Abstract
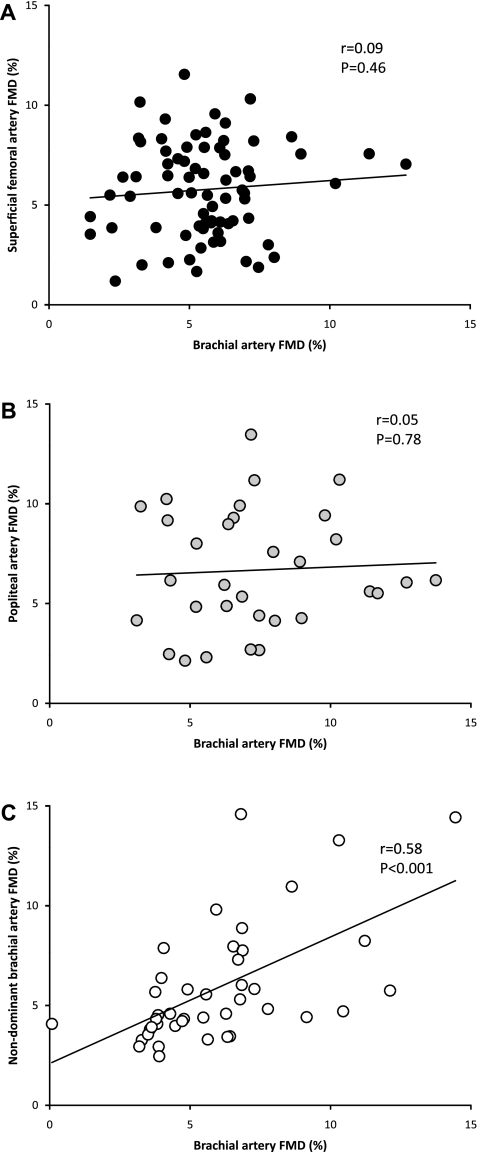
Brachial artery flow-mediated dilation (FMD) is a strong predictor of future cardiovascular disease and is believed to represent a “barometer” of systemic endothelial health. Although a recent study [Padilla et al. Exp Biol Med (Maywood) 235: 1287–1291, 2010] in pigs confirmed a strong correlation between brachial and femoral artery endothelial function, it is unclear to what extent brachial artery FMD represents a systemic index of endothelial function in humans. We conducted a retrospective analysis of data from our laboratory to evaluate relationships between the upper (i.e., brachial artery) vs. lower limb (superficial femoral n = 75; popliteal artery n = 32) endothelium-dependent FMD and endothelium-independent glyceryl trinitrate (GTN)-mediated dilation in young, healthy individuals. We also examined the relationship between FMD assessed in both brachial arteries (n = 42). There was no correlation between brachial and superficial femoral artery FMD (r2 = 0.008; P = 0.46) or between brachial and popliteal artery FMD (r2 = 0.003; P = 0.78). However, a correlation was observed in FMD between both brachial arteries (r2 = 0.34; P < 0.001). Brachial and superficial femoral artery GTN were modestly correlated (r2 = 0.13; P = 0.007), but brachial and popliteal artery GTN responses were not (r2 = 0.08; P = 0.11). Collectively, these data indicate that conduit artery vasodilator function in the upper limbs (of healthy humans) is not predictive of that in the lower limbs, whereas measurement of FMD in one arm appears to be predictive of FMD in the other. These data do not support the hypothesis that brachial artery FMD in healthy humans represents a systemic index of endothelial function.
Keywords: flow-mediated dilation, nitroglycerine, arterial diameter, high-resolution ultrasound, heterogeneity
endothelial dysfunction is implicated in the etiology of atherosclerosis (32). Given that the endothelium is considered a final common pathway of the impact of traditional and novel cardiovascular risk factors, assessment of endothelial (dys)function presents an attractive strategy as a surrogate marker of cardiovascular risk (44). In this regard, flow-mediated dilation (FMD) is a frequently used, noninvasive technique that measures bioactivity of endothelium-derived nitric oxide (NO) (15, 21) in peripheral conduit arteries and predicts cardiovascular events (9, 16, 20, 22, 30).
