed with low VT. BP and blood gases were well maintained throughout, with a decline in BP and Po2 toward the end of high-VT ventilation.
P < 0.05 vs. 0 h;
P < 0.05 vs. 1 h.
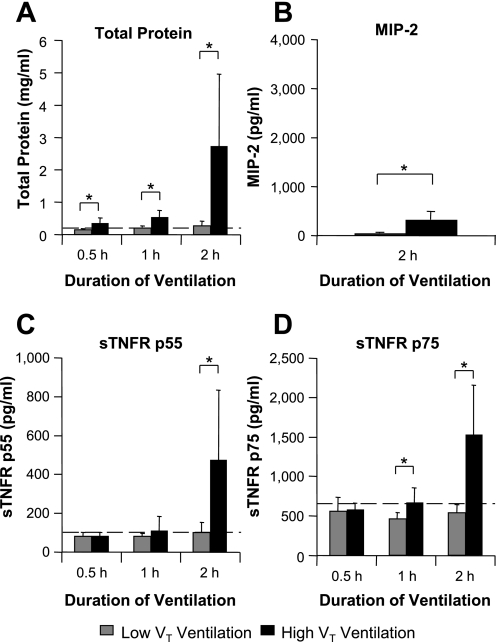
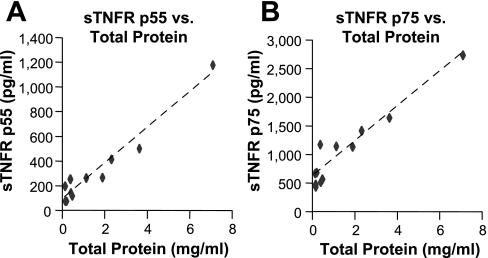
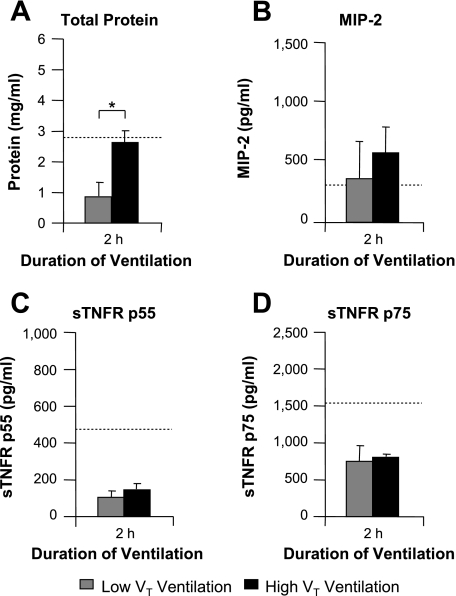
High-VT ventilation induced small increases in BALF protein at 0.5 and 1 h, with a much larger increase at 2 h (P < 0.05; Fig. 3A) compared with low-VT ventilation, consistent with the time courses of PIP and respiratory mechanics (Table 1). Increased alveolar epithelial permeability was further confirmed by increased lung wet:dry weight ratio at 2 h (7.71 ± 0.52 for high VT vs. 5.03 ± 0.09 for low VT; P < 0.05; n = 4). Significant increases in BALF MIP-2 levels were observed following 2 h of high-VT vs. low-VT ventilation (P < 0.05; Fig. 3B), although increases were relatively small compared with the intratracheal LPS model, suggesting a lesser degree of intra-alveolar inflammation at this time point during the progression of VILI. High-VT ventilation increased BALF sTNFR p75 slightly at 1 h (P < 0.05) with a similar tendency for p55 and at 2 h induced substantial increases in both soluble receptors (P < 0.05, Fig. 3, C and D). This pattern was very different from the LPS model and appeared to follow increases in alveolar epithelial permeability, which was confirmed by very strong correlations between BALF total protein and each receptor at 2 h (r = 0.974 for p55 and r = 0.956 for p75; n = 12; P < 0.0001, Fig. 4).
Fig. 3.
