Abstract
Previous studies show that transient increases in both blood flow and magnetic resonance image signal intensity (SI) occur in human muscle after brief, single contractions, and that the SI increases are threefold larger in physically active compared with sedentary subjects. This study examined the relationship between these transient changes by measuring anterior tibial artery flow (Doppler ultrasound), anterior muscle SI (3T, one-shot echo-planar images, TR/TE = 1,000/35), and muscle blood volume and hemoglobin saturation [near-infrared spectroscopy (NIRS)] in the same subjects after 1-s-duration maximum isometric ankle dorsiflexion contractions. Arterial flow increased to a peak 5.9 ± 0.7-fold above rest (SE, n = 11, range 2.6–10.2) within 7 s and muscle SI increased to a peak 2.7 ± 0.6% (range 0.0–6.0%) above rest within 12 s after the contractions. The peak postcontractile SI change was significantly correlated with both peak postcontractile flow (r = 0.61, n = 11) and with subject activity level (r = 0.63, n = 10) estimated from 7-day accelerometer recordings. In a subset of 7 subjects in which NIRS data acquisition was successful, the peak magnitude of the postcontractile SI change agreed well with SI calculated from the NIRS blood volume and saturation changes (r = 0.80, slope = 1.02, intercept = 0.16), confirming the blood-oxygenation-level-dependent (BOLD) mechanism underlying the SI change. The magnitudes of postcontractile changes in blood saturation and SI were reproduced by a simple one-compartment muscle vascular model that incorporated the observed pattern of postcontractile flow, and which assumed muscle O2 consumption peaks within 2 s after a brief contraction. The results show that muscle postcontractile BOLD SI changes depend critically on the balance between O2 delivery and O2 consumption, both of which can be altered by chronic physical activity.
Keywords: blood flow, exercise, magnetic resonance imaging
the blood-oxygenation-level-dependent (BOLD) effect refers to the dependence of tissue signal intensity (SI) in T2-weighted functional magnetic resonance images (MRI) on the tissue's hemoglobin saturation and blood volume (15, 31, 40). In brain studies increased MRI SI due to the BOLD effect is now commonly exploited to map the location and extent of neural activity noninvasively and in real-time in response to a variety of stimuli. BOLD contrast in these studies is not a direct measure of neural activity per se, but depends on increased blood flow and oxygenation spatially and temporally correlated with the neural activity. Of course, coupling between cellular activity and blood flow is not exclusive to the brain. Skeletal muscle and other tissues also undergo changes in flow and oxygen consumption as part of their normal physiological function, the pattern of which may be altered by pathology or in response to habitual physical activity (5, 11, 58).
BOLD effects were previously demonstrated in skeletal muscle, and the magnitude and time course of these effects were clearly related to changes in hemoglobin saturation (31, 32, 40). For example, a robust postcontractile BOLD-based increase in MRI signal can be detected in skeletal muscle following single, very brief 1-s-duration contractions (40). This may come as somewhat of a surprise given the transient nature of the contraction; however, previous studies show that blood flow can increase by as much as sixfold after a single brief muscle contraction (6, 63). This postcontractile flow increase is an order of magnitude greater than the local blood flow increases typically reported in brain functional MRI studies (9, 10). The underlying mechanism for the postcontractile flow increase is not fully understood, but is thought to result from a combination of factors including the muscle pump, i.e., contraction-induced widening of the arterial-venous pressure gradient (29, 30, 64), the myogenic effect, i.e., vascular smooth muscle relaxation induced by a change in transmural pressure (14, 43), and a host of vasodilators, including K+ (12, 13, 21).
Previously we reported that the magnitude of the postcontractile BOLD response is threefold greater in active vs. sedentary individuals (61). The reason for this difference may be one of the many well-established adaptations to the peripheral vasculature that result from habitual physical activity (7, 28, 38, 66), and that could increase the flow response to a brief contraction. These adaptations include increased collateral blood flow (67, 68), increased microvascular density (7, 24), and increased contraction-induced dilation of the microvasculature (28). Although activity-dependent differences in the flow response to single brief contractions have not been reported, training has been shown to increase the “on-response” (58) of blood flow to exercise and the initial flow response to repeated contractions is greater in trained vs. sedentary controls (33). The “on-response” is the blood flow increase measured in the first few seconds of exercise and the factors that control this response may be similar to those involved for brief contractions (12).
Therefore, the purposes of this study were 1) to compare the postcontractile blood flow responses in muscle of habitually active vs. inactive subjects, 2) to determine if the magnitude of the MRI-measured postcontractile BOLD response is quantitatively explained by the magnitude and time course of changes in blood volume and hemoglobin saturation measured by near-infrared spectroscopy (NIRS) across the range of subject activity levels, and 3) to examine using a simple model whether the postcontractile flow response and estimates of postcontractile oxygen consumption together explain the observed blood volume and saturation changes, and hence the MRI-measured BOLD response.
METHODS
Subjects.
Eleven subjects [3 women, age 24.5 ± 5.6 (SD) yr] participated in the study. The study was approved by the University's Committee on Research Involving Human Subjects, and all subjects gave informed, written consent. A pretest screening, including a magnetic material safety questionnaire and a medical history questionnaire, were completed by all subjects prior to enrollment. All subjects were apparently healthy and with no family history of cardiovascular disease and no musculoskeletal disorder that would preclude participation. Subjects were selected to cover a range of self-reported physical activity levels, ranging from sedentary (no regular exercise program) to very active (>6 h/wk of intense aerobic training).
General methods.
Subjects reported to the laboratory for testing on two separate occasions, one day for Doppler flow and NIRS testing and a second day for MRI testing. The testing days, either Doppler and NIRS or MRI, were randomized to avoid an order effect. Testing was scheduled at approximately the same time of day (±1 h) and at least 1 wk apart. To limit the potential effects of food or caffeine on blood flow subjects reported to the laboratory following an overnight fast. Subjects were asked to refrain from taking any medications including over the counter medications on the mornings of testing. In addition subjects were asked to refrain from any intense physical activity for at least 24 h prior to their visits. Compliance with pretest criteria was determined by a questionnaire. In the event that pretest criteria were not met the subject was rescheduled for a later date. All women were tested in the early follicular phase of the menstrual cycle, within 4 days of the end of their most recent menses.
Blood pressure was measured in the brachial artery using a standard arm blood pressure cuff and a sphygmomanometer. Ankle blood pressure was measured in the posterior tibial artery using a portable handheld ultrasound device and a leg blood pressure cuff positioned around the calf. Blood pressure was measured in duplicate and the highest value for systolic blood pressure in the brachial and posterior tibial artery was used to calculate the ankle brachial index (ABI, ratio of ankle systolic blood pressure to brachial blood pressure). An ABI of <1.0 is a indicator of compromised peripheral vascular function (37).
Physical activity levels of 10 of the 11 subjects were confirmed using a physical activity accelerometer (Model GT1M, ActiGraph, LLC. Pensacola, FL). The accelerometer measures changes in acceleration in an intensity-dependent manor at 30 samples/s and records the accelerations as counts which are summed and stored over user defined time periods. The counts recorded by the accelerometer are highly correlated with independent measures of metabolic rate in both laboratory settings and a free-living environment (50). Subjects wore the physical activity monitor on a belt around their waist during all waking hours for 7 days. Total accumulated counts were averaged over the total days the subjects wore the monitors and this value is reported as average counts/day.
Doppler blood flow.
Subjects lay quietly on a patient table in a supine position with arms folded across their chest and with both legs extended. Subjects rested quietly in this position for 15 min prior to data collection. The subjects' right leg was positioned at approximately heart level, supported by foam pads and secured in place using nylon straps. The subject's right foot was placed in a custom-built foot device for measuring the force of isometric ankle dorsiflexion. The footplate of the device was fixed at an angle of 120°, and the foot was strapped to the footplate using a nylon strap with Velcro closures. A strain-gauge force transducer (Interface, model SSM-EV-250, Scottsdale, AZ) was mounted to the underside of the footplate and force during the isometric exercise was digitized (DATAQ Instruments, model DI-195B, Akron, OH), sampled at 120 Hz, and recorded on a personal computer.
