Abstract
The pathogenesis of the development of sclerotic lesions in focal segmental glomerulosclerosis (FSGS) remains unknown. Here, we selectively tagged podocytes or parietal epithelial cells (PECs) to determine whether PECs contribute to sclerosis. In three distinct models of FSGS (5/6-nephrectomy + DOCA-salt; the murine transgenic chronic Thy1.1 model; or the MWF rat) and in human biopsies, the primary injury to induce FSGS associated with focal activation of PECs and the formation of cellular adhesions to the capillary tuft. From this entry site, activated PECs invaded the affected segment of the glomerular tuft and deposited extracellular matrix. Within the affected segment, podocytes were lost and mesangial sclerosis developed within the endocapillary compartment. In conclusion, these results demonstrate that PECs contribute to the development and progression of the sclerotic lesions that define FSGS, but this pathogenesis may be relevant to all etiologies of glomerulosclerosis.
Focal segmental glomerulosclerosis (FSGS) is one of the most common glomerular pathologies. It was first described in 1925 by Fahr.1 The diagnosis of FSGS relies on histopathologic findings characterized by the presence of adhesions between the glomerular tuft and Bowman's capsule (BC), focal and segmental lesions with mesangial sclerosis, and obliteration of glomerular capillaries with hyalinosis.2 FSGS is considered primary or idiopathic when no etiology can be identified. Secondary FSGS is associated with infections, obesity, chronic hypertension, immunologic processes (e.g., IgA nephropathy and immune complex nephritis), and drug abuse. Up to 18% of primary FSGS cases are attributed to genetic mutations.3
FSGS is not a specific disease entity, but rather a pattern of specific histologic changes with quite diverse clinical behavior. Recently, various morphologic variants have been defined and a classification has been proposed to better address the diversity.4 Five light microscopic variants of FSGS were defined: the perihilar variant (lesions predominantly located at the vascular pole), the tip variant (for lesions located at the urinary pole), the cellular variant (characterized by endocapillary hypercellularity), the collapsing variant (collapse of the glomerular tuft associated with epithelial cell hypertrophy and hyperplasia), and finally, FSGS not otherwise specified (NOS) or classic FSGS, if lesions do not fit into one of the above mentioned categories. All morphologic variants are accompanied by some degree of epithelial hyperplasia, being less prominent in the perihilar variant and sometimes very prominent in the cellular and collapsing variant. It is still unclear whether the morphologic appearance of the glomerular lesions is associated with a specific cause or pathomechanism or the degree or developmental stage of the disease.
It is now generally accepted that loss of podocytes beyond a certain threshold triggers glomerulosclerosis.5–7 In addition, almost any glomerular disease, which ultimately leads to end-stage renal failure, triggers the formation of FSGS lesions. For this reason, FSGS has been called “the final common pathway” in the development of end-stage renal disease.
So far, the pathogenesis of a segmental glomerular lesion defining FSGS is not completely resolved. Understanding the sequence of events resulting in a sclerotic lesion is an important prerequisite for the development of specific therapeutical approaches. Great advances have been made in seminal studies of Kriz and coworkers. In these studies, it was shown that the classic FSGS lesion was associated with the formation of adhesions (synechia) between BC and the glomerular tuft.8 Nonetheless, it is still unclear how an adhesion progresses toward a segmental or even global sclerotic lesion of the glomerulus. In previous studies, it has been suggested that parietal epithelial cells (PECs) can proliferate to form cellular glomerular lesions observed in crescentic glomerulonephritis and collapsing glomerulopathy.9–15 Le Hir and Kriz proposed that cellular adhesions were formed predominantly by injured podocytes.16 In this study, we employed a transgenic approach and cell lineage tracing experiments to determine whether PECs are involved in the formation of glomerular sclerotic lesions in models with different underlying etiologies. For this purpose, podocytes and PECs were specifically traced in models of FSGS induced by maladaptation to chronic hypertension, or by a primary injury to the podocytes. The findings were validated in human biopsies diagnosed with FSGS or nephrosclerosis.