Injurious mechanical ventilation increases BALF levels of both sTNFR p55 and p75. Mice were ventilated with low tidal volume [VT; 8–9 ml/kg, with the initial peak inspiratory pressure (PIP) set at ∼10 cmH2O] or high-VT (36–41 ml/kg, with the initial PIP set at ∼35 cmH2O) ventilation for up to 2 h. On termination, BALF was analyzed for sTNFRs, MIP-2, and total protein. A: high-VT ventilation produced small marginal increases in BALF total protein at 0.5 and 1 h, followed by a large increase at 2 h, indicating progressive increases in alveolar epithelial permeability. B: high-VT ventilation also produced a significant increase in BALF MIP-2 at 2 h, although levels were considerably lower than for intratracheal LPS (Fig. 1B). C: high-VT ventilation substantially increased BALF sTNFR p55 levels at 2 h. D: high-VT ventilation for 1 h produced a small increase in BALF sTNFR p75 levels, with a larger increase at 2 h. Note that increased sTNFR levels were not apparent before the substantial increase in BALF total protein. Dashed lines indicate baseline values from untreated mice. *P < 0.05, high VT vs. low VT; n = 5–7/each group.
Fig. 4.
BALF sTNFR levels strongly correlated with total protein after 2 h of ventilation. Association between BALF levels of each sTNFR and total protein in the in vivo ventilator-induced lung injury (VILI) model were evaluated by Pearson's correlation analysis using the data from 2 h ventilation groups. Strong positive correlations were detected between BALF sTNFR p55 and total protein (A, r = 0.974, P < 0.0001), and between BALF sTNFR p75 and total protein (B, r = 0.956, P < 0.0001), suggesting leakage from the plasma as the mechanism underlying increased BALF sTNFRs. Each data point represents one animal; n = 12.
Injurious ventilation in ex vivo IPL does not increase BALF levels of sTNFRs.
Our in vivo injurious ventilation model suggests that sTNFRs were elevated in BALF primarily due to alveolar epithelial permeability changes and leakage from the plasma, where comparatively high levels of both sTNFRs are present under baseline conditions (2,420 ± 775 pg/ml for p55 and 6,660 ± 1,740 pg/ml for p75, untreated mice, n = 6). To test this hypothesis, we performed ex vivo VILI experiments utilizing a previously described IPL system (22, 34). In this model, lungs were perfused in a nonrecirculating manner with a buffer devoid of sTNFRs, and ventilated with either low or high VT.
High-VT ventilation in the IPL displayed a similar pattern of alveolar epithelial permeability changes to the in vivo high-VT experiments, with progressive increases in PIP (data not shown) and increased BALF total protein at 2 h (Fig. 5A). Similarly, BALF MIP-2 levels tended to increase with high-VT ventilation (Fig. 5B), suggesting our IPL preparation was viable and able to produce soluble mediators in response to mechanical stretch. However, in contrast to the in vivo model, ex vivo high-VT ventilation did not significantly increase BALF sTNFR levels compared with low VT (Fig. 5, C and D), suggesting that circulating sTNFRs are required for increased BALF sTNFR levels in pure mechanical VILI.
Fig. 5.
Injurious ventilation in ex vivo isolated buffer-perfused lungs does not increase BALF levels of sTNFRs. The mechanisms of sTNFR increases in BALF during VILI were further investigated in an isolated buffer-perfused mouse lung system. Lungs were perfused with a buffer devoid of sTNFRs, and subjected to low-VT (8 ml/kg, with the initial PIP set at ∼10 cmH2O) or high-VT (20–25 ml/kg, with the initial PIP set at ∼25 cmH2O) ventilation for 2 h. On termination, BALF was analyzed for sTNFRs, MIP-2, and total protein levels. A: high-VT ventilation produced increased alveolar epithelial permeability, as reflected by increases in BALF total protein, consistent with our in vivo VILI model (Fig. 3A). B: similarly, MIP-2 levels in BALF tended to increase following 2 h of high-VT ventilation, although this was not statistically significant. C: in contrast to our in vivo data, no increase was observed for BALF sTNFR p55. D: equally, no increases were observed for sTNFR p75. These data support our hypothesis that in pure VILI, plasma leakage is the primary source of enhanced BALF sTNFR levels. Dotted lines indicate respective values from 2 h in vivo high-VT experiments from Fig. 3 for comparison. *P < 0.05, high VT vs. low VT; n = 4/each group.
