Abstract
The kidney contains receptors for the cytokine IL-25, but the effects of IL-25 in CKD are unknown. Here, we induced adriamycin nephropathy in both BALB/c mice and severe combined immunodeficient (SCID) mice, and we injected IL-25 for 7 consecutive days starting at day 5 after adriamycin administration. BALB/c mice treated with IL-25 had less glomerulosclerosis, tubular atrophy, interstitial expansion, and proteinuria than control mice at day 28. IL-25 increased the levels of IL-4 and IL-13 in serum, kidney, renal draining lymph nodes, and CD4+ lymphocytes. IL-25 also directly suppressed effector macrophages in vitro and in vivo and induced alternatively activated (M2) macrophages in vivo. However, in SCID mice and in BALB/c mice treated with IL-4/13–neutralizing antibody, IL-25 failed to protect against renal injury and did not induce M2. In conclusion, IL-25 protects against renal injury in adriamycin nephropathy in mice by, at least in part, inducing Th2 immune responses.
IL-25 is a member of the IL-17 family of cytokines capable of inducing Th2-associated cytokine production, but is distinctly different in function from other members of this family that act to promote rather than ameliorate proinflammatory responses in various tissues.1 IL-25 is produced by activated Th2 cells, mast cells, eosinophils, basophils, and cells in lung and intestine.2–4 Administration of IL-25 to mice has been shown to induce immune responses characterized by the overproduction of Th2 cytokines such as IL-4, IL-5, and IL-13.5 IL-25 is an important regulator of Th2 responses and host defense. IL-25 dampens counterproductive Th1 responses in helminth infection and limits chronic inflammation in the gastrointestinal tract,6 independent of its effects on Th2 cell differentiation. It has been reported that endogenous IL-25 is required to limit IL-23 expression and number of Th17 cells in large intestine.4 Furthermore, IL-25 not only inhibits the development of experimental autoimmune encephalomyelitis (EAE) by suppressing IL-17 function but also suppresses episodes of relapsing-remitting EAE.4,7 Recently another study showed that IL-25 is able to reduce proinflammatory cytokine production of CD14+ monocytes/macrophages in vitro and to protect against LPS-induced lethal endotoxemia in mice.8 Additionally, IL-25 increases serum levels of IgE, IgG1, IgA, blood eosinophilia, and eosinophilic infiltrates in lungs and plays an important role in the development and exacerbation of asthma and allergic inflammation.9 IL-25 has also been shown to activate NF-κB and to induce the production of IL-8 in a renal cell line.10 The role of IL-25 in kidney disease is unknown.
IL-25's biologic effects are mediated by the IL-25 receptor (IL-25R), which is constitutively expressed in kidney, liver, and intestine.10 IL-25R is not only highly expressed by T cells (particularly Th2 memory cells) but is also expressed by human blood monocytes (CD14 cells).8 In kidney disease, macrophages are essential mediators of chronic inflammatory reactions.11 Macrophage and T cell accumulation is a common feature of human and animal renal disease and correlates with the degree of histologic and functional injury.12,13 Adriamycin nephropathy (AN) is a model of chronic proteinuric renal disease induced by adriamycin that resembles human focal segmental glomerular sclerosis.14,15 In AN, inflammatory infiltrates are also composed largely of macrophages and T cells. It has been reported that administration of IL-4 ameliorates experimental GN.16 An adenovirus expressing IL-13 injected into kidney also increases renal IL-13 levels and attenuates acute kidney allograft injury.17 These studies have suggested that enhancing Th2 responses in kidney may reduce renal inflammation and renal damage. Therefore, given its ability to induce Th2 immune responses and to inhibit macrophage-derived inflammatory cytokines, IL-25 is a potential candidate for treating kidney disease. We thus examined the role of IL-25 in AN and tested its dependence on Th2 immune responses.
In this study, IL-25 was administered to mice with AN. We evaluated its ability to protect against renal functional and structural injury in vivo and examined possible mechanisms underlying its effect on macrophages and T cells. Here, we provide evidence that IL-25 is a critical cytokine in both promoting Th2 immune responses and inhibiting renal injury in murine AN. Notably, in addition to its role in promotion of Th2 immune responses and deactivation of macrophages, a novel mechanism underlying IL-25's protective effects against renal injury has been uncovered: that of its ability to induce alternatively activated macrophages in vivo.
RESULTS
IL-25 Protected against Renal Injury in AN BALB/c Mice
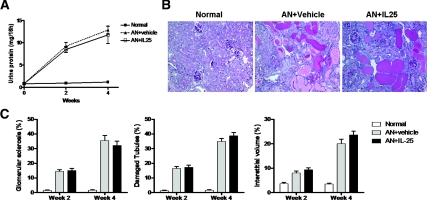
Body weight was significantly reduced in AN mice compared with that of normal mice and was significantly improved in AN mice treated with IL-25 (Figure 1, A and B). Similarly, urine protein was significantly increased in AN mice compared with that of normal mice and was significantly improved in AN mice treated with IL-25 (Figure 1C). In AN, renal injury was characterized by glomerular sclerosis, tubular atrophy, and interstitial fibrosis. Glomerular sclerosis was significantly reduced in AN mice treated with IL-25 compared with that of control AN mice. Damaged tubules was significantly improved in AN mice with IL-25 compared with that of control AN mice. Similarly interstitial volume was significantly reduced in mice given IL-25 compared with that of control AN mice (Figure 1, D and E).
Figure 1.