RESULTS
Induction of the 5/6 Nephrectomy + Deoxycorticosterone Acetate–Salt Model for FSGS in Triple Transgenic Mouse Lines
The 5/6 nephrectomy (Nx) + deoxycorticosterone acetate–salt (DOCA-salt) model was induced in transgenic male mice after podocytes or PECs had been irreversibly labeled by administration of doxycycline (dox) (Figure 1). Proteinuria, as measured by dipstick on spot urine samples, occurred 2 to 4 weeks after implantation of the DOCA pellets. After 12 weeks, histology revealed typical sclerotic lesions in 20 to 50% of all glomeruli. The histopathology of the 5/6 Nx + DOCA-salt model showed a focal and segmental sclerosis of the glomeruli characterized by formation of adhesions between the Bowman's capsule and the glomerular convolute and obliteration of the capillaries due to hyalinosis and extracellular matrix accumulation in a segment of the glomerular convolute (Figure 1, B through F). Sclerotic lesions and adhesions were seen along the entire periphery of the glomerulus, from the vascular stalk to the glomerular urinary pole. The phenotype of the glomerulosclerotic lesions was heterogenous in this model, and next to the glomerular sclerotic lesions as described above, which were the most prevalent, the kidneys also showed more “active glomerulosclerosis,” which describe lesions with scarring and/or hyalinosis but also a marked hypertrophy and/or hyperplasia of the glomerular epithelium which lead to the formation of pseudocrescents. Representative examples of active lesions are shown in Figure 1. In summary, focal and segmental lesions were successfully induced in all our transgenic mouse lines required to tag podocytes or PECs.
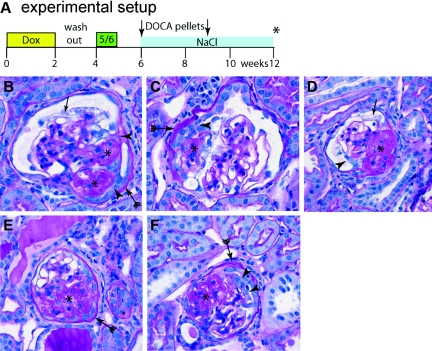
Figure 1.
The 5/6 Nx + DOCA-salt model induces glomerular lesions characteristic of focal and segmental glomerulosclerosis. (A) Schematic of the experimental set-up. After 14 days of treatment with doxycycline (dox) to induce the irreversible genetic labeling and a washout phase of 7 days, a subtotal nephrectomy (5/6) was performed. After a recovery phase, two 25-mg DOCA pellets were implanted subcutaneously (arrows) and 0.1 to 0.9% NaCl was added to the drinking water. Animals were killed after 12 weeks (asterisk). (B through F) PAS-stained paraffin section showing segmental glomerulosclerosis induced in the 5/6 Nx + DOCA-salt model. (B) Lesion near the vascular stalk, where two adhesions (arrowheads), segmental sclerosis (asterisks), a thickened BC (arrow with tails), and vacuolization of podocytes (arrow), is present. (C,D,F) Glomerular lesions with extracapillary proliferations (arrowheads) were also observed. Thickening of the basement membrane of BC (arrow with tails) was observed, mostly in association with an adhesion (B, arrowheads) and/or segmental sclerosis of the glomerular tuft (asterisk). (D and F) More advanced sclerotic lesions (asterisk).
Tracing Podocytes in the 5/6 Nx + DOCA-Salt Model for FSGS
First, podocytes were irreversibly genetically tagged in triple transgenic Pod-rtTA/LC1/R26R mice (Figure 2, A and B). Usage of a dox-inducible labeling system assured that no additional direct genetic labeling occurred during the disease models.17 As shown in Figure 2, C and D, genetically labeled podocytes were absent from sclerotic lesions of affected glomeruli. Stainings for β-galactosidase (i.e., the genetic tag of podocytes) and the podocyte marker protein synaptopodin on serial sections confirmed that sclerotic lesions were devoid of podocytes (Figure 2, E1 through F2, asterisk). Because in studies so far the genetic label β-galactosidase (β-gal) was independent of the differentiation status in podocytes,17,18 this result showed that even no viable dedifferentiated podocytes were present within sclerotic lesions.
