Abstract
Amino acid transporters and mammalian target of rapamycin complex 1 (mTORC1) signaling are important contributors to muscle protein anabolism. Aging is associated with reduced mTORC1 signaling following resistance exercise, but the role of amino acid transporters is unknown. Young (n = 13; 28 ± 2 yr) and older (n = 13; 68 ± 2 yr) subjects performed a bout of resistance exercise. Skeletal muscle biopsies (vastus lateralis) were obtained at basal and 3, 6, and 24 h postexercise and were analyzed for amino acid transporter mRNA and protein expression and regulators of amino acid transporter transcription utilizing real-time PCR and Western blotting. We found that basal amino acid transporter expression was similar in young and older adults (P > 0.05). Exercise increased L-type amino acid transporter 1/solute-linked carrier (SLC) 7A5, CD98/SLC3A2, sodium-coupled neutral amino acid transporter 2/SLC38A2, proton-assisted amino acid transporter 1/SLC36A1, and cationic amino acid transporter 1/SLC7A1 mRNA expression in both young and older adults (P < 0.05). L-type amino acid transporter 1 and CD98 protein increased only in younger adults (P < 0.05). eukaryotic initiation factor 2 α-subunit (S52) increased similarly in young and older adults postexercise (P < 0.05). Ribosomal protein S6 (S240/244) and activating transcription factor 4 nuclear protein expression tended to be higher in the young, while nuclear signal transducer and activator of transcription 3 (STAT3) (Y705) was higher in the older subjects postexercise (P < 0.05). These results suggest that the rapid upregulation of amino acid transporter expression following resistance exercise may be regulated differently between the age groups, but involves a combination of mTORC1, activating transcription factor 4, eukaryotic initiation factor 2 α-subunit, and STAT3. We propose an increase in amino acid transporter expression may contribute to enhanced amino acid sensitivity following exercise in young and older adults. In older adults, the increased nuclear STAT3 phosphorylation may be indicative of an exercise-induced stress response, perhaps to export amino acids from muscle cells.
Keywords: mammalian target of rapamycin complex 1, L-type amino acid transporter 1, activating transcription factor 4, proton-assisted amino acid transporter 1, sarcopenia
age-related sarcopenia is characterized by a gradual, but progressive, loss in skeletal muscle mass and strength (7, 14). The molecular and cellular mechanisms of sarcopenia are complex and result from many interacting physiological events (40). A factor that contributes to the loss of lean mass in older adults may be the inability to fully activate components of the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway following resistance exercise (15, 34, 38). The mTORC1 pathway is sensitive to changes in nutritional, hormonal, contractile, and energy status, and its activity notably leads to downstream phosphorylation of ribosomal S6 kinase 1 and 4E binding protein 1 (10). mTORC1 can also indirectly upregulate the transcription of several genes associated with amino acid transport (1, 37, 44, 45). This function appears to be partly mediated by activating transcription factor 4 (ATF4/cAMP response element binding protein 2) (1, 16, 31, 42), but the mechanisms of mTORC1-regulated amino acid transporter expression in young and older adult skeletal muscle are not yet understood.
Changes in amino acid availability can profoundly alter protein metabolism (50). Amino acid transporters have a key role in the regulation of muscle protein metabolism because of their ability to activate the mTORC1 signaling pathway. Amino acid transporters can influence mTORC1 activity, either by increasing the delivery of substrates (i.e., leucine) to an unknown upstream nutrient sensor, or by relaying signals to downstream targets, independent of amino acid transport (e.g., transceptor) (17, 24). Specifically, system L [L-type amino acid transporter 1 (LAT1)/solute-linked carrier (SLC) 7A5 and CD98/SLC3A2 heterodimeric complex] and system A [i.e., sodium-coupled neutral amino acid transporter 2 (SNAT2)/SLC38A2] amino acid transporters have received special attention because of their role in sensing changes in amino acid availability (3, 13, 26, 39, 41), transporting large neutral amino acids (i.e., branch-chain amino acids) into the cell to activate mTORC1 (2, 6, 27), and are transcriptionally regulated by a mTORC1-dependent mechanism (1, 37, 43–45). More recently, the proton-assisted amino acid transporters (i.e., PAT1) have been credited with regulating cell size and may also be important players in transmitting nutrient signals to mTORC1 (17–19, 21). The role of cationic amino acid transporter 1 (CAT1)/SLC7A1 in skeletal muscle is less clear, although it is a modulator of arginine transport (28, 48). To add further complexity, amino acid availability influences CAT1 expression (25, 36), while downregulation of CAT1 transporters by RNA interference in neuronal cells caused a marked reduction of mTORC1 activity and nerve growth (23). In support of the link between mTORC1 signaling and amino acid transporter expression, we have recently reported that an increase in amino acid availability increases mTORC1 signaling and protein synthesis as well as system L, system A, and PAT1 expression in human skeletal muscle (12).
Resistance exercise in fasted adults also increases amino acid transport and, subsequently, muscle protein synthesis, as assessed with the stable isotope tracer technique (4). To date, some information is available describing the role of specific amino acid transporters in rodent (20, 32), but not human, skeletal muscle following exercise. Since an unaccustomed bout of resistance exercise stimulates mTORC1 signaling and muscle protein synthesis in young but not older adults (15, 34, 38), we postulated that signaling and transcriptional events associated with amino acid transporter expression, downstream of mTORC1, may be reduced in older adults.