IL-25 protected against renal injury in AN BALB/c mice. (A) BALB/c mice were injected daily with IL-25 from day 5 to day 12 after ADR injection. Mice were killed on day 14 and day 28. (B) Body weight in normal, AN + vehicle, and AN + IL-25 groups on days 0 to 28. (C) Proteinuria at weeks 2 and 4. (D) PAS-stained sections of renal cortices at week 4 (×200). (E) Quantitative assessment of glomerular sclerosis, tubular damage, and interstitial volume. The values represent the mean ± SEM of evaluations from each group (n = 8 per group). *P < 0.05, and **P < 0.01 versus AN + vehicle.
IL-25 Induced Th2 Responses in the Periphery
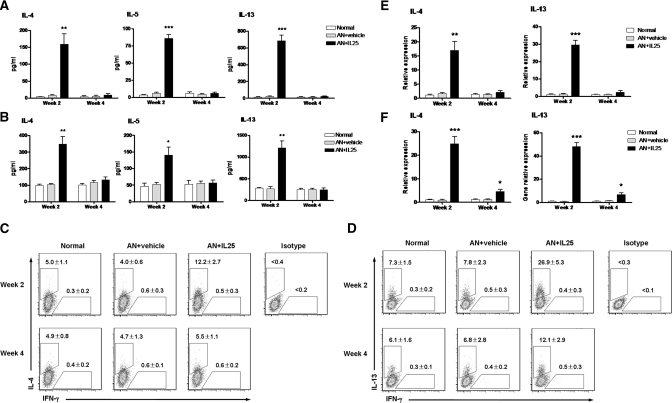
In AN mice treated with IL-25, serum levels of the Th2 cytokines IL-4, IL-5, and IL-13 were significantly increased compared with those of normal and control AN mice (Figure 2A). Splenic CD4+ T cells separated from AN mice treated with IL-25 produced high levels of IL-4, IL-5, and IL-13 in culture supernatant with CD3/CD28 stimulation (Figure 2B). Similarly, by flow cytometry assessment, the numbers of IL-4+ and IL-13+ cells were increased significantly among splenic CD4+ T cells from AN mice treated with IL-25 (Figure 2, C and D). Furthermore, the mRNA expression of IL-4 and IL-13 in kidney and renal draining lymph nodes (RDLN) were significantly increased in AN mice treated with IL-25 compared with normal and control AN groups (Figure 2, E and F). The increase of Th2 cytokines in AN mice treated with IL-25 was transient. Th2 cytokine levels were high at week 2 and fell by week 4 but were still significantly higher than those of normal and control AN mice at the same time point.
Figure 2.
IL-25 induced peripheral Th2 responses. (A) IL-4, IL-5, and IL-13 levels in serum were assessed in normal, AN + vehicle, and AN + IL-25 groups at weeks 2 and 4. (B) The levels of cytokine expression were measured by ELISA in splenic CD4+ T cells stimulated for 3 days with anti-CD3/CD28 (1 μg/ml) and then restimulated for 24 hours with 1 μg/ml of anti-CD3. (C and D) Intracellular IL-4 and IL-13 expression was analyzed by flow cytometry in CD4+ T cells stimulated with ionomycin and PMA in the presence of GolgiStop for 5 hours. Numbers above the gates indicate the percentage of cells stained positive for each respective cytokine. (E and F) The mRNA expression of IL-4 and IL-13 in kidney (E) and RDLN (F) was examined by real-time PCR and expressed relative to the control of each experiment. The values represent the mean ± SEM of evaluations from each group (n = 8 per group). *P < 0.05, **P < 0.01, and ***P < 0.001 versus AN + vehicle.
IL-25 Suppressed Macrophage Activation In Vivo and In Vitro
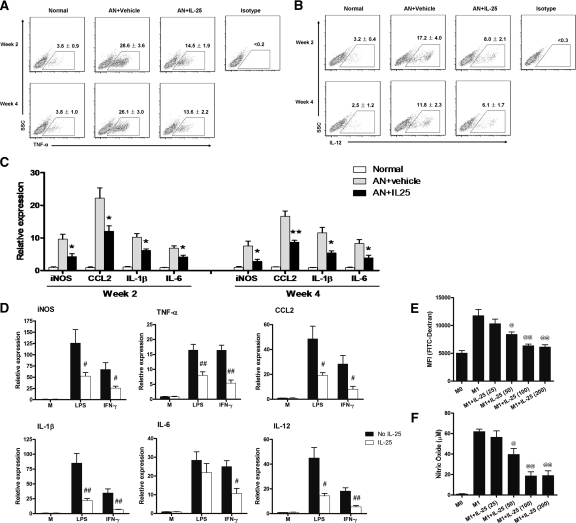
To define the mechanisms underlying the protective effect of IL-25 against renal injury, we examined the activation status of endogenous macrophages in the kidney. A significantly lower percentage of macrophages expressed TNF-α and IL-12 in AN mice treated with IL-25 compared with that from control AN mice (Figure 3, A and B). In addition, mRNA expression of inducible nitric oxide synthase (iNOS), chemokine ligand 2 (CCL2), IL-1β, and IL-6 was significantly lower in the macrophages from AN mice treated with IL-25 compared with that from control AN mice (Figure 3C). Similarly, in vitro studies showed that preincubation of activated macrophages with IL-25 significantly reduced their mRNA expression of iNOS and proinflammatory cytokines TNF-α, CCL2, IL-1β, IL-6, and IL-12 (Figure 3D). Furthermore, IL-25 significantly suppressed phagocytosis and NO production of LPS-activated macrophages (Figure 3, E and F).