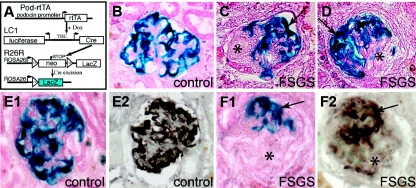
Figure 2.
Genetically labeled podocytes are absent from sclerotic lesions. (A) Schematic of the podocyte-specific inducible irreversible genetic tagging. For a detailed description see text. (B) Normal β-gal staining of genetically labeled podocytes (blue), as can be observed in control animals after dox administration (control), or in unaffected glomeruli of animals after 5/6 Nx + DOCA-salt treatment. (C and D) In experimental animals, β-gal staining is absent from segmental sclerotic lesions (asterisks) but still preserved within the unaffected portion of the same glomerulus (arrows, X-gal/eosin stained cryosections). (E and F) Serial cryosections were stained for the genetic marker β-gal (E1, F1, blue staining, eosin counterstain) or synaptopodin (E2, F2, brown staining). β-gal and synaptopodin staining colocalized within the tuft of healthy glomeruli (E1, E2) and outside of sclerotic lesions (F1, F2). Within sclerotic lesions neither staining was detected (F1, F2, asterisk).
Tracing PECs in the 5/6 Nx + DOCA-Salt Model
Next, PECs were labeled in an irreversible fashion by administration of dox using triple transgenic PEC-rtTA/LC1/R26R mice (Figure 3A). It was verified in the removed right kidneys that PECs were genetically labeled with 50 to 66% efficiency as described before.19 In the left remnant kidneys, weak speckled β-gal staining was observed within glomerular tufts of animals subjected to the 5/6 Nx + DOCA-salt model. Because this staining was weak and also observed in glomeruli without evidence for sclerotic lesions (Figure 3B), it was not interpreted as a specific cellular staining.
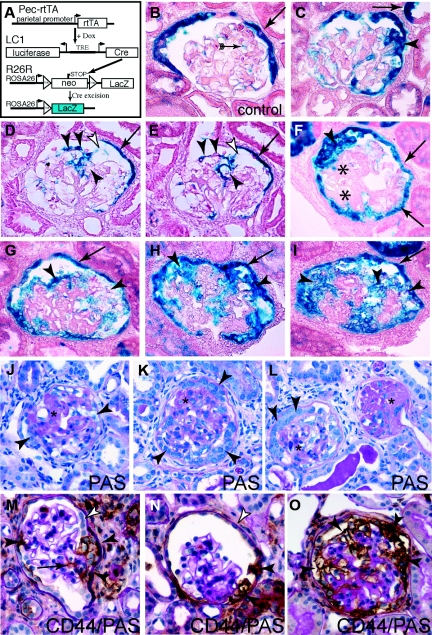
Figure 3.
