Abstract
Diabetic nephropathy (DN) increases podocyte cyclooxygenase-2 (COX-2) expression, and COX-2 inhibition reduces proteinuria and glomerular injury in animal models of diabetes. To investigate the role of podocyte COX-2 in development of diabetic nephropathy, we employed a streptozotocin model of diabetic mellitus in wild-type and transgenic mice expressing COX-2 selectively in podocytes. Progressive albuminuria developed only in diabetic COX-2 transgenic mice despite hyperglycemia, BP, and GFR being similar to those in wild-type mice. Transgenic mice also manifested significant foot-process effacement, moderate mesangial expansion, and segmental thickening of the glomerular basement membrane. In cultured podocytes overexpressing COX-2, high glucose induced cell injury and increased both expression of the pro(renin) receptor and activation of the renin-angiotensin system. Downregulation of the (pro)renin receptor attenuated the injury induced by high glucose. In vivo, podocyte pro(renin) receptor expression increased in diabetic COX-2–transgenic mice, and treatment with a COX-2 inhibitor abrogated the upregulation of (pro)renin receptor and reduced albuminuria, foot-process effacement, and mesangial matrix expansion. In summary, these results demonstrate that increased expression of podocyte COX-2 predisposes to diabetic glomerular injury and that the (pro)renin receptor may be one mediator for this increased susceptibility to injury.
Hyperglycemia-mediated metabolic abnormalities, hemodynamic abnormalities, and oxidative stress have all been implicated in the pathogenesis of diabetic nephropathy.1 Diabetes can affect numerous cell types in the kidney, including glomerular podocytes, mesangial and endothelial cells, tubular epithelia, interstitial fibroblasts, and vascular endothelia.2 Altered podocyte function occurs early in the development of diabetic nephropathy and can affect cell-cell interactions, attachment to the glomerular basement membrane, and apoptosis.2–10
In humans, COX-2 expression is readily detectable in glomerular podocytes of adults,11,12 and expression levels have been reported to increase during acute renal allograft rejection.13,14 Occasional COX-2–positive podocytes are also detectable in adult rats,15,16 and we have previously reported that in rat models of diabetes there is increased COX-2 expression in podocytes, and also in mesangial cells and macula densa cells, and have shown that COX-2 inhibition can attenuate proteinuria and retard diabetic nephropathy progression.15 In this study, we investigated the role of increased COX-2 in diabetes-induced podocyte injury. Our results indicate that increased podocyte expression of COX-2 increases susceptibility to development of diabetic nephropathy and that the podocyte injury is due in part to increased expression and activity of the (pro)renin receptor.
RESULTS
Podocyte COX-2 Expression in Diabetes
The level of basal renal COX-2 expression varies among species, and minimal COX-2 immunoreactivity is detectable in nonstressed adult mice of most strains.17 Furthermore, in most mouse strains, diabetes induces relatively minor glomerular pathology.18 In this regard, our preliminary studies indicated that in wild-type B6/D2 mice, the strain used to generate the transgenic animals, low-dose streptozotocin-induced (STZ-induced) diabetes did not induce significant diabetic nephropathy, and renal COX-2–positive cells were rare under both basal and diabetic conditions. Therefore, in initial studies, we examined renal COX-2 expression in a mouse model that does develop significant and progressive nephropathy, the eNOS−/− db/db (eNOS, endothelial nitric oxide synthase) mouse.19,20 In these mice, renal COX-2 was significantly upregulated, not only in the macula densa but also in the glomerulus, in podocytes, and in mesangial cells (Figure 1A).
Figure 1.
Increased COX-2 and (pro)renin receptor in db/db mice with eNOS deficiency. (A) Increased COX-2 and (pro)renin receptor expression in the kidney from db/db mice with eNOS deficiency. Immunohistochemical staining with a specific COX-2 antibody demonstrated that COX-2 is hardly detectable in normal mouse kidney (wild type in left panel); it is increased in db/db mice with eNOS deficiency (eNOS−/− db/db in right panel), not only in the macula densa (indicated by arrow head) but also at podocytes (indicated by arrows) and mesangial cells. (B) Increased (pro)renin receptor mRNA in glomeruli from eNOS−/− db/db mice. n = 4, P < 0.05. (C) Immunoreactive (pro)renin receptor cells included podocytes (indicated by arrow), endothelial cells (indicated by double head arrow), and mesangium (indicated by arrow head) within the glomeruli of eNOSKO db/db mice. KO, knockout.
To investigate the potential role of increased podocyte COX-2 expression in diabetic glomerular injury, we induced diabetes in wild-type mice on the B6/D2 background and in transgenic mice (tg) selectively expressing COX-2 in podocytes (COX-2 tg). Blood glucose was not different between groups at baseline or after induction of diabetes (Figure 2A). After 16 weeks of diabetes, BP and GFR also remained equivalent between groups (Figure 2, B and C), but albuminuria was significantly greater in diabetic COX-2 tg mice (96.1 ± 9.1 versus 28.3 ± 5.3 μg albumin/mg creatinine, n = 5 to 6, P < 0.05) (Figure 2D). The diabetic COX-2 tg mice had moderate mesangial expansion and segmental GBM thickness compared with minimal mesangial expansion and no increase in GBM thickness in diabetic wild-type mice; COX-2 tg mice also demonstrated significantly increased foot process effacement (Figure 2, E and F).
