Abstract
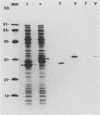
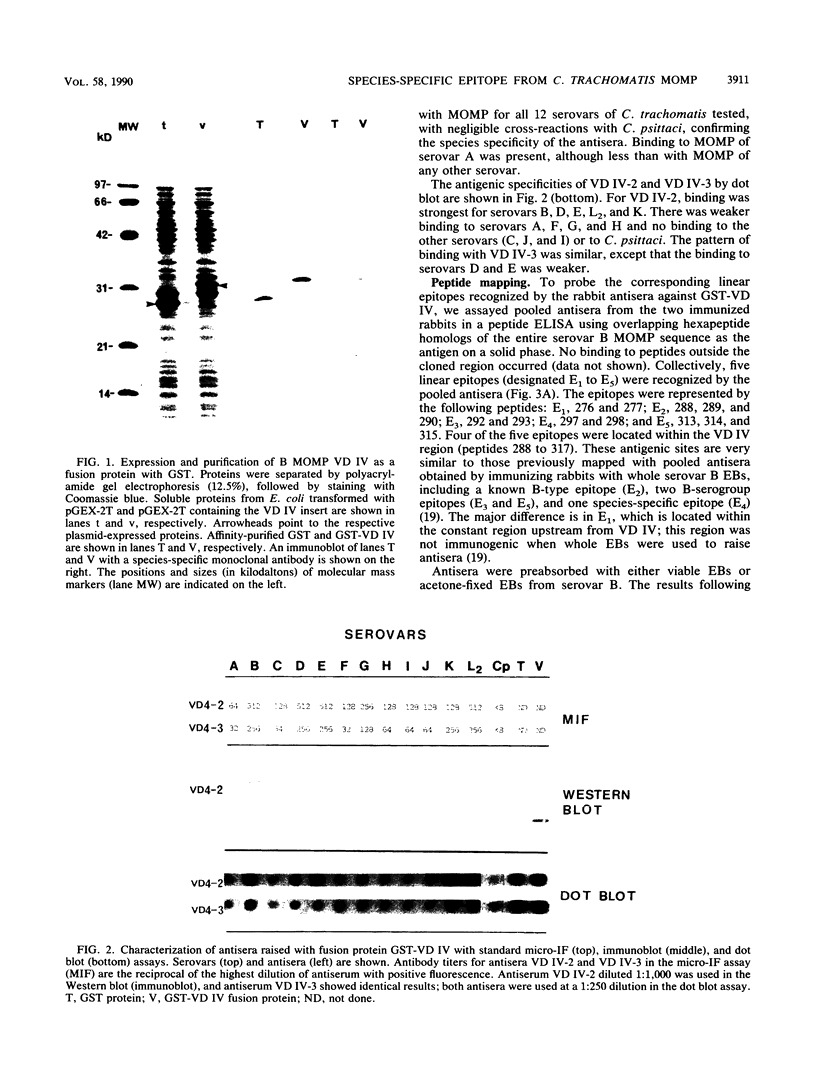
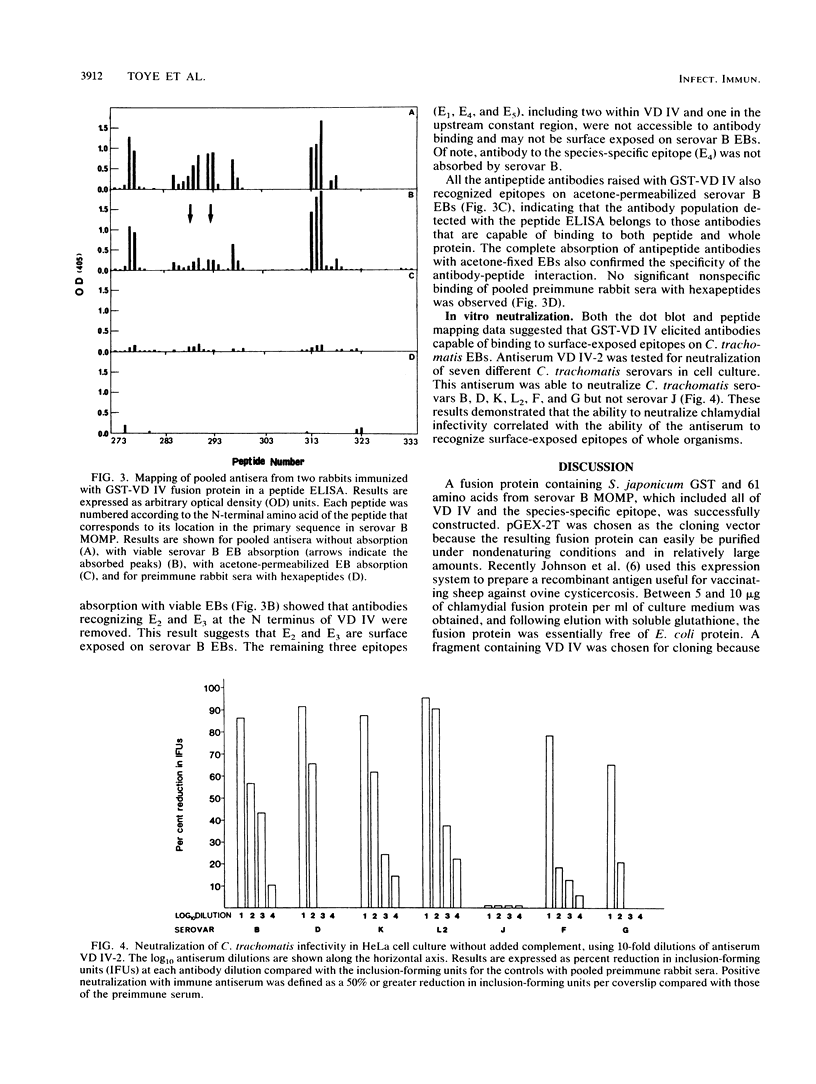
A 183-bp fragment encoding variable domain IV (VD IV) of Chlamydia trachomatis serovar B major outer membrane protein (MOMP) (amino acids 273 to 333) and containing the species-specific epitope was cloned and expressed in Escherichia coli as a fusion protein with Schistosoma japonicum glutathione S-transferase (GST-VD IV). The fusion protein was affinity purified under nondenaturing conditions and used to immunize rabbits. Antisera were characterized by microimmunofluorescence, immunoblot, dot blot, peptide enzyme-linked immunosorbent, and in vitro neutralization assays. Antisera recognized MOMP from all 12 tested serovars of C. trachomatis but not from Chlamydia psittaci. In a dot blot assay, antisera bound to elementary bodies of serovars B, D, E, L2, and K in a strong fashion and to elementary bodies of serovars F, G, A, and H in a weak fashion but not to elementary bodies of serovars C, J, and I. High-resolution peptide mapping with synthetic overlapping serovar B MOMP peptides in a solid-phase enzyme-linked immunosorbent assay showed that immunization with GST-VD IV produced a serologic response that closely mimicked the response produced with purified serovar B elementary bodies. Antipeptide antibodies with strong binding to species- and subspecies-specific epitopes were elicited. Antisera were able to neutralize only those C. trachomatis serovars that bound antibodies in the dot blot assay. These results suggest that antigenic fragments from VD IV containing the species-specific epitope may be useful in the construction of a chlamydial vaccine for some but not all C. trachomatis serovars.