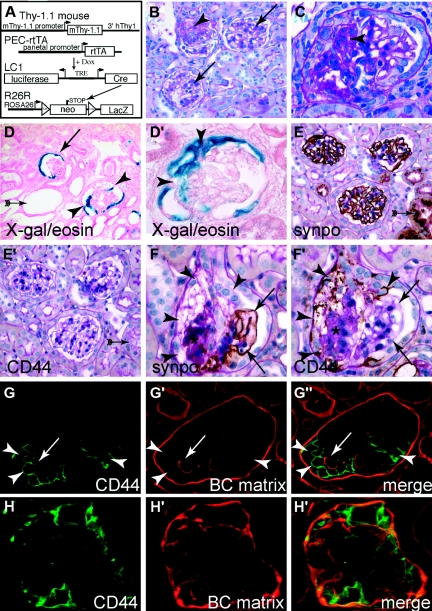
Parietal cells contribute to glomerulosclerotic lesions in the 5/6 Nx + DOCA-salt model. (A) Schematic of inducible irreversible genetic tagging of PECs. (B) Normal β-gal staining of genetically labeled PECs (blue, arrow). Note that, after 5/6 Nx + DOCA-salt treatment, weak nonspecific β-gal staining can also be observed along the tissue interfaces within the glomerular tuft (arrow with tails), which is not present directly after genetic labeling. (C through J) Representative examples of glomeruli affected by segmental or global sclerotic lesions. (C) Small segmental lesion showing bridging and migration of β-gal–positive PECs in the involved tuft segment (arrowhead). Note that single tubular cells can also be labeled in this mouse (arrow). (D and E) Serial sections show a β-gal–positive cellular adhesion (white arrowhead) from where β-gal–positive cells invade a segment of the glomerular tuft (arrowheads; arrows, labeled PECs). (F) A cellular lesion (arrowhead) consisting of labeled PECs (arrows) associated with segmental sclerosis (asterisks). (G through I) Late stages with global sclerosis of the glomerular tuft. β-gal–positive PECs cover a large part, or the entire surface, of the tuft [arrowheads; (B through J) X-gal/eosin stained cryosections]. The arrows mark the genetically labeled PECs on BC. (J through L) Corresponding later stages of largely sclerosed glomeruli are shown on PAS-stained paraffin sections (asterisks). Bowman's space as well as the glomerular tuft is populated by multiple, often polygonal, cells (arrowheads). Fewer cells can be found in very late stages of globally sclerosed glomeruli [panel (L), on the right]. (M) Activated PECs participate in the formation of the sclerotic lesion. Parietal cells express the activation marker CD44 (brown staining) only at the site or in close proximity of the adhesion to BC (black arrowheads) but not at other sites (white arrowhead). A thickened basement membrane of BC also indicates PEC activation. CD44-positive cells invade the glomerular tuft using the adhesion as entry site (arrow). (N) Parietal cells express higher levels of CD44 close to the sclerotic lesion at the vascular stalk (black arrowheads). (O) Example of a glomerulus showing global sclerosis. The glomerular tuft is surrounded by CD44-positive presumptive parietal cells [arrowheads; (A through O)] 5/6 Nx + DOCA-salt model of the mouse; (M through O) CD44 immunhistologic staining (brown) costained with PAS).
When the left remnant kidneys were examined, genetically labeled PECs were detected on sclerotic segments of the capillary tuft (Figure 3, C through E, black arrowheads). There was a clear association of such cells with cellular adhesions to BC (Figure 3, D and E, white arrowheads). Some of the sclerotic lesions were associated with hyperplasia in Bowman's space (Figure 3F). In these cases, the cells in Bowman's space were genetically tagged, indicating their origin from PECs. In glomeruli affected by global sclerosis, genetically labeled cells were present on Bowman's capsule and covered large segments of the glomerular tuft (Figure 3, G through I), indicating that the PECs remained viable also in these glomeruli. For comparison, the morphology of glomeruli affected by more advanced sclerotic lesions is shown in periodic acid–Schiff (PAS)-stained paraffin sections of the same animals (Figure 3, J through L). Multiple cells—presumably of parietal cells origin—can be seen in advanced sclerotic glomeruli, even on PAS-stained sections (arrowheads). Fewer cells were present in glomeruli affected by global sclerosis (Figure 3L, asterisk).
Focal Expression of the Activation Marker CD44 by Parietal Cells
CD44 has been identified previously to be expressed de novo by activated PECs but not by podocytes.17 In addition, it was verified also in a murine model of FSGS that CD44 is specifically expressed on activated PECs and not on podocytes or any other resident glomerular cells (see Supplemental Figure 1). Apart from activated PECs, CD44 is also expressed on leukocytes. To test whether PECs were also activated in the 5/6 Nx + DOCA-salt model, sclerotic lesions were costained with CD44 and PAS (Figure 3, M through O). As shown in Figure 3M, CD44 was expressed by a subpopulation of cells in close association with an adhesion between the glomerular tuft and BC (black arrowheads). PECs along other parts of BC were mostly negative for CD44. Morphologic changes of some PECs were interpreted as signs of activation (i.e., increased cellular volume, increased density of cells, and thickened BC), mostly close to cellular adhesions (white arrowheads). Not all of these PECs were positive for CD44, suggesting that it is a late marker for activation.