Prior to data collection each subject performed a series of practice contractions and the highest force recorded during the practice contractions was used as the subject's maximal voluntary contraction (MVC). To ensure consistent contraction intensity and duration, visual feedback of force was provided to each subject by a computer monitor and target force was indicated by a red line bisecting the screen at the appropriate force level. To allow for sufficient recovery of blood flow to preexercise levels a 10-min rest period followed the practice contractions.
During the exercise protocol each subject performed 1-s-duration maximal isometric ankle dorsiflexion contractions at 60- to 80-s intervals. Subjects were instructed to perform the contractions at maximal effort and to exhale during the contraction to avoid performing a Valsalva maneuver. Blood flow was measured continuously in the anterior tibial artery (ATA), 2–3 cm distal to the head of the fibula, using a duplex Doppler ultrasound scanner (LOGIQ Book, GE Medical Systems, Milwaukee, WI) mounted with an 8-MHz linear probe (Model 8L-RS). The sampling depth and gate width were optimized to sample the vessel along a 1- to 2-cm length during the data acquisition. The probe was held securely in place at an insonation angle of 60° or less, and was angle corrected to 60°. Data were acquired and stored for data processing in cinematic loops of 30- to 60-s duration, and were analyzed using the GE software available on the LOGIQ Book system.
Prior to the exercise high-resolution B-mode images were acquired to measure the resting diameter of the ATA. All diameter measurements were made during diastole by the same investigator. In a subset of subjects the ATA diameter was measured in 10 images before and 10 images after the exercise. In these subjects resting ATA diameter was not significantly different from postexercise diameter; therefore for all subjects the preexercise value was used to calculate blood flow. ATA blood flow (BF) in milliliters per minute was calculated by multiplying the cross-sectional area (CSA) of the ATA by the mean blood velocity over the duration of the pulse-waveform (time average mean, TAMEAN) according to the following equation:
Flow was normalized to anterior compartment muscle volume, estimated for each individual from MR images (see below), and is reported as milliliters per minutes per 100 ml muscle. To average data from subjects with different heart rates, and therefore different sampling intervals, results for each subject were linearly interpolated to 1-s intervals.
Near infrared spectroscopy.
NIRS data were acquired simultaneously with the Doppler measures of blood velocity using a LEDI NIRS imager (Near Infrared Monitoring, Philadelphia PA). This device has two separate probes of the following dimensions, 11.5 × 7 × 2 cm. Each probe has 6 detectors and 2 light sources with a detector-light source separation of 3 cm. The system measures absorption of light at two different wave lengths, 730 and 850 nm, and provides relative measures of oxyhemoglobin (HbO2), deoxyhemoglobin (Hb), and blood volume (BV, sum of HbO2 + Hb). The NIRS device gain was standardized prior to each data collection using a muscle tissue phantom provided by NIM. The probes were positioned, one on each leg (left leg as a control), over the belly of the anterior compartment muscles, primarily the anterior tibialis muscle. In the case of the right leg the probe was positioned just distal to the site of flow measurement, and lateral to the anterior border of the tibia. Each probe was secured to the leg using an ACE bandage wrapped firmly enough around the leg to prevent movement and pollution by ambient light during the exercise, however not so tight as to compromise blood flow. NIRS data were acquired continuously during the protocol, sampled at 3 Hz, and stored on a personnel computer for postprocessing.
NIRS data were analyzed using the LEDI imager analyzer program. To calibrate the recorded, relative NIRS signals an ischemia-reactive hyperemia protocol was performed immediately following the exercise (36). A large contoured vascular cuff (model CC22 24 × 122.5 cm, Hokanson, Bellevue, WA) was placed around the thigh just proximal to the knee joint. During the ischemia protocol the cuff was rapidly inflated to 240 mmHg using a rapid cuff inflator (Model E20; Hokanson, model 666). This pressure was maintained for 5–6 min, at which time the cuff was released to obtain peak reperfusion values.
The changes in HbO2 and Hb during the exercise were expressed as a percentage of the maximum changes in HbO2 and Hb observed during the ischemia (assumed to fully desaturate all the heme groups), and reactive hyperemia (assumed to fully saturate the heme groups) (36). Percent saturation of hemoglobin during exercise was calculated from
where the constant (0.4) accounts for the contribution of myoglobin heme groups to the HbO2 signal. This correction assumes that myoglobin is fully saturated in resting muscle (31, 53) and therefore during the postcontractile transients, when Po2 is further increased, and that 40% of the maximum HbO2 signal recorded during ischemia-reperfusion is due to myoglobin. [Human muscle contains ∼0.2 mM heme groups from myoglobin (16) and ∼0.3 mM from hemoglobin, assuming 3% blood volume and 15 g/dl hemoglobin in blood]. For BV a resting value of 3% was assumed and the changes during exercise were normalized relative to this value (42, 51, 52).
MRI measurements.
On a separate day, at least 1 wk apart from the flow and NIRS testing day, and at approximately the same time of day (±1 h), subjects reported to the MRI center. All MR images were acquired using a standard quadrature extremity coil on a 3.0-T GE Horizon system (GE Medical Systems, Milwaukee, WI). Subjects were fitted with a four-lead ECG used to monitor heart rate and to gate the acquisition of phase-contrast flow images (61) for measurement of resting ATA flow. The leg was positioned in the imaging coil such that the same portion of the right leg that was under the NIRS probe was contained within the imaging coil. In the case of individuals that performed the MRI testing first the maximal CSA of the ankle dorsiflexors, as determined by visual inspection, was positioned in the center of the coil. Soft foam positioners were placed in the coil and around the leg to minimize motion of the leg during the exercise. The same foot device was used for both protocols and the foot was positioned in the device as described above.
A set of axial gradient-recalled-echo time-of-flight (TOF) flow images was acquired to identify a suitable axial/oblique plane for resting ATA flow measurements, as described previously (61). Based on these images, an oblique slice was selected which transected the anterior and posterior tibial arteries 1–3 cm below their bifurcation from the popliteal artery. Flow velocity images (TR 18 ms, TE 6 ms, 20° pulse, 1-cm slice thickness, 14-cm FOV, 256 × 160 acquisition matrix, 1 NEX, 100 cm/s VENC) of the selected slices were acquired in retrospectively ECG-gated CINE mode as described previously (61). Retrospective gating of the data acquired over 128 heart beats (total acquisition time 2–4 min, depending on the subject's heart rate) yielded 32 cardiac-gated flow-velocity and magnitude images. Flow (ml/min) was calculated by integrating velocity (cm/s) across the area (cm2) of ATA as described previously (61). Mean flow in the ATA was then calculated from the mean across all 32 images. Flow images were acquired at rest, just prior to the exercise protocol.
The largest CSA of the ankle dorsiflexor muscles was located from a series of T1-weighted [3-plane, TR 500 ms, TE 1.32 ms, 22-cm FOV, 5-mm slice thickness, 7 slices per plane, 256 × 160 acquisition matrix, 1 NEX] and T2-weighted (axial fast spin echo, TR 500 ms, TE 12.3 ms, echo train length 4, 256 × 256 acquisition matrix, 18-cm FOV, 1-cm slice, 1 cm slice separation, 1 NEX) images. Muscle volume was estimated from the T2-weighted images. The CSA of anterior compartment muscle in each slice was measured and multiplied by the slice thickness to obtain muscle volume for that slice. The volume of the muscle between slices was estimated by linear interpolating the CSA from the two adjacent slices and multiplying this value by the slice spacing. Total muscle volume was calculated by adding the volume of each slice over the entire length of the muscle. In cases where the images did not extend over the whole length of an individual's muscle, the additional volume was estimated assuming a linear tapering of the muscle to the externally measured length of the anterior compartment.