Therefore, the goal of this study was twofold: 1) to determine whether a single bout of resistance exercise alters amino acid transporter mRNA and protein expression in human skeletal muscle; and 2) to determine whether aging is associated with a differential response following exercise. We hypothesized that an acute bout of high-intensity resistance exercise would increase amino acid transporter mRNA and protein expression (LAT1/SLC7A5, CD98/SLC3A2, SNAT2/SLC38A2, PAT1/SLC36A1, and CAT1/SLC7A1) and ATF4 nuclear protein expression to a greater extent in younger than in older adults.
MATERIALS AND METHODS
Subjects.
We studied young (n = 13; 8 male, 5 female) and older (n = 13; 8 male, 5 female) subjects, who were a subset from a larger trial (15). Subjects only differed in age (young 28 ± 2 yr; old 68 ± 2 yr) and leg strength (one-repetition maximum: young 102 ± 9 kg; old 69 ± 5 kg) (P < 0.05). The subjects were considered physically active, but did not engage in any regular exercise training program at the time of enrollment. Screening of subjects was performed with clinical history, physical exam, and laboratory tests, including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance test, hepatitis B and C screening, human immuodeficiency virus test, TSH, lipid profile, urinalysis, and drug screening. Older subjects underwent additional screening that included an electrocardiogram and stress test on a treadmill. On a separate day, a dual-energy X-ray absorptiometry scan (Hologic QDR 4500W, Bedford, MA) was performed to measure lean and fat mass. Subjects were also tested for maximal strength by performing a one-repetition maximum on a leg extension machine (Cybex-VR2, Medway, MA) during the initial screening. A second one-repetition maximum testing was performed at least 1 wk before study participation. The higher of the two maximum tests was recorded as the subjects' one-repetition maximum. All subjects gave written, informed consents before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (which is in compliance with the Declaration of Helsinki).
Experimental design.
Subjects were admitted to the Institutional Translational Sciences-Clinical Research Center of the University of Texas Medical Branch the day before the study and refrained from exercise for 48 h before study participation. Subjects were fed a standard dinner and a snack at 2200 and were studied following an overnight fast. Experiments were conducted at the same approximate time of day to minimize subject-to-subject variation due to circadian rhythms and lengths of fasting. Further details involving tracer methodologies within the current experiment can be found elsewhere (15). The morning of the study (day 1), a muscle biopsy was obtained from the vastus lateralis using a 5-mm Bergström biopsy needle with suction under local anesthesia (1% lidocaine). Following the basal biopsy, subjects were transported to the exercise laboratory in which each subject performed 8 sets of 10 repetitions of bilateral leg extension exercise (Cybex International) at 70% of their one-repetition maximum. The rest period between sets was 3 min. On completion of exercise (∼40 min), subjects returned to their hospital bed for the remainder of the study. Muscle biopsies were sampled at 3 and 6 h following the completion of resistance exercise. Following the 6-h postexercise muscle biopsy, the subjects were fed a standardized lunch and dinner and a snack at 2200 as on the prior night. The next day after an overnight fast (day 2), a final biopsy was performed at 24 h postexercise.
On day 1, the basal biopsy was sampled out of a single incision, while the 3- and 6-h postexercise muscle biopsies were sampled from another incision site on the same leg ∼7 cm proximal to the previous incision site. To minimize trauma to muscle due to the previous biopsy sampling, muscle biopsies were angled in such a way that ∼5 cm separated each sampling location (49). On day 2, the 24-h postexercise muscle biopsy was sampled from a single incision on the opposite leg. Muscle tissue was immediately blotted and frozen in liquid nitrogen and stored at −80°C until analysis.
Phenylalanine and leucine concentrations.
Blood and muscle intracellular free concentrations of phenylalanine and leucine were determined via the internal standard method, which includes measurement of tracer enrichments for l-[ring-13C6]phenylalanine and [1-13C]leucine and appropriate internal standards (l-[13C9, 15N]phenylalanine and [5,5,5-2H3]leucine). Measurements were determined by gas chromatography-mass spectrometry (6890 Plus CG, 5973N MSD, 7683 autosampler, Agilent Technologies, Palo Alto, CA) as previously described (51).
RNA extraction and semiquantitative real-time PCR.
Total RNA, cDNA synthesis, and real-time PCR were conducted as previously reported (12). The average RNA integrity number for 104 total isolated RNA samples was 8.40 ± 0.03 (1–10 scale; 10 highest) and a 1.30 ± 0.01 28S-to-18S ratio. All isolated RNA and cDNA samples were stored at −80°C until further analysis. A majority of the primer sequences used in this experiment has been published previously (12). Unpublished sequences and the accession number for CAT1/SLC7A1 (NM_003045) are the following: Fwd: CCCACTCTACTTATCATCCC, Rev: ATACATTTAGCGACCTTTCTG. β2-Microglobulin was used as a normalization gene, as it did not change over time or between age groups. Relative fold changes were determined from the Ct values using the 2−ΔΔCt method (35).