DISCUSSION
This study aimed to clarify the sources of increased BALF sTNFR levels observed during clinical ALI/ARDS. Elevated BALF and plasma levels of sTNFRs have been proposed as markers in ARDS (24, 25), but interpretation of these increases is difficult without first understanding the underlying mechanisms. Both inflammation-induced intra-alveolar production and passive movement of plasma sTNFRs across a permeable alveolar-capillary barrier have been suggested as potential mechanisms (24) but have never been properly investigated clinically as the two pathophysiologies rarely exist in isolation.
ARDS is a complex syndrome characterized by common features, including neutrophilic alveolitis, pulmonary edema, tissue injury, abnormal gas exchange, and altered lung mechanics, and currently no single animal model simulates all pathophysiological features (18). Analysis integrating findings from multiple models, reflecting different aspects of the disease process, is therefore required. We investigated intra-alveolar sTNFR levels in two in vivo mouse models that have distinct features reflecting different pathophysiologies of ALI. Consistent with the literature (18), intratracheal LPS induced substantial acute intra-alveolar inflammation, but negligible permeability changes with the doses and lengths of exposure used here. Conversely, injurious mechanical ventilation produced acute alveolar epithelial permeability changes with a comparatively smaller inflammatory component after 2 h, consistent with previous findings (36, 38).
LPS-induced pulmonary inflammation showed a specific increase in BALF sTNFR p75. The substantial attenuation of LPS-induced increases in p75 by intratracheal clodronate-liposome pretreatment suggests the involvement of alveolar macrophages. Clodronate-liposomes induce apoptosis in phagocytic cells (23), thus targeting alveolar macrophages when delivered intratracheally. Clodronate could also potentially influence our findings through its action as a broad-spectrum inhibitor of matrix metalloproteinases (MMPs) (28). This is unlikely though as it has been reported (6) that clodronate does not inhibit catalytic activity of the putative TNFR sheddase, TNF-α converting enzyme (TACE) (32) in vitro, and the endogenous MMP inhibitors TIMP-1 and -2 are ineffective at preventing TNF-induced TNFR shedding (8). It is also possible that clodronate-liposomes could produce nonspecific intra-alveolar toxicity/inflammation. However, this would be expected to enhance BALF p75 levels whereas they were clearly decreased in our treatment group. This suggests that any deleterious/toxic effects of clodronate-liposomes were relatively small compared with their influence on macrophage depletion. Finally, alveolar macrophages could mediate release of sTNFR p75 from other cell types such as the alveolar epithelium, but it is controversial whether these cells express TNFR p75 in substantial quantities (11). Thus alveolar macrophages are likely the major source for intra-alveolar sTNFR p75 production in this model.
The most likely mechanism for macrophage release of sTNFR p75 is by cell-surface receptor shedding (33). The apparent maximal release of p75, as suggested by only minor influences of a higher LPS dose and longer exposure, combined with the rapid initial upregulation, is consistent with the usual kinetics of such a process. While secretion of stored (16) or de novo synthesized full-length receptors has been shown for constitutive (but not inducible) sTNFR p55 production (16, 17), to the best of our knowledge there is currently no evidence that sTNFR p75 is released in this manner.
Our in vivo VILI model displayed significant increases in both sTNFR p55 and p75 in BALF that correlated very strongly with alveolar epithelial permeability. This suggests that the majority of sTNFRs may have leaked into the alveolar space from the plasma, where circulating levels are readily detectible even under resting conditions. From our data, the ratio of p55:p75 in BALF following high-VT ventilation was very similar to that of plasma (∼1:3), which further supports passive leakage of receptors. This was confirmed using an ex vivo IPL system, where sTNFRs were absent from the perfusate. High-VT ventilation in this ex vivo VILI model induced a similar increase in permeability to the in vivo VILI model, but produced no significant increases in BALF sTNFR levels. We therefore conclude that leakage from plasma is likely the primary mechanism underlying the increases in both BALF sTNFRs during VILI, and potentially whenever alveolar epithelial permeability is significantly increased.
