Abstract
Octreotide is a somatostatin analog that constricts the splanchnic circulation, thereby improving orthostatic tolerance. We tested the hypotheses that octreotide improves orthostatic tolerance by 1)increasing cardiac filling (right atrial) pressure via reductions in vascular capacity; 2) by causing an upward (i.e., cranial) shift of the hydrostatic indifferent point; and 3) by increasing arterial pressure via a reduction in total vascular conductance. Studies were carried out in acepromazine-sedated, hexamethonium-treated atrioventricular-blocked conscious dogs lightly restrained in lateral recumbency. Beat-by-beat cardiac output was held constant via computer-controlled ventricular pacing at rest and during 30 s of 30° head-up tilt. Octreotide (1.5 μg/kg iv) raised right atrial pressure by 0.5 mmHg and raised mean arterial pressure by 11 mmHg by reducing total vascular conductance (all P < 0.05). Right atrial pressure fell by a similar amount in response to tilting before and after octreotide, thus there was no difference in location of the hydrostatic indifferent point. These data indicate that octreotide improves orthostatic tolerance by decreasing total vascular conductance and by increasing cardiac filling pressure via a reduction in unstressed vascular volume and not by eliciting a cranial shift of the location of the hydrostatic indifferent point.
Keywords: vascular capacity, orthostatic tolerance, hydrostatic indifferent point, octreotide
in humans, the majority of the blood volume is located a significant distance caudal of the heart, and quiescent standing and/or a sudden shift to upright posture can challenge the ability of the cardiovascular system to maintain an adequate cerebral perfusion pressure (21). Indeed, upright posture is characterized by immediate decreases in cerebral perfusion pressure and cardiac filling pressure because the brain and the heart are both located above the hydrostatic indifferent point. During quiet standing, cardiac filling pressure is further reduced in a time-dependent manner by the displacement of blood volume from the chest as the dependent vessels fill with blood (21). If not counteracted by the muscle pump, the progressive decrease in cardiac filling pressure leads to a fall in cardiac output and cerebral perfusion pressure resulting in syncope.
The splanchnic circulation is the most compliant vascular bed in humans and it receives a large fraction (∼25%) of the total cardiac output at rest (21). Constriction of the splanchnic circulation either by the baroreflexes or pressor drugs, and the subsequent decrease in splanchnic vascular capacity, has the potential to buffer the fall in cardiac filling pressure that attends orthostasis. The somatostatin analog, octreotide, has been purported to be a viable pharmacological agent to slow orthostatic hypotension and thus improve orthostatic tolerance (5, 10–13) and has recently been shown to cause an upward shift of the location of the volume indifferent point as determined via segmental bioelectrical impedance (14). The therapeutic benefit of octreotide is thought to be due to selective constriction of the splanchnic circulation (4, 18, 23, 29). In patients with autonomic neuropathy, octreotide has been shown to be effective in attenuating postprandial hypotension via increases in cardiac output and splanchnic resistance (11, 12). Furthermore, octreotide has been show to blunt the excessive tachycardia observed during upright posture in patients with postural orthostatic tachycardia syndrome and to decrease total effective vascular compliance in patients with posthepatic cirrhosis (10, 17). The extent to which octreotide is effective in improving orthostatic tolerance has been largely limited to subjects with an underlying pathology. However, recent data from Curren et al. (5) found that octreotide significantly improves orthostatic tolerance in young, healthy humans as demonstrated by an increase in median time completed in a tilt-table test. The data from Curren and colleagues (5) provide strong evidence to suggest the therapeutic benefits of octreotide may extend beyond portal hypertension to orthostatic intolerance in young otherwise healthy humans. In addition to the observed relationship between improvements in tilt-table time and reductions in splanchnic vascular conductance (5), other potential mechanisms may underlie octreotide-induced improvements in orthostatic tolerance.
The purpose of this study was to evaluate the contribution of three potential hemodynamic mechanisms by which octreotide might improve orthostatic tolerance. First, we sought to test the hypothesis that octreotide improves orthostatic tolerance by decreasing total vascular conductance, thereby increasing mean arterial pressure at a fixed cardiac output. Second, we sought to test the hypothesis that octreotide improves orthostatic tolerance by increasing cardiac filling pressure via reductions in vascular capacitance (i.e., in both the horizontal and head-up postures). Finally, we sought to test the hypothesis that octreotide improves orthostatic tolerance by raising the location of the hydrostatic indifferent point, the point in the cardiovascular system where pressure remains constant despite changes in posture. The closer the heart lies to the hydrostatic indifferent point [located near the level of the diaphragm in humans (9)] the smaller the fall in cardiac filling pressure during head-up tilt.