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. S., Harrison G. B., Lightowlers M. W., O'Hoy K. L., Cougle W. G., Dempster R. P., Lawrence S. B., Vinton J. G., Heath D. D., Rickard M. D. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature. 1989 Apr 13;338(6216):585–587. doi: 10.1038/338585a0. [DOI] [PubMed] [Google Scholar]
- Maclean I. W., Peeling R. W., Brunham R. C. Characterization of Chlamydia trachomatis antigens with monoclonal and polyclonal antibodies. Can J Microbiol. 1988 Feb;34(2):141–147. doi: 10.1139/m88-028. [DOI] [PubMed] [Google Scholar]
- Peeling R., Maclean I. W., Brunham R. C. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect Immun. 1984 Nov;46(2):484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Zhong G. M., Carlson E., de la Maza L. M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988 Apr;56(4):885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Kuo C. C., Newport G., Agabian N. Molecular cloning and expression of Chlamydia trachomatis major outer membrane protein antigens in Escherichia coli. Infect Immun. 1985 Mar;47(3):713–718. doi: 10.1128/iai.47.3.713-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Schoolnik G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988 Mar 1;167(3):817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. Formalinized Chlamydia trachomatis organisms as antigen in the micro-immunofluorescence test. J Clin Microbiol. 1979 Aug;10(2):259–261. doi: 10.1128/jcm.10.2.259-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- Zhong G. M., Reid R. E., Brunham R. C. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect Immun. 1990 May;58(5):1450–1455. doi: 10.1128/iai.58.5.1450-1455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]