Abstract
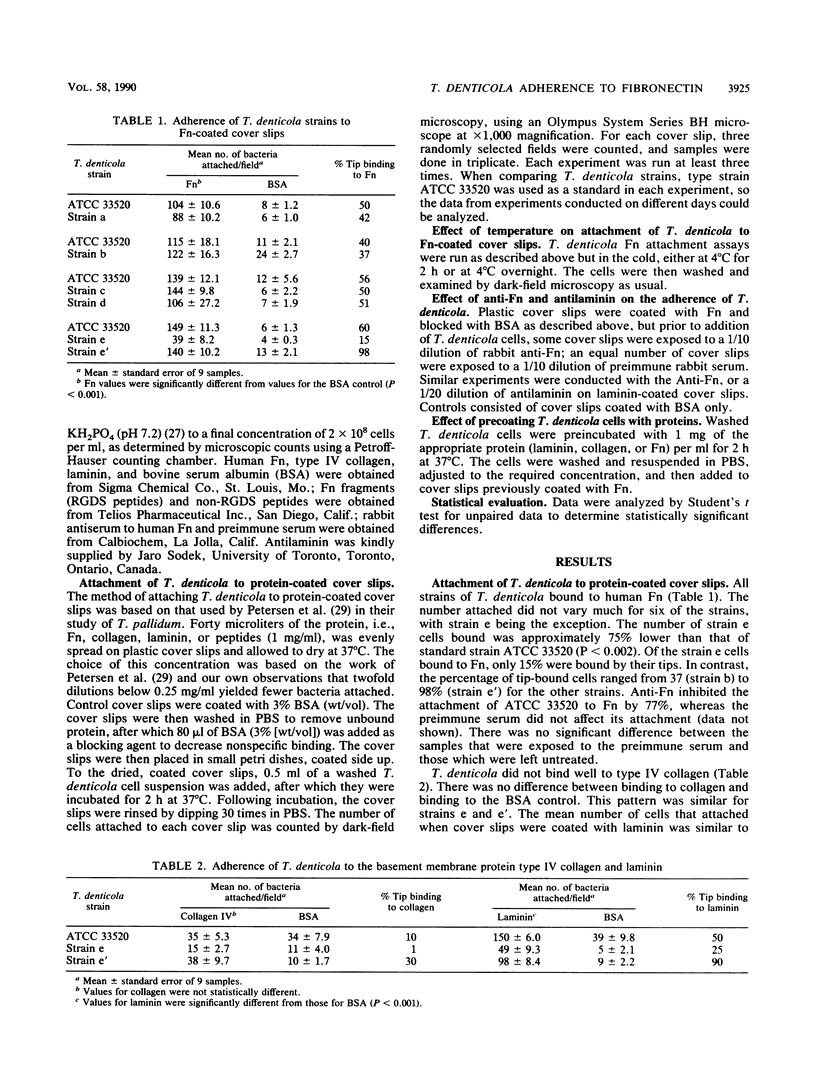
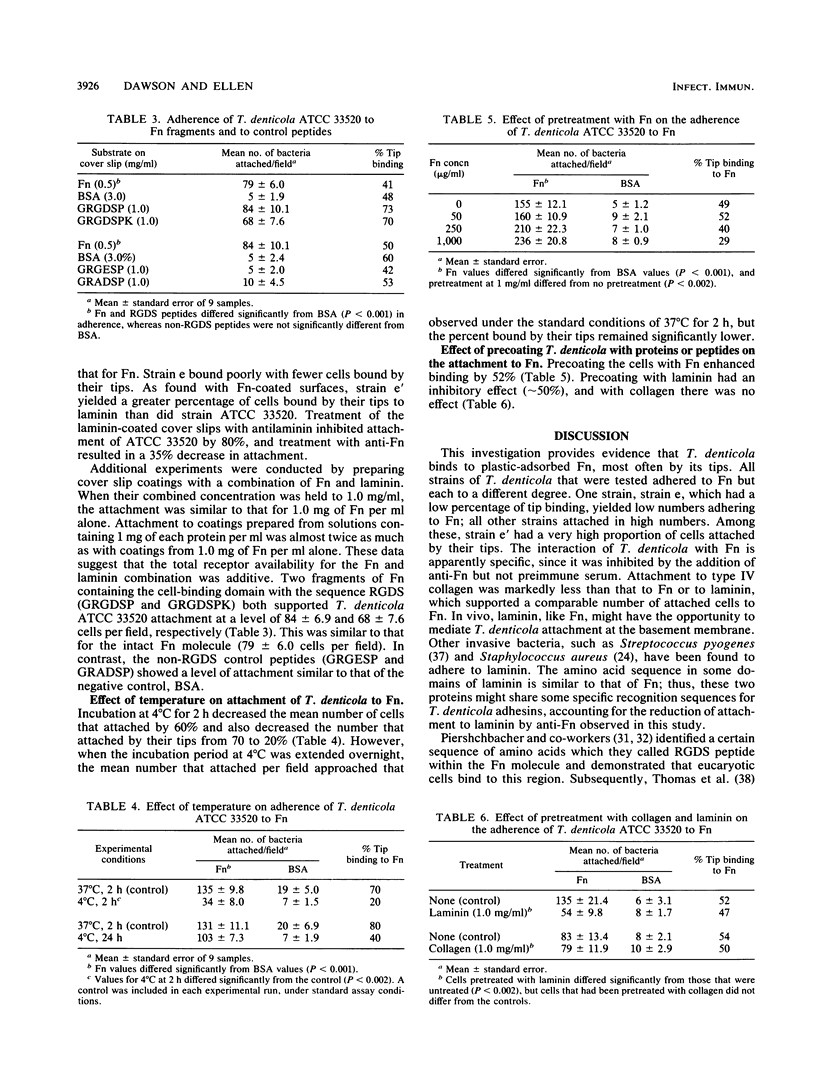
The adherence of Treponema denticola to ligands on cell surfaces or in basement membranes of periodontal tissues might play an important role in its pathogenicity. A direct microscopic assay was used to examine the binding of T. denticola to fibronectin and other protein substrates adsorbed on plastic cover slips. All strains of T. denticola that were tested adhered to fibronectin but to different degrees. The strains which bound in high numbers frequently bound by their tips. Type strain ATCC 33520 bound to fibronectin in high numbers (149 +/- 11.3 bacteria per microscopic field), with 60% bound by the tips. Strain e' bound in high numbers (140 +/- 10.2) and had the highest percentage of tip binding (98%); strain e bound in lowest numbers (39 +/- 8.2) and had the lowest percentage of tip binding (15%). Laminin supported binding at a level similar to that of fibronectin, as did fibronectin fragments which contained the cell binding domain peptides, RGDS. Type IV collagen and non-RGDS peptides did not support binding. Binding to fibronectin and laminin was inhibited by the addition of antifibronectin and antilaminin antibodies. By lowering the incubation temperature from 37 to 4 degrees C, the number of cells that attached decreased by 60% and tip binding was reduced by 50%. Pretreatment of the cells with collagen did not affect binding, whereas fibronectin pretreatment enhanced binding by 50% and laminin pretreatment resulted in a decrease of 60%. T. denticola adheres by its tips to fibronectin-coated surfaces, which suggests that fibronectin-specific adhesins cluster at the tips.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Cobb C. M., Taylor R. E., Fullmer H. M. Trypsin activation of latent collagenase from several mammalian sources. Scand J Dent Res. 1975 Sep;83(5):302–305. doi: 10.1111/j.1600-0722.1975.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Boehringer H., Berthold P. H., Taichman N. S. Studies on the interaction of human neutrophils with plaque spirochetes. J Periodontal Res. 1986 May;21(3):195–209. doi: 10.1111/j.1600-0765.1986.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Boehringer H., Taichman N. S., Shenker B. J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984 Jul;45(1):155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. L., Chan E. C. The routine isolation, growth, and maintenance of the intermediate-size anaerobic oral spirochetes from periodontal pockets. J Periodontal Res. 1983 Jul;18(4):362–368. doi: 10.1111/j.1600-0765.1983.