The widespread belief that brachial artery FMD represents a “barometer” of systemic endothelial health (44) is founded on studies that have reported correlations between brachial artery FMD and coronary artery vasomotor function (2, 37, 38). However, no such data are available for relationships between lower limb and coronary arterial function, and it is also unclear whether FMD responses are associated between the upper and lower limbs.
In a recent study in pigs, Padilla et al. (28) reported that brachial artery vasomotor function correlates well with femoral artery function, thus supporting the notion that endothelial function in the brachial artery might reflect function in other conduit arteries. FMD in the brachial (13, 18, 21) and superficial femoral artery (15) in humans are both NO mediated, which indicates that these arteries may indeed exhibit similar dilator responses. However, locomotor activity in quadrupeds is the result of muscles perfused by both brachial and femoral arteries, whereas this is the case only for the femoral arteries in humans. While both upper and lower limb arteries of humans can develop atherosclerosis (36, 43), the lower limbs demonstrate a higher incidence of clinical vascular disease and claudication. Upper and lower limb vasculatures also demonstrate different vasomotor responses to shear (26, 29) and pharmacological vasoactive substances (23, 24) in humans. Although comparisons of FMD between upper and lower limbs have been previously reported (26, 29, 41), to our knowledge the degree to which brachial and femoral/popliteal artery endothelial function is correlated in humans has not been examined.
In this paper, we present a retrospective analysis undertaken to evaluate the strength of relationships in vasomotor function between the upper (i.e., brachial artery) and lower (i.e., superficial femoral and popliteal arteries) limbs of humans. Based on recent evidence in pigs (28), we hypothesized that a correlation in endothelium-dependent dilation would exist between conduit arteries in the upper and lower limbs. Furthermore, we reasoned that the relationship in vasomotor function between contralateral limbs (i.e., brachial arteries) would be stronger than that between upper and lower limbs.
METHODS
Subjects
We conducted a retrospective analysis of vascular function data from our laboratory. In the present investigation, we selected data in which endothelium-dependent and -independent dilation of the brachial and femoral, brachial, and popliteal arteries, or both brachial arteries, were assessed in the same subjects (Table 1). All subjects were healthy and free of diagnosed cardiovascular disease, diabetes, insulin resistance, or cardiovascular risk factors, such as hypercholesterolemia or hypertension. Subjects who smoked or were on medications of any type that could interfere with the cardiovascular system were excluded from the analysis. The study procedures were approved by the Ethics Committee of Liverpool John Moores University and adhered to the Declaration of Helsinki, and all subjects gave written consent before participation.
Table 1.
Subject characteristics of the subgroups for the analyses on BA-SFA, BA-PA, and BA-BA
| BA-SFA | BA-PA | BA-BA | |
|---|---|---|---|
| n | 75 | 32 | 42 |
| Age, yr | 26 ± 6 | 29 ± 6 | 24 ± 5 |
| Men, % | 100 | 81 | 98 |
| BMI, kg/m2 | 24.0 ± 2.7 | 24.2 ± 3.1 | 23.8 ± 2.7 |
| Mean arterial pressure, mmHg | 87 ± 7 | 82 ± 7 | 84 ± 13 |
Values are means ± SD; n, no. of subjects. BA-SFA, brachial artery vs. superficial femoral artery; BA-PA, brachial artery vs. popliteal artery; BA-BA, brachial artery vs. contralateral brachial artery; BMI, body mass index.
Experimental Design
Most subjects attended the laboratory on two different occasions, separated by at least 1 and 7 days maximally. On one visit, vascular function measures were performed at the brachial artery, whereas on the other visit measures were performed at the superficial femoral artery (n = 75), popliteal artery (n = 32), or the contralateral brachial artery (n = 42). The order of visits was randomized. Some participants underwent assessment of brachial and superficial femoral (n = 51) or popliteal artery (n = 11) vascular function on a single day.