One-shot gradient-recalled echo-planar images (TR 1000 ms, TE 35 ms, 60° pulse, 16-cm FOV, 1-cm slice thickness, 62.5-kHz bandwidth, 64 × 64 acquisition matrix) were acquired from a single axial slice transecting the largest CSA of the ankle dorsiflexor muscles. Echo planar images were acquired continuously for 7 min, during which time subjects performed a single, 1-s isometric MVC ankle dorsiflexion every 60 s (total of 6 contractions). Signal intensity (SI) in a fixed region-of -interest in the anterior muscle was measured across all images, and the peak percent change in SI after contraction was computed from the average of the postcontractile responses as described previously (61).
Calculations and modeling.
The effects of blood volume and blood saturation on muscle signal intensity were calculated as follows. The intravascular BOLD effect was estimated from the oxygen saturation dependence of blood R2* at 3.0 T, as reported by Zhao et al. (69):
where Y is the fractional oxygen saturation. The intravascular BOLD effect depends on the relative relaxation rates of blood vs. muscle according to
| (1) |
where SI is the total MR signal and BV is the fractional blood volume. For this calculation, TE was 35 ms, and muscle R2* was assumed to be constant at 38 s−1 at 3.0 T (40).
The potential contribution of the extravascular BOLD effect was estimated using the parallel vessel model as described previously (26, 40). In brief, the microvasculature was modeled as a rectangular array of parallel cylindrical vessels with a base diameter of 5 μm, spaced initially at 25.6 μm (for a vascular volume of 3%), and at an angle of 15 degrees relative to the main field (27). As before (40), the assumed mean diffusion coefficient of extravascular spins was 2 × 10−5 cm2/s and the susceptibility difference between tissue and fully deoxygenated blood was assumed to be 8 × 10−2 ppm. The simulation was run for 30,000 spins randomly distributed in the extracellular volume, and phase accumulation was followed to an echo time of 35 ms, and for blood saturation values ranging from 0 to 100%. The simulation was repeated with the vascular volume varied from 2.5 to 6% either by decreasing the spacing between vessels or by increasing the volume of the vessels. Results of both intravascular and extravascular calculations were normalized to the calculated values of muscle SI at 50% saturation and 3% blood volume.
To explore the effects of blood flow and oxygen consumption changes on muscle postcontractile BOLD response, a dynamic model of muscle oxygen delivery, consumption, and efflux was developed using Stella software (ISEE systems, Lebanon, NH). In this model input time courses of arterial blood flow and muscle oxygen consumption were used to calculate the time course and magnitude of changes in blood saturation and blood volume, and from these the time course and magnitude of the BOLD effect. The model assumed a single, well-mixed vascular chamber within the muscle, and delays in the development of blood volume and saturation changes due to vascular transit time were implemented by applying simple smoothing functions. Further details of the model are provided in appendix.
Statistics.
Comparisons between the calculated and measured values for the peak muscle BOLD, resting vs. peak blood flow, blood volume and %hemoglobin saturation changes were made using a paired t-test. Correlations between various parameters were made by linear regression. The level of significance was set at P < 0.05.
RESULTS
The subjects were college age with normal blood pressure and ABI > 1.0 (Table 1). These subjects were recruited to cover a wide range of physical activity levels, and this was confirmed in 10 of the 11 subjects by the wide range in accelerometer counts (from 118 × 103 to 1157 × 103 counts/day) recorded during the 7-day monitoring.
Table 1.
Subject characteristics
| Subject Characteristic | Value |
|---|---|
| Age yr | 24.5 ± 5.6 |
| Height, cm | 172.0 ± 9.2 |
| Weight, kg | 71.1 ± 14.6 |
| Blood pressure (SBP/DBP), mmHg | 113.9 / 74.6 ± 11.0 / 7.5 |
| ABI | 1.2 ± 0.1 |
| Physical activity, 103 counts/day (n = 10) | 432.5 ± 315.3 |
| Muscle CSA, cm2 | 12.1 ± 2.3 |
| MVC, N/cm2 | 20.8 ± 2.8 |
Values are means SD; n = 11 subjects (3 women), except where noted; ABI, ankle brachial index. SBP, systolic blood pressure; DBP, diastolic blood pressure; CSA, cross-sectional area; MVC, maximal voluntary contraction.
Postcontractile flow vs. activity level and the MRI SI response.
Figure 1 shows an example Doppler ultrasound recording of blood flow velocity in the anterior tibial artery of a subject before and after a single 1-s-duration ankle dorsiflexion MVC. Following the contraction (indicated by the yellow arrow), there was a large increase in flow that peaked 5 cardiac cycles after the contraction. Noticeably, the retrograde flow was absent following the contraction as blood flow greatly increased during both systole and diastole. These changes are comparable to those reported by others in the brachial artery after short contractions of forearm muscles (6, 63). Figure 2 shows the mean time course of anterior tibial artery blood flow in response to 1-s MVCs in all 11 subjects. Peak flow was elevated by 5.9 ± 0.7 fold (SE, n = 11, range 2.6–10.2) above rest, and occurred between the 4th and 7th heartbeat after contraction in all subjects, i.e., an average of 6 s after the contraction. After this rapid response, there was a more modest, slowly developing, secondary phase of flow increase which peaked 40–50 s after the contraction.
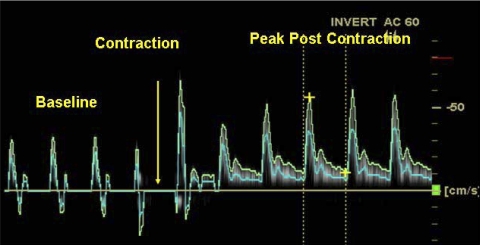
Fig. 1.
Doppler ultrasound recording of blood velocity (cm/s) in the proximal anterior tibial artery over 10 cardiac cycles. A single 1-s-duration maximal voluntary contraction (MVC) ankle dorsiflexion was performed at the time marked by the yellow arrow. The blue line is the mean velocity, and the vertical dashed lines illustrate a region over one cardiac cycle used to calculate the time-averaged mean velocity (TAMEAN) and blood flow. The first cardiac cycle coincident with or immediately after the contraction (as in this example) was distorted by probe motion and was not included in the analysis.
Fig. 2.
Time course of anterior tibial artery flow (mean ± SD) before and after 1-s-duration MVC (from −1 to 0 s). Vertical lines indicate when data collection stopped for an individual due to movement of the ultrasound probe. Thus, from −10 to 12 s, n = 11; from 12 to 23 s, n = 10, etc.
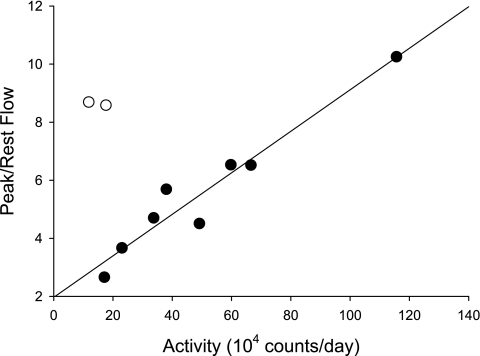
Figure 3 shows the relationship between physical activity of the subjects, as estimated from the 7 day activity monitor recordings, vs. the peak postcontractile flow change. Two subjects (open circles in Fig. 3) with a large flow response reported relatively low physical activity. One of these subjects had been a competitive soccer player for several years, but had recently stopped participating in the sport. Thus, even assuming good subject compliance, 7 days of activity monitoring does not always accurately reflect a subject's prior chronic physical activity level. Including both these subjects, the correlation coefficient between peak post contractile flow change and physical activity level was not significant (r = 0.46, P = 0.18). However, excluding these two anomalous subjects, the correlation rose to 0.97 (P < 0.001). Inasmuch as no subject had a high reported activity level but a low flow response, we conclude that the postcontractile flow response, like the postcontractile SI response (61), is greater in more physically active than in sedentary subjects.