Although the latter two of the three forgoing hypothesis might both work to improve cardiac filling pressure during orthostatic stress, the distinction between the two is important; i.e., they are not redundant statements. When cardiac output and total blood volume are both fixed, a reduction in vascular capacitance will increase cardiac filling pressure. The reduction in vascular capacitance can be achieved by conceptually distinct mechanisms. For example, vascular capacitance can be reduced by 1) a reduction in unstressed vascular volume, i.e., a downward shift in the venous pressure:volume relationship resulting in a change in the volume intercept of the relationship with no change in slope of the relationship (i.e., no change in compliance), and/or 2) a reduction in venous compliance (i.e., a reduction of the slope of the venous pressure:volume relationship). We sought to test the manner by which octreotide might alter vascular capacity; namely, does octreotide reduce unstressed venous volume and/or does octreotide reduce venous (principally splanchnic) compliance? This question was addressed by examining the effects of octreotide on the location of the hydrostatic indifferent point. The rationale was that the location of the hydrostatic indifferent point is determined by the distribution of venous compliance (9); thus, if octreotide acts principally to reduce splanchnic venous compliance, then octreotide should raise right atrial pressure and shift the hydrostatic indifferent point upward (cranially). Alternatively, if octreotide acts principally to reduce venous unstressed volume without changing compliance, then octreotide should raise right atrial pressure without altering the location of the hydrostatic indifferent point. We studied the effect of octreotide in chronically instrumented dogs in the horizontal position and during 30° head-up tilt while beat-by-beat cardiac output was held constant via computer-controlled ventricular pacing such that the effect of changes in cardiac output on right atrial pressure would not confound the results. We blocked autonomic reflexes with hexamethonium in order that the effects of octreotide could be studied in isolation. Our aim was to isolate potentially confounding factors (baroreflexes and changes in cardiac output) to more definitely establish the effects of octreotide on circulatory function in a mechanistic and quantifiable manner.
METHODS
All procedures were approved by the Institutional Animal Care and Use Committee at The University of Iowa and conformed to the guidelines for animal research as set forth by the National Institutes of Health. Experiments were performed on five 23- to 29-kg mongrel hound-type dogs (Oak Hill Genetics, Ewing, IL).
Surgical preparation.
A total of five dogs were prepared in a series of two aseptic surgical procedures as described previously (26, 27). For both procedures, anesthesia was induced with thiopental sodium and maintained with isoflurane. In one procedure, a right thoracotomy was performed and a blood flow transducer (Transonic, Ithaca, NY) was placed on the ascending aorta. Pacing electrodes were sutured to the apex of the left ventricle and recording electrodes were sutured to the right atrial appendage. Atrioventricular block was achieved via injection of formalin into the area of the atrioventricular node. Following induction of atrioventricular block and between experiments, a pacemaker carried by the dog paced the heart at ∼70 beats/min. A skin patch delivering 50 μg/h of fentanyl was placed on the dog the evening before the surgical procedure or a skin patch delivering 50 μg/h of fentanyl was placed on the dog after induction of anesthesia and the animal was given 50 μg fentanyl subcutaneously before the onset of the surgical procedure to control postoperative pain. The animal was given 50 μg fentanyl subcutaneously again at the end of the procedure. The fentanyl patches were left in place for 3 days. Additional 25 μg/h patches were applied as indicated for the control of postoperative pain.
In another procedure, a mid-abdominal incision was made and a blood flow transducer was placed around the terminal aorta (for use in separate experiments). A flow probe was implanted on the caudal mesenteric artery in one dog to verify that octreotide induced splanchnic constriction in this model. For the measurement of arterial pressure, a catheter was placed in a side branch of the femoral artery and advanced into the abdominal aorta. A second catheter was placed in a side branch of the femoral vein and advanced into the abdominal vena cava for delivery of drugs. A third catheter was placed in the right jugular vein and advanced to the caval-right atrial junction for the measurement of right atrial pressure. All cables and catheters were tunneled subcutaneously to exit sites on the back. A skin patch delivering 25 μg/h of fentanyl was placed on the dog the evening before the surgical procedure or a skin patch delivering 25 μg/h of fentanyl was placed on the dog after induction of anesthesia and the animal was given 25 μg/h fentanyl subcutaneously before the onset of the surgical procedure to control postoperative pain. The animal was given 25 μg/h fentanyl subcutaneously again at the end of the procedure. The fentanyl patches were left in place for 3 days. Additional 25 μg/h patches were applied as indicated for the control of postoperative pain. Prior to each surgical procedure the animals were treated with cephazolin (1 g iv) and with cephalexin (500 mg po, twice daily) for 1 wk postoperatively. The animals were allowed at least 1 wk recovery between surgical procedures. Data collection commenced after the animals recovered from the second surgical procedure (∼1 wk) and were afebrile, active, and of good appetite.
Experimental procedures.
Animals were sedated with acepromazine (20–30 mg iv) so that the results would not be confounded by the effects of excitement and/or muscular activity on the cardiovascular system during tilting. For all protocols, dogs were lightly restrained in lateral recumbency on a padded tilt table and autonomic transmission was inhibited via hexamethonium (5 mg/kg iv) to investigate the effects of octreotide in the absence of changes in autonomic activity.