Experimental Procedures
All tests were performed in a temperature-controlled room and according to recent guidelines for assessment of the FMD (40). Measurements were always performed at the same time of day to prevent diurnal variation in the FMD response (14). Female participants were tested in the first week of the menstrual cycle (40). All measures were performed following a ≥6 h fast, ≥8 h abstinence from caffeine, and at least 24 h after strenuous physical activity (40). Before each FMD test, subjects rested in the supine (for the brachial and superficial femoral arteries) or prone (for the popliteal artery) position for at least 30 min. Heart rate and mean arterial pressure were assessed with an automated sphygmomanometer (Dinamap; GE Pro 300V2, Tampa, FL). For assessment of the FMD response, a rapid inflation/deflation pneumatic cuff (Hokanson, Bellevue, WA) was positioned distal to the imaged artery and inflated for 5 min (>200 mmHg) to provide the stimulus for reactive hyperemia. A 10-MHz multifrequency linear array probe attached to a high-resolution ultrasound machine (T3000, Terason, Burlington, MA) was used to image the arteries. Ultrasound parameters were set to optimize longitudinal, B-mode images of the lumen/arterial wall interface. Continuous Doppler velocity assessment was also obtained using the Terason and was collected using the lowest possible insonation angle (always <60°), which did not vary during each study.
Brachial artery FMD.
Subjects were positioned supine with the right arm extended and immobilized with foam, supported at an angle of ∼80° from the torso. The pneumatic cuff was placed distal to the olecranon process, and the brachial artery was scanned 2–5 cm above the antecubital fossa. Postdeflation diameter and blood velocity were recorded continuously until 3 min after cuff deflation.
Superficial femoral artery FMD.
Subjects were positioned supine with the lower leg slightly elevated and supported on an ∼15-cm-thick foam. The pneumatic cuff was placed ∼15 cm below the inguinal ligament. The superficial femoral artery was scanned in the proximal one-third of the thigh, at least 5 cm distal from the bifurcation and above the cuff position. Post-deflation diameter and blood velocity were recorded continuously until 5 min after cuff deflation.
Popliteal artery FMD.
Subjects were positioned prone with the right knee supported at an angle of ∼20°. The pneumatic cuff was placed immediately distal to the popliteal fossa, and the popliteal artery was scanned in the popliteal fossa. Post-deflation diameter and blood velocity were recorded continuously until 5 min after cuff deflation.
Endothelium-independent dilation.
After a resting period of 30 min to allow arterial diameter to return to baseline, another 1 min baseline recording of the conduit artery was made. Subsequently, endothelium-independent dilation was examined using a single spray of sublingual glyceryl trinitrate (GTN, 400 μg), a NO donor, followed by at least 10-min recording of the diameter.
Conduit Artery Diameter and Blood Flow Analysis
Posttest analysis was performed using custom-designed, edge-detection, and wall-tracking software, which is independent of investigator bias (45). Briefly, the echo Doppler signal was real-time encoded and stored as a digital file. Subsequent software analysis of this data was performed at 30 Hz using an icon-based graphical programming language and toolkit (LabVIEW 6.02, National Instruments, Austin, TX). The initial phase of image analysis involved the identification of regions of interest on the first frame of every individual study. These regions of interest allowed automated calibration for diameters on the B-mode image and velocities on the Doppler strip. Details of this analysis technique can be found elsewhere (4, 45). Ultimately, from this synchronized diameter and velocity data, shear rate (4 times velocity divided by diameter) was calculated at 30 Hz (45). All data were written to file and retrieved for analysis in a custom-designed analysis package. We have shown that reproducibility of diameter measurements using this semiautomated software is significantly better than manual methods, reduces observer error significantly, and possesses an intraobserver coefficient of variation (CV) of 6.7%. Furthermore, our method of blood flow assessment is closely correlated with actual flow through a “phantom” arterial flow system (10).