Fig. 3.
Relationship between peak postcontractile anterior tibial artery flow vs. subject activity level as measured by 7-day activity monitoring. The regression line (y = 0.07x +1.96, r = 0.97) excludes the two subjects (open circles) with low activity and large postcontractile flow (see text).
Figure 4 shows example images and the SI changes in anterior tibial muscle of a subject during a sequence of 1-s-duration isometric MVC performed at 1-min intervals. The mean peak postcontractile SI was 2.73 ± 0.59% (SE, n = 11). As expected from our previous study (61), there was a significant correlation between subject activity level and the peak magnitude of the postcontractile SI response (r = 0.63, n = 10, P = 0.05). Figure 5 shows the relationship between the peak postcontractile flow vs. the peak magnitude of the MRI SI response in the same subjects. Although statistically significant (P = 0.048), the relationship is not strong (r2 = 0.37), indicating that other factors besides the postcontractile flow increase are important in determining the MRI response. Thus, as also argued by others (17), BOLD measurements in skeletal muscle are not simply a surrogate for blood flow measurements.
Fig. 4.
Representative anatomic T2-weighted (A) and echo-planar (B) images acquired from a subject during the MRI protocol. The green marks in the echo-planar image enclose the region-of-interest in anterior tibial muscle from which the time course of signal intensity (SI) (panel C) was extracted. The spikes in SI at 1-min intervals are T1-related motion artifacts caused by movement of unsaturated muscle into and out of the imaging plane during and immediately after the contraction (40).
Fig. 5.
Relationship between peak anterior tibial artery flow vs. peak MRI-measured muscle SI increase after 1-s MVC of the ankle dorsiflexors. The line is a linear regression (r =0.61, n = 11, P = 0.048). Note: the two outliers in Fig. 3 (low activity, but peak flow 8- to 9-fold above rest) are included in this relationship.
Postcontractile blood volume and hemoglobin saturation vs. MRI SI response.
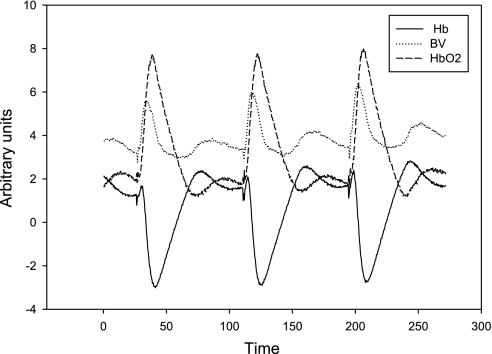
We next examined if postcontractile changes in hemoglobin saturation and blood volume quantitatively explain the magnitude of the MRI SI response, irrespective of the flow response. Figure 6 shows representative data from the NIRS device acquired during a sequence of three 1-s-duration contractions performed at 80-s intervals. The postcontractile changes in Hb, HbO2, and BV occur over multiple phases. There is an initial increase in all three that evolves over a slightly different time course for each. The small initial increase in Hb (black solid line) peaks at ∼2 s while both the initial increase in blood volume (dotted gray line, 6 s) and HbO2 (dashed line, 8–10 s) peak later at approximately the nadir of Hb (8–10 s). In addition there is a secondary increase in both Hb and blood volume above baseline values that peaks 40–50 s after the contraction, coincident with the second phase flow response noted above.
Fig. 6.
Illustrative NIRS data (arbitrary units) acquired at 3 Hz from anterior muscle of a relatively active subject during a sequence of three 1-s-duration MVCs separated by 80 s of rest. The peak in blood volume (dotted gray line) occurs before the peak in oxyhemoglobin (dashed) and the nadir of deoxyhemoglobin (solid).
Unfortunately, NIRS acquisitions were not successful in 4 of the 11 subjects (2 women) due to inadequate penetration of light through the skin and subcutaneous fat. Table 2 shows the mean resting and peak postcontractile increases in blood flow, blood volume, hemoglobin saturation, and MRI SI in response to single 1-s-duration MVCs for the 7 subjects for whom all modalities are available. Resting blood flow measured by Doppler ultrasound was not significantly different than resting blood flow measured by CINE-PC MR angiography in these same seven subjects (3.77 ± 0.5 vs. 5.06 ± 0.9 ml·min−1·100 ml muscle−1, SE, P = 0.38).
Table 2.
Anterior tibial artery blood flow, NIRS-measured blood volume, and percent hemoglobin saturation in the ankle dorsiflexors at rest and peak after a 1-s MVC
| Rest | Peak Postcontraction | |
|---|---|---|
| Blood flow, ml ·min−1 ·100 ml muscle−1 | 3.77 ± 0.5 | 23.2 ± 2.2 |
| Blood volume, % | 3.0 | 6.29 ± 0.7 |
| Hemoglobin saturation, % | 51.6 ± 2.8 | 73.2 ± 5.6 |
| Peak MRI SI, % rest | 100 | 103.4 ± 0.8 |
Values are means ± SE ; n = 7. Blood volume as rest was assumed to be 3% for all subjects, and MRI data are normalized to the signal intensity (SI) at rest. NIRS, near-infrared spectroscopy. The increases in blood flow, blood volume, hemoglobin saturation, and MRI signal intensity from rest to peak post are all significant (P < 0.05).
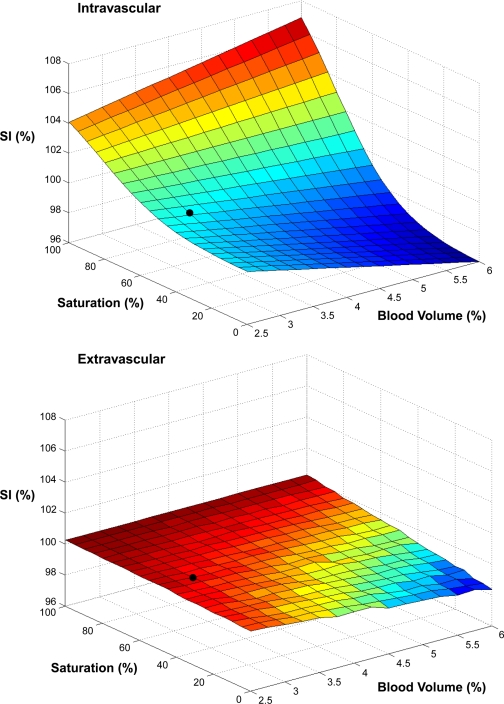
Figure 7 shows the theoretical dependence of MRI SI on blood volume and saturation for both the intravascular and extravascular BOLD effects, computed as described in methods. It is apparent from these calculations that, assuming a fiber angle of 15 degrees relative to the main field for the anterior tibial muscle (27), the contribution of the extravascular effect is minor compared with the intravascular effect over the range considered. Therefore, in the following calculations of BOLD responses from NIRS-measured blood volume and saturation changes, the minor extravascular contribution is ignored.
Fig. 7.
Intravascular (top) and extravascular (bottom) BOLD effects for gradient-echo images at echo time (TE) of 35 ms over a range of blood volumes and saturations, calculated as described in methods. SI is normalized relative to the value at baseline conditions of 50% hemoglobin saturation and a 3% blood volume (black dot locations on the surfaces). For the extravascular calculation, blood volume was increased by adding more vessels. Increasing blood volume by increasing vessel size had even smaller extravascular effects on calculated SI.
Figure 8 shows the time course of changes in blood volume and saturation (top panel), and MRI-measured SI (bottom panel, open symbols) after single contractions in the most highly active subject studied (115 × 103 counts/day, peak flow response 10.2-fold above rest). Also shown in the bottom panel is the mean time course of the MRI SI response calculated from the blood volume and saturation time courses from the same subject. The agreement between the calculated MRI response and the response actually measured by MRI is close, both with respect the peak magnitudes (calculated 105.9% vs. observed 105.6%) and the decay rate (time from peak to half-peak amplitude 13 vs. 11 s). The time course of the MRI response clearly depends on the time courses of both the blood volume and the saturation changes. For example, both the calculated and measured MRI time course peak after the peak in volume but before the peak in saturation.