Figure 2.
STZ induced diabetes in wild-type and COX-2 transgenic mice. (A) STZ induced hyperglycemia in both wild-type and COX-2 transgenic mice. Wt, wild type; tg, transgenic mice with selectively increased COX-2 expression in podocytes. *P < 0.05, compared with control. There was no significant difference between Wt and COX-2 tg either at basal levels or after STZ injection. n = 5 to 6. (B) Systolic BP was not increased after 16 weeks of STZ administration. n = 5, NS. (C) GFRs were not different between groups after 16 weeks of diabetes. n = 5, NS. (D) Albuminuria was significantly increased in diabetic COX-2 tg mice. n = 6, *P < 0.05. (E) Foot process effacement was significantly increased in diabetic COX-2 tg mice. The lower right panel (HP) represents the GBM from diabetic COX-2 tg mice under higher magnification. (F) Percentage of foot process effacement. n = 3, *P < 0.05.
As expected, COX-2 tg mice had increased glomerular COX-2 expression at baseline, and expression further increased in the diabetic animals, whereas no significant increase in glomerular COX-2 was detected in wild-type mice (Figure 3A). With use of primers specific for endogenous and transgene COX-2 mRNA, real-time PCR demonstrated increases predominantly in endogenous rather than the transgene COX-2 expression (Figure 3, B and C). The increased glomerular COX-2 was largely restricted to podocytes and was associated with a 50% decrease in nephrin expression (fold wild-type [Wt] control: Wt + STZ: 0.9 ± 0.2; COX-2 tg: 0.9 ± 0.1; COX-2 tg + STZ: 0.5 ± 0.2, n = 3) (Figure 3D).
Figure 3.
Diabetes led to upregulation of endogenous COX-2 in podocytes of COX-2 transgenic mice. (A) Immunoblotting demonstrated that COX-2 was significantly increased after STZ administration in glomeruli from COX-2 transgenic mice. n = 4, *P < 0.05, compared with wild type; #P < 0.05, compared with the transgenic control mice. (B and C) RT-PCR indicated that diabetes induced significant upregulation of endogenous COX-2 in COX-2 tg mice. n = 4, **P < 0.01 compared with wild control or basal level (B), but did not alter COX-2 transgene expression, n = 5, NS (C). (D) Increased glomerular COX-2 was primarily located in podocytes. The upper panel is representative immunohistochemical staining. COX-2 immunofluorescence is green and nephrin is red.
High-Glucose Medium Induced More Severe Injury in Podocytes Overexpressing COX-2
Differentiated podocytes from wild-type or COX-2 tg mice were exposed for 48 hours to medium with normal glucose (5.5 mM), mannitol (30 mM), or high glucose (30 mM). The high-glucose medium induced more striking cytoskeleton disorganization (Figure 4A), decreased α-actinin 4 expression (Figure 4B), and induced more apoptosis (COX-2 tg versus wild type: 18.7 ± 2.9 versus 7.7 ± 0.9%, n = 3, P < 0.05). These effects were unrelated to increased medium osmolarity per se because they were not seen with exposure to mannitol.
Figure 4.
High-glucose medium induced more injury in cultured COX-2 tg podocytes. (A) High glucose induced cytoskeleton reorganization. The upper panel is from wild type and the lower from COX-2 tg podocytes. High glucose, but not mannitol, caused cytoskeleton disorganization in both wild-type and COX-2 tg podocytes but more stress fibers were seen in COX-2 tg podocytes. The lower right panel is enlarged from a single COX-2 tg podocyte exposed to high glucose. The arrows indicate cytoskeleton reorganization with loss of stress fibers and cytoplasmic aggregates. (B) High glucose decreased α-actinin 4 expression in COX-2 tg podocytes. n = 4, *P < 0.05. (C) High glucose induced more severe apoptosis in COX-2 tg podocytes. Apoptosis was determined by the percentage of TUNEL-positive cells. *P < 0.05 compared with basal level; #P < 0.05 compared with wild type in high-glucose medium. NG, normal glucose medium (5.5 mM glucose); M, 30 mM mannitol; HG, high glucose (30 mM).