Data Analysis
Baseline diameter and shear rate were calculated as the mean of data acquired across the 1 min preceding the cuff inflation period. Following cuff deflation, peak diameter following cuff deflation was automatically detected according to an algorithm that identified the maximum bracket of data subsequent to performance of a moving window smoothing function. This smoothing routine calculates the median value from 100 consecutive samples, before the window shifts to the next bracket of data, which shares 20% overlap with the preceding bracket. The maximum value of all of the calculated median values is then automatically detected and chosen to represent the peak of the diameter curve. FMD was calculated as the percentage of rise of this peak diameter from the preceding baseline diameter. Calculation of FMD was, therefore, observer independent and based on standardized algorithms applied to data that had undergone automated edge detection and wall tracking. The post-deflation shear rate data, derived from simultaneously acquired velocity and diameter measures at 30 Hz, was used to calculate the area under the shear rate curve for data up to the point of maximal post-deflation diameter (FMD) for each individual. Reproducibility of the brachial artery FMD using this semiautomated software possesses a CV of 6.7–10.5% (42, 45), while the superficial femoral artery FMD has a CV of 15% (7).
Statistics
Statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL) software. All data are reported as means (±SD), and statistical significance was assumed at P < 0.05. Pearson's correlation coefficients and paired t-tests were used to assess the relationship and differences between upper limb (brachial artery) and lower limb (superficial femoral and popliteal artery) conduit artery FMD. Based on the potential impact of resting diameter (34, 41) and post-deflation shear rate (31) on FMD responses, we also performed a partial correlation with statistical correction for these parameters. We also examined the relation between body weight and body mass index (BMI) with vascular function (FMD) and structure (baseline diameter). We used Bonferroni's correction for the multiple correlations (n = 3 for the primary aims of the study) (6).
RESULTS
Table 1 summarizes subject characteristics of three subgroups. Body weight significantly correlated with superficial femoral, popliteal, and brachial artery diameter, but not with superficial femoral and popliteal artery FMD (Table 2). A weak inverse correlation was present between body weight and brachial artery FMD (Table 2). BMI correlated with superficial femoral and brachial artery diameter, but not with popliteal artery diameter or with superficial femoral, popliteal, and brachial artery FMD (Table 2). Mean arterial pressure was not different between the three groups (Table 2) and did not differ between testing days (data not shown).
Table 2.
Correlation between weight (in kg) or BMI (kg/m2) and SFA, PA, or BA diameter or FMD
| SFA |
BA |
PA |
||||
|---|---|---|---|---|---|---|
| Diameter | FMD | Diameter | FMD | Diameter | FMD | |
| Weight, kg | R = 0.54 | R = 0.02 | R = 0.40 | R = −0.18 | R = 0.52 | R = −0.01 |
| P < 0.001 | P = 0.85 | P < 0.001 | P = 0.04 | P = 0.003 | P = 0.96 | |
| BMI, kg/m2 | R = 0.38 | R = 0.12 | R = 0.23 | R = −0.01 | R = 0.17 | R = 0.17 |
| P = 0.001 | P = 0.32 | P = 0.01 | P = 0.89 | P = 0.37 | P = 0.37 | |
FMD, flow-mediated dilation.
Brachial Artery vs. Superficial Femoral Artery
Brachial artery baseline diameter was significantly smaller than the superficial femoral artery (Table 3) and was modestly correlated between both arteries (r = 0.29, P = 0.013). The time to peak diameter in the brachial artery was significantly shorter than that in the superficial femoral artery (Table 3). We found no difference (Table 3) or correlation (Fig. 1A) between brachial and superficial femoral artery FMD. Furthermore, when controlling for resting diameter and shear rate area under the curve (AUC), the partial correlation did not reveal significance (r = −0.06, P = 0.65). Brachial artery endothelium-independent dilation to GTN was significantly larger than that observed in the superficial femoral artery (Table 3). A correlation was observed between the brachial and superficial femoral artery endothelium-independent response to GTN (r = 0.37, P = 0.007).
Table 3.