Figure 3.
IL-25 suppressed endogenous renal macrophages in vivo and M1 macrophages in vitro. After administration of IL-25, CD11b+ endogenous renal macrophages at week 2 and week 4 were purified by flow cytometry. The expression of TNFα and IL-12 by endogenous renal macrophages was analyzed by flow cytometry (A and B) and the mRNA expression of iNOS, CCL2, IL-1β, and IL-6 was examined by real-time PCR (C). The values represent the mean ± SEM of evaluations from each group (n = 8 per group). *P < 0.05, and **P < 0.01 versus AN + vehicle. (D) The mRNA expression of iNOS, TNFα, CCL2, IL-1β, IL-6, and IL-12 by bone marrow macrophages preincubated with medium (M) or IL-25 (100 ng/ml) for 1 hour and stimulated or not with LPS or IFNγ for a further 6 h in vitro. The values represent the mean ± SEM of evaluations from each group (n = 4 per group). #P < 0.05 and ##P < 0.01 versus No IL-25. (E and F) Resting macrophages (M0) were cultured with LPS (to become M1 macrophages) in the presence of various concentrations (ng/ml) of mouse recombinant IL-25 for 24 hours. Cells were co-cultured with FITC-labeled dextran for 45 minutes. The uptake of fluorescence beads (mean fluorescence intensity) was determined by flow cytometry. In parallel, macrophages were cultured with LPS in the presence of various concentrations (ng/ml) of mouse recombinant IL-25 for 48 hours, and NO was measured in the culture supernatant. The values represent the mean ± SEM of evaluations from each group (n = 4 per group). @P < 0.05 and @@P < 0.01 versus M1.
IL-25 Induced Alternatively Activated Macrophages In Vivo
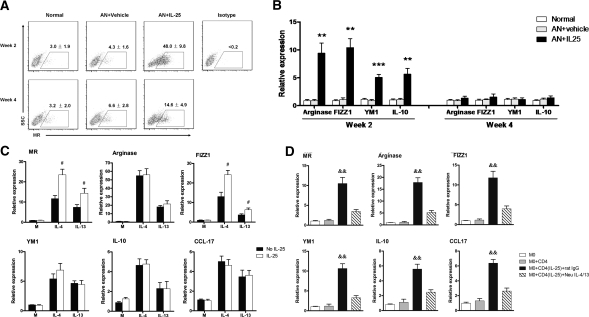
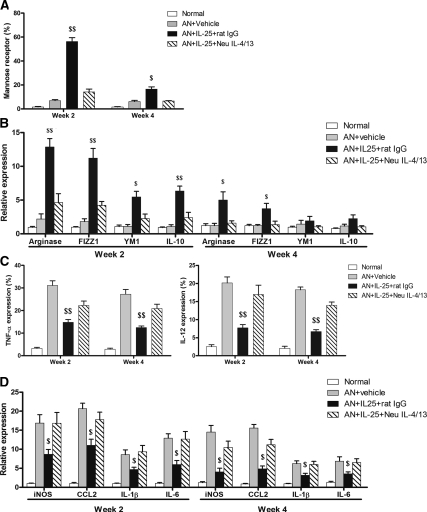
To further study the mechanisms of IL-25's protective effects, we examined the phenotype and cytokine profile of endogenous macrophages in kidney. Interestingly, the macrophages in kidneys from AN mice treated with IL-25 had elevated levels of mannose receptor (MR)—10 times higher expression at week 2 and 2 times higher expression at week 4 compared with those from normal and control AN mice (Figure 4A). MR is expressed on and has been used as a marker for alternatively activated macrophages (M2). Correspondingly, other genes also recognized as M2 markers, arginase, FIZZ1, YM1, and IL-10, showed increased expression at week 2 in renal macrophages from AN mice treated with IL-25 (Figure 4B). In vitro, macrophages isolated from bone marrow and cultured with IL-25 alone did not express increased mRNA levels of MR, arginase, FIZZ1, YM1, IL-10, or CCL-17. This showed that IL-25 did not induce M2 macrophages directly. However, after stimulation with IL-25 and IL-4 or IL-13, macrophages increased their expression of MR and FIZZ1, but not arginase, YM1, IL-10, and CCL-17, compared with macrophages exposed to IL-4 or IL-13 alone (Figure 4C and Supplementary Figure S1). In addition, IL-25 induced expression of IL-4/13 in CD4+ T cells in vitro after 3-day stimulation (Figure S2). These IL-4/13 expressing CD4 T cells after IL-25 stimulation induced M2 phenotype in co-cultured macrophages. The effect on macrophages of IL-25–induced T cells was blocked by IL-4/13 neutralizing antibodies (Figure 4D).
Figure 4.