(Pro)renin Receptors Expression in Podocytes Overexpressing COX-2
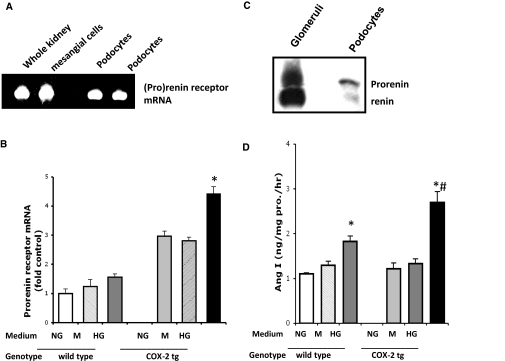
Our previous studies demonstrated interactions between COX-2 and the renin-angiotensin system (RAS) in the kidney,21 and podocytes have been shown to express components of RAS,22,23 which increase in response to high glucose.24 The (pro)renin receptor was first localized to the mesangium of glomeruli,25 but recent reports have also detected it in cultured human podocytes.26 Reverse transcription–PCR confirmed expression of (pro)renin receptor in cultured mouse differentiated podocytes (Figure 5A). In COX-2 tg podocytes, high glucose led to significant increases in receptor mRNA expression (Figure 5B).
Figure 5.
High glucose increased (pro)renin receptor mRNA expression in COX-2 tg podocytes. (A) (Pro)renin receptor was expressed in cultured podocytes. Mouse whole kidney and primary cultured mesangial cells were used as positive controls. (B) High glucose upregulated (pro)renin receptor mRNA in COX-2 tg podocytes. n = 4, *P < 0.05. (C) Immunoreactive renin was detectable in cultured podocytes. (D) High-glucose medium stimulated renin activity, especially in COX-2 tg podocytes. n = 6, *P < 0.05 compared with basal level; #P < 0.05 COX-2 tg versus Wt.
Cultured podocytes expressed predominantly immunoreactive prorenin, although there was also low but detectable renin, (Figure 5C); there were no significant differences in levels of expression among groups (data not shown). However, high glucose stimulated cellular renin activity (from 1.1 ± 0.1 to 1.8 ± 0.1 ng Ang I/mg prorenin per hour in wild-type podocytes and from 1.2 ± 0.1 to 2.7 ± 0.2 ng Ang I/mg (pro)renin per hour in COX-2 tg podocytes, n = 6, P < 0.05 compared with basal levels in each group and between wild-type versus COX-2 tg podocytes in high glucose) (Figure 5D). There were similar differences in renin activity measured in the media. In COX-2 tg podocytes, high glucose also induced phospho-ERK (from 1.0 ± 0.1- to 3.7 ± 0.6-fold of wild-type control, P < 0.05) (Supplemental Figure 1A) and phospho-p38 (from 0.9 ± 0.1- to 2.6 ± 0.1-fold of wild-type control, P < 0.05) (Supplemental Figure 1B) while minimally increasing expression in wild-type podocytes. Inhibitors of MEK (PD-98059) or p38 (SB203580) reduced high-glucose–induced apoptosis (to 7 ± 1 and 8 ± 2%, respectively; n = 4, P < 0.05 compared with untreated group).
Inhibition of COX-2 in COX-2 tg podocytes prevented the increases in high-glucose–stimulated (pro)renin receptor mRNA (from 4.6 ± 0.2- to 2.1 ± 0.2-fold control [n = 4, P < 0.05]) (Figure 6A), as well as inhibition of renin activity (from 2.7 ± 0.2 to 1.6 ± 0.2 ng Ang I/mg (pro)renin per hour, n = 4 to 6, P < 0.05). In addition, the COX-2 inhibitor also partially inhibited high-glucose–induced increases in phospho-ERK and phospho-p38 (2.1 ± 0.5-fold control, n = 4, P < 0.05, and 1.9 ± 0.1-fold control, n = 4, P < 0.05, respectively) (Figure 6B).
Figure 6.
COX-2 inhibition and (pro)renin receptor mRNA knockdown attenuated high-glucose–induced COX-2 tg podocyte injury. (A) High-glucose–stimulated (pro)renin receptor mRNA upregulation in COX-2 tg podocytes was significantly blunted by the COX-2 inhibitor, SC58236. n = 4, *P < 0.05 compared with wild type; #P < 0.05 compared with SC58236 treatment. (B) SC58236 also inhibited high-glucose–mediated pERK and p38 expression. Representative photograph from three separate experiments. (Pro)renin receptor mRNA knockdown partially inhibited mitogen-activated protein kinase activation (C), with scrambled SiRNA as a negative control and attenuated high-glucose–induced α-actinin 4 downregulation (D) protected from apoptosis in COX-2 tg podocytes by high glucose (E). Blue indicates DAPI staining. DAPI, 4,6-diamidino-2-phenylindole; ERK, extracellular signal–regulated kinase.