Details of BA and SFA flow-mediated, endothelium-dependent dilation and GTN-mediated, endothelium-independent dilation in a group of healthy subjects
| BA | SFA | P Value | |
|---|---|---|---|
| Diameter, mm | 4.5 ± 0.6 | 7.0 ± 0.7 | <0.001 |
| Peak FMD diameter, mm | 4.8 ± 0.6 | 7.5 ± 0.8 | <0.001 |
| Time to peak diameter, s | 76 ± 33 | 130 ± 71 | <0.001 |
| FMD, % | 5.6 ± 2.0 | 5.8 ± 2.4 | 0.56 |
| SRAUC*, s (103) | 15.2 ± 8.0 | 10.9 ± 6.7 | <0.001 |
| Peak GTN diameter, mm | 5.2 ± 0.6 | 7.8 ± 0.9 | <0.001 |
| GTN, % | 17.1 ± 5.3 | 9.9 ± 3.6 | <0.001 |
Values are means ± SD; n = 75 FMD and n = 53 glyceryl trinitrate (GTN) subjects. The shear rate area-under-the-curve (SRAUC) is presented, being the stimulus for arterial dilation during the FMD response.
Due to technical problems, we could not record the postdeflation shear rate in 5 subjects.
Fig. 1.
Correlation of the brachial artery flow-mediated dilation (FMD) and superficial femoral artery FMD (n = 75; A), popliteal artery FMD (n = 32; B), and nondominant brachial artery FMD (n = 42; C) in a group of healthy, young subjects. The regression analysis is shown.
Brachial Artery vs. Popliteal Artery
Brachial artery baseline diameter was significantly smaller than the popliteal artery (Table 4) and was correlated between both arteries (r = 0.44, P = 0.011). We found no difference (Table 4) or correlation (Fig. 1B) between the brachial and popliteal artery FMD. Also, when controlling for resting diameter and shear rate AUC, the partial correlation did not reveal significance (r = −0.11, P = 0.63). The time to peak diameter in the brachial artery was shorter than that in the popliteal artery (Table 4). Brachial artery dilation to GTN was significantly larger than that observed in the popliteal artery (Table 4). We found no correlation between brachial and popliteal artery response to GTN (r = 0.28, P = 0.11).
Table 4.
Details of BA and PA flow-mediated, endothelium-dependent dilation and GTN-mediated, endothelium-independent dilation in a group of healthy subjects
| BA | PA | P Value | |
|---|---|---|---|
| Diameter, mm | 4.2 ± 0.6 | 5.9 ± 1.0 | <0.001 |
| Peak FMD diameter, mm | 4.5 ± 0.6 | 6.3 ± 1.0 | <0.001 |
| Time to peak diameter, s | 72 ± 28 | 151 ± 79 | <0.001 |
| FMD, % | 7.3 ± 2.7 | 6.7 ± 3.0 | 0.38 |
| SRAUC*, s (103) | 15.3 ± 9.2 | 14.0 ± 7.7 | 0.36 |
| Peak GTN diameter, mm | 5.0 ± 0.7 | 6.5 ± 0.9 | <0.001 |
| GTN, % | 18.1 ± 6.1 | 9.1 ± 3.5 | <0.001 |
Values are means ± SD; n = 32 subjects. The SRAUC is presented, being the stimulus for arterial dilation during the FMD response.
Due to technical problems, we could not record the PA SRAUC in 6 subjects.
Brachial Artery vs. Contralateral Brachial Artery
No difference was found between both brachial arteries in baseline diameter or FMD (Table 5). A significant correlation in baseline diameter was found between both arteries (r = 0.68, P < 0.001) and FMD responses (Fig. 1C). The correlation remained significant after correcting for resting diameter and the shear rate AUC (r = 0.55, P < 0.001).
Table 5.
Details of BA flow-mediated, endothelium-dependent dilation and GTN-mediated, endothelium-independent dilation in the dominant and nondominant arm in a group of healthy subjects
| BA |
|||
|---|---|---|---|
| Dominant | Nondominant | P Value | |
| Diameter, mm | 4.0 ± 0.5 | 4.1 ± 0.6 | 0.76 |
| Peak FMD diameter, mm | 4.3 ± 0.5 | 4.3 ± 0.6 | 0.80 |
| Time to peak diameter, s | 60 ± 37 | 66 ± 37 | 0.25 |
| FMD, % | 6.0 ± 2.8 | 5.9 ± 3.0 | 0.80 |
| SRAUC*, s (103) | 20.6 ± 13.8 | 20.4 ± 8.9 | 0.30 |
Values are means ± SD; n = 42 subjects. The SRAUC is presented, being the stimulus for arterial dilation during the FMD-response.