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Cheng S. L., Siboo R., Quee T. C., Johnson J. L., Mayberry W. R., Chan E. C. Comparative study of six random oral spirochete isolates. Serological heterogeneity of Treponema denticola. J Periodontal Res. 1985 Nov;20(6):602–612. doi: 10.1111/j.1600-0765.1985.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Cimasoni G., McBride B. C. Adherence of Treponema denticola to modified hydroxyapatite. J Dent Res. 1987 Dec;66(12):1727–1729. doi: 10.1177/00220345870660120601. [DOI] [PubMed] [Google Scholar]
- Condacci I., Cimasoni G., Ahmad-Zadeh C. Alpha 2-macroglobulin in sulci from healthy and inflamed human gingivae. Infect Immun. 1982 Apr;36(1):66–71. doi: 10.1128/iai.36.1.66-71.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn N. E., Westergaard J. Nutrient and environmental growth factors for eight small-sized oral spirochetes. Scand J Dent Res. 1986 Jun;94(3):208–218. doi: 10.1111/j.1600-0722.1986.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect Immun. 1985 Oct;50(1):77–81. doi: 10.1128/iai.50.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhe J., Liljenberg B., Listgarten M. Some microbiological and histopathological features of periodontal disease in man. J Periodontol. 1980 May;51(5):264–269. doi: 10.1902/jop.1980.51.5.264. [DOI] [PubMed] [Google Scholar]
- Lindhe J., Socransky S. S. Chemotaxis and vascular permeability produced by human periodontopathic bacteria. J Periodontal Res. 1979 Mar;14(2):138–146. doi: 10.1111/j.1600-0765.1979.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Subgingival microbiological differences between periodontally healthy sites and diseased sites prior to and after treatment. Int J Periodontics Restorative Dent. 1984;4(1):27–34. [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Schmidt E., Morrison E. C. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985 Aug;56(8):447–456. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. The role of spirochetes in periodontal disease. Adv Dent Res. 1988 Nov;2(2):275–283. doi: 10.1177/08959374880020021201. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Lowrance J. H., Hasty D. L., Simpson W. A. Adherence of Streptococcus sanguis to conformationally specific determinants in fibronectin. Infect Immun. 1988 Sep;56(9):2279–2285. doi: 10.1128/iai.56.9.2279-2285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman H. N. The apical border of plaque in chronic inflammatory periodontal disease. Br Dent J. 1976 Aug 17;141(4):105–113. doi: 10.1038/sj.bdj.4803800. [DOI] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. Attachment of Treponema denticola to cultured human epithelial cells. Scand J Dent Res. 1984 Feb;92(1):55–63. doi: 10.1111/j.1600-0722.1984.