Specific (pro)renin receptor siRNA inhibited prorenin receptor mRNA expression by 50 to 70% (Supplemental Figure 2). Compared with scrambled control siRNA, (pro)renin receptor mRNA knockdown in COX-2 tg cells decreased the high-glucose–stimulated renin activity (from 2.7 ± 0.2 to 1.0 ± 0.1 ng Ang I/mg (pro)renin per hour, n = 4 to 6, P < 0.05) without significantly affecting renin expression. It also significantly inhibited high-glucose–induced phospho-ERK (to 1.6 ± 0.1-fold wild control, n = 4, P < 0.05) and phospho-p38 expression (to 1.4 ± 0.1-fold wild control, n = 4, P < 0.05) (Figure 6C). In addition, downregulation of (pro)renin receptor expression partially restored α-actinin 4 expression (Figure 6D) and reduced apoptosis (from 19 ± 3 to 6 ± 2%, n = 4, P < 0.05) (Figure 6E and Supplemental Figure 2B). To determine the potential role of angiotensin II in these responses, we utilized the specific AT1 receptor antagonist, losartan (0.1 μM), which partially inhibited high-glucose–induced phospho-ERK (to 2.0 ± 0.1-fold wild control, n = 4, P < 0.05) and phospho-p38 (to 1.7 ± 0.1-fold wild control, n = 4, P < 0.05) (Supplement Figure 3A) and decreased apoptosis (to 7 ± 1%, n = 4, P < 0.05) (Figure 6E). The combination of (pro)renin receptor mRNA interference and AT1R blockade with losartan further reduced high-glucose–induced apoptosis to 4 ± 1% (n = 4).
Elevated (Pro)renin Receptor Expression in Glomeruli of Diabetic COX-2 tg Mice
(Pro)renin receptor mRNA expression increased significantly in isolated glomeruli from diabetic COX-2 tg mice (to 3.9 ± 0.2-fold control, n = 4, P < 0.05) (Figure 7A), with localization to podocytes and mesangial cells (Figure 7B) and colocalization of increased COX-2 and (pro)renin receptor in podocytes of diabetic COX-2 tg mice (Figure 7C). Increased (pro)renin receptor mRNA and protein expression was also detected in glomeruli of eNOS−/− db/db mice (Figure 1, B and C).
Figure 7.
Diabetes stimulated (pro)renin receptor up-regulation in COX-2 tg. (A) Diabetes induced (pro)renin receptor mRNA elevation in COX-2 tg mice. n = 4, *P < 0.05 versus basal; #P < 0.05 versus Wt. (B) Diabetes led to minor increases in immunoreactive (pro)renin receptor in wild-type mice, predominantly in the mesangial area but markedly increased (pro)renin receptor in COX-2 tg mice in podocytes as well as mesangial cells. (C) Immunoflurorescence indicated colocalization of COX-2 (red) and (pro)renin (green).
Effect of COX-2 Inhibition in STZ-Induced Diabetic COX-2 tg Mice
Consistent with our previous studies in diabetic rats,15 treatment of diabetic COX-2 tg mice with the selective COX-2 inhibitor, SC58236, significantly attenuated albuminuria (from 96.1 ± 9.2 to 50.7 ± 4.1 μg albumin/mg creatinine, n = 6, P < 0.05) (Figure 8A) and foot process effacement (from 70 ± 10 to 40 ± 2%, n = 3, P < 0.05) (lower panel in Figure 8, B and C). COX-2 inhibitor treatment did not alter glomerular COX-2 expression (Figure 8, D and E) but did attenuate the increases in glomerular (pro)renin receptor mRNA (from 3.9 ± 0.2- to 1.7 ± 0.5-fold control, n = 4, P < 0.05) (Figure 8E) and protein (Figure 8F).
Figure 8.
COX-2 inhibition ameliorated diabetic renal injury in COX-2 tg mice. (A) The COX-2 inhibitor, SC58236, attenuated albuminuria in diabetic COX-2 tg mice. n = 5 to 6, *P < 0.05. (B and C) SC58236 treatment prevented diffuse foot process effacement in diabetic COX-2 tg mice. (D) SC58236 administration did not alter glomerular COX-2 expression. (E and F) SC58236 treatment inhibited glomerular (pro)renin receptor mRNA (n = 4, P < 0.05) (E) as well as immunoreactive protein staining (F). SC, SC58236.
DISCUSSION
Our previous studies indicated that selective overexpression of COX-2 in podoctes increased susceptibility to adriamycin or puromycin-induced glomerular injury.27,28 The current studies demonstrated that in streptozotocin-induced diabetes, mice with selective increases in podocyte COX-2 expression exhibited significant albuminuria, foot process effacement, and GBM thickening. In cultured podocytes, COX-2 overexpression led to more severe cytoskeleton disorganization and apoptosis in response to high-glucose stimulation. These changes were ameliorated by treatment with a specific COX-2 inhibitor, indicating that podocyte COX-2 expression increases susceptibility to development of diabetic nephropathy. The current studies also indicate that increased podocyte (pro)renin receptor expression may partially mediate the observed increased susceptibility to diabetic injury of COX-2 overexpressing podocytes.