Due to technical problems, we could not record the BA SRAUC in 4 subjects.
DISCUSSION
Previous studies in humans have reported a relationship in vasomotor function between the brachial and coronary arteries (2, 19, 37–39), and recent data from pigs suggest that a relationship also exists between the brachial and femoral arteries (28). The results of the present study, which was performed in a healthy, homogeneous cohort of young adults, demonstrated no correlation between the FMD in conduit arteries of the upper (i.e., brachial artery) and lower limbs (superficial femoral or popliteal artery). In contrast, a significant correlation was observed when examining the FMD between both brachial arteries. These data suggest that conduit artery vasodilator function in the upper limbs of healthy humans is not predictive of that in the lower limbs, whereas measurement of FMD in one arm appears to be predictive of FMD in the contralateral arm.
Previous studies have supported the concept that impaired FMD represents an early and integral manifestation of vascular disease, which predicts cardiovascular events (9, 16, 20, 22, 30), and that endothelial vasomotor function can be regarded as a “barometer” of systemic endothelial health (44). Accordingly, the present dogma is that FMD responses obtained at the brachial artery can be extrapolated to other vascular beds. However, our findings clearly challenge this view in that virtually no correlation was found in FMD between upper and lower limb conduit arteries of humans. There are a number of potential physiological explanations for this. From a methodological standpoint, the fact that we focused on a cohort without clinical evidence of atherosclerosis and/or cardiovascular disease may be a factor. Previous studies reporting significant correlations between brachial artery FMD and coronary artery vasomotor function used patients with recognized cardiovascular risk factors or disease (2, 19, 37–39). Thus it is possible that inclusion of subjects with a larger range of vascular (dys)function (e.g., presence of cardiovascular risk factors) would have increased our chances for detecting a correlation between vascular beds. It is also possible that inclusion of subjects with endothelial dysfunction reveals a relationship between upper and lower limbs in terms of the degree of impairment.
Our data also differ from those of a recent study in pigs (28). There may be several explanations for this discrepancy in findings between studies. Unlike quadrupeds, upper and lower limb artery size differ substantially in humans (41), and it is known that artery size and wall thickness can impact on functional responses (8, 34, 41). Structural variation in the vascular wall in humans may, therefore, contribute to the lack of correlation in FMD observed between limb vasculatures (8). In addition, previous studies in healthy humans have established that upper and lower limb vasculatures exhibit different vasomotor responses to shear (26, 29) and pharmacological vasoactive substances (23, 24).
These differences in responsiveness between limbs may also contribute to the lack of correlation in conduit artery endothelial function. Another possible explanation for our findings, relative to those of Padilla et al. (28), relates to the presence of distinct hemodynamic stimuli across arteries. Unlike pigs, the upper and lower limbs of humans are subjected to different hydrostatic pressures for a significant part of each day. In addition, upper and lower limb conduit arteries are also exposed to different magnitudes and patterns of shear stress at rest and during physical activity (25, 46). Growing evidence from in vitro and in vivo studies in animals and humans indicates that the endothelium is sensitive to intraluminal pressure, cyclic strain, and shear stress (17, 33). It is plausible that different patterns of pressure and shear stress influence endothelial cell phenotype and conduit artery responsiveness in the arms and legs, therefore contributing to the lack of correlation in FMD. Conversely, the exposure of brachial and femoral arteries to presumably similar hemodynamic forces in quadrupeds may explain the correlation in vasomotor function between these vascular beds in pigs (28).