Activated Parietal Cells Deposit Extracellular Matrix
To test whether activated PECs participate in the formation of sclerotic lesions, a costaining with the single-chain antibody LKIV69 and CD44 was performed. The LKIV69 antibody recognizes specifically the extracellular matrix of BC (i.e., specific heparan sulfate moieties) but not of the glomerular tuft.20 Bowman's capsule type matrix (BC matrix) was detected as thin deposits on the glomerular tuft in early segmental sclerotic lesions (Figure 4A). BC-type matrix was always found in association with CD44-positive PECs. Advanced sclerotic lesions contained more BC-type matrix depositions on the glomerular tuft (Figure 4, B through D). In glomeruli with global sclerosis, the capillary tuft was surrounded by CD44-positive cells and contained BC-type matrix along its entire circumference (Figure 4D). Again, there was colocalization of CD44-positive cells and BC-type matrix. To analyze the microanatomical relationship between the BC-type matrix and the GBM, both types of matrixes were selectively stained (Figure 5). Within small lesions (Figure 5, A and B), BC-type matrix is deposited along the outer surface of the GBM, which remains preserved also within the lesion. Note that BC-type matrix is not always present at the site of the adhesion (Figure 5B, white arrow), indicating that early adhesions may be purely cellular. BC-type matrix remains confined to the outer aspect of the GBM also in more advanced sclerotic lesions (Figure 5C), consistent with the notion that endocapillary matrix (i.e., sclerosis) is not deposited by parietal cells.
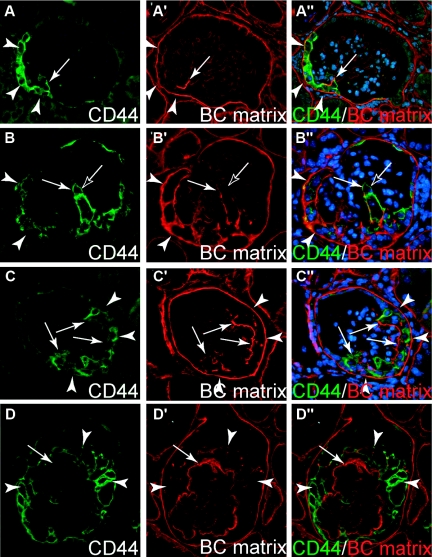
Figure 4.
Activated parietal cells deposit extracellular matrix. (A through D) Serial sections of four glomeruli with segmental (A through C) to global (D) sclerosis stained for the parietal cell activation marker CD44 and BC-type matrix (LKIV69). (A) Small early lesions showing expression of CD44 in a subpopulation of the PECs at the BC (arrowheads). A few CD44-positive cells bridge Bowman's space and are attached to the glomerular tuft. The involved segment of the glomerular tuft is covered by BC type matrix which colocalizes with CD44-positive cells (A′ and A″, arrow). (B and C) More advanced lesions in which segments of the glomerular tuft are involved. CD44 is expressed by most PECs along BC (arrowheads) and also by many cells on the glomerular tuft (arrows). BC-type matrix is deposited in association with CD44-positive PECs on the glomerular tuft the BC (B″ and C″, arrows). Occasionally, CD44-positive cells were seen without evidence for BC-type matrix (B, black arrow). (D) In a glomerulus with global sclerosis, CD44-positive cells (arrowheads) and BC-type matrix (arrow) are present along the entire circumference of the capillary.
Figure 5.