The renin-angiotensin system is a well-recognized mediator of diabetic nephropathy, and RAS blockade can slow progression in both type I and type II diabetes.29,30 In addition to the systemic RAS, there is increasing evidence that local tissue and/or cell type–specific RAS are potentially important mediators of injury. Recent studies have shown that components of the RAS exist in podocytes,23 suggesting that podocytes not only may be a target for the deleterious effects of angiotensin II but also may serve as a local source of angiotensin II production.31
In differentiated podocytes, high-glucose–increased (pro)renin receptor expression, along with increased renin activity, and knockdown of (pro)renin receptor mRNA in COX-2 tg podocytes blocked the increased renin activity, partially reversed the increased ERK and p38 activation, and decreased cell injury induced by high glucose. Inhibition of COX-2 decreased the high-glucose–induced (pro)renin receptor upregulation in the cultured podocytes and in the diabetic COX-2 transgenic mice. Furthermore, AT1 receptor blockade indicated that at least part of the mechanism by which the (pro)renin receptor mediates podocyte injury results from local activation of the podocyte renin-angiotensin system. In addition, the current studies demonstrated that in eNOS knockout (KO) db/db mice, a model with significant diabetic glomerulopathy, podocyte COX-2 expression was significantly increased, as was podocyte (pro)renin expression. In this regard, we have found that RAS inhibition significantly decreases glomerulopathy in eNOS−/− db/db mice (Zhang et al. unpublished). Durvasula et al. have also recently reported increased (pro)renin receptor mRNA expression in cultured podocytes exposed to high-glucose medium and in podocytes of diabetic rats.24
Diabetic patients have lower circulating renin levels but higher prorenin levels than normal healthy patients,32 and increased plasma prorenin levels have been linked to an increased risk of microvascular complications such as retinopathy and nephropathy.33,34 Prorenin is generally considered to be an inactive precursor of renin that becomes catalytically active after proteolytic cleavage of its prosegment.35 The (pro)renin receptor can bind prorenin as well as renin, and binding of prorenin may induce renin activity without proteolytic cleavage of its prosegment;25,36 it may also trigger intracellular signaling pathways independent of angiotensin II.25 In the kidney, receptor expression has been reported in mesangium, macula densa, and tubular cells, and also in cultured differentiated podocytes.25,26,37 Overexpression of the (pro)renin receptor has been implicated in hypertensive and diabetic kidney injury.38,39 Receptor blockade with a handle region peptide (HRP) has been reported not only to inhibit the progression of nephropathy but also even to reverse established diabetic glomerular injury.40 However, these results have not necessarily been confirmed by other investigators.41
Recent studies have indicated that glucose promotes cultured mesangial COX-2 production via enhanced (pro)renin receptor expression,42 and in transgenic rats overexpressing the human (pro)renin receptor gene globally, increased COX-2 was found predominantly in the macula densa cells.43 These results suggest that the (pro)renin receptor may directly or indirectly contribute to the regulation of COX-2 expression in mesangial and macula densa cells. In this regard, we did observe that downregulation of (pro)renin receptor also partially inhibited high-glucose–mediated podocyte COX-2 expression. Therefore, it is possible that COX-2 metabolites promote (pro)renin receptor activation, which may then mediate further increases of COX-2.
In summary, we found that transgenic mice with selectively increased podocyte COX-2 expression were predisposed to development of more severe glomerular injury in response to streptozotocin-induced diabetes. Increased podocyte COX-2 expression induced podocyte (pro)renin receptor expression in response to high glucose both in vitro and in vivo. Inhibition of COX-2 activity or (pro)renin expression partially protected COX-2–overexpressing podocytes from high-glucose–induced injury. Further studies will be required to elucidate underlying molecular mechanisms.
CONCISE METHODS
Materials
STZ was purchased from Sigma Chemical Co. (St. Louis). Goat polyclonal ATP6IP2 antibody was purchased from Novus Biologicals, LLC (Littleton, CO). Rabbit anti-COX-2 antibodies were from Cayman Chemical Co. (Ann Arbor, MI); rabbit anti-α-actinin 4 antibody was from Invitrogen (Camarillo, CA); anti-mouse renin-1 antibody was from R&D Systems (Minneapolis); Cy3-labeled anti-goat antibody and anti-rabbit antibody were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Angiotensin I EIA Kit was from Phoenix Pharmaceuticals, Inc. (Burlingame, CA); siPORT NeoFX Transfection Agent was from Ambion (Austin, TX). Fluorescence phallotoxin was from Molecular Probes/Invitrogen Detection Technologies (Eugene, OR), and TUNEL apoptosis detection kit was from Upstate (Lake Placid, NY). Dynabeads (M-450 tosylactivated) were purchased from Dynal Biotec Inc. (Lake Success, NY). Nylon cell strainers were purchased from BD Biosciences (Bedford, MA). SC58236 was a gift from Pfizer (St Louis). Other reagents were purchased from Sigma Chemical Co. (St. Louis).