Efficacy of autonomic blockade.
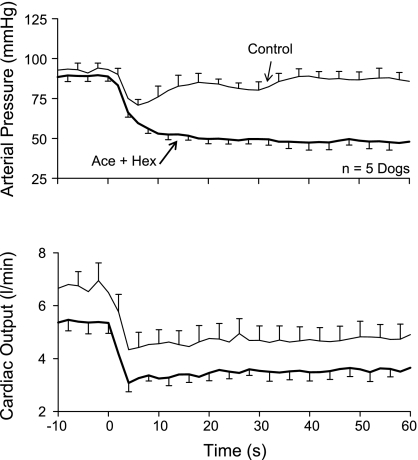
Baroreflex function was tested by imposing a 50- to 75-beat/min step reduction in ventricular pacing rate. Figure 1 depicts the response of arterial pressure (Fig. 1, top) and cardiac output (Fig. 1, bottom) under two conditions: 1) control (no drugs) with the dogs standing quietly and 2) following acepromazine and hexamethonium with the dogs in lateral recumbency. In the control condition, arterial pressure initially fell and then was restored toward the baseline level by compensatory vasoconstriction (Fig. 1, top, thin line) as total vascular conductance fell from 73 ± 7 to 55 ± 3 ml·min−1·mmHg−1 (P < 0.05). This compensatory vasoconstriction was absent following acepromazine and hexamethonium; arterial pressure simply fell monotonically to its new lower value (Fig. 1, top, thick line). In fact, following acepromazine and hexamethonium, total vascular conductance rose from 58 ± 4 to 75 ± 3 ml·min−1·mmHg−1 (P < 0.05) when pacing rate was reduced. Thus acepromazine and hexamethonium inhibited baroreflex function in that it reversed the vasoconstriction that normally accompanies a reduction in cardiac output to vasodilation.
Fig. 1.
Arterial pressure (top) and cardiac output (bottom) responses to a step reduction in pacing rate in conscious dogs with atrioventricular block. Control (thin solid lines), responses in standing dogs. Ace + Hex (thick solid lines), responses in recumbent dogs following acepromazine and hexamethonium. Ace and Ace + Hex reversed the compensatory vasoconstriction that accompanies a reduction in cardiac output (Control) to vasodilation. Means ± SE for n = 7 dogs.
As changes in cardiac output are known to alter right atrial pressure (27), cardiac output in each animal was held constant via computer-controlled ventricular pacing (19, 25–27, 31) for the duration of the experiment.
Hemodynamic response to head-up tilt.
Following baseline measurements (∼3–5 min), animals were subjected to 30 s of 30° (+0.50 Gz) head-up tilt. Movement time was ∼1 s.
Effect of octreotide on hemodynamics in the horizontal posture.
Following the control (predrug) tilt, and once all hemodynamic variables returned to baseline, octreotide (1.5 μg/kg iv) was administered to each animal and data were collected for at least 5 min before repeating the tilting protocol (see Effect of octreotide on hemodynamic response to head-up tilt). We selected this approach based on the following factors: 1) similar absolute and relative doses of octreotide have been shown to elicit sustained cardiovascular responses (1, 5, 13, 18); 2) octreotide has been shown to elicit cardiovascular responses for as long as 3–6 h following a single bolus dose of similar concentration used in the present investigation (13); 3) multiple bolus doses have been shown to result in tachyphylaxis (8); 4) a bolus dose followed by a continuous dose of octreotide has been shown to have no further benefit compared with bolus dose alone (8); and 5) vasoconstriction has been observed in response to bolus, but not continuous, infusions of octreotide (18).
Effect of octreotide on hemodynamic response to head-up tilt.
To determine the effect of octreotide on the hemodynamic response to orthostatic stress, animals were subjected to the same gravitational stress as described above following octreotide. In two animals, the tilt protocol following octreotide needed to be repeated because of animal movement during the first attempted tilt.
Data collection.
Blood flow transducers were connected to flowmeters (T106, Transonic, Ithaca, NY). Arterial and jugular catheters were connected to pressure transducers (PE10 EZ, Ohmeda, Madison, WI) aligned with the spine at the level of the right atrium. Water-filled tubing connected to a pressure transducer was mounted on the tilt table to measure the angle of tilt. All signals were digitized at 1 kHz and beat-by-beat mean values were calculated and stored on a computer for subsequent analysis.
Data analysis.
Data analysis was performed on 2-s averages of the digitized beat-by-beat data. Stroke volume was calculated as cardiac output divided by heart rate. Total vascular conductance was calculated by dividing cardiac output by the heart-level arterial − right atrial pressure difference. The location of the hydrostatic indifferent point from the right atrium was calculated using equation 1 (3): Distance (cm) = ΔRight Atrial Pressure (cmH2O) ÷ sine tilt (θ), where ΔRight Atrial Pressure denotes the change in right atrial pressure in going from horizontal to head-up tilt. In this scheme, a fall in right atrial pressure in response to head-up tilt produces a negative distance, which indicates that the hydrostatic indifferent point lies caudal of the heart. When cardiac output and total blood volume are constant, a decrease in vascular capacitance (e.g., venoconstriction) results in an increase in cardiac filling (right atrial) pressure, and the effective volume shift induced by octreotide infusion was estimated from the compliance data in dogs (∼3 ml·mmHg−1·kg−1) from Bennett et al. (2).