IL-25 induced alternatively activated macrophages. CD11b+ endogenous renal macrophages were sorted by flow cytometry at weeks 2 and 4. The expression of MR was assessed by flow cytometry (A), and the mRNA expression of arginase, FIZZ1, YM1, and IL-10 was quantified by real-time PCR (B). The values represent the mean ± SEM of evaluations from each group (n = 8 per group). **P < 0.01 and ***P < 0.001 versus AN + vehicle and normal. (C) The mRNA expression of MR, arginase, FIZZ1, YM1, IL-10, and CCL-17 was examined by real-time PCR in bone marrow macrophages preincubated for 1 hour with medium (M) or IL-25 (100 ng/ml) combined or not with IL-4 (10 ng/ml) or IL-13 (10 ng/ml) for a further 6 hours in vitro. The values represent the mean ± SEM of evaluations from each group (n = 4 per group). #P < 0.05 versus No IL-25. (D) Naïve CD4+ T cells preincubated or not with mouse recombinant IL-25 were co-cultured with bone marrow–derived macrophages (M0) in the presence of IL-4/13 neutralizing antibodies or control rat IgG1 for 24 hours. The mRNA expression of MR, arginase, FIZZ1, YM1, IL-10, and CCL-17 in bone marrow macrophages was examined by real-time PCR. The values represent the mean ± SEM of evaluations from each group (n = 4 per group). &&P < 0.01, versus the other three groups.
IL-4/13 Was Required for IL-25–Mediated Protection
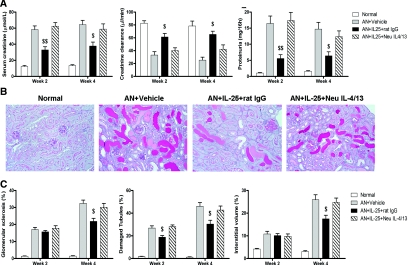
To test whether IL-25 renoprotection was Th2 cytokine dependent, IL-4/13 neutralizing antibodies were administered to AN mice treated with IL-25. The protective effects of IL-25 on renal function and histology were blocked by IL-4/13 neutralizing antibodies (Figure 5, A–C). IL-4/13 neutralizing antibodies also blocked IL-25–mediated induction of M2 macrophages in AN mice. The number of MR-positive macrophages in AN mice treated with IL-25 was decreased at week 2 and week 4 by IL-4/13 neutralizing antibodies compared with AN mice treated with IL-25 alone (Figure 6A). Correspondingly, other markers of M2 macrophages including arginase, FIZZ1, YM1, and IL-10 were decreased at week 2 and week 4 (Figure 6B). Cellular protein levels of TNF-α and IL-12 and mRNA expression of iNOS, CCL2, IL-1, and IL-6 in renal macrophages from AN mice treated with IL-25 were increased by IL-4/13 neutralizing antibodies at week 2 and week 4 (Figure 6, C and D).
Figure 5.
IL-25 protected against injury in AN in an IL-4/13–dependent manner. (A) Serum creatinine, creatinine clearance, and proteinuria were assessed at weeks 2 and 4. (B) PAS-stained sections of renal cortices at week 4 (×200). (C) Quantitative assessment of glomerular sclerosis, tubular damage, and interstitial volume expansion. The values represent the mean ± SEM of evaluations from each group (n = 8 per group). $P < 0.05 and $$P < 0.01 versus the other three groups.
Figure 6.
IL-25 induced M2 macrophages and suppressed endogenous renal macrophages in an IL-4/13–dependent manner. The percentage of endogenous renal macrophages expressing MR, TNFα, and IL-12 was measured by flow cytometry (A and C) and the mRNA expression of arginase, FIZZ1, YM1, IL-10, iNOS, CCL2, IL-1β, and IL-6 was quantified by real-time PCR (B and D). The values represent the mean ± SEM of evaluations from each group (n = 8 per group). $P < 0.05 and $$P < 0.01 versus the other three groups.
Effects of IL-25 on Inflammatory Infiltrates
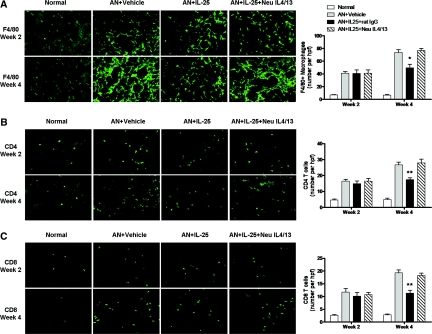
Interstitial infiltration with macrophages, CD4+, and CD8+ T cells was significantly reduced in renal cortex of AN mice treated with IL-25 compared with that of control AN mice at week 4 but not at week 2. Reduction of inflammatory infiltrates by IL-25 was blocked by IL-4/13 neutralizing antibodies (Figure 7).
Figure 7.
IL-25 reduced inflammatory infiltrates in a IL4/13-dependent manner. Numbers of F4/80+ macrophages (A) and CD4+ and CD8+ T cells (B and C) were assessed by immunofluorescence staining in renal cortex of mice at week 2 and week 4. The values represent the mean ± SEM of evaluations from each group (n = 8 per group). $P < 0.05, and $$P < 0.01 versus the other three groups.
IL-25 Did Not Protect against Renal Injury in Severe Combined Immunodeficient Mice
To test whether IL-25–induced renoprotection was lymphocyte dependent, the effect of IL-25 administration in severe combined immunodeficient (SCID) mice with AN (AN SCID mice) was examined. Serum IL-4, IL-5, and IL-13 levels and mRNA expression of IL-4 and IL-13 in kidney were not increased in AN SCID mice treated with IL-25 at both weeks 2 and 4 (Supplementary Figure S3, A and B). However, IL-4 and IL-13 expression in RDLN was significantly increased at week 2 but not at week 4 in AN SCID mice treated with IL-25 (Supplementary Figure S3C).