Experimental Animals
Nephrin-driven COX-2 transgenic mice on the B6/D2 background (COX-2 tg) were genotyped by PCR and Southern blotting as described previously.27 Diabetes was induced with the low-dose STZ Induction Protocol of the Animal Models of Diabetes Complications Consortium (AMDCC, http://www.amdcc.org). Briefly, mice were starved for 4 hours, then anesthetized with isoflurane, and injected intraperitoneally with 50 mg/kg of STZ for 5 consecutive days. We divided age- and strain-matched (10- to 12-weeks old) male mice into the following groups: (1) Wild type (citrate buffer only) (Wt); (2) Wt + STZ; (3) COX-2 transgenic control (COX-2 tg); (4) COX-2 tg + STZ; (5) COX-2 tg + STZ treated with the COX-2 selective inhibitor, SC58236 (6 mg/L in drinking water). Blood glucose was monitored and mice were sacrificed at the end of 16 weeks. We studied eNOS−/− db/db mice on the BKS background at 26 weeks.19
All animal procedures were approved by the Animal Care and Use Committee of Vanderbilt University Medical Center.
Assessment of Glomerular Injury
Glomerular injury was assessed histologically with light microscopy and electron microscopy (EM). Systolic BP was measured by tail cuff sphygmometry and GFR was determined as described previously.19 Urinary albumin levels were determined by ELISA using a murine microalbuminuria ELISA kit, AlbuwellM (Exocell Inc., Philadelphia). The urine creatinine concentration was measured with a microplate assay kit, Creatinine Companion (Exocell Inc., Philadelphia). All measurements were performed in duplicate and albuminuria was determined as the ratio of urinary albumin (μg/ml) to creatinine (mg/ml).
Isolation of Glomeruli
Glomeruli were isolated immediately after sacrifice, using the Dynabeads method modified from Takemoto et al.44 Briefly, mice were anesthetized by an intraperitoneal injection of Nembutal (0.05 mg/g body wt) and perfused through the descending aorta with 8 × 107 Dynabeads diluted in phosphate-buffered saline. The kidneys were removed, minced into 1 mm3 pieces, and digested in collagenase (1 mg/ml collagenase A, 100 U/ml deoxyribonuclease I in HBSS) at 37°C for 30 minutes with gentle agitation. The collagenase-digested tissue was gently pressed through a 100-μm cell strainer using a flattened pestle, and the cell strainer was then washed with 5 ml of cold HBSS. The filtered cells were passed through a new cell strainer without pressing and the cell strainer washed with 5 ml of cold HBSS. The cell suspension was then centrifuged at 200g for 5 minutes at 4°C. The supernatant was discarded and the cell pellet was resuspended in 2 ml of cold HBSS. Finally, glomeruli-containing Dynabeads were gathered by a magnetic particle concentrator and washed three times with cold HBSS. During the procedure, kidney tissues were kept at 4°C except for the collagenase digestion at 37°C. The preparation consisted of >90% glomeruli.
Culture of Mouse Podocytes
We used immortal podocyte cell lines with or without COX-2 overexpression generated as described previously,45 maintained in medium with mouse recombinant-IFNγ (Sigma, St. Louis) (10 U/ml) for permissive conditions (33°C). Podocytes were characterized by specific markers (e.g., nephrin, and WT1 for podocytes and synaptopodin for differentiated podocytes). Cells between passage 10 and 15 were used for all experiments. Differentiation was induced by switching the incubation temperature to 37°C (nonpermissive conditions) and removing IFNγ from the culture media for 10 to 14 days.
After quiescence for 16 to 24 hours, a subgroup of cells were grown in high-glucose (30 mM) medium or osmolality control medium (30 mM mannitol) for 48 hours before experimentation.
RNA Extraction and Real Time RT-PCR
Total glomerular and podocyte RNA was extracted using TRI-reagent (Molecular Research Center Inc., Cincinnati, OH) and chloroform extraction and further purified with an RNeasy kit (Qigen Inc., Valencia, CA). Upper primer: 5′-tct ccg aac tgc aag tgc ta; lower primer: 5′-ctg caa act ttt gga gag ca. Real-time PCR was performed with specific primers and IQ SYBR Green Supermix Kit (Bio-RAD Laboratories, Inc., Hercules, CA) at 95°C for 3 minutes and then 95°C for 20 seconds, 62°C for 20 seconds, and 72°C for 60 seconds in 40 cycles. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Comparative CT and statistical analysis were calculated as per instructions of User Bulletin #2 from ABI (Applied Biosystems, Hammonton, NJ).
(Pro)renin Receptor Knockdown by siRNA
The single-stranded 19-nt RNA duplexes 5′-CCUACAACCUUGCGUAUAA-3′ and siPORT NeoFX Transfection Agent were purchased from Ambion (Austin, TX). Experiments followed the manufacturer's suggested protocol with 100 nM siRNA duplex in six well plates. Fresh growth medium was replaced after 8 hours in subsequent experiments. A maximal suppression of (pro)renin receptor mRNA was shown for 48 hours and protein expression for 72 hours. We used scrambled SiRNA as a negative control.