Unexpectedly, injurious mechanical ventilation induced no significant intra-alveolar sTNFR production, despite the fact that alveolar macrophage-mediated inflammation and toll-like receptor-4 signaling (of which LPS is a potent ligand) have been implicated in inflammation/injury induced by mechanical ventilation (14, 31). It is possible that a small degree of sTNFR production is present, and masked by pulmonary edema and influx of plasma receptors, but our IPL data do not support this. Thus mechanical stretch appears to be a weak stimulator of alveolar macrophages with respect to sTNFR p75 shedding/production compared with endotoxin. While the VILI model utilized here involves use of ventilator settings that may be viewed as having limited clinical relevance, it is important to note that mouse respiratory mechanics are vastly different from humans, i.e., much more compliant at higher lung volumes (29), which makes direct extrapolation of VT to humans misleading. The ability of VILI models to reproduce many of the important features of clinical ALI makes them among the most clinically applicable models in the field (18).
Neither model in the present study induced detectable intra-alveolar sTNFR p55 production (even with longer exposure to a higher LPS dose). This was also the case with intratracheal administration of lipoteichoic acid (data not shown). Some in vivo studies have reported increased BALF sTNFR p55 following various insults (21, 26), but none have excluded plasma leakage of receptors as an underlying mechanism. In vitro, preferential release of sTNFR p75 vs. p55 in response to LPS has previously been suggested in alveolar macrophages (7), but the underlying mechanisms behind such differential cleavage remain unclear. The physiological sheddase responsible for in vivo cleavage of each of the TNFRs is not well defined. TACE is capable of shedding both TNFR p55 and p75 (4), while neutrophil elastase can preferentially cleave TNFR p75 (15), although it is unlikely that neutrophils played any significant role within this study as p75 increases occurred prior to their migration into the alveolar space.
Intriguingly, in contrast to the intratracheal LPS challenge used in this study, intravenous LPS challenge has been shown to induce rapid (<2 h, i.e., within the time frame of our study) shedding of TNFR p55 from pulmonary endothelial cells (3) and increases in soluble p55 in plasma (5). Such discrepancies in the TNFR shedding pattern between the alveolar and intravascular space may suggest that activity of the physiological sheddase(s) for p55 is significantly lower within cells in the alveoli compared with pulmonary endothelial cells, or that some other aspect of the alveolar milieu is inhibitory to TNFR p55 shedding/release. If true, this would have substantial implications for the biology of TNF signaling within the alveolar space. Previous studies have reported that stimulated pulmonary epithelial cells can release sTNFR p55 (17, 20, 25), suggesting that increased intra-alveolar sTNFR p55 may reflect inflammation in ARDS, but such in vitro studies may be subject to significant artifacts relating to the use of cell lines and/or the absence of important regulatory mechanisms present in vivo (41). Although our data do not exclude the possibility that a substantially greater stimulus (e.g., higher magnitude stretch or longer periods of inflammatory challenge) may induce some degree of local production/shedding of sTNFRs, we conclude that increased BALF levels of sTNFR p55 during ALI primarily reflect increased alveolar epithelial permeability.
Although physiological significance of this differential regulation of alveolar sTNFR levels remains to be further investigated, our results may have some clinical implications. Analysis of relative levels of sTNFR p55 and p75 in BALF, compared with plasma levels, may give useful information about the etiology and impact of inflammation vs. permeability/pulmonary edema. In clinical ALI, significant intra-alveolar inflammation is accompanied by increased alveolar permeability and pulmonary edema formation. From our findings it is clear that local sTNFR p75 release is more sensitive than p55 to the presence of intra-alveolar inflammation. Conversely, BALF sTNFR p55 appears to mainly reflect increased alveolar permeability, which can be caused by either direct or indirect pulmonary challenges. We could therefore speculate that during ALI of primarily direct pulmonary origin, the BALF sTNFR p75:p55 ratio would be substantially increased, whereas during indirect ALI (where any inflammatory locus is extrapulmonary) the ratio of receptors in BALF would be similar to that found in plasma. These speculations warrant further clinical investigation.