Baseline values were established by averaging the signals over a 20-s period before the infusion of octreotide. Infusion of octreotide resulted in rapid and large changes within ∼1 min. The peak or nadir values that occurred over this period were identified. Steady-state hemodynamic values in response to octreotide were established by averaging the signals over a 20-s period 3 min following octreotide infusion. Because a primary goal was to determine the location of the hydrostatic indifferent point, the hemodynamic responses to head-up tilt were determined by averaging the signals over a 10-s period immediately prior to head-up tilt and during a 10-s period starting 20 s after head-up tilt when right atrial pressure had fallen to a stable level.
Statistical analysis.
We compared the peak (or nadir) values in response to octreotide to the peak (or nadir) values from the 20-s baseline period immediately before octreotide infusion using Wilcoxon signed-ranks tests. We compared the steady-state values in response to octreotide to the baseline values using Wilcoxon signed-ranks tests. We compared the location of the hydrostatic indifferent point before and after octreotide using a Wilcoxon signed-ranks test. Tests of significance on the responses to tilting, and of the affects of octreotide on the responses to tilting, were done by multiple linear regression (analogous to repeated-measures ANOVA; 28). The specific influence of octreotide on the response to head-up tilt was evaluated by testing for a statistically significant interaction of octreotide treatment and gravitational stress (O × HUT in Table 1); using arterial pressure as an example, the test for interaction would reveal whether octreotide exerted an exaggerated effect during gravitational stress compared with control (no drug) gravitational stress (either working to lessen or worsen the fall in arterial pressure during gravitational stress). A P value <0.05 was considered statistically significant, and all data are presented as medians ± SE except where otherwise noted.
Table 1.
Hemodynamic response to head-up tilt before and after octreotide
| MAP, mmHg | RAP | CO, l/min | HR, beats/min | SV, ml | TVC, ml ·min−1 ·mmHg−1 | HIP, cm | |
|---|---|---|---|---|---|---|---|
| No Drug | |||||||
| Control | 68 ± 4 | 1.2 ± 1.1 | 4.5 ± 0.3 | 72 ± 5 | 59 ± 4 | 68 ± 4 | |
| HUT | 70 ± 5 | −7.3 ± 1.1 | 4.4 ± 0.3 | 87 ± 8 | 49 ± 4 | 58 ± 2 | −17.9 ± 1.9 |
| Octreotide | |||||||
| Control | 79 ± 4 | 0.8 ± 1.1 | 4.4 ± 0.3 | 72 ± 4 | 62 ± 3 | 58 ± 6 | |
| HUT | 81 ± 4 | −8.3 ± 1.3 | 4.5 ± 0.3 | 94 ± 8 | 41 ± 5 | 52 ± 4 | −19.2 ± 2.0 |
| Octreotide | * | * | |||||
| HUT | * | * | * | * | |||
| O × HUT |
Values are medians ± SE; n = 5. MAP, mean arterial pressure; RAP, right atrial pressure; CO, cardiac output; HR, heart rate; SV, stroke volume; TVC, total vascular conductance; HIP; hydrostatic indifferent point (negative values indicate caudal of right atrium); HUT, 30° head-up tilt; O, octreotide.
P < 0.05.
RESULTS
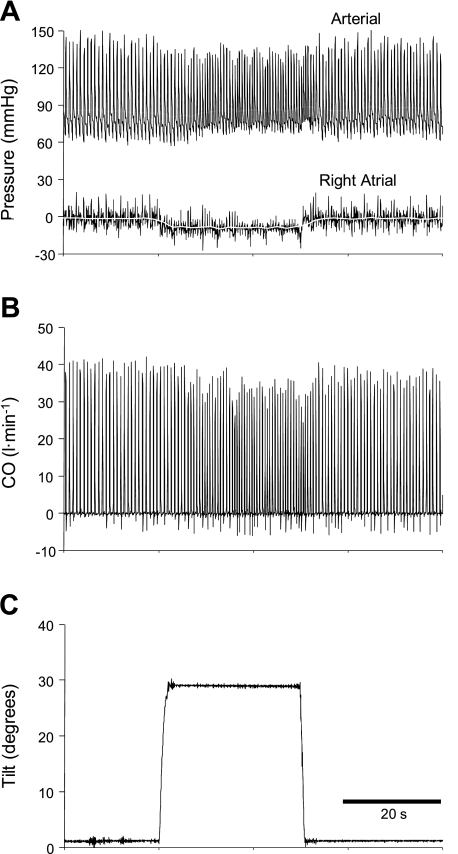
Figure 2 illustrates the protocol and depicts a representative example of the response to octreotide and to tilting before and after octreotide.