Fig. 8.
Blood volume and hemoglobin saturation (top), and calculated and measured muscle SI response (bottom) to a 1-s MVC of the ankle dorsiflexors. These data are from the most active individual in the study, who also exhibited the largest postcontractile increases in blood flow (10.2-fold above rest).
Figure 9 shows the comparison between calculated vs. MRI-measured BOLD responses in 3 other subjects: a moderately active subject (subject A, 600 × 103 counts/day, peak flow 6.53-fold above resting) with a large BOLD response (top panel), in another moderately active subject (subject B, 660 × 103 counts/day, peak flow 6.52-fold above rest) with a smaller BOLD response (middle), and in a sedentary subject (170 × 103 counts/day, peak flow 2.7-fold above rest) with no positive response. In all three cases the calculated and MRI-measured changes have a comparable pattern, but these patterns differ greatly between subjects. In particular, as the peak SI change is diminished, this peak is preceded by a decrease in signal intensity below the precontraction baseline. In the most sedentary subject, the initial decrease in SI after the contraction is dominant, and no increase in SI above the baseline is observed. Including all seven subjects, a linear regression of MRI-measured vs. calculated peak SI magnitude was significant (r = 0.80, P = 0.03), with slope and intercept not significantly different from the line of identity (Fig. 10). Thus changes in hemoglobin saturation and blood volume together quantitatively account for the magnitude of the postcontractile SI response across a wide range of individual responses, confirming that the BOLD mechanism underlies the SI changes. Finally, despite the marked differences in response between subjects, the MRI-measured SI response averaged across all seven subjects also agreed well with the averaged response calculated from the individual blood volume and saturation changes (Fig. 11).
Fig. 9.
Comparison of calculated (●) vs. measured (○) muscle BOLD response from three different individuals (see text).
Fig. 10.
Linear regression (solid line, y = 1.02x + 0.16, r = 0.80, P = 0.03) between peak postcontractile SI increase calculated from NIRS blood volume and saturation data vs. SI increase observed by MRI in the same subjects. The dashed line is the line of identity.
Fig. 11.
Mean blood flow (top panel, data linearly interpolated to 1 Hz); blood volume (●) and %saturation (○) (middle); and model-calculated (●) and measured (○) muscle BOLD (bottom) for 7 subjects for whom ultrasound, NIRS, and MRI are all available.
Modeling of the BOLD response from flow and oxygen consumption.
The similarity between the calculated and observed MRI postcontractile BOLD responses indicates that postcontractile changes in blood volume and saturation are the main factors which directly determine the MRI responses. However, how are the changes in volume and saturation, and hence in MRI response, related to the observed postcontractile flow response? To explore this question, a dynamic model of muscle blood flow, vascular volume, oxygen delivery, and oxygen consumption was implemented (see appendix for details). The model includes an idealized two-phase arterial flow response similar to the measured mean arterial flow waveform (Fig. 2 and Fig. 11, top). Blood volume was assumed to vary in proportion to arterial flow after a delay as observed above (e.g., Fig. 11). The time course of muscle oxygen consumption was based on the observed rate of phosphcreatine (PCr) recovery in human ankle dorsiflexor muscle after similar brief contractions (59).
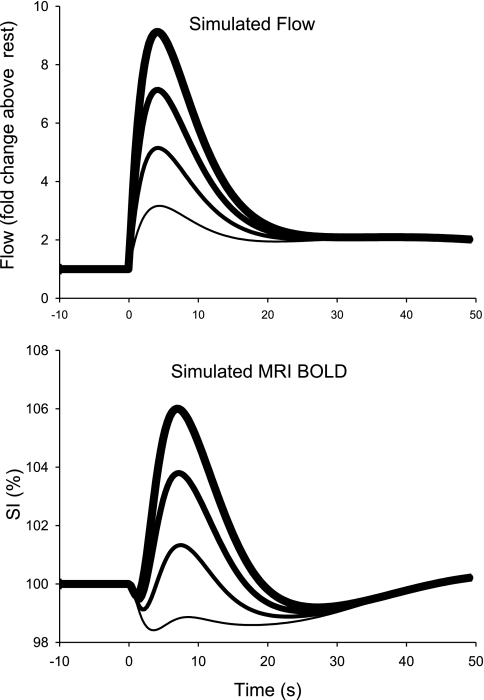
Figure 12 shows the input arterial flow (top panel), input oxygen consumption (second panel), and output blood volume, saturation (third panel), and MRI SI response (bottom panel) for the base model. Although the computed time courses do not perfectly match the observed group average result in Fig. 11, the main characteristics are clearly reproduced by this relatively simple model. For example, in the base model blood saturation increases from a baseline of 51.8% to a peak of 79%, compared with the measured change from 51.6% to 73.2% (Table 2). There is an initial dip in the computed SI, followed by a peak at 103.8%, vs. the MRI-measured peak SI of 103.4%.
Fig. 12.
Dynamic model of muscle blood flow (top panel), oxygen consumption (second panel), blood volume and oxygenation (third panel), and MRI SI change (bottom panel), as described in appendix. The modeled postcontractile increase in blood flow is idealized from the mean flows reported in Figs. 2 and 11. The modeled postcontractile increase in muscle oxygen consumption is based the extent of depletion and the rate of recovery of phosphocreatine in human muscle after short contractions (59).
Table 3 shows the sensitivity of the peak postcontractile SI magnitude of the base model shown in Fig. 12 to small (1%) variations in model parameters. These computations show that the first phase flow amplitude and time course, the O2 cost of the contraction, and the time constant of recovery O2 consumption are the key variables which determine the peak magnitude of the MRI response. Thus the exact results of the model depend critically on the balance between oxygen delivery and oxygen consumption. For example, Fig. 13 shows the effect of varying the magnitude of the first, fast phase of arterial flow increase after contraction on the computed SI. For these calculations, the ATP cost of contraction and rate of PCr recovery were held constant. Decreased flow amplifies the initial dip in SI, and decreases the magnitude of the peak SI change after the contraction. At the lowest input peak flow (12 ml·min−1·100 ml−1, or 3.2 fold above resting flow), the positive BOLD effect is eliminated. On the other hand, at the highest peak flow (34.5 ml·min−1·100 ml−1, or 9.1 fold above resting) the initial dip in SI is nearly eliminated (and would be obscured by the contraction artifact in the MRI data), and SI is near the maximum observed SI of 106% (e.g., Fig. 8). The opposite changes can be produced in the model by holding flow constant and varying the assumed ATP cost of the contraction, or the time constant of PCr recovery, and hence the peak postcontractile oxygen consumption. If peak oxygen consumption is decreased, the dip decreases and the amplitude of the SI peak increases. Thus individuals with similar postcontractile flows (e.g., subjects A and B in Fig. 9), can have substantially different MRI-measured BOLD transients.
Table 3.
Base model parameters and MRI BOLD sensitivity
| Model Parameter | Base Model Value | Sensitivity |
|---|---|---|
| Fast flow time constant (τ1 | 4 s | 2.38 |
| Fast flow amplitude (A1) | 15 ml ·min−1 ·100 ml muscle−1 | 2.02 |
| ATP cost of contraction | 1.7 mM/s | −1.30 |
| PCr recovery time constant | 40 s | 1.15 |
| Resting blood volume (BV) | 3 ml/100 ml muscle | 0.71 |
| Resting arterial flow (FArest) | 3.7 ml ·min−1 ·100 ml muscle−1 | 0.35 |
| Flow dependence of blood volume (k) | 0.19 | 0.29 |
| Resting O2 consumption (Vo2rest) | 0.38 ml O2 ·min−1 ·100 ml muscle−1 | 0.24 |
| Slow flow amplitude (A2) | 4 ml ·min−1 ·100 ml muscle−1 | 0.08 |
| Slow flow time constant (τ2) | 40 s | 0.01 |
MRI BOLD sensitivity values are %change in computed peak postcontractile SI with 1% change in parameter value. See appendix for details.