In conclusion, our data strongly suggest that pulmonary inflammation during ALI induces alveolar macrophage-mediated production of sTNFR p75 alone, whereas increased alveolar epithelial permeability upregulates both sTNFR p75 and p55 passively via plasma leakage. While the precise roles of sTNFRs within the alveoli remain to be investigated, these data help elucidate the differential regulation of intra-alveolar sTNFRs and offer new insights into the pathophysiological events within the alveoli during ALI.
GRANTS
This work was supported by the Westminster Medical School Research Trust and Wellcome Trust (Grant No. #081208).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med 175: 323–329, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong L, Thickett DR, Christie SJ, Kendall H, Millar AB. Increased expression of functionally active membrane-associated tumor necrosis factor in acute respiratory distress syndrome. Am J Respir Cell Mol Biol 22: 68–74, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bertok S, Wilson MR, Dorr AD, Dokpesi JO, O'Dea KP, Marczin N, Takata M. Characterization of TNF receptor subtype expression and signaling on pulmonary endothelial cells in mice. Am J Physiol Lung Cell Mol Physiol 300: L781–L789, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black RA. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol 34: 1–5, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Carpenter A, Evans TJ, Buurman WA, Bemelmans MH, Moyes D, Cohen J. Differences in the shedding of soluble TNF receptors between endotoxin-sensitive and endotoxin-resistant mice in response to lipopolysaccharide or live bacterial challenge. J Immunol 155: 2005–2012, 1995 [PubMed] [Google Scholar]
- 6. Ceponis A, Waris E, Monkkonen J, Laasonen L, Hyttinen M, Solovieva SA, Hanemaaijer R, Bitsch A, Konttinen YT. Effects of low-dose, noncytotoxic, intraarticular liposomal clodronate on development of erosions and proteoglycan loss in established antigen-induced arthritis in rabbits. Arthritis Rheum 44: 1908–1916, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Dai H, Guzman J, Chen B, Costabel U. Production of soluble tumor necrosis factor receptors and tumor necrosis factor-alpha by alveolar macrophages in sarcoidosis and extrinsic allergic alveolitis. Chest 127: 251–256, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Dri P, Gasparini C, Menegazzi R, Cramer R, Alberi L, Presani G, Garbisa S, Patriarca P. TNF-Induced shedding of TNF receptors in human polymorphonuclear leukocytes: role of the 55-kDa TNF receptor and involvement of a membrane-bound and non-matrix metalloproteinase. J Immunol 165: 2165–2172, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Ebach DR, Riehl TE, Stenson WF. Opposing effects of tumor necrosis factor receptor 1 and 2 in sepsis due to cecal ligation and puncture. Shock 23: 311–318, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Eissner G, Kirchner S, Lindner H, Kolch W, Janosch P, Grell M, Scheurich P, Andreesen R, Holler E. Reverse signaling through transmembrane TNF confers resistance to lipopolysaccharide in human monocytes and macrophages. J Immunol 164: 6193–6198, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Ermert M, Pantazis C, Duncker HR, Grimminger F, Seeger W, Ermert L. In situ localization of TNFalpha/beta, TACE and TNF receptors TNF-R1 and TNF-R2 in control and LPS-treated lung tissue. Cytokine 22: 89–100, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Ewart S, Levitt R, Mitzner W. Respiratory system mechanics in mice measured by end-inflation occlusion. J Appl Physiol 79: 560–566, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci 22: RC216, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291: L1191–L1198, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Gasparini C, Menegazzi R, Patriarca P, Dri P. Evidence that elastase is the TNF-R75 shedding enzyme in resting human polymorphonuclear leukocytes. FEBS Lett 553: 360–364, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA 101: 1297–1302, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Islam A, Adamik B, Hawari FI, Ma G, Rouhani FN, Zhang J, Levine SJ. Extracellular TNFR1 release requires the calcium-dependent formation of a nucleobindin 2-ARTS-1 