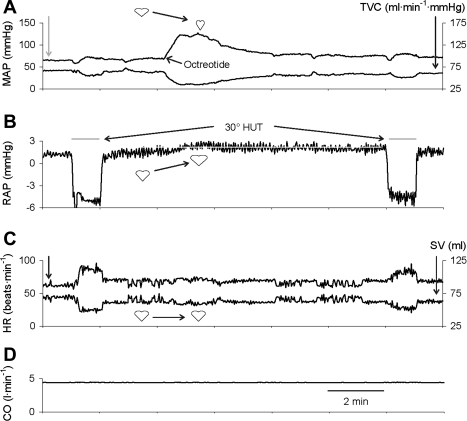
Fig. 2.
Hemodynamic responses to octreotide, and to tilting before and after octreotide, while cardiac output (CO; D) is maintained constant. Octreotide raised mean arterial pressure (MAP; A) and right atrial pressure (RAP; B). Because CO was constant, total vascular conductance (TVC; A) mirrors MAP, and heart rate (HR; C) and stroke volume (SV; C) mirror one another. Head-up tilt (HUT) reduced RAP and SV and increased HR similarly before and after octreotide. Heart-shaped symbols indicate that the tendency for an increase in afterload (MAP; A) to reduce SV and an increase in preload (RAP; B) to augment SV offset one another such that octreotide did not alter SV (C). White line in B highlights the sustained increase in RAP induced by octreotide. Heart symbols denote the expected effect of the change in each variable on stroke volume.
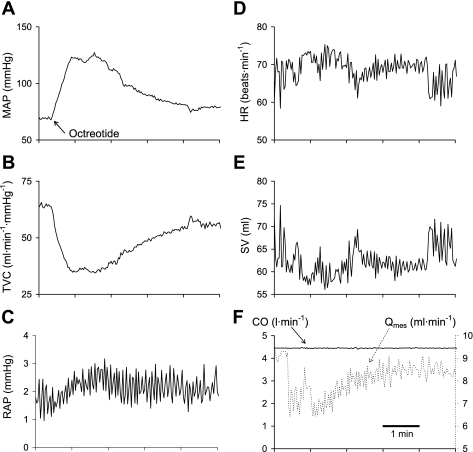
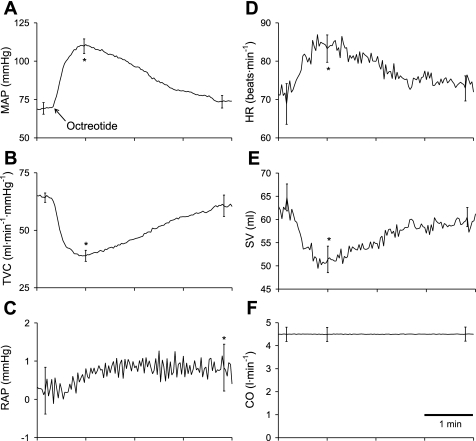
The time course of the hemodynamic response to octreotide infusion in one dog is shown in Fig. 3. Octreotide caused a large initial rise in mean arterial pressure after which pressure fell to a steady level that exceeded baseline pressure (Fig. 3A). Octreotide raised right atrial pressure (Fig. 3C). Because cardiac output was maintained constant (Fig. 3F), total vascular conductance (Fig. 3B) and mean arterial pressure (Fig. 3A) mirrored one another, as did heart rate (Fig. 3D) and stroke volume (Fig. 3E). Octreotide caused a large initial decrease in mesenteric blood flow after which flow rose to a steady level remaining below baseline flow (Fig. 3F). The group mean responses to octreotide are presented in Fig. 4 and are in all ways similar to the responses presented in Fig. 3. Octreotide elicited significant initial increases in mean arterial pressure of 42 mmHg (Fig. 4A) and heart rate (Fig. 4D) and decreases in total vascular conductance (Fig. 4B) and stroke volume (Fig. 4E). Octreotide raised right atrial pressure (Fig. 4C). The 0.5-mmHg increase in right atrial pressure indicates that octreotide decreased vascular capacity by ∼1.5 ml/kg.
Fig. 3.
Time course of the hemodynamic responses to octreotide while CO was maintained constant. Arrow denotes the onset to the rise in MAP induced by octreotide. Octreotide also raised RAP and lowered TVC, SV, and mesenteric flow (Qmes). Qmes is from one dog, and all of the other data is from another representative dog.
Fig. 4.
Group mean time course of the hemodynamic responses to octreotide while CO was maintained constant. Arrow denotes the onset to the rise in MAP induced by octreotide. Octreotide transiently lowered TVC and SV. Octreotide necessitated an increase in heart rate to maintain CO constant. Octreotide also raised RAP *P < 0.05 for peak (or nadir) response to octreotide; †P < 0.05 for steady-state response to octreotide. Data are means ± SE for selected time points of interest.