Fig. 13.
Effect of variation in the peak of the first phase of the postcontractile increase in blood flow on the simulated SI change in muscle. The time course of muscle O2 consumption and other model parameters were the same as in the base model shown in Fig. 11. These simulated flow variations (between 3- and 9-fold) result in simulated peak postcontractile muscle SI over the entire range observed in subjects (from 100 to 106%).
DISCUSSION
The main results of this study are 1) the magnitude of the postcontractile increase in blood flow following a single 1-s contraction is greater in physically active subjects, 2) there are two phases of the postcontractile flow response, an initial fast phase that peaks near 6 s after the contraction and a secondary phase that peaks at near 40 s, 3) blood volume and oxygenation changes together quantitatively explain the peak magnitude of postcontractile BOLD response, and 4) the larger postcontractile flow response in active subjects contributes to the larger BOLD response in these subjects, but the BOLD response also depends critically on the kinetics of oxygen consumption.
Although we are the first to report that the magnitude of the fast postcontractile flow response depends on physical activity, this finding should come as no surprise. Habitual physical activity results in adaptations to the peripheral vasculature at all sites along the vascular tree (29). Chronic endurance training increases collateral blood flow (68), and microvascular density including increased density of small arterioles (28) and capillaries (7). In addition to these structural changes habitual physical activity improves the functional capacity of the peripheral vasculature. Training increases flow-mediated dilation and endothelium-independent dilation of small arteries (18, 34), and improves contraction-induced dilation of small arterioles (28).
Habitual physical activity has also been shown to alter the kinetics of blood flow during the transition from rest to work. As little as 10 days of cycle ergometer training is sufficient to increase both the initial rate of blood velocity and the amplitude of the blood velocity increase at 10 s, as measured in the femoral artery in response to a knee extension exercise (58). Although in that study the initial kinetics (called the “on-response”) of blood velocity were altered with training, the steady state blood velocity did not change with training and was not different compared with the control group. Similarly, in rats a 5-wk treadmill training program resulted in greater initial (first 30 s) flow responses to exercise, but no differences in the maximal flow (33). Conversely, the profound muscle disuse caused by spinal cord injury dramatically slows the initial kinetics of blood flow at the start of repetitive muscle stimulation, but has no effect on the steady-state flow (48). Thus the major muscle blood flow adaptation to regular use or training appears to be the speed of the initial flow response, rather than the flow achieved during steady-state exercise.
Dilation of the small arterioles and in particular the terminal arterioles regulates the blood flow response in the early stages of exercise and in response to a brief muscle contraction (2, 41, 47, 55, 57). Arteriolar dilation precedes changes in blood flow in the feed arteries which are located external to the muscle and do not dilate in response to brief muscle contraction (6, 62, 64). The control of blood flow by feed arteries may be greater during steady state exercise (56, 65) although there appear to be species differences (56). Therefore the blood flow response to brief contractions represents a response controlled at the microvascular level, and may be used as an indicator of microvascular reactivity.
Although the anatomical location of flow control has been clearly defined, the underlying mechanism is still being debated (12, 62). The increase in blood flow is likely due to a variety of mechanisms including the muscle pump, myogenic response, and rapid vasodilation resulting from a contraction-induced increase in interstitial K+. The muscle pump effect is a contraction-induced widening of the arterial-venous pressure gradient due to the emptying of the venous vasculature. Although the muscle pump has been shown to impact the magnitude of the postcontractile flow response, its contribution is relatively small, less than 20% to the overall blood flow response (64). Based on the time course of the flow response in this study, as well as on the time course of the BOLD response, it is unlikely that the muscle pump makes a major contribution to the fast flow. The muscle pump effect would be greatest during the first 1–2 s postcontraction when the pressure gradient is largest, whereas the flow response measured in this (Fig. 2) and other studies (6, 30, 64) peaked at 6 s after the contraction. Furthermore the peak flow response, up to a 10-fold increase, is much larger than could be attributed solely to refilling of the muscle venous volume.
The initial flow response to a brief muscle contraction has been linked to both the myogenic response (14, 43) and K+-induced vasodilation (12, 21). The myogenic response refers to the mechanism by which the vascular smooth muscle tone is altered in response to a change in transmural pressure. K+ released during depolarization of the muscle membrane is thought to initiate vasodilation by hyperpolarizing the smooth muscle membrane and closing of voltage-gated calcium channels, resulting in relaxation (8, 25, 44, 46). Changes in K+ concentration in the venous effluent following a contraction follow a time course similar to that of the flow response (44, 45) and the flow response is almost completely prevented when the preparation is pretreated with K+ channel blockers (2) or smooth muscle membrane potential is clamped to prevent hyperpolarization (21).
Although the mechanisms for the initial phase of the flow response have been well studied, our data clearly show a secondary flow response that peaks near 40 s after the contraction. To our knowledge we are the first to report such a finding in humans. A biphasic contraction-induced vascular response has been shown previously in intact preparations of canine and rodent muscles (19, 35, 45) and in situ rodent preparations (3, 19). Mohrman and Sparks (43) showed a biphasic change in vascular conductance with the initial response peaking at near 10 s and the second phase at 25–35 s. The authors attributed the first phase to K+ induced vasodilation and suggested the second phase was due entirely to low Po2 as K+ was near baseline by 15 s (44). A biphasic pattern of arteriolar dilation was also reported by Gorczynski and Duling (19) and the magnitude of the secondary dilation was significantly reduced when the preparation was superfused with elevated Po2. Our findings are consistent with a link between low tissue Po2 and the secondary flow response. Deoxyhemoglobin reached a peak at near 40 s and the nadir of hemoglobin saturation was at approximately the same time point as the peak of the secondary flow response. This suggests an oxygen sensor or vasodilator linked to muscle oxidative metabolism. An attractive candidate is hemoglobin itself, which has been implicated in hypoxic vasodilation. Under conditions of low Po2 during hemoglobin desaturation, hemoglobin is thought to increase NO bioactivity which can act as a potent vasodilator of the peripheral vasculature (1).
Blood volume in this study increased by twofold after the contractions. This change in volume was not fully considered in our previous study (40) of the postcontractile BOLD response, largely because the NIRS device employed in that study was limited compared with the device used in this study. These new results, in combination with the modeling, support an important role for blood volume in determining the postcontractile BOLD effect, and are in line with the findings of Damon et al. (15). However, it is important to note that the contribution of blood volume to the BOLD contrast is saturation dependent. If saturation remains at or below baseline values near 50%, an increase in blood volume results in a decrease in total muscle SI (see Fig. 7, top). At higher saturations an increase in blood volume results in an increase in total SI. At near 45 s, when blood volume is near its secondary peak and saturation has returned to baseline, the BOLD signal is near or slightly below baseline. These dynamic relationships between blood flow, blood volume, and saturation can result in a poststimulus undershoot seen in some of our subjects, and in some brain functional MRI studies (9, 10). In fact, the similarity between the magnitude and time course of our muscle BOLD and NIRS data and those of some brain functional studies is striking. This similarity in the response suggests common mechanisms coupling blood flow to activation in brain and skeletal muscle.
The dynamic model used in this study reproduces the main features of postcontractile changes in blood volume, saturation, and muscle BOLD, despite some obvious simplifications. For example, blood volume in our model assumes a delayed linear relationship between flow and volume. However, blood volume changes are more likely related to microvascular compliance and pressure than to flow per se. It would be more appropriate to model the flow relationship by incorporation of compliance of the capacitance vessels, similar to the balloon model proposed by Buxton et al. (10). The use of a single well-mixed vascular compartment is also a major simplification. A more complete model would consider the incremental extraction of oxygen as blood travels through the capillaries, as well as the diffusion of oxygen within the muscle cells, and possible heterogeneity of perfusion rate and oxygen consumption across the muscle.