Immunoblotting
Cultured cells or isolated glomeruli were homogenized as described previously.46 Proteins were resuspended in SDS-sample buffer, diluted in SDS buffer containing 2-mercaptoethanol (Sigma Chemical Co.), and boiled for 10 minutes before loading. The samples were run on 8% SDS-PAGE gels under reducing conditions and transferred onto polyvinylidene fluoride membrane (Immobilion-P; Millipore Co., Bedford, MA). After blocking with 5% nonfat milk in Tris Buffered Saline (TTBS), the membranes were exposed to the primary antibody overnight at 4°C, followed by HRP-conjugated secondary antibodies. The HRP signal was enhanced using the ESL method and the images were developed on high-performance autoradiography film, Hyperfilm MP (Amersham Biosciences, Buckinghamshire, UK). Membranes were rehybridized with goat anti–β-actin antibody (Santa Cruz, CA) to normalize protein loading.
Immunohistochemistry
Under deep anesthesia with Nembutal (70 mg/kg intraperitoneally), mice were exsanguinated with 50 ml/100 g heparinized saline (0.9% NaCl, 2 U/ml heparin, 0.02% sodium nitrite) through a transcardial aortic cannula and fixed with glutaraldehyde-periodate acid saline as described previously. Glutaraldehyde-periodate acid saline contains final concentrations of 2.5% glutaraldehyde, 0.011 M sodium metaperiodate, 0.04 M sodium phosphate, 1% acetic acid, and 0.1 M NaCl and provides excellent preservation of tissue structure and antigenicity. Antigens were retrieved in 0.01 M citrate buffer pH 6.0 by microwave for 2 minutes, followed by steam for 25 minutes. The second antibody was localized using Vectastain ABC-Elite (Vector, Burlingame, CA) with diaminobenzidine as the chromogen, followed by a light counterstain with toluidine blue. The fixed kidneys were dehydrated through a graded series of ethanols, embedded in paraffin, sectioned at 4-μm thickness, and mounted on glass slides.
Immunofluorescence
Freshly removed kidneys were embedded in Cryo Embedding Medium (OCT) compound for frozen sections and were stored at −80°C. Five-micrometer sections were fixed with acetone and 4% paraformaldehyde, followed by blocking with the corresponding serum and incubation with primary and second antibodies. Cy3 donkey anti-goat antibody and anti-rabbit antibody were used as second antibodies. Images were semiquantified with Image J.
Phallodin Binding Assay
Cultured differentiated podocytes on glass cover slips were washed in PBS, fixed in 3.7% paraformaldehyde in PBS for 20 minutes, and extracted with acetone at −20°C for 3 to 5 minutes. After podocytes were blocked with 1% BSA, they were incubated with 0.1% saponin in PBS containing a saturating amount (0.4 μM) of Orange Green 488 phalloidin (Invitrogen, Carlsbad, CA) for 30 minutes at room temperature in darkness and examined at 500 to 520 nm.
Apoptosis Detection
Measurements utilized a TUNEL Apoptosis detection kit (Upstate). 4′,6-diamidino-2-phenylindole (DAPI) was used for counterstain. The percentage of apoptotic cells in 400 total cells from the same field was determined for quantification.
Renin Activity
Renin activity was measured as described previously.47,48 Briefly, cells were homogenized in 0.1 M Tris-HCl, pH 7.4, containing 3.4 mM 8-hydroxyquinolone sulfate, 0.25 mM EDTA, 0.1 mM phenylmethysulfonyl fluoride, 1.6 mM dimercaprol, 5 mM sodium tetrathionate, and 0.1% Triton X-100. Angiotensin I was detected using an Angiotensin I detection kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA), according to the manufacturer's instructions, and excess exogenous renin substrate was provided as described previously.49 The concentration of protein was determined with a BCA protein assay kit (Pierce, Rockford, IL).
Statistical Analysis
All values are presented as mean ± SEM. ANOVA and Bonferroni t tests were used for statistical analysis, and differences were considered significant when P < 0.05.
DISCLOSURES
None.