ATF4 and STAT3 nuclear and cytoplasmic protein isolation.
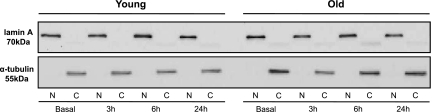
Isolation of protein subfractions was conducted using a commercially available kit (NE-PER; Thermo Fisher Scientific). Muscle samples were weighed and washed in 1× PBS. Samples were homogenized in a cytoplasmic extraction reagent (I) that contained an appropriate volume of protease inhibitors (Thermo-Scientific Halt Protease Inhibitor Cocktail). Muscle samples were then vortexed and incubated on ice. A second cytoplasmic extraction reagent was added (II), the sample was vortexed and then centrifuged, and the supernatant (cytoplasmic fraction) was collected. The pellet was resuspended in a nuclear extraction reagent, then vortexed and incubated on ice for a total of four times. Afterwards, the slurry was centrifuged, and the supernatant collected (nuclear subfraction). Total protein was determined for both of the protein subfractions using the Bradford assay. Nuclear and cytoplasmic protein was then subjected to Western blotting. Effective separation of the protein subfraction was accomplished by minimal/undetectable levels of α-tubulin (a cytoplasmic protein) in the nuclear protein compartment and lamin A (a nuclear protein) in the cytoplasmic protein compartment (Fig. 1).
Fig. 1.
Representative Western blot image from a young and older adult at basal and 3, 6, and 24 h postexercise. Gel was loaded alternately with nuclear (N) and cytoplasmic (C) subfractions and then probed with lamin A (a nuclear protein) and α-tubulin (a cytoplasmic protein).
Western blotting.
Protein expression analysis was performed as previously reported (9). Western blot data were normalized to an internal control (loaded on every gel to compare across blots). Antibodies used were the following: LAT1 (cat. no. 5347; Cell Signaling), CD98 (cat. no. sc-9160; Santa Cruz Biotechnology, Santa Cruz, CA), SNAT2 (cat. no. sc-67081; Santa Cruz Biotechnology), ribosomal protein S6 (rpS6) (S240/244; cat. no. 2215; Cell Signaling, Beverley, MA), eukaryotic initiation factor 2 α-subunit (eIF2α) (S52; cat. no. 44728G; Invitrogen, Carlsbad, CA), total eIF2α (cat. no. 9722; Cell Signaling), ATF4 (cat. no. sc-200; Santa Cruz Biotechnology); STAT3 (Y705; cat. no. 9131; Cell Signaling), total STAT3; (cat no. 9132; Cell Signaling), lamin A (cat. no. MAB3540; Millipore, Billerica, MA), and α-tubulin (cat. no. F2168; Sigma-Aldrich, St. Louis, MO). Appropriate secondary antibodies were used, dependent on the primary antibody of interest. Due to limited muscle tissue, only a subset of subjects was used for Western analysis (see results and Fig. 1–6 legends).
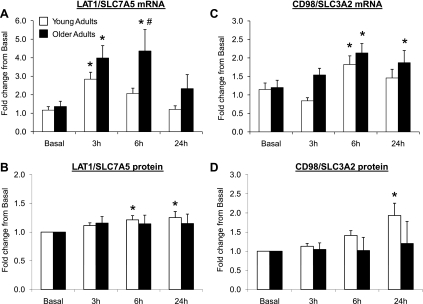
Fig. 2.
Data (means ± SE) represent expression for L-type amino acid transporter 1 (LAT1)/solute-linked carrier (SLC) 7A5 mRNA (A), LAT1/SLC7A5 protein (B), CD98/SLC3A2 mRNA (C), and CD98/SLC3A2 protein (D) before (basal) and after (3, 6, and 24 h) a single bout of resistance exercise in skeletal muscle of young and older adults. *Significantly different than basal (P < 0.05). #Significantly different between the age groups at indicated postexercise time point (P < 0.05). mRNA fold change was calculated using the 2−ΔΔCt method (35). Note: 13 young and 13 older adults were included in analysis, except for LAT1 (young, n = 13; old, n = 11) and CD98 protein expression (young, n = 12; old, n = 12).
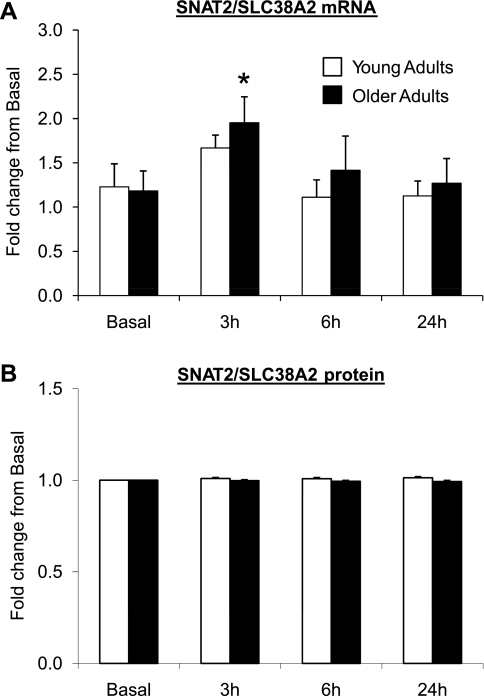
Fig. 3.