Furthermore, IL-25 failed to induce alternatively activated macrophages in the kidneys of AN SCID mice. IL-25 did not increase the expression of MR, arginase, FIZZ1, YM1, and IL-10 in renal macrophages from AN SCID mice (Supplementary Figure S4, A and B). In addition, IL-25 did not suppress endogenous renal macrophages in AN SCID mice. Cellular protein levels of TNF-α and IL-12 and mRNA expression of iNOS, CCL2, IL-1, and IL-6 were not reduced in renal macrophages from AN SCID mice treated with IL-25 compared with those from normal and AN SCID mice (Supplementary Figure S4, C–E).
IL-25 did not protect against renal injury in AN SCID mice. There were no differences in urine protein levels of AN SCID mice treated with or without IL-25 (Figure 8A). There were also no differences in glomerular sclerosis, tubular atrophy, and interstitial expansion between AN SCID mice treated with IL-25 and those treated with saline (Figure 8, B and C).
Figure 8.
IL-25 failed to protect against renal injury in AN SCID mice. (A) Proteinuria at weeks 2 and 4. (B) PAS-stained sections of renal cortices at week 4 (×200). (C) Quantitative assessment of glomerular sclerosis, tubular damage, and interstitial volume. The values represent the mean ± SEM of evaluations from each group (n = 8 per group).
DISCUSSION
In this study, IL-25 induced a Th2 immune response and reduced renal injury in immunocompetent mice with AN. The protective effect of IL-25 could be caused by its initiation of Th2 responses, induction of alternatively activated (M2) macrophages, and deactivation of effector macrophages in kidney. Furthermore, induction of M2 macrophages by IL-25 was shown to depend on Th2 immune responses, because IL-25 failed to induce M2 macrophages and protect renal injury either in AN BALB/c mice treated with IL-4/13 neutralizing antibodies or in AN SCID mice.
IL-25 has been shown to regulate cognate immunity in experimental models of autoimmune diseases including type I diabetes18 and EAE,7 whereas there is much less known about its potential role in innate immune mechanisms of chronic inflammatory diseases. AN is produced by injection of adriamycin, which leads to a chronic inflammatory kidney disease similar to human focal segmental glomerular sclerosis. Innate immunity seems to be the dominant pathway of renal injury induction and progression in this model. AN mice treated with IL-25 at an early stage of disease had less glomerular sclerosis, tubular atrophy, and interstitial expansion than control AN BALB/c mice. AN mice infused with IL-25 also had significantly reduced proteinuria compared with control AN mice. This is the first report to show the ability of IL-25 to reduce renal injury in chronic kidney disease. The mechanisms underlying the renoprotective effects of IL-25 could involve its initiation and augmentation of Th2 cell–mediated immune responses. However, it is uncertain whether IL-25 would be protective against disease progression in this model if administered at a later stage of disease.
IL-25 is able to induce Th2 cytokine production in vitro directly from naive and memory T cells stimulated with anti-CD3/CD28.2,19 In this study, IL-25 administration increased serum levels of IL-4, -5, and -13 and mRNA expression of IL-4 and IL-13 in kidney and draining lymph nodes in comparison to normal and untreated AN mice. Similarly, cultured T cells from spleens of AN mice treated with IL-25 showed a high percentage of IL-4+ and IL-13+ cells and higher level secretion of IL-4, -5, and -13. These data show that IL-25 was able to induce high levels of IL-4 and IL-13 in both the periphery and within the kidneys of AN mice. It has been shown that enhancing Th2 immunity with exogenous IL-4 and/or IL-10 and IL-13 reduced renal injury in a model of experimental immune-mediated GN.16,17,20 Thus, the protective effect of IL-25 in this study could be caused by its induction of Th2 responses.
A most interesting finding in this study was the induction by IL-25 of alternatively activated (M2) macrophages in vivo, shown here for the first time. The number of renal M2 macrophages was significantly greater at 2 and 4 weeks in AN mice treated with IL-25 than in control mice, whereas the elevation of Th2 cytokines by IL-25 was apparent at week 2 but had vanished by week 4. A persistent effect to induce M2 macrophages may be an important mechanism underlying IL-25's protective role in AN. Adoptive transfer of macrophages would be a useful approach to prove the role of these M2 macrophages in AN. Indeed, in our previous study, either M2a or M2c macrophage administration ameliorated renal injury in AN.21,22 Therefore, it is likely that IL-25 induction of M2 is involved in the reduction of renal injury in AN mice.
Data from our study indicate that IL-25 is capable of suppressing effector macrophages, leading to reduced proinflammatory cytokine production in vitro. This finding is consistent with the report by Caruso et al.,8 in which IL-25 was a negative regulator of monocyte proinflammatory cytokine responses in vitro and protected against LPS-induced lethal endotoxemia in mice. Inhibition of cytokine responses by IL-25 occurred via a p38 MAP kinase–driven Socs-3–dependent mechanism.8 A novel and interesting observation uncovered by our study was that IL-25 indeed acted on endogenous macrophages in situ within the inflamed kidney. Thus, monocytes/macrophages responded to IL-25 in vivo both by down-regulating their synthesis of proinflammatory cytokines and up-regulation of anti-inflammatory cytokines. Furthermore, our in vitro study showed that IL-25 significantly suppressed other functions of LPS-activated macrophages, including phagocytosis and NO production.