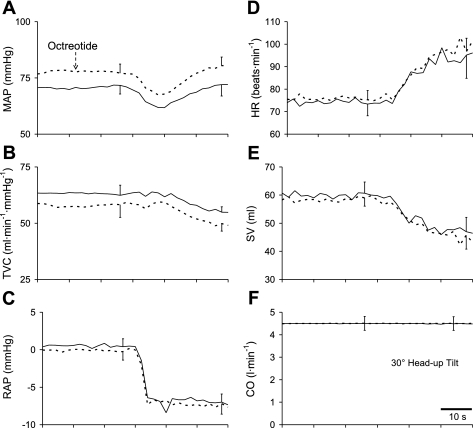
The time course of the hemodynamic responses to tilting from one dog following octreotide are shown in Fig. 5, and the group mean responses are presented in Fig. 6. The group mean values of the hemodynamic variables in response to tilting before and after octreotide are provided in Table 1. Octreotide raised mean arterial pressure by lowering total vascular conductance. Head-up tilt lowered right atrial pressure, stroke volume, and total vascular conductance and raised heart rate. Octreotide did not alter the location of the hydrostatic indifferent point nor was there any specific influence of octreotide on the responses to head-up tilt [no interaction (O × HUT in Table 1)].
Fig. 5.
Time course of hemodynamic responses to tilting from one representative dog following octreotide while beat-by-beat CO was maintained constant. B: black line depicts the continuous measurement of ascending aortic flow and the white line depicts the time-averaged CO.
Fig. 6.
Group mean time course of hemodynamic responses to tilting before (solid lines) and after octreotide (dashed lines) while beat-by-beat CO was maintained constant. Octreotide raised MAP (A) by lowering TVC (B). RAP (C) fell similarly before and after octreotide.
DISCUSSION
The major new finding of this study is that when cardiac output is held constant octreotide raises mean arterial pressure by reducing total vascular conductance. Octreotide increases cardiac filling (right atrial) pressure under steady-state conditions with animals in the horizontal position. The 0.5-mmHg rise in right atrial pressure corresponds to a ∼1.5 ml/kg decrease in total vascular capacity. Furthermore, octreotide did not alter the location of the hydrostatic indifferent point. Taken together, the data from the present investigation suggest octreotide improves orthostatic tolerance by producing a reduction in total vascular conductance and raising cardiac filling pressure more so than by altering the location of the hydrostatic indifferent point in sedated, autonomically blocked dogs with atrioventricular block when cardiac output is maintained constant beat-by-beat.
Octreotide is a somatostatin analog that has been shown to induce arteriolar and venular constriction of the splanchnic circulation in both humans and dogs (4, 23, 29). As the splanchnic circulation is the most compliant vascular bed in humans and receives a large fraction of the total cardiac output at rest, octreotide has the potential to mobilize a large volume of blood and help maintain an adequate cardiac filling pressure during upright posture (21). Indeed, octreotide has been shown to induce sustained increases in right atrial pressure and has been used as an effective treatment in patients with orthostatic hypotension and postprandial orthostatic hypotension (5, 10–13, 17, 18). Recently, Curren et al. (5) demonstrated that 0 of 14 subjects completed a 45-min, 70° tilt-table test under control conditions, whereas 7 of 14 completed the same test following administration of octreotide (100 mg iv). Furthermore, these investigators found a direct relationship between reductions in splanchnic vascular conductance and improvements in tilt-table time (5). Inasmuch as reductions in systemic arterial and cerebral perfusion pressure lead to syncope, the ability to maintain systemic arterial and cerebral perfusion pressure are crucial for preventing syncopal events. Our current data appear to further support the notion that octreotide can improve orthostatic tolerance and, at least, minimize syncopal events as we observed a higher systemic arterial pressure and reduced total vascular conductance following octreotide. Importantly, the increase in arterial pressure and reduction in total vascular conductance induced by octreotide in the present study were unopposed by the arterial baroreflex because the animals were treated with hexamethonium to inhibit ganglionic autonomic neurotransmission (24, 26, 30).
Our dose of octreotide raised right atrial pressure by 0.5 mmHg. By comparison, the muscle pump can raise right atrial pressure by 5 mmHg (27), the arterial baroreflex can raise right atrial pressure by 0.8 mmHg (2), and the muscle chemoreflex can raise right atrial pressure by 10 mmHg (25). Although the increase in right atrial pressure induced by octreotide is modest it is not insignificant, owing to the steep slope of the ventricular function curve; for example; this rise in right atrial pressure can raise stroke volume by 10% following prolonged bed rest deconditioning (15). We observed little change in stroke volume in response to octreotide, likely owing to the rise in arterial pressure (afterload) as depicted in Fig. 2 when the influence of octreotide was unbuffered by the arterial baroreflexes. Recent evidence indicates that stroke volume is quite sensitive to changes in arterial pressure even within the normal range of pressure (7). Also, the rise in right atrial pressure induced by octreotide was not sustained throughout the postoctreotide tilting procedures, likely owing to animal movement as mentioned above. That is, the movement of these two animals during the first attempted tilt following octreotide led to the need to reposition the animals, and this further altered the baseline right atrial pressure in addition to the effects of octreotide alone.