Data (means ± SE) represent sodium-coupled neutral amino acid transporter 2 (SNAT2)/SLC38A2 mRNA (A) and protein (B) expression before (basal) and after (3, 6, and 24 h) a single bout of resistance exercise in skeletal muscle of young (n = 13) and older (n = 13) adults. *Significantly different than basal (P < 0.05). mRNA fold change was calculated using the 2−ΔΔCt method (35).
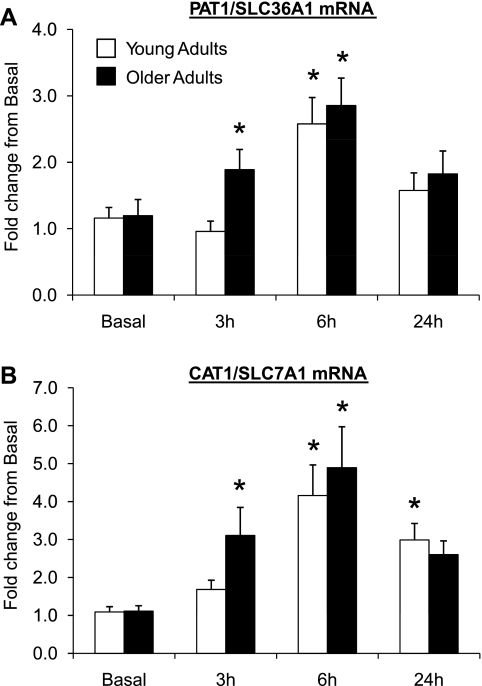
Fig. 4.
Data (means ± SE) represent mRNA expression for proton-assisted amino acid transporter 1 (PAT1)/SLC36A1 (A) and cationic amino acid transporter 1 (CAT1)/SLC7A1 (B) before (basal) and after (3, 6, and 24 h) a single bout of resistance exercise in skeletal muscle of young (n = 13) and older (n = 13) adults. *Significantly different than basal (P ≤ 0.05). mRNA fold change was calculated using the 2−ΔΔCt method (35).
Fig. 5.
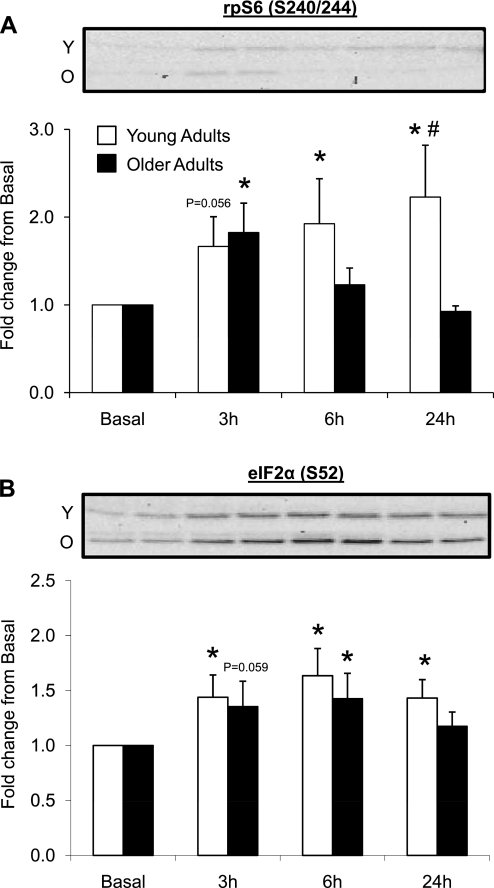
Data (means ± SE) represent phosphorylation of ribosomal protein S6 (rpS6) at Ser240/244 (A) and eukaryotic initiation factor 2 α-subunit (eIF2α) at Ser52 (B) before (basal) and after (3, 6, and 24 h) a single bout of resistance exercise in skeletal muscle of young and older adults. Western blot insets are representative images corresponding to time points below (in duplicate) from a single young and older adult. *Significantly different than basal (P < 0.05). #Significantly different between the age groups at indicated postexercise time point (P < 0.05). Note: 13 young and 13 older adults were included in analysis, except for eIF2α (S52) expression (young, n = 13; old, n = 12).
Fig. 6.