It is also possible that the lack of correlation in FMD between limbs, as observed in our study, is inherently determined. Gene expression differences have been observed between brachial and femoral arteries of newborn (3) and adult (28) pigs, hence suggesting that a dissociation in endothelial cell phenotype may exist, despite the presence of apparently similar hemodynamic signals. In this context, it was recently demonstrated that in vivo interarterial differences in endothelial gene expression do not fully disappear after removal of environmental cues through the exposure of the cells to static culture (5). Thus, while there is strong evidence that hemodynamic forces play a key role in determining vascular cell phenotype, it is possible that some aspects of endothelial cell phenotype/genotype in the arm and leg vasculatures are dictated by inherent factors [e.g., epigenetic modifications (1)], which themselves are unrelated. Further research in this area is needed to elucidate whether between-limb differences in endothelial gene expression are present in humans. The involvement of cutting edge techniques capable of in vivo endothelial cell sampling (35) may be required to address some of these interesting questions.
If between-limb differences in physiological stimuli (e.g., shear stress, blood pressure, physical activity level) or inherent factors contribute to the lack of correlation in FMD between the upper and lower extremities, we reasoned that “elimination” of these extrinsic and intrinsic differences would result in the presence of a correlation in vasomotor function between two vessels. In keeping with this hypothesis, we found a significant correlation in brachial artery FMD between the dominant and nondominant side. A portion of the unexplained variance in the correlation between arms could be attributed to the influence of arm dominance; however, we found no significant effect of arm dominance on baseline diameter or FMD. Alternatively, given that there were no apparent structural or functional differences between vessels of the upper limbs, it is also possible that a portion of the unexplained variance between arms (as well as between limbs) is due to the measurement error associated with the FMD technique. Regardless, these data suggest that vascular function of conduit arteries of similar arterial size and comparable in exposure to hemodynamic stimuli and inherent factors show relative good agreement in humans.
We observed a strong correlation between body weight or BMI and conduit artery diameter of the upper and lower limbs. A previous study from Olive et al. (27) demonstrated that conduit arterial diameter is closely related to muscle volume, suggesting a close link between vascular and muscular adaptations. Although we did not measure individual lean mass, our results reinforce previous findings indicating that structural characteristics of vessels (i.e., diameter) relate to (lean) body weight. Moreover, we found that the superficial femoral artery has a stronger correlation with body weight and BMI than the brachial artery. This finding may relate to the weight-bearing function of the lower limbs. These observations may add further support to the implication of bipedal locomotion and upright posture in explaining the vasomotor function differences between the upper and lower limbs of humans, which are not apparent in quadrupeds (28).
A large number of previous studies have demonstrated the (independent) predictive value of brachial artery FMD in asymptomatic subjects, as well as in those with cardiovascular disease and/or risk (9, 16, 20, 22, 30). Indeed, a recent meta-analysis found that 1% decrease in FMD, independent of the group studied, is associated with a 13% change in cardiovascular risk (12). To our knowledge, there are no extant data pertaining to the prognostic role of FMD derived from the femoral or popliteal arteries. Our findings, that the relationships between brachial and lower limb artery function are absent, suggest that caution is warranted in extrapolating global arterial function or health from lower limb arterial function testing. Notwithstanding the absence of correlation in FMD between limbs, it is possible that other markers of endothelial (dys)function (e.g., vasodilator responses to pharmacological stimuli) may be related between arms and legs. Also, our data relate to healthy young men without clinical presentation of atherosclerosis or cardiovascular disease only. Subjects with cardiovascular disease or risk may present a different relation between upper and lower limb FMD (or in the degree of impairment), especially since the prognostic role of FMD seems to differ between subjects without and those with cardiovascular disease (40). Future studies should include subjects with cardiovascular disease to further examine this question.
Collectively, our data indicate that conduit artery vasodilator function in the upper limbs (of healthy humans) is not predictive of that in the lower limbs. The agreement in FMD responses between brachial arteries of contralateral arms suggests that differences between upper and lower limb arteries in humans may be attributed to discrepancies in artery structure and/or vascular cell phenotype, possibly induced by differences in local hemodynamics and/or inherent factors. Although the present study does not invalidate the prognostic value of brachial artery FMD (11, 12), these data indicate that the measure of FMD at the brachial artery cannot be extrapolated to all arteries in healthy young men.