Acknowledgments
This work was supported by funds from the Department of Veterans Affairs, the Vanderbilt George O'Brien Kidney and Urologic Diseases Center (DK-79341), DK-62794, DK61018 (AMDCC), and Vanderbilt Diabetes Research and Training Center (DK20593).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Tan AL, Forbes JM, Cooper ME: AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol 27: 130–143, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR: Diabetic nephropathy: Mechanisms of renal disease progression. Exp Biol Med (Maywood) 233: 4–11, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Marshall SM: The podocyte: A major player in the development of diabetic nephropathy? Horm Metab Res 37 [Suppl 1]: 9–16, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Marshall SM: The podocyte: A potential therapeutic target in diabetic nephropathy? Curr Pharm Des 13: 2713–2720, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Reddy GR, Kotlyarevska K, Ransom RF, Menon RK: The podocyte and diabetes mellitus: Is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens 17: 32–36, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Coimbra TM, Janssen U, Grone HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J: Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 57: 167–182, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC, 3rd: Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: Prevention by lipoic acid treatment. BMC Nephrol 7: 6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Susztak K, Raff AC, Schiffer M, Bottinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 9. Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P: Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52: 1031–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G: Nephrin expression is reduced in human diabetic nephropathy: Evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 52: 1023–1030, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Komhoff M, Grone HJ, Klein T, Seyberth HW, Nusing RM: Localization of cyclooxygenase-1 and -2 in adult and fetal human kidney: Implication for renal function. Am J Physiol 272: F460–F468, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Adegboyega PA, Ololade O: Immunohistochemical expression of cyclooxygenase-2 in normal kidneys. Appl Immunohistochem Mol Morphol 12: 71–74, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Rangel EB, Moura LA, Franco MF, Pacheco-Silva A: Up-regulation of cyclooxygenase-2 during acute human renal allograft rejection. Clin Transplant 19: 543–550, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Rangel EB, Moura LA, Franco M, Pacheco-Silva A: Expression of cyclooxygenases during renal allograft rejection. Transplant Proc 36: 838–839, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Cheng HF, Wang CJ, Moeckel GW, Zhang MZ, McKanna JA, Harris RC: Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int 62: 929–939, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S: Immunohistochemical and functional correlations of renal cyclooxygenase- 2 in experimental diabetes. J Clin Invest 107: 889–898, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yabuki A, Taniguchi K, Yamato O: Immunohistochemical examination of cyclooxygenase-2 and renin in a KK-A(y) mouse model of diabetic nephropathy. Exp Anim 59: 479–486, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM: Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC: Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brosius FC, 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T: Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris RC, Zhang MZ, Cheng HF: Cyclooxygenase-2 and the renal renin-angiotensin system. Acta Physiol Scand 181: 543–547, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, Pichler R, Griffin S, Couser WG, Shankland SJ: Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 65: 30–39, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG: Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol 293: F398–F407, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Durvasula RV, Shankland SJ: Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol 294: F830–F839, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD: Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Itoh H: The (pro)renin receptor and the kidney. Semin Nephrol 27: 524–528, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Cheng H, Wang S, Jo YI, Hao CM, Zhang M, Fan X, Kennedy C, Breyer MD, Moeckel GW, Harris RC: Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol 18: 551–559, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Jo YI, Cheng H, Wang S, Moeckel GW, Harris RC: Puromycin induces reversible proteinuric injury in transgenic mice expressing cyclooxygenase-2 in podocytes. Nephron Exp Nephrol 107: e87–e94, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK: Aliskiren combined with losartan in type 2-diabetes and nephropathy. N Engl J Med 358: 2433–2446, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Durvasula RV, Shankland SJ: The renin-angiotensin system in glomerular podocytes: Mediator of glomerulosclerosis and link to hypertensive nephropathy. Curr Hypertens Rep 8: 132–138, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Derkx FH, Schalekamp MA: Human prorenin: Pathophysiology and clinical implications. Clin Exp Hypertens A 10: 1213–1225, 1988 [PubMed] [Google Scholar]
- 33. Deinum J, Ronn B, Mathiesen E, Derkx FH, Hop WC, Schalekamp MA: Increase in serum prorenin precedes onset of microalbuminuria in patients with insulin-dependent diabetes mellitus. Diabetologia 42: 1006–1010, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Wilson DM, Luetscher JA: Plasma prorenin activity and complications in children with insulin-dependent diabetes mellitus. N Engl J Med 323: 1101–1106, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Wilkinson-Berka JL: Prorenin and the (pro)renin receptor in ocular pathology. Am J Pathol 173: 1591–1594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F: Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med 18: 483–488, 2006 [PubMed] [Google Scholar]
- 37. Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W: Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Burckle CA, Jan Danser AH, Muller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G: Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47: 552–556, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T: Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi H, Ichihara A, Kaneshiro Y, Inomata K, Sakoda M, Takemitsu T, Nishiyama A, Itoh H: Regression of nephropathy developed in diabetes by (Pro)renin receptor blockade. J Am Soc Nephrol 18: 2054–2061, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Muller DN, Klanke B, Feldt S, Cordasic N, Hartner A, Schmieder RE, Luft FC, Hilgers KF: (Pro)renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension 51: 676–681, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Huang J, Siragy HM: Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology 150: 5557–5565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T: Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int 70: 641–646, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng H, Fan X, Guan Y, Moeckel GW, Zent R, Harris RC: Distinct roles for basal and induced COX-2 in podocyte injury. J Am Soc Nephrol 20: 1953–1962, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheng HF, Harris RC: Cyclooxygenase-2 expression in cultured cortical thick ascending limb of Henle increases in response to decreased extracellular ionic content by both transcriptional and post-transcriptional mechanisms. Role of p38-mediated pathways. J Biol Chem 277: 45638–45643, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA: High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 286: F1039–F1045, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Singh R, Singh AK, Alavi N, Leehey DJ: Mechanism of increased angiotensin II levels in glomerular mesangial cells cultured in high glucose. J Am Soc Nephrol 14: 873–880, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Cheng HF, Wang JL, Zhang MZ, Wang SW, McKanna JA, Harris RC: Genetic deletion of COX-2 prevents increased renin expression in response to ACE inhibition. Am J Physiol Renal Physiol 280: F449–F456, 2001 [DOI] [PubMed] [Google Scholar]