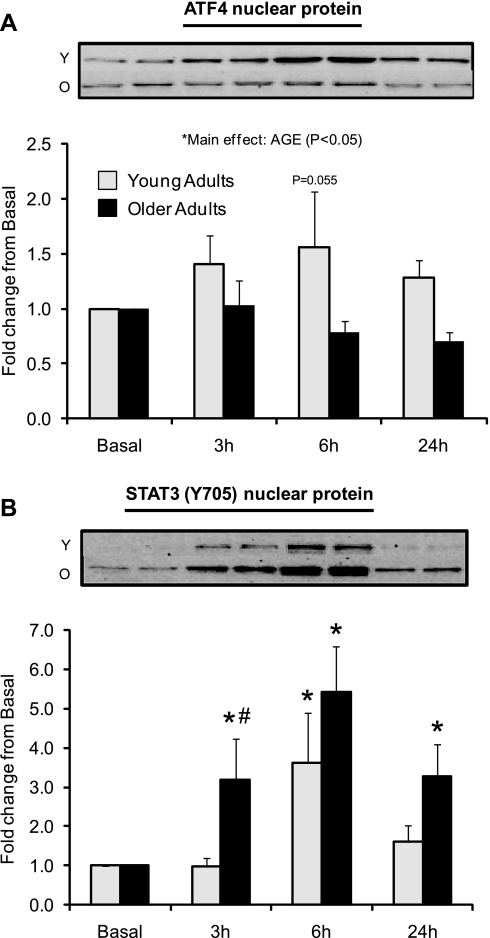
Data (means ± SE) represent protein expression for nuclear activating transcription factor 4 (ATF4; A) and STAT3 phosphorylation at Thr705 (B) before (basal) and after (3, 6, and 24 h) a single bout of resistance exercise in skeletal muscle of young and older adults. Western blot insets are representative images corresponding to time points below (in duplicate) from a single young and older adult. *Significantly different than basal (P < 0.05). #Significantly different between the age groups at indicated postexercise time point (P ≤ 0.05). Note: a subset of the enrolled subjects was included for nuclear ATF4 (young, n = 12; old, n = 12) and STAT3 (young, n = 11; old, n = 12) analysis.
Statistical analysis.
To measure differences across time and between age groups, data were statistically analyzed using a two-way repeated-measures ANOVA. When a main effect existed, post hoc tests (Fisher least significant difference) were conducted to assess specific interactions. Subject characteristic analysis was conducted using an unpaired t-test. Significance was set at P < 0.05, unless otherwise noted. All values are presented as means ± SE. All analyses were performed with SigmaStat software (version 3.5).
RESULTS
Blood and muscle intracellular phenylalanine and leucine concentrations.
Phenylalanine and leucine concentrations from young and older adults before and after resistance exercise are presented in Table 1. Blood leucine concentrations were decreased at 3 h postexercise in young and older subjects compared with basal levels (P < 0.05). Muscle intracellular phenylalanine concentrations decreased across all postexercise time points in young and older adults compared with basal levels (P < 0.05). There was a main effect for time for blood phenylalanine (P < 0.05). When the age groups were collapsed, there was a tendency for blood phenylalanine to decrease at 3 h (P = 0.06), while there was a decrease at 6 h postexercise compared with basal levels (P < 0.05). There were no changes in muscle intracellular leucine concentrations following resistance exercise compared with basal levels (P > 0.05). There were no differences between the age groups for any of the phenylalanine or leucine parameters (P > 0.05).
Table 1.
Blood and muscle intracellular phenylalanine and leucine concentration before and after resistance exercise in young and older subjects
| Basal | 3 h Post | 6 h Post | 24 h Post | |
|---|---|---|---|---|
| Blood phenylalanine, μmol/l | ||||
| Young | 65 ± 3 | 62 ± 2 | 60 ± 3 | 68 ± 4 |
| Old | 66 ± 4 | 60 ± 3 | 60 ± 3 | 66 ± 4 |
| Blood leucine, μmol/l | ||||
| Young | 157 ± 6 | 136 ± 4* | 147 ± 5 | 157 ± 9 |
| Old | 144 ± 9 | 126 ± 8* | 144 ± 9 | 138 ± 5 |
| Muscle intracellular phenylalanine, μmol/l | ||||
| Young | 65 ± 5 | 54 ± 3* | 48 ± 2* | 50 ± 4* |
| Old | 70 ± 4 | 59 ± 3* | 57 ± 5* | 57 ± 4* |
| Muscle intracellular leucine, μmol/l | ||||
| Young | 170 ± 18 | 153 ± 15 | 150 ± 18 | 165 ± 18 |
| Old | 176 ± 12 | 168 ± 21 | 148 ± 15 | 144 ± 12 |
Values are means ± SE; n = 13 young and n = 13 older subjects, except for muscle intracellular leucine (young, n = 12; old, n = 12). Post, after resistance exercise.
Significantly different from basal (P < 0.05). Time main effect at 6 h for blood phenylalanine (P < 0.05).
Amino acid transporter mRNA and protein expression.