There is an inherent limitation in most studies on human subjects seeking to quantify the effect of various stressors or pharmacological agents on cardiac filling pressure owing to the dependence of right atrial pressure on cardiac output in the intact circulation (16, 21, 27). That is, if any stressor or agent that alters cardiac filling pressure is also permitted to alter cardiac output, whether directly via the change in filling pressure or by other means such as a change in heart rate, the net result will reflect the action of the stressor or agent on cardiac filling pressure as modified by the change in cardiac output so induced. For example, Echt and coworkers (6) were careful to characterize the rise in central venous pressure in response to saline infusion as reflecting the “effective” vascular compliance, inasmuch as cardiac output was permitted to rise in their study. This is so because central venous pressure would have risen by more, reflecting a lower vascular capacity, had cardiac output not been permitted to rise. Therefore cardiac output must be maintained constant for a change in right atrial pressure to accurately reflect a change in vascular capacity. For these reasons, the rise in right atrial pressure in response to octreotide in our study faithfully reflects a change in total vascular capacity, likely induced via a reduction in splanchnic vascular capacity. How might a change in vascular capacity be produced?
A change in vascular capacity (total or regional) can be produced via a change in vascular compliance (the slope of a pressure:volume relationship) and/or via a change in unstressed vascular volume (the intercept of the pressure:volume relationship; 20). A common approach to discern between the two previously required technically demanding, simultaneous measures of pressure and volume while volume was experimentally manipulated (20). Herein we sought to exploit a fundamental characteristic of the circulation, namely the dependence of the location of the hydrostatic indifferent point on the distribution of vascular compliance, to use tilting as a tool to determine the manner in which, in this particular case, a pharmacologic agent induced a change in vascular capacity. Octreotide did not alter the location of the hydrostatic indifferent point as indicated by the observation that right atrial pressure fell similarly during tilting before and after octreotide. Thus octreotide raised right atrial pressure by reducing the unstressed vascular volume, a commonly found mechanism for a variety of factors that alter vascular capacity (20). Our finding that octreotide alters regional unstressed vascular volume suggests that the cranial shift in the location of the volume indifferent point (14) may be produced via an alteration in unstressed volume as well.
We performed relatively low angle, brief tilts in autonomically blocked quadrupeds having a human-sized trunk length but much shorter legs. Thus extrapolation of our results to the problem of orthostatic intolerance that often only occurs after more prolonged periods in the completely upright posture in autonomically intact humans is problematic. It is possible that sympathetic splanchnic constriction and octreotide-induced constriction work synergistically to provide greater protection then the sum of the two working independently, but this awaits further investigation. If octreotide exerts marked splenic constriction our results might exaggerate the effects of this drug compared with humans having a smaller spleen-to-body weight ratio compared with dogs. Right atrial pressure stabilized quickly at the new lower level following head-up tilt (Fig. 6) as seen previously (24), indicating that 30 s appears sufficient for the major volume shifts to occur. Longer duration tilt in humans will of course lead to slow, progressive filtration in the legs and perhaps a further reduction in right atrial pressure, but it is unlikely that octreotide would specifically provide protection against this phenomenon.
Summary.
The data from this study demonstrate that, in the horizontal position, octreotide induces significant increases in mean arterial pressure (11 mmHg) and right atrial pressure (0.5 mmHg), which corresponds to a decrease in total vascular capacity of ∼1.5 ml/kg. While the octreotide-induced increase in right atrial pressure and decrease in total vascular capacity in the horizontal position are potential mechanisms explaining the beneficial effect of octreotide on orthostatic tolerance, the data from the present study suggests octreotide improves orthostatic tolerance by reducing total vascular conductance and thereby raising mean arterial pressure when cardiac output is maintained constant in autonomically blocked dogs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-46314.