To determine whether the protective effect of IL-25 in AN BALB/c mice was dependent on Th2 lymphocytes or caused by a direct effect of IL-25 on macrophages, the effects of IL-25 were further examined in AN SCID mice that are deficient in T and B cells. IL-25 administration neither induced M2 macrophages nor protected against renal structural and functional injury in AN SCID mice. Thus, the lymphocyte-dependent protective role of IL-25 was shown by its failure to down-regulate production of inflammatory cytokines from effector macrophages or to induce M2 macrophages and furthermore by its failure to protect against renal injury in this innate immune model of chronic renal disease. In immunocompetent AN mice, we found that IL-25 did induce Th2 cytokines in CD4+ T cells in vivo and that blockade of Th2 responses by IL-4 and IL-13 neutralizing antibodies abolished the protective effects of IL-25 on renal function and histology. Therefore, Th2 responses induced by IL-25 have a central protective effect against renal injury. However, to determine whether IL-4 or IL-13 alone play an individual role in IL-25's renoprotective effect, further studies are required using anti-IL-4 or anti-IL-13 separately.
In this study, we also found no direct effect of IL-25 to induce of M2 macrophages in vitro, except synergistically with IL-4 or IL-13. An indirect effect of IL-25 on M2 macrophages was shown by co-culture of IL-25–modulated Th2 cells and macrophages; in these experiments, IL-4/13 expressing CD4+ T cells generated by IL-25 did induce M2 macrophages. The effects on macrophages of T cells modified by IL-25 were blocked by IL-4/13 neutralizing antibodies. Although IL-25 suppression of macrophages was shown in vitro by a reduction of their proinflammatory cytokine production, this direct effect is not seen in vivo in AN SCID mice. The predominant effects of IL-25 on macrophages in vivo in immunocompetent AN mice seem to be through the modulation of Th2 responses and induction of M2 macrophages. Lack of interaction of Th2 cells and M2 macrophages with effector macrophages and absence of an increase in M2 macrophages in kidney of SCID AN mice could explain why renal endogenous macrophages from SCID AN mice expressed similar levels of TNF-α and IL-12 with or without IL-25 treatment. In addition to the effects of IL-25 on T cells, IL-25 could regulate the production of Th2 cytokines and M2 macrophages in vivo via the assistance of other cells. It is possible that renal tubular epithelial cells may be a cellular target of IL-25, because they express the IL-25 receptor. However, we found that renal tubular epithelial cells stimulated with IL-25 do not induce Th2 cytokines (unpublished data). It is also possible that IL-25 acts on other innate immune cells including mast, NKT, and dendritic cells, which play an important role in early Th2 cytokine production and M2 macrophage formation. The (transient) production of Th2 cytokines in renal draining lymph nodes in SCID mice is consistent with this.
Taken together, our data showed that IL-25, via its induction of Th2 responses, is able to induce protective macrophages, suppress effector macrophages, and protect against renal injury in AN.
CONCISE METHODS
AN Murine Model and IL-25 Administration
Six- to 8-week-old male BALB/c and SCID mice obtained from the Animal Resources Centre (Perth, Australia) were used in this study. The Animal Ethics Committee of Westmead Hospital approved all procedures. Dose-finding studies defined an optimal dose of 10.4 mg/kg body weight of Adriamycin (ADR; doxorubicin; Pharmacia & Upjohn Pty Ltd) for BALB/c mice and 5.4 mg/kg body weight of ADR for SCID mice. ADR was injected once via the tail vein of each mouse. All mice were weighed twice daily.
BALB/c and SCID mice were divided into three groups: normal, AN with saline, and AN with IL-25 treatment. For IL-25 treatment alone, mice were administered 0.5 μg mouse recombinant IL-25 (R&D Systems) intraperitoneally on days 5 to 12 after ADR injection. The dose and duration were selected according to previous published studies.4,6 Control animals received PBS only. In another study, BALB/c mice were divided into four groups: normal, AN with saline, AN with IL-25 and rat IgG1, and AN with IL-25 and neutralizing anti-IL-4/13 antibodies. For IL-25 and neutralizing anti-IL-4/13 antibody treatment, mice were administered 0.5 μg mouse recombinant IL-25 intraperitoneally on days 5 to 12 after administration of 200 μg neutralizing antibodies IL-4/13 (eBiosicence) or control rat IgG1 (eBiosicence) on days 8 and 12 after ADR injection. Mice were killed at weeks 2 and 4 after ADR injection. Blood, urine, spleen, renal draining lymph nodes, and kidneys were harvested for analysis. All urine and blood specimens were analyzed by the Institute of Clinical Pathology and Medical Research (Westmead Hospital), using a BM/Hitachi 747 analyzer (Tokyo, Japan).
T Cell Culture and Assays
CD4+ T cells were isolated from spleens of BALB/c mice by FACS sorting at week 2 and week 4 after ADR injection. Cells were stimulated with anti-CD3/CD28 (1 μg/ml), anti-IFNγ (10 μg/ml; BD Biosciences), and 50 U/ml of mouse IL-2 for 3 days. Cells were washed and restimulated with 1 μg/ml of anti-CD3 for 24 hours, and culture supernatants were analyzed for cytokines by ELISA (IL-4, IL-5, and IL-13 kits; eBioscience). For intracellular cytokine analysis, after 4 days of stimulation, CD4+ T cells were restimulated with 500 ng/ml ionomycin and 50 ng/ml PMA (Sigma-Aldrich) in the presence of GolgiStop (BD Biosciences) for 5 hours. Cells were stained with FITC-conjugated anti-mouse CD4 (BD Biosciences) and permeabilized with Cytofix/Cytoperm (BD Biosciences). Intracellular staining with antibodies against IL-4, IL-13, and IFNγ (BD Biosciences) was performed and analyzed by flow cytometry. Cell viability was assessed by staining with 7-amino-actinomycin D and Annexin V according to the manufacturer's protocol. Cell viability of CD4+ T cells was >95%.