LAT1/SLC7A5 mRNA expression (Fig. 2A) was elevated at 3 h postexercise for both age groups compared with basal (P < 0.05). However, LAT1/SLC7A5 mRNA expression in the older adults remained elevated and was higher at 6 h postexercise than in the younger adults (P < 0.05). LAT1/SLC7A5 protein expression (Fig. 2B) (young: n = 13, old: n = 11) was elevated ∼25% in the young adults at 6 and 24 h postexercise compared with basal (P < 0.05). There were no significant changes in LAT1/SLC7A5 protein expression from basal at any time point in the older adults (3 h, P = 0.16; 6 h, P = 0.19; 24 h, P = 0.18). CD98/SLC3A2 mRNA expression (Fig. 2C) (young: n = 12, old: n = 12) was elevated at 6 h postexercise in young and older adults compared with basal levels (P < 0.05). CD98/SLC3A2 protein expression (Fig. 2D) was elevated only in young adults at 24 h postexercise (∼100%) compared with basal (P < 0.05). There were no changes in CD98/SLC3A2 protein expression at any time point compared with basal in the older adults (P > 0.05). SNAT2/SLC38A2 mRNA expression (Fig. 3A) was elevated at 3 h postexercise only for the older adults compared with basal levels (P < 0.05). SNAT2/SLC38A2 protein expression (Fig. 3B) was unchanged in young and older adults postexercise compared with basal levels (P > 0.05). PAT1/SLC36A1 mRNA expression (Fig. 4A) tended to be higher at 3 h only in the older adults (P = 0.053) and was elevated at 6 h postexercise in both younger and older adults compared with basal levels (P < 0.05). CAT1/SLC7A1 mRNA expression (Fig. 4B) was elevated at 3 and 6 h postexercise in the older adults compared with basal (P < 0.05), while CAT1/SLC7A1 mRNA expression increased at 6 and 24 h postexercise in younger adults compared with basal levels (P < 0.05). There were no differences in basal amino acid transporter mRNA and protein expression levels between young and older adults (P > 0.05; data not shown).
rpS6 (S240/244) and eIF2α (S52) phosphorylation.
rpS6 phosphorylation (S240/244) (Fig. 5A) tended to be elevated at 3 h (P = 0.056), but was elevated at 6 and 24 h postexercise in the younger adults compared with basal levels (P < 0.05). rpS6 phosphorylation was elevated at 3 h postexercise in older adults compared with basal levels (P < 0.05). In addition, younger adults exhibited a higher rpS6 phosphorylation at 24 h compared with older adults (P < 0.05). eIF2α (S52) phosphorylation (Fig. 5B) (young: n = 13, old: n = 12) was elevated 3–24 h postexercise in younger adults compared with basal levels (P < 0.05). eIF2α (S52) phosphorylation tended to be elevated at 3 h (P = 0.059), but was elevated at 6 h in the older adults compared with basal levels (P < 0.05). There were no differences at baseline between young and older adults for rpS6 or eIF2α phosphorylation (P > 0.05; data not shown).
ATF4 and STAT3 (Y705) nuclear expression.
Nuclear protein expression of ATF4 (Fig. 6A) (young: n = 12, old: n = 12) was modestly higher in younger vs. the older adults [main effect for age (P < 0.05)]. There was a tendency for ATF4 nuclear expression to be higher in the younger adults 6 h postexercise compared with basal levels (P = 0.055). Phospho-STAT3 (Y705) nuclear expression (Fig. 6B) (young: n = 11, old: n = 12) was elevated at 6 h postexercise in the younger adults (P < 0.05) and elevated at 3, 6, and 24 h postexercise in the older adults compared with basal levels (P < 0.05). Older adults tended to have higher STAT3 (Y705) nuclear expression at 3 h postexercise compared with younger adults (P = 0.054). There were no differences at baseline between young and older adults for nuclear ATF4 expression or STAT3 nuclear phosphorylation (P > 0.05; data not shown).
DISCUSSION
The novel findings of this study were that 1) a single bout of high-intensity resistance exercise increased the expression of several skeletal muscle amino acid transporters (LAT1/SLC7A5, SNAT2/SLC38A2, CD98/SLC3A2, PAT1/SLC36A1, and CAT1/SLC7A1) in healthy men and women independent of age; 2) resistance exercise increased rpS6 phosphorylation (S240/244) and the nuclear protein expression of ATF4 primarily in young adults; and 3) nuclear protein expression of STAT3 (Y705) was increased to a greater extent in older adults. These data suggest that, in young adults, the upregulation of amino acid transporter mRNA and protein expression following exercise may be associated with enhanced mTORC1 signaling and nuclear ATF4 expression. However, in older adults, the increase in amino acid transporter expression may be regulated by a different mechanism, which may involve enhanced STAT3 phosphorylation. We propose that increased amino acid transporter mRNA and protein expression following a bout of resistance exercise may be an adaptive mechanism to increase muscle amino acid sensitivity to increase amino acid import/export during postexercise recovery or to amplify the muscle protein synthesis response when combined with a subsequent anabolic stimulus (i.e., protein-rich meal). Future work is required to determine the precise role and contribution of the exercise-induced upregulation of amino acid transporter expression on amino acid sensitivity and the regulation of intracellular amino acid availability.