The success of our simple model depends critically on the assumed kinetics of O2 consumption following the contraction. We implemented a fast increase in O2 consumption after the contraction with a time constant of 0.5 s, such that peak O2 consumption is reached within 2 s. This assumption is supported by many previous studies. For example, Territo et al. (60) showed that isolated mitochondria increase respiration rate to a new steady state in response to step changes in ADP or inorganic phosphate within 3 s. A recent NMR study of PCr kinetics shows that PCr recovery rate reaches a maximum with 3 s (the earliest time measured) after short MVC of human anterior tibial muscle (59). Furthermore, studies of mouse muscle with genetic knockout of creatine kinase demonstrate that muscle O2 consumption can increase very rapidly during short bursts of twitch contractions (54).
In contrast, it has been argued that there is an inherent delay of up to 15 s in the activation of mitochondrial respiration in muscle. This delay is typically referred to as “metabolic inertia” and has been variously attributed to delayed activation of the pyruvate dehydrogenase, delayed supplementation of TCA cycle intermediates, or delayed diffusion of metabolites to and within mitochondria (4, 20). In fact, the results of this study provide further evidence that there is no major “inertial” delay in the onset of muscle respiration after contractions. For example, had we used a much longer time constant for activation of respiration in our model (e.g., 7–15 s), blood saturation would rapidly increase in parallel with the fast phase of postcontractile blood flow, there would be no initial dip in the BOLD response, and the peak BOLD response would be higher, and would occur much earlier than the actual measured BOLD response.
The importance of postcontractile O2 consumption kinetics in determining the BOLD response may explain why, although there is a relationship between peak postcontractile flow and the BOLD magnitude, the correlation is not strong (Fig. 5). The time course of O2 consumption is likely to vary between individuals, both because of individual variations in fiber type, which determines the ATP cost of contraction (22), and because of variations in mitochondrial content, which determines PCr recovery rate (49). Thus the muscle BOLD response is an integrated measure of at least three related but distinguishable aspects of an individual's muscle aerobic fitness: microvascular dynamics, fiber type, and mitochondrial content.
In summary, this study shows that physical activity influences the magnitude of the postcontractile flow response, and that this flow response has two distinct phases: a fast initial phase and a slower, secondary phase. The postcontractile muscle BOLD response can be quantitatively explained by changes in blood volume and saturation. These are ultimately determined by the balance between muscle blood flow and O2 consumption.
GRANTS
This study was supported by National Institutes of Health Grant AR-043903.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Harvey V. Sparks, Jr., for many helpful comments on regulation of blood flow in skeletal muscle.
Present address of T. F. Towse: Institute of Imaging Science, Vanderbilt Univ. School of Medicine, Nashville, TN 37232.
APPENDIX
The dynamic model consisted of a single, well-mixed muscle vascular compartment with initial, resting volume equal to 3% of muscle volume. The time course of postcontractile arterial blood flow [FA(t)] was simulated by a two-phase idealized waveform mimicking the amplitude and shape of the measured flow changes:
where A1 and τ1 together determine the amplitude and shape of the larger, first phase of postcontractile flow, and A2 and τ2 determine the amplitude and peak time of the smaller, secondary phase of postcontractile flow. Venous blood flow [FV(t)] was equal to arterial blood flow, minus the change in muscle blood volume, which was modeled as proportional to the change in arterial flow according to a simple linear function:
The resulting d(BV)/dt function was smoothed with an exponential averaging time of 1 s to mimic the observed delay between peak flow and blood volume changes, and the arbitrary constant k was selected to mimic the observed peak change in BV at the observed peak arterial flow rates (Table 2).
The modeled time course of muscle oxygen consumption was based on the following assumptions. First, the mean ATP cost of maximum voluntary contractions in human anterior tibial muscle in young adult subjects is 1.7 mM/s (59). During the short contraction this ATP utilization is provided entirely by PCr depletion, which is resynthesized by mitochondrial ATP production after the contraction. Furthermore, PCr resynthesis rate after short contractions peaks within 2 s, and thereafter follows an exponential time course with an average time constant of 40 s (59). Therefore,
where ΔPCr is the change in PCr compared with the resting state. The last term prevents an instantaneous increase in PCr recovery rate after the contraction and is consistent with the observation that respiration in isolated mitochondria reaches a new steady state within less than 3 s after step changes in ADP and inorganic phosphate (60). Assuming the contraction-induced recovery oxygen consumption depends on the rate of PCr recovery and recovery P/O2 ratio = 6 (23), the postcontraction oxygen consumption (Q̇o2, in mM/s) is therefore
Converting to milliliters O2 per minute per 100 ml muscle:
Arterial O2 content (Vo2A) was fixed at 20 ml O2/100 ml blood, and the Stella model computed muscle and venous blood O2 content [Vo2M(t) and Vo2V(t), in ml O2/100 ml blood] over time according to
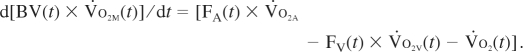 |
O2 saturation was calculated from Vo2M(t) according to the standard clinical formula, assuming 14 g Hb/100 ml blood:
To simulate the effect of transit time delays through the vasculature, saturation was exponentially smoothed to produce a 4-s delay relative to the peak flow. Finally, muscle SI was computed from blood volume and saturation according to Eq. I. Table 3 lists values for the key parameters in the base model. Also shown in Table 3 is the sensitivity of the computed peak postcontraction SI to small (1%) variations in these key parameters.
REFERENCES
- 1. Allen BW, Piantadosi CA. How do red blood cells dilate blood vessels? The SNO-hemoglobin paradigm. Am J Physiol Heart Circ Physiol 291: H1507–H1512, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol 581: 841–852, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armstrong ML, Dua AK, Murrant CL. Time course of vasodilation at the onset of repetitive skeletal muscle contractions. Am J Physiol Regul Integr Comp Physiol 292: R505–R515, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bangsbo J. Muscle oxygen uptake in humans at onset of and during intense exercise. Acta Physiol Scand 168: 457–464, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Berry KL, Skyrme-Jones RA, Cameron JD, O'Brien RC, Meredith IT. Systemic arterial compliance is reduced in young patients with IDDM. Am J Physiol Heart Circ Physiol 276: H1839–H1845, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol 85: 2249–2254, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Brodal P, Ingjer F, Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol Heart Circ Physiol 232: H705–H712, 1977 [DOI] [PubMed] [Google Scholar]
- 8. Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 11: 279–293, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buxton RB, Uludaæg K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. NeuroImage 23: S220–S233, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med 39: 855–864, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Candido R, Allen TJ. Haemodynamics in microvascular complications in type 1 diabetes. Diabetes Metab Res Rev 18: 286–304, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Clifford PS, Jasperse JL. Feedforward vasodilatation at the onset of exercise. J Physiol 583: 811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damon BM, Hornberger JL, Wadington MC, Lansdown DA, Kent-Braun JA. Dual gradient-echo MRI of post-contraction changes in skeletal muscle blood volume and oxygenation. Magn Reson Med 57: 670–679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duteil S, Bourrilhon C, Raynaud JS, Wary C, Richardson RS, Leroy-Willig A, Jouanin JC, Guezennec CY, Carlier PG. Metabolic and vascular support for the role of myoglobin in humans: a multiparametric NMR study. Am J Physiol Regul Integr Comp Physiol 287: R1441–R1449, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Duteil S, Wray C, Reynaud JS, Lebon V, Lesage D, Leroy-Willig A, Carlier PG. Influence of vascular filling and perfusion on BOLD contrast during reactive hyperemia in human skeletal muscle. Magn Reson Med 55: 450–454, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Fuchsjèager-Mayrl G, Pleiner J, Wiesinger GF, Sieder AE, Quittan M, Nuhr MJ, Francesconi C, Seit HP, Francesconi M, Schmetterer L, Wolzt M. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care 25: 1795–1801, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Gorczynski RJ, Duling BR. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am J Physiol Heart Circ Physiol 235: H505–H515, 1978 [DOI] [PubMed] [Google Scholar]