Macrophage Isolation and Culture
Kidneys were perfused with saline before removal and digested with collagenase and DNase as described previously.23 Kidneys were cut into 1- to 2-mm3 pieces and placed in DMEM containing 1 mg/ml collagenase D (Sigma Aldrich) and 100 μg/ml DNase I (Roche) for 40 minutes at 37°C with intermittent agitation. Mononuclear cells from kidneys were separated using a step-gradient sucrose separation procedure and stained with FITC-conjugated anti-mouse CD11b. CD11b+ endogenous renal macrophages were sorted by FACS. For further purification of CD11b+ macrophages, CD11b+ cells were incubated at 37°C for 40 minutes, and the culture supernatant that contained floating cells (e.g., T cells, natural killer cells, dendritic cells) was discarded. The adherent cells were 96 ± 2.1% CD11b positive (macrophage marker), 94 ± 3.5% F4/80 positive (macrophage marker), and 2.1 ± 0.62% CD11c positive (dendritic cell marker). Cell viability was >95%. Sorted cells were used for real-time PCR analyses to detect phenotypes of these macrophages. Some sorted cells were stained with AF647 conjugated anti-mouse mannose receptor (Biolegend) and analyzed by flow cytometry. For intracellular cytokine analysis, CD11b+ endogenous renal macrophages from each group were stimulated with LPS (100 ng/ml; Sigma Aldrich) in the presence of GolgiStop (BD Biosciences) for 5 hours and examined for TNF-α and IL-12 intracellular staining using flow cytometry.
Primary cultures of murine macrophages were obtained from bone marrow of BALB/c mice by a technique previously described.24 Macrophages derived from bone marrow were cultured in RPMI 1640 medium, supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml), plus 10 ng/ml macrophage colony-stimulating factor for 6 days, and CD11b+ macrophages were sorted by flow cytometry. Cell viability was >95%. Sorted cells were seeded onto 12-well culture plates (2 × 105 cells/well), incubated with mouse recombinant IL-25 (100 ng/ml; R&D Systems) for 1 hour, and stimulated with IL-4 (10 ng/ml, Invitrogen), IL-13 (10 ng/ml; Invitrogen), LPS (100 ng/ml; Sigma Aldrich), or IFN-γ (100 U/ml; Roche) for 6 hours. Cells were used for real-time PCR analyses to detect macrophage phenotype. In parallel, cells were preincubated with IL-25 for 1 hour and stimulated with IL-4/IL-13 for a further 20 hours. The resulting cells were stained with AF647-conjugated anti-mouse MR and analyzed by flow cytometry.
CD4 T Cell Co-Culture with Macrophages
CD4+ T cells isolated from spleens of BALB/c mice were stimulated with or without mouse recombinant IL-25 in the presence of anti-CD3/CD28 (1 μg/ml), anti-IFN-γ (10 μg/ml; BD Biosciences), and 50 U/ml of mouse IL-2 for 3 days. Cell viability was >95%. CD4+ T cells were washed and co-cultured with bone marrow–derived macrophages in the presence of IL-4/13 neutralizing antibodies (eBioscience) or control rat IgG1 (eBioscience) for 24 hours. Macrophages were used for real-time PCR analyses to detect macrophage phenotype. In parallel, CD4+ T cells were restimulated with 1 μg/ml of anti-CD3 for 24 hours, and culture supernatants were analyzed for cytokines by ELISA (IL-4 and IL-13 kits; eBioscience).
ELISA of Cytokines
IL-4, IL-5, and IL-13 levels in sera and culture supernatants were assayed using an ELISA kit purchased from eBioscience. ELISA was performed according to the manufacturer's protocol.
Phagocytic Activity
Macrophages derived from bone marrow were cultured with LPS (100 ng/ml; Sigma Aldrich) in the presence of various concentrations (25, 50, 100, and 200 ng/ml) of mouse recombinant IL-25 for 24 hours. Cells were co-cultured with 1 mg/ml FITC-labeled dextran (40,000 kD; Molecular Probes) for 45 minutes. In controls for nonspecific dextran attachment, cells were added to 0.02% azide or cultured at 4°C to stop energy-dependent cellular functions. Cell viability was >95%. To determine phagocytic activity, the uptake of FITC-labeled dextran was detected by multicolor flow cytometry.
NO Production
NO production by macrophages was determined by the measurement of the nitrite concentration with Griess assay.23 Briefly, macrophages derived from bone marrow were cultured with LPS (100 ng/ml; Sigma Aldrich) in the presence of various concentrations (25, 50, 100, and 200 ng/ml) of mouse recombinant IL-25. After 48 hours, 50 μl of 14 mM 4′4-diaminodiphenylsulfone and 50 μl of 4 mM N-ethylenediamine were added to the culture supernatant and incubated at room temperature for 10 minutes, and NO was detected by measuring the OD at 560 nm on a Dynatech MR5000 reader (Dynatech, Chantilly, VA).
Quantitative RT-PCR
One microgram of RNA isolated by the RNeasy Mini Kit (Qiagen) was reverse-transcribed with the First Strand cDNA Synthesis Kit (Fermantas), and real-time PCR was performed on the Rotogene-6000 Real-Time Thermo cycler (Corbett Research) using the SYBR mastermix (Invitrogen). The analysis method was as described before,25 and the PCR primer sequences are presented in Supplementary Table S1.