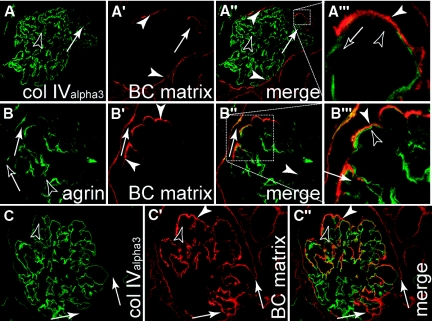
Parietal cells deposit matrix onto the GBM. Human biopsies of patients diagnosed with FSGS were costained for BC-type matrix or the GBM [(A and C) collagenIValpha3; or (B) agrin]. (A) CollagenIValpha3 was specifically detected within the capillary convolute (black arrowheads). An early lesion (white arrow) is in direct continuation with the BC-type matrix of Bowman's capsule (white arrowheads). (A‴) Higher magnification shows that BC-type matrix is deposited on top of the GBM. (B) The same results are obtained in early sclerotic lesions when staining for agrin, which stains the GBM (black arrowheads) as well as Bowman's capsule (black arrow). (C) Advanced sclerotic lesion, where most of the glomerular tuft is covered by BC-type matrix (white arrowheads). Within the endocapillary compartment, no BC-type matrix is deposited (black arrowhead). At the site of the adhesion, BC-type matrix is in continuity from Bowman's capsule onto the capillary convolute (white arrows) while no additional GBM is formed at this site.
Tracing Parietal Cells in the Spontaneous Thy1.1 Mouse Model for FSGS
To trace PECs in the chronic Thy1.1 mouse model, the transgenic Thy1.1 mouse was crossed to the triple transgenic PEC-rtTA/LC1/R26R mouse (Figure 6A). Thy1.1 transgenic mice develop glomerular FSGS lesions as they age.21,22 Proteinuria could be observed beginning from 8 to 10 weeks after birth. Sixteen to 24 weeks after birth, about 10 to 25% of all glomeruli showed the characteristic histologic changes of segmental glomerulosclerosis of the glomerular tuft (Figure 6, B and C, arrows; normal glomeruli, arrows, arrowheads). Because the PECs were labeled by irreversible somatic recombination, the spontaneous Thy1.1 model for FSGS could be analyzed despite its long course. Aberrant genetic labeling of other glomerular cells could occur due to the spontaneous phenotype of the Thy1.1 transgene; the labeling was verified by a uninephrectomy after a washout period of the dox. The specificity of the labeling was also confirmed in Supplemental Figure 1.
Figure 6.
Parietal cells contribute to glomerulosclerotic lesions in the spontaneous Thy-1.1 mouse model. (A) The triple transgenic PEC-rtTA/LC1/R26R mouse was crossbred to the Thy1.1 transgenic mouse model, which expresses the rat Thy1.1 antigen on podocytes. Expression of the Thy1.1 antigen causes spontaneous FSGS in aging mice. (B) Representative histologic section of the kidney of aged male Thy1.1/PEC-rtTA/LC1/R26R mice. Glomerulosclerotic lesions occur in a focal fashion (arrow) next to unaffected glomeruli (arrowheads). (C) Higher magnification of a segmental sclerotic lesion (asterisk). (D and D′) Representative sclerotic lesions. Parietal cells are genetically labeled in normal glomeruli (arrow). Adhesions between BC and the glomerular tuft can be observed in sclerotic glomeruli (arrowheads). Genetically labeled cells are present on the glomerular tuft in close association with the adhesion. Multiple tubular dilatations can be seen as a sign for chronic proteinuria and renal injury (arrow with tails). (E and E′) Serial sections of three unaffected glomeruli showing preserved synaptopodin staining and absent glomerular CD44 staining. Note the nonspecific staining of protein resorption vacuoles in a neighboring tubule as a sign for significant focal proteinuria [panel (E), arrow with tails]. (F and F′) Within a segmental sclerotic lesion (asterisk), the podocyte marker synaptopodin is absent (F, arrowheads) but CD44 is expressed de novo (F′, arrowheads). Bowman's capsule is thickened in a segmental fashion close to the CD44-positive cells. Along the unaffected part of the glomerular tuft (arrows), the podocyte marker synaptopodin is preserved. (G through G″) Segmental sclerotic lesion with CD44-positive cells (arrowheads, activated PECs). Deposition of BC-type extracellular matrix (arrow, BC matrix) is observed exclusively in direct association with the CD44-positive cells. (H through H″) Advanced sclerotic lesion. The glomerular tuft is surrounded by CD44-positive cells. BC-type matrix is deposited ubiquitously on all segments of the sclerotic glomerular tuft.