- 20. Grassi B. Regulation of oxygen consumption at exercise onset: is it really controversial? Exerc Sport Sci Rev 29: 134–138, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol 557: 1013–1020, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harkema SJ, Adams GR, Meyer RA. Acidosis has no effect on the ATP cost of contraction in cat fast- and slow-twitch skeletal muscles. Am J Physiol Cell Physiol 272: C485–C490, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Harkema SJ, Meyer RA. Effect of acidosis on control of respiration in skeletal muscle. Am J Physiol Cell Physiol 272: C491–C500, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Hermansen L, Wachtlova M. Capillary density of skeletal muscle in well-trained and untrained men. J Appl Physiol 30: 860–863, 1971 [DOI] [PubMed] [Google Scholar]
- 25. Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation 12: 113–127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med 31: 9–21, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Lansdown DA, Ding Z, Wadington M, Hornberger JL, Damon BM. Quantitative diffusion tensor MRI-based fiber tracking of human skeletal muscle. J Appl Physiol 103: 673–681, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lash JM, Bohlen HG. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol 72: 2052–2062, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Laughlin MH, Korthuis RJ, Dunker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 16, p. 705–769, 1996 [Google Scholar]
- 30. Laughlin MH, Schrage WG. Effects of muscle contraction on skeletal muscle blood flow: when is there a muscle pump? Med Sci Sports Exerc 31: 1027–1035, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Lebon V, Brillault-Salvat C, Bloch G, Leroy-Willig A, Carlier PG. Evidence of muscle BOLD effect revealed by simultaneous interleaved gradient-echo NMRI and myoglobin NMRS during leg ischemia. Magn Reson Med 40: 551–558, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Lebon V, Carlier PG, Brillault-Salvat C, Leroy-Willig A. Simultaneous measurement of perfusion and oxygenation changes using a multiple gradient-echo sequence: application to human muscle study. Magn Reson Imaging 16: 721–729, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Mackie BG, Terjung RL. Influence of training on blood flow to different skeletal muscle fiber types. J Appl Physiol 55: 1072–1078, 1983 [DOI] [PubMed] [Google Scholar]
- 34. Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38: 860–866, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Marshall JM, Tandon HC. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol 350: 447–459, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCully KK, Hamaoka T. Near-infrared spectroscopy: what can it tell us about oxygen saturation in skeletal muscle? Exerc Sport Sci Rev 28: 123–127, 2000 [PubMed] [Google Scholar]
- 37. McDermott MM, Kerwin DR, Liu K, Martin GJ, O'Brien E, Kaplan H, Greenland P. Prevalence and significance of unrecognized lower extremity peripheral arterial disease in general medicine practice. J Gen Intern Med 16: 384–390, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meredith CN, Frontera WR, Fisher EC, Hughes VA, Herland JC, Edwards J, Evans WJ. Peripheral effects of endurance training in young and old subjects. J Appl Physiol 66: 2844–2849, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Meyer RA, Foley JM, Harkema SJ, Sierra A, Potchen EJ. Magnetic resonance measurement of blood flow in peripheral vessels after acute exercise. Magn Reson Imaging 11: 1085–1092, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Meyer RA, Towse TF, Reid RW, Jayaraman RC, Wiseman RW, McCully KK. BOLD MRI mapping of transient hyperemia in skeletal muscle after single contractions. NMR Biomed 17: 392–398, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol 82: 282–287, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Mizuno M, Kimura Y, Iwakawa T, Oda K, Ishii K, Ishiwata K, Nakamura Y, Muraoka I. Regional differences in blood volume and blood transit time in resting skeletal muscle. Jpn J Physiol 53: 467–470, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Mohrman DE, Sparks HV. Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol 227: 531–535, 1974 [DOI] [PubMed] [Google Scholar]
- 44. Mohrman DE, Sparks HV. Role of potassium ions in the vascular response to a brief tetanus. Circ Res 35: 384–390, 1974 [DOI] [PubMed] [Google Scholar]
- 45. Mohrman DE, Sparks HV. Dynamics of exercise hyperemia. J Dynam Syst Measurements Control: 285–287, 1973 [Google Scholar]
- 46. Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar dilations. Am J Physiol Regul Integr Comp Physiol 282: R969–R978, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Murrant CL, Sarelius IH. Coupling of muscle metabolism and muscle blood flow in capillary units during contraction. Acta Physiol Scand 168: 531–541, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Olive JL, Slade JM, Dudley GA, McCully KK. Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J Appl Physiol 94: 701–706, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol Cell Physiol 272: C501–C510, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity 15: 2371–2379, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Raitakari M, Knuuti MJ, Ruotsalainen U, Laine H, Makea P, Teras M, Sipila H, Niskanen T, Raitakari OT, Iida H. Insulin increases blood volume in human skeletal muscle: studies using [15O]CO and positron emission tomography. Am J Physiol Endocrinol Metab 269: E1000–E1005, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Raitakari M, Nuutila P, Knuuti J, Raitakari OT, Laine H, Ruotsalainen U, Kirvela O, Takala TO, Iida H, Yki-Jarvinen H. Effects of insulin on blood flow and volume in skeletal muscle of patients with IDDM: studies using [15O]H2O, [15O]CO, and positron emission tomography. Diabetes 46: 2017–2021, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Richardson RS, Newcomer SC, Noyszewski EA. Skeletal muscle intracellular PO(2) assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol 91: 2679–2685, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Roman BB, Meyer RA, Wiseman RW. Phosphocreatine kinetics at the onset of contractions in skeletal muscle of MM creatine kinase knockout mice. Am J Physiol Cell Physiol 283: C1776–C1783, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Segal SS. Microvascular recruitment in hamster striated muscle: role for conducted vasodilation. Am J Physiol Heart Circ Physiol 261: H181–H189, 1991 [DOI] [PubMed] [Google Scholar]
- 56. Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand 168: 511–518, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Shoemaker JK, Phillips SM, Green HJ, Hughson RL. Faster femoral artery blood velocity kinetics at the onset of exercise following short-term training. Cardiovasc Res 31: 278–286, 1996 [PubMed] [Google Scholar]
- 59. Slade JM, Towse TF, Delano MC, Wiseman RW, Meyer RA. A gated 31P NMR method for the estimation of phosphocreatine recovery time and contractile ATP cost in human muscle. NMR Biomed 19: 573–580, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVo2, NADH, AND light scattering. J Biol Chem 276: 2586–2599, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Towse TF, Slade JM, Meyer RA. Effect of physical activity on MRI-measured blood oxygen level-dependent transients in skeletal muscle after brief contractions. J Appl Physiol 99: 715–722, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol 97: 739–747, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Tschakovsky ME, Shoemaker JK, Hughson RL. Beat-by-beat forearm blood flow with Doppler ultrasound and strain-gauge plethysmography. J Appl Physiol 79: 713–719, 1995 [DOI] [PubMed] [Google Scholar]
- 64. Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol 271: H1697–H1701, 1996 [DOI] [PubMed] [Google Scholar]
- 65. Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol 463: 631–646, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R987–R991, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Yang HT, Laughlin MH, Terjung RL. Prior exercise training increases collateral-dependent blood flow in rats after acute femoral artery occlusion. Am J Physiol Heart Circ Physiol 279: H1890–H1897, 2000 [DOI] [PubMed] [Google Scholar]
- 68. Yang HT, Ogilvie RW, Terjung RL. Training increases collateral-dependent muscle blood flow in aged rats. Am J Physiol Heart Circ Physiol 268: H1174–H1180, 1995 [DOI] [PubMed] [Google Scholar]
- 69. Zhao JM, Clingman CS, Narvainen MJ, Kauppinen RA, van Zijl PC. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med 58: 592–597, 2007 [DOI] [PubMed] [Google Scholar]