complex. J Biol Chem 281: 6860–6873, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 108: 1303–1314, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Nakamura H, Hino T, Kato S, Shibata Y, Takahashi H, Tomoike H. Tumour necrosis factor receptor gene expression and shedding in human whole lung tissue and pulmonary epithelium. Eur Respir J 9: 1643–1647, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Natarajan S, Kim J, Remick DG. Acute pulmonary lipopolysaccharide tolerance decreases TNF-alpha without reducing neutrophil recruitment. J Immunol 181: 8402–8408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Dea KP, Wilson MR, Dokpesi JO, Wakabayashi K, Tatton L, van Rooijen N, Takata M. Mobilization and margination of bone marrow Gr-1high monocytes during subclinical endotoxemia predisposes the lungs toward acute injury. J Immunol 182: 1155–1166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Dea KP, Young AJ, Yamamoto H, Robotham JL, Brennan FM, Takata M. Lung-marginated monocytes modulate pulmonary microvascular injury during early endotoxemia. Am J Respir Crit Care Med 172: 1119–1127, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, 2nd, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 164: 1896–1903, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 288: L426–L431, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Pryhuber GS, Huyck HL, Bhagwat S, O'Reilly MA, Finkelstein JN, Gigliotti F, Wright TW. Parenchymal cell TNF receptors contribute to inflammatory cell recruitment and respiratory failure in Pneumocystis carinii-induced pneumonia. J Immunol 181: 1409–1419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282: 54–61, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Sasanelli R, Boccarelli A, Giordano D, Laforgia M, Arnesano F, Natile G, Cardellicchio C, Capozzi MA, Coluccia M. Platinum complexes can inhibit matrix metalloproteinase activity: platinum-diethyl[(methylsulfinyl)methyl]phosphonate complexes as inhibitors of matrix metalloproteinases 2, 3, 9, and 12. J Med Chem 50: 3434–3441, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Soutiere SE, Mitzner W. On defining total lung capacity in the mouse. J Appl Physiol 96: 1658–1664, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Vaneker M, Joosten LA, Heunks LM, Snijdelaar DG, Halbertsma FJ, van Egmond J, Netea MG, van der Hoeven JG, Scheffer GJ. Low-tidal-volume mechanical ventilation induces a toll-like receptor 4-dependent inflammatory response in healthy mice. Anesthesiology 109: 465–472, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 10: 45–65, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Weimann J, Bloch KD, Takata M, Steudel W, Zapol WM. Congenital NOS2 deficiency protects mice from LPS-induced hyporesponsiveness to inhaled nitric oxide. Anesthesiology 91: 1744–1753, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369: 1553–1564, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Wilson MR, Choudhury S, Goddard ME, O'Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol 95: 1385–1393, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Wilson MR, Choudhury S, Takata M. Pulmonary inflammation induced by high-stretch ventilation is mediated by tumor necrosis factor signaling in mice. Am J Physiol Lung Cell Mol Physiol 288: L599–L607, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Wilson MR, Goddard ME, O'Dea KP, Choudhury S, Takata M. Differential roles of p55 and p75 tumor necrosis factor receptors on stretch-induced pulmonary edema in mice. Am J Physiol Lung Cell Mol Physiol 293: L60–L68, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Wilson MR, O'Dea KP, Dorr AD, Yamamoto H, Goddard ME, Takata M. Efficacy and safety of inhaled carbon monoxide during pulmonary inflammation in mice. PLoS ONE 5: e11565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson MR, O'Dea KP, Zhang D, Shearman AD, van Rooijen N, Takata M. Role of lung-marginated monocytes in an in vivo mouse model of ventilator-induced lung injury. Am J Respir Crit Care Med 179: 914–922, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wissinger E, Goulding J, Hussell T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol 21: 147–155, 2009 [DOI] [PubMed] [Google Scholar]