When histologic sections of aged transgenic mice were stained with X-gal, focal sclerosis of the glomeruli and signs of chronic renal damage, such as dilated and atrophic tubules, were observed (Figure 6D, arrow with tails). Adhesions of BC to the glomerular tuft were frequently observed (Figure 6, D and D′, arrowheads, labeled PECs, arrow). Adhesions stained positive for β-gal, confirming that the adhesions were formed by PECs. In addition, β-gal–positive PECs populated the glomerular tuft. PECs were mostly observed in continuity with cellular adhesions, supporting the notion that adhesions provide the entry site to the glomerular tuft.
To test whether PECs migrated onto the glomerular tuft and replaced podocytes, serial paraffin sections were stained for CD44 as a marker for activated PECs and synaptopodin as a marker for podocytes. In unaffected glomeruli, synaptopodin expression remained preserved throughout the entire glomerular tuft and no CD44 expression was observed (Figure 6, E and E′). In glomeruli with sclerotic lesions, CD44 expression was always observed in association with the affected sclerotic segment whereas synaptopodin expression was excluded from this area. Synaptopodin expression remained preserved along the intact part of the glomerular tuft (Figure 6, F and F′). These results confirmed our results in the 5/6 Unx + DOCA model that differentiated podocytes were absent from the sclerotic lesions.
Finally, it was tested whether activated PECs also participate in the formation of the matrix within sclerotic segmental lesions similar to the 5/6 Nx + DOCA-salt model. BC matrix was detected as thin deposits on the glomerular tuft of early segmental sclerotic lesions (Figure 6G, arrow). Again, BC-type matrix was always found in association with CD44-positive PECs on the glomerular tuft. In glomeruli with global sclerosis, the capillary tuft was surrounded by CD44-positive cells and contained BC-type matrix along its entire circumference (Figure 6H).
Analysis of the Munich-Wistar-Froemter Rat Model for FSGS
To confirm our results, a third genetic model of spontaneous FSGS was analyzed in 16-month-old Munich-Wistar-Froemter (MWF) rats. Because of an unknown genetic defect, these rats develop glomerulosclerotic lesions around the age of 16 to 20 weeks.23 In this model, similar results were obtained as in the above-described mouse models: Serial sections were stained consecutively for synaptopodin and CD44. Within glomeruli without evidence for sclerotic lesions, the podocyte marker synaptopodin was expressed throughout the entire capillary tuft (Figure 7A1). CD44 was not expressed in these glomeruli. However, a de novo expression of CD44 was observed in a subpopulation of single PECs (Figure 7A, black arrowhead). Within glomeruli with mild to moderate segmental sclerotic lesions, differentiated podocytes were absent within the lesions, as detected by staining for synaptopodin (Figure 7B1, arrowhead). CD44 was expressed de novo within the sclerotic lesions, indicating the presence of activated PECs (Figure 7B2). Similar findings were observed in advanced sclerotic lesions (Figure 7, C1 and C2). Of note, the histology of these sclerotic lesions did not show hyperplasia and therefore did not suggest the presence of additional or different cells. Nonetheless, the use of the specific markers claudin-1 (not shown) and CD44 revealed the presence of activated PECs on the affected tuft segments.
Figure 7.