GRANTS
D. J. Green received research funding support from the National Heart Foundation of Australia and the Australian Research Council. N. Rowley's research is funded by the charity Cardiac Risk in the Young (United Kingdom). D. H. J. Thijssen is the recipient of the E. Dekker stipend (Netherlands Heart Foundation). J. Padilla is supported by the American Heart Association Postdoctoral Fellowship (11POST5080002). G. H. Simmons is supported by National Institutes of Health (NIH) Grant T32-AR048523. M. H. Laughlin is supported by NIH Grants HL36088 and HL52490.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aird WC. Spatial and temporal dynamics of the endothelium. J Thromb Haemost 3: 1392–1406, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bahls M, Bidwell CA, Juan H, Granada JF, Krueger CG, Reed JD, VanAlstine WG, Newcomer SC. Gene expression differences in the brachial and femoral arteries of Rapacz Familial Hypercholesterloemic swine at birth are related to atherosusceptibility (Abstract). FASEB J 24: 1039.1, 2010 [Google Scholar]
- 4. Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Burridge KA, Friedman MH. Environment and vascular bed origin influence differences in endothelial transcriptional profiles of coronary and iliac arteries. Am J Physiol Heart Circ Physiol 299: H837–H846, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curtin F, Schulz P. Multiple correlations and Bonferroni's correction. Biol Psychiatry 44: 775–777, 1998 [DOI] [PubMed] [Google Scholar]
- 7. de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol 287: H374–H380, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Folkow B. Physiological aspects of primary hypertension. Physiol Rev 62: 347–504, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105: 1567–1572, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Jones H, Green DJ, George K, Atkinson G. Intermittent exercise abolishes the diurnal variation in endothelial-dependent flow-mediated dilation in humans. Am J Physiol Regul Integr Comp Physiol 298: R427–R432, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Rand WM, Udelson JE, Karas RH. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol 38: 1843–1849, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol 78: 1210–1214, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Matsuo S, Matsumoto T, Takashima H, Ohira N, Yamane T, Yasuda Y, Tarutani Y, Horie M. The relationship between flow-mediated brachial artery vasodilation and coronary vasomotor responses to bradykinin: comparison with those to acetylcholine. J Cardiovasc Pharmacol 44: 164–170, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 40: 505–510, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 86: 207–210, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol 556: 1001–1011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Newcomer SC, Sauder CL, Kuipers NT, Laughlin MH, Ray CA. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol 294: H1833–H1839, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Olive JL, Dudley GA, McCully KK. Vascular remodeling after spinal cord injury. Med Sci Sports Exerc 35: 901–907, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Padilla J, Simmons GH, Newcomer SC, Laughlin MH. Relationship between brachial and femoral artery endothelial vasomotor function/phenotype in pigs. Exp Biol Med (Maywood) 235: 1287–1291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol 291: H3043–H3049, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Shin HY, Gerritsen ME, Bizios R. Regulation of endothelial cell proliferation and apoptosis by cyclic pressure. Ann Biomed Eng 30: 297–304, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J Am Coll Cardiol 38: 1859–1865, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res 47: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sorensen KE, Kristensen IB, Celermajer DS. Atherosclerosis in the human brachial artery. J Am Coll Cardiol 29: 318–322, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Takase B, Hamabe A, Satomura K, Akima T, Uehata A, Ohsuzu F, Ishihara M, Kurita A. Close relationship between the vasodilator response to acetylcholine in the brachial and coronary artery in suspected coronary artery disease. Int J Cardiol 105: 58–66, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 82: 1535–1539, A1537–A1538, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Teragawa H, Ueda K, Matsuda K, Kimura M, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol 28: 460–466, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol 295: H1927–H1934, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Vink A, Schoneveld AH, Poppen M, de Kleijn DP, Borst C, Pasterkamp G. Morphometric and immunohistochemical characterization of the intimal layer throughout the arterial system of elderly humans. J Anat 200: 97–103, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: an in vivo MRI study. J Magn Reson Imaging 19: 188–193, 2004 [DOI] [PubMed] [Google Scholar]