Histology and Immunofluorescence
Coronal sections of renal tissue were stained with periodic acid–Schiff (PAS). Glomerulosclerosis, tubular damage, and interstitial volume were evaluated using methods described previously.26 Briefly, images were digitalized using a video camera and analyzed using image analysis software (ImageJ; NIH). The degree of glomerulosclerosis was measured using a quantitative method. The outline of the glomerular capillary tuft was traced, and the computed area was used as a measure of total glomerular area. The area covered by PAS-positive staining in the same glomerulus was determined. The percentage of glomerulosclerosis for each glomerulus was calculated by dividing the total PAS-positive area by the total glomerular area. The mean value of 20 randomly selected glomeruli was determined for each section. Damaged tubules were identified by the presence of diffuse tubular dilation, intraluminal casts, and/or tubular cell vacuolization and detachment in cortex and medulla in 10 to 15 high power fields (×200 magnification) per PAS-stained section, in a blinded fashion. The number of damaged tubules was divided by the number of the total tubules in the same field to obtain the percentage of damaged tubules. The degree of interstitial expansion was determined by quantitation of the relative interstitial volume in 10 to 15 high power fields (×200 magnification) per PAS-stained section. The percentage of relative interstitial volume was calculated by dividing the total interstitial area by the total area. To avoid selection bias, the areas to be viewed for morphometric analysis were anatomically identical for each section and positioned before microscopic visualization.
For immunofluorescence staining, rat anti-mouse F4/80 (1/100; eBiosciences), CD4 (1/50), or CD8 (1/50; BD Biosciences) was used as the primary antibody and AF488 goat anti-rat IgG (1/800; Invitrogen) as the secondary antibody. Control rat IgG to primary antibodies was included in staining. The number of interstitial F4/80+, CD4+, and CD8+ cells was quantitated in 10 nonoverlapping cortical fields (×400).
Statistical Analysis
Renal functional data (serum creatinine, creatinine clearance, and proteinuria) were log-transformed before analysis to stabilize the variance. Statistical tests included unpaired, two-tailed t test using Welch's correction for unequal variances and one-way ANOVA with Tukey's multiple comparison test. Statistical analyses were done using Prism (Version 4; GraphPad). Results are expressed as the mean ± SEM. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Grants 457345 and 632665 to Y.W. and D.H.) and Johnson & Johnson Research Pty Ltd (focused funding to D.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Moseley TA, Haudenschild DR, Rose L, Reddi AH: Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14: 155–174, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C: Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med 204: 1509–1517, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I: Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood 101: 3594–3596, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D: Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med 205: 2191–2198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM: IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15: 985–995, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN: Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 203: 1105–1116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, Kastelein RA, Cua DJ: IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med 204: 161–170, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caruso R, Stolfi C, Sarra M, Rizzo A, Fantini MC, Pallone F, MacDonald TT, Monteleone G: Inhibition of monocyte-derived inflammatory cytokines by IL-25 occurs via p38 Map kinase-dependent induction of Socs-3. Blood 113: 3512–3519, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H: IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol 118: 606–614, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL: IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem 276: 1660–1664, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Noronha IL, Kruger C, Andrassy K, Ritz E, Waldherr R: In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int 43: 682–692, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Ferrario F, Castiglione A, Colasanti G, Barbiano di Belgioioso G, Bertoli S, D'Amico G: The detection of monocytes in human glomerulonephritis. Kidney Int 28: 513–519, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Schreiner GF: Macrophages and cellular immunity in experimental nephrosis and glomerulonephritis. Contrib Nephrol 45: 115–122, 1985 [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Wang YP, Tay YC, Harris DC: Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int 58: 1797–1804, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Sandovici M, Henning RH, van Goor H, Helfrich W, de Zeeuw D, Deelman LE: Systemic gene therapy with interleukin-13 attenuates renal ischemia-reperfusion injury. Kidney Int 73: 1364–1373, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Tam FW, Smith J, Karkar AM, Pusey CD, Rees AJ: Interleukin-4 ameliorates experimental glomerulonephritis and up-regulates glomerular gene expression of IL-1 decoy receptor. Kidney Int 52: 1224–1231, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Sandovici M, Deelman LE, van Goor H, Helfrich W, de Zeeuw D, Henning RH: Adenovirus-mediated interleukin-13 gene therapy attenuates acute kidney allograft injury. J Gene Med 9: 1024–1032, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM: Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ: IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 204: 1837–1847, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR: Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol 27: 530–537, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC: Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int 72: 290–299, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Cao Q, Wang Y, Zheng D, Sun Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC: IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol 21: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kruger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Muller-Newen G, Floege J, Kurts C: Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 15: 613–621, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Johnson CR, Kitz D, Little JR: A method for the derivation and continuous propagation of cloned murine bone marrow macrophages. J Immunol Methods 65: 319–332, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Cao Q, Wang L, Du F, Sheng H, Zhang Y, Wu J, Shen B, Shen T, Zhang J, Li D, Li N: Downregulation of CD4+CD25+ regulatory T cells may underlie enhanced Th1 immunity caused by immunization with activated autologous T cells. Cell Res 17: 627–637, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Rangan GK, Tesch GH: Quantification of renal pathology by image analysis. Nephrology (Carlton) 12: 553–558, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.