REFERENCES
- 1. Baik SK, Jeong PH, Ji SW, Yoo BS, Kim HS, Lee DK, Kwon SO, Kim YJ, Park JW, Chang SJ, Lee SS. Acute hemodynamic effects of octreotide and terlipressin in patients with cirrhosis: a randomized comparison. Am J Gastroenterol 100: 631–635, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bennett TD, Wyss CR, Scher AM. Changes in vascular capacity in awake dogs in response to carotid sinus occlusion and administration of catecholamines. Circ Res 55: 440–453, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Buckner PS, Quail AW, Cottee DB, White SW. Venous hydrostatic indifference point as a marker of postnatal adaptation to orthostasis in swine. J Appl Physiol 94: 882–888, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Clarke DL, McKune A, Thomson SR. Octreotide lowers gastric mucosal blood flow in normal and portal hypertensive stomachs. Surg Endosc 17: 1570–1572, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Curren MJ, Florian JP, Jarvis SS, Pawelczyk J. Effect of a somatostatin analog on splanchnic hemodynamics and tilt-table tolerance. FASEB J 20: A1253–A1253, 2006 [Google Scholar]
- 6. Echt M, Düweling J, Gauer OH, Lange L. Effective compliance of the total vascular bed and the intrathoracic compartment derived from changes in central venous pressure induced by volume changes in man. Circ Res 40: 61–68, 1974 [DOI] [PubMed] [Google Scholar]
- 7. Elstad M, Nådland IH, Toska K, Walløe L. Stroke volume decreases during mild dynamic and static exercise in supine humans. Acta Physiol 195: 289–300, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Escorsell A, Bandi JC, Andreu V, Moitinho E, Garcia-Pagan JC, Bosch J, Rodes J. Desensitization to the effects of intravenous octreotide in cirrhotic patients with portal hypertension. Gastroenterology 120: 161–169, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Gauer OH, Thron HL. Postural changes in the circulation. In: Handbook of Physiology. Circulation. Bethesda, MD: Am Physiol Soc, 1965, sect. 2, vol. III, chapt. 67, p. 2409–2439 [Google Scholar]
- 10. Hoeldtke RD, Davis KM. The orthostatic tachycardia syndrome: evaluation of autonomic function and treatment with octreotide and ergot alkaloids. J Clin Endocrinol Metab 73: 132–139, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Hoeldtke RD, Davis KM, Joseph J, Gonzales R, Panidis IP, Friedman AC. Hemodynamic effects of octreotide in patients with autonomic neuropathy. Circulation 84: 168–176, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Hoeldtke RD, Israel BC. Treatment of orthostatic hypotension with octreotide. J Clin Endocrinol Metab 68: 1051–1059, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Hoeldtke RD, O'Dorisio TM, Boden G. Treatment of autonomic neuropathy with a somatostatin analogue SMS-201–995. Lancet 2: 602–605, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Jarvis SS, Pawelczyk JA. The location of the human volume indifferent point predicts orthostatic tolerance. Eur J Appl Physiol 109: 331–341, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96: 517–525, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Levy MN. The cardiac and vascular factors that determine systemic blood flow. Circ Res 44: 739–747, 1979 [DOI] [PubMed] [Google Scholar]
- 17. Lin HC, Tsai YT, Yang MCM, Lee FY, Hou MC, Chen LS, Lee SD. Effect of octreotide on total effective vascular compliance in patients with posthepatitic cirrhosis. J Hepatol 24: 81–87, 1996 [DOI] [PubMed] [Google Scholar]
- 18. McCormick PA, Chin J, Greenslade L, Karatapanis S, Dick R, McIntyre N, Burroughs AK. Cardiovascular effects of octreotide in patients with hepatic cirrhosis. Hepatology 21: 1255–1260, 1995 [PubMed] [Google Scholar]
- 19. O'Leary DS, Scher AM, Bassett JE. Effects of steps in cardiac output and arterial pressure in awake dogs with AV block. Am J Physiol Heart Circ Physiol 256: H361–H367, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Rothe CF. Mean circulatory filling pressure: its meaning and measurement. J Appl Physiol 74: 499–509, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Rowell LB. Human Cardiovascular Control. New York: Oxford University Press, 1993 [Google Scholar]
- 22. Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford University Press, 1986 [Google Scholar]
- 23. Scarpignato C, Pelosini I. Somatostatin for upper gastrointestinal hemorrhage and pancreatic surgery—A review of its pharmacology and safety. Digestion 60: 1–16, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Sheriff DD. Hypotensive effect of push-pull gravitational stress occurs after autonomic blockade. J Appl Physiol 95: 167–171, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol 265: H1227–H1234, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Sheriff DD, Zhou XP, Scher AM, Rowell LB. Dependence of cardiac filling pressure on cardiac output during rest and dynamic exercise in dogs. Am J Physiol Heart Circ Physiol 265: H316–H322, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Slinker BK, Glantz SA. Multiple linear-regression is a useful alternative to traditional analyses of variance. Am J Physiol Regul Integr Comp Physiol 255: R353–R367, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Tarnasky PR, Kovacs TOG, Leung FW, Hirabayashi K, Jensen DM. Octreotide decreases canine gastric-mucosal blood-flow—a controlled assessment by endoscopic reflectance spectrophotometry. Gastrointest Endosc 40: 56–61, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Wong BJ, Sheriff DD. Hemodynamic consequences of rapid changes in posture in awake, A-V blocked dogs following autonomic blockade. J Appl Physiol 105: 1837–1844, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wyss CR, Bennett TD, Scher AM. Beat-by-beat control of cardiac output in awake dogs with atrioventricular block. Am J Physiol Heart Circ Physiol 242: H1118–H1121, 1982 [DOI] [PubMed] [Google Scholar]