Our findings clearly show that amino acid transporter mRNA expression (LAT1/SLC7A5, CD98/SLC3A2, SNAT2/SLC38A2, and CAT1/SLC7A1) increased between 3 and 24 h postexercise in fasted adults and, surprisingly, was not different between the age groups (Figs. 2–4). Although we noticed an increase in LAT1 and CD98 protein expression that was limited to young subjects, the inability to detect changes in transporter protein expression in older adults may be due to an underpowered sample size. In the present study design, we were unable to gather information on amino acid kinetics to clarify the direction of amino acid transport following exercise (Table 1); however, we propose that increased amino acid transporter expression may be a mechanism to increase the sensitivity of muscle cells to amino acids. An increase in amino acid transporters postexercise could be important for 1) acutely increasing amino acid influx/efflux for muscle protein synthesis/breakdown (4); 2) increasing amino acid sensitivity for a protein-rich meal (5, 11); and/or 3) increasing amino acid sensitivity in response to a second bout of exercise. Evidence consistent with this “sensitizing” hypothesis is the observation that a bout of exercise in rats improves insulin and IGF-I-stimulated system A transport (i.e., SNAT2/SLC38A2) activity during postexercise recovery (20, 52), while an anabolic mixture provided after resistance exercise in humans enhances the rate of muscle protein synthesis compared with exercise independent of feeding (5, 8, 11). We have recently reported that resistance exercise increased muscle protein synthesis in young but not older adults over a 24-h postexercise period (15). Therefore, our present data, in light of Fry et al. (15), would infer that an upregulation of amino acid transporters may not regulate muscle protein synthesis in the immediate postexercise period when in the fasted state (at least in older adults), but instead may influence amino acid sensitivity for a subsequent anabolic stimulus. In support of this notion, Drummond et al. (11) showed that a single bout of exercise when followed with 20 g of essential amino acids increased muscle protein synthesis similarly in healthy, young and older adults 1–6 h postexercise. Regardless, whether or not increased amino acid transporter expression is partly responsible for improving muscle cell sensitization to amino acids, an increase in amino acid transporter expression is an adaptive response to an acute bout of unaccustomed resistance exercise and may be an important player in the overall protein anabolic response following resistance exercise.
Following resistance exercise, we found that rpS6 phosphorylation (S240/244) (Fig. 5A) and nuclear ATF4 (Fig. 6A) expression was significantly increased primarily in young but not older subjects. ATF4 has been implicated in regulating several genes associated with amino acid transport (i.e., LAT1/SLC7A5, CD98/SLC3A2, SNAT2/SLC38A2, and CAT1/SLC7A1) (1, 31, 37). Additionally, mTORC1 signaling has been proposed to be a regulator of ATF4 and amino acid transporter expression, as demonstrated by cell culture experiments utilizing the chemical inhibitor rapamycin (1, 37, 43–45). However, to the best of our knowledge, no data have been reported identifying a regulatory role for mTORC1 signaling on PAT1 expression. The possibility of mTORC1 playing a part in driving ATF4 expression is modeled well in our present findings and is in agreement with our previous report showing robust increases in amino acid transporter expression following amino acid ingestion in young subjects in association with increased rpS6 phosphorylation and ATF4 expression (12).
ATF4 expression can also be positively regulated by eIF2α Ser52 phosphorylation although, paradoxically, eIF2α (S52) inhibits protein synthesis (22, 33). In our study, we show a similar age-related increase in phosphorylation, whereas the increase in ATF4 nuclear expression was limited to young adults, suggesting that ATF4 may be regulated by eIF2α phosphorylation in young adults. Therefore, amino acid transporter expression may be linked to mTORC1 signaling (e.g., rpS6), ATF4, and eIF2α (S52) expression in young adults following resistance exercise, while this relationship is less obvious in older subjects.
We found that STAT3 (Y705) nuclear protein expression was elevated to a greater extent in older subjects compared with the young subjects postexercise (Fig. 6B). Recently, two independent studies by Jones et al. (29, 30) showed that IL-6, TNF-α, and adiponectin increased system A transporter expression (i.e., SNAT2) in placental trophoblast cells, and this was mediated through STAT3 transcriptional activity. Elevated STAT3 phosphorylation (Y705) is in agreement with previous resistance exercise studies in older subjects (46, 47) and implies that stress-mediated damage may elevate nuclear STAT3 levels and perhaps increase amino acid transporter expression for amino acid export. Although the increase in SNAT2/SLC38A2 expression was small in our experiment, we cannot rule out that STAT3 (Y705) impacts the expression of other amino acid transporters. Our initial data would suggest that ATF4 may play a regulatory role (at least in the young subjects) controlling amino acid transporter expression, but clearly is not the only mechanism, as additional factors such as the muscle stress response following resistance exercise may also be involved.
We conclude that, following a single bout of resistance exercise, 1) the mRNA and/or protein expression of several amino acid transporters is elevated in both young and older adults; and 2) amino acid transporter expression is likely regulated differently in young and older subjects. The specific mechanism(s) that regulates transporter expression remains to be elucidated, but may involve mTORC1 signaling, ATF4, eIF2α, and STAT3 expression. An increase in amino acid transporter expression following resistance exercise may be an adaptive mechanism to increase amino acid sensitivity within the cell during anabolic conditions of muscle growth and/or to export amino acids when protein turnover increases during muscle remodeling.
GRANTS
This study was supported in part by National Institutes of Health (NIH) Grants R01-AR-049877 and P30-AG-024832. Additional support came from the NIH/National Center for Research Resources (1UL1RR029876-01) and NIH T32-HD007539.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the nursing staff at the Institute for Translational Sciences Clinical Research Center and the Claude E. Pepper Older Americans Independence Center recruitment coordinators, and Dr. Shaheen Dhanani for assistance in screening, admitting, and assisting with the subjects during data collection. We also thank Shelley Medina, Junfang Hao, and Ming Zheng for help with the data analysis.