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Baseman J. B., Alderete J. F. Treponema pallidum receptor binding proteins interact with fibronectin. J Exp Med. 1983 Jun 1;157(6):1958–1970. doi: 10.1084/jem.157.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew D. W., Sodek J., Wang H. M., Brunette D. M. Inhibitors of collagenolytic enzymes synthesized by fibroblasts and epithelial cells from porcine and macaque periodontal tissues. Arch Oral Biol. 1980;25(4):269–274. doi: 10.1016/0003-9969(80)90033-3. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M., Hayman E. G., Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjens F. M., Mikx F. H., Wolters-Lutgerhorst J. M., Maltha J. C. Adherence of oral treponemes and their effect on morphological damage and detachment of epithelial cells in vitro. Infect Immun. 1986 Feb;51(2):642–647. doi: 10.1128/iai.51.2.642-647.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Domain structure of human plasma fibronectin. Differences and similarities between human and hamster fibronectins. J Biol Chem. 1983 Mar 25;258(6):3967–3973. [PubMed] [Google Scholar]
- Sela M. N., Weinberg A., Borinsky R., Holt S. C., Dishon T. Inhibition of superoxide production in human polymorphonuclear leukocytes by oral treponemal factors. Infect Immun. 1988 Mar;56(3):589–594. doi: 10.1128/iai.56.3.589-594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Enhanced levels of attachment of fibronectin-primed Treponema pallidum to extracellular matrix. Infect Immun. 1986 Jun;52(3):736–741. doi: 10.1128/iai.52.3.736-741.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto V. J., Chan E. C., Quee T. C. Initial characterization of neutral proteinases from oral spirochetes. J Periodontal Res. 1986 Mar;21(2):95–100. doi: 10.1111/j.1600-0765.1986.tb01442.x. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Grenier D., Chan E. C., McBride B. C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988 Oct;56(10):2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto V. J., Raeste A. M. Activation of latent collagenase of human leukocytes and gingival fluid by bacterial plaque. J Dent Res. 1978 Jul-Aug;57(7-8):844–851. doi: 10.1177/00220345780570071401. [DOI] [PubMed] [Google Scholar]
- Vinh T., Faine S., Adler B. Adhesion of leptospires to mouse fibroblasts (L929) and its enhancement by specific antibody. J Med Microbiol. 1984 Aug;18(1):73–85. doi: 10.1099/00222615-18-1-73. [DOI] [PubMed] [Google Scholar]