REFERENCES
- 1. Adams CM. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem 282: 16744–16753, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of system A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab 297: E822–E829, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bevington A, Brown J, Butler H, Govindji S, KMK, Sheridan K, Walls J. Impaired system A amino acid transport mimics the catabolic effects of acid in L6 cells. Eur J Clin Invest 32: 590–602, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141: 568–573, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem 277: 9952–9957, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412–423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 106: 1374–1384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104: 1452–1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298: E1011–E1018, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signaling to protein synthesis in L6 muscle cells. J Am Soc Nephrol 18: 1426–1436, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ 2005: pe24, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skeletal Muscle 1: ?–?, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gjymishka A, Palii SS, Shan J, Kilberg MS. Despite increased ATF4 binding at the C/EBP-ATF composite site following activation of the unfolded protein response, system A transporter 2 (SNAT2) transcription activity is repressed in HepG2 cells. J Biol Chem 283: 27736–27747, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goberdhan DC. Intracellular amino acid sensing and mTORC1-regulated growth: new ways to block an old target? Curr Opin Investig Drugs 11: 1360–1367, 2010 [PMC free article] [PubMed] [Google Scholar]
- 18. Goberdhan DC, Meredith D, Boyd CA, Wilson C. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development 132: 2365–2375, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Goberdhan DC, Ogmundsdottir MH, Kazi S, Reynolds B, Visvalingam SM, Wilson C, Boyd CA. Amino acid sensing and mTOR regulation: inside or out? Biochem Soc Trans 37: 248–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henriksen EJ, Louters LL, Stump CS, Tipton CM. Effects of prior exercise on the action of insulin-like growth factor I in skeletal muscle. Am J Physiol Endocrinol Metab 263: E340–E344, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene 29: 4068–4079, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59: 407–450, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, Snyder SH. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J Neurosci 27: 449–458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296: E603–E613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hyatt SL, Aulak KS, Malandro M, Kilberg MS, Hatzoglou M. Adaptive regulation of the cationic amino acid transporter-1 (Cat-1) in Fao cells. J Biol Chem 272: 19951–19957, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Hyde R, Christie GR, Litherland GJ, Hajduch E, Taylor PM, Hundal HS. Subcellular localization and adaptive up-regulation of the System A (SAT2) amino acid transporter in skeletal-muscle cells and adipocytes. Biochem J 355: 563–568, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates system A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19: 461–463, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J 373: 1–18, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones HN, Jansson T, Powell TL. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes 59: 1161–1170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol 297: C1228–C1235, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. King PA. Effects of insulin and exercise on amino acid transport in rat skeletal muscle. Am J Physiol Cell Physiol 266: C524–C530, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Kubica N, Jefferson LS, Kimball SR. Eukaryotic initiation factor 2B and its role in alterations in mRNA translation that occur under a number of pathophysiological and physiological conditions. Prog Nucleic Acid Res Mol Biol 81: 271–296, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–8, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Lopez AB, Wang C, Huang CC, Yaman I, Li Y, Chakravarty K, Johnson PF, Chiang CM, Snider MD, Wek RC, Hatzoglou M. A feedback transcriptional mechanism controls the level of the arginine/lysine transporter cat-1 during amino acid starvation. Biochem J 402: 163–173, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malmberg SE, Adams CM. Insulin signaling and the general amino acid control response. Two distinct pathways to amino acid synthesis and uptake. J Biol Chem 283: 19229–19234, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol 107: 1655–1662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McDowell HE, Christie GR, Stenhouse G, Hundal HS. Leucine activates system A amino acid transport in L6 rat skeletal muscle cells. Am J Physiol Cell Physiol 269: C1287–C1294, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95: 139–159, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Palii SS, Kays CE, Deval C, Bruhat A, Fafournoux P, Kilberg MS. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids 37: 79–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palii SS, Thiaville MM, Pan YX, Zhong C, Kilberg MS. Characterization of the amino acid response element within the human sodium-coupled neutral amino acid transporter 2 (SNAT2) System A transporter gene. Biochem J 395: 517–527, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol 22: 5575–5584, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol 296: C142–C150, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Roos S, Lagerlof O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol 297: C723–C731, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Thalacker-Mercer AE, Dell'Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trenerry MK, Carey KA, Ward AC, Farnfield MM, Cameron-Smith D. Exercise-induced activation of STAT3 signaling is increased with age. Rejuvenation Res 11: 717–724, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Arch 447: 532–542, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Volpi E, Chinkes DL, Rasmussen BB. Sequential muscle biopsies during a 6-h tracer infusion do not affect human mixed muscle protein synthesis and muscle phenylalanine kinetics. Am J Physiol Endocrinol Metab 295: E959–E963, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wolfe RR. Regulation of muscle protein by amino acids. J Nutr 132: 3219S–3224S, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research Principles and Practice of Kinetic Analysis. Hobokon, NJ: Wiley-Liss, 2005 [Google Scholar]
- 52. Zorzano A, Balon TW, Garetto LP, Goodman MN, Ruderman NB. Muscle alpha-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol Endocrinol Metab 248: E546–E552, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.