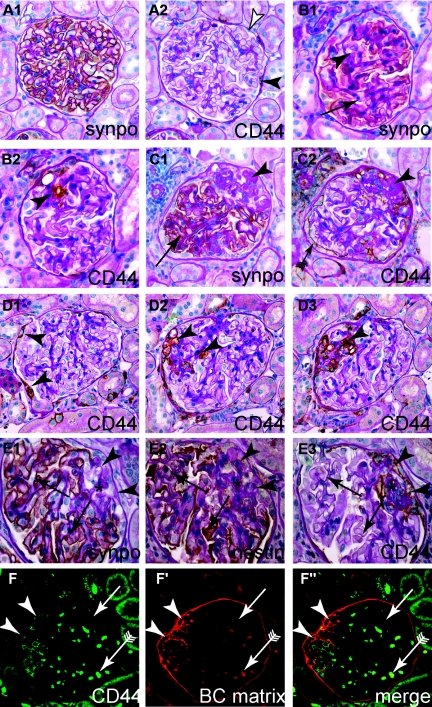
Activated parietal cells are present within sclerotic lesions of the MWF rat. (A through C) Representative serial sections of aged female MWF rats, consecutively stained for synaptopodin as podocyte marker or CD44 as marker for activated PECs. (A1 and A2) Normal glomerulus. Although most PECs are CD44-negative (white arrowhead), focal CD44 expression can be observed in PECs (black arrowhead). (B1 and B2) A small sclerotic lesion, where synaptopodin staining is reduced and replaced by CD44-positive cells (arrowhead). (C1 and C2) Advanced sclerotic lesion, where podocytes are absent (arrowhead) and replaced by matrix or CD44-positive cells. Note that CD44-positive cells on BC show a more activated phenotype (more cuboid, arrow with tale). (D1 through D3) Serial sections of a glomerulus affected by a segmental sclerotic lesion (arrowheads D2 and D3). Note that on section D1 the lesion cannot be seen. Focally activated CD44-positive PECs are the only sign of a sclerotic lesion in another plane of sectioning (arrowheads, D1). (E1 through E3) To exclude a potential loss of the podocyte marker synaptopodin, serial sections were also stained for the podocyte marker nestin. Nestin always colocalized with synaptopodin (arrows). Nestin was not expressed in sclerotic lesions (asterisk), which was populated by CD44-positive cells [arrowheads; (A through E) immunohistologic stainings on paraffin sections counterstained with PAS]. (F through F″) BC-type matrix was deposited in sclerotic lesions (arrowheads, CD44-positive) but not along the intact glomerular tuft (arrow). Erythrocytes show autofluorescence in all channels (arrow with tails; immunofluorescent double staining on paraffin sections).
Segmental sclerotic lesions were often not detectable on all serial sections of the affected glomerulus. In these cases, CD44 expression of PECs was the only sign of a sclerotic lesion of the affected glomerulus (Figure 7D1, arrowheads). To verify that the absence of synaptopodin staining from sclerotic lesions did not occur as a result of podocyte dedifferentiation, serial sections were also stained for nestin, which has been proposed as a more stable marker for podocytes.24 As shown in Figure 7, E1 through E3, nestin staining colocalized with synaptopodin staining (arrows) and was also excluded from sclerotic lesions (arrowheads). Finally, it was tested whether CD44-positive cells again colocalize with BC-type basement membrane in the MWF model. As depicted in Figure 7, F through F″, BC-type matrix was deposited de novo exclusively within the segmental sclerotic lesions. There, the matrix colocalized with CD44-positive activated PECs (arrowheads; arrow with tails, autofluorescence erythrocytes).
Analysis of Sclerotic Lesions in Human Renal Biopsies
To test whether activated PECs are also involved in the formation of sclerotic lesions of human patients, 16 biopsies of native kidneys and 2 biopsies of transplanted kidneys were selected because they contained FSGS lesions, as diagnosed by a renal pathologist. A representative example of a patient diagnosed with primary FSGS or with hypertensive nephrosclerosis is shown in Figure 8, A through A‴ and B through B″, respectively. In all sclerotic lesions, claudin-1–positive PECs were observed on the glomerular tuft. Most of the lesions coexpressed CD44 and/or BC-type matrix. As shown in Figure 8B, sclerotic lesions may easily be missed on light microscopy if specific stainings for claudin-1 or BC-type matrix are not performed. In summary, these results showed that the sclerotic lesions in our animal models recapitulated the pathomorphology of sclerotic lesions in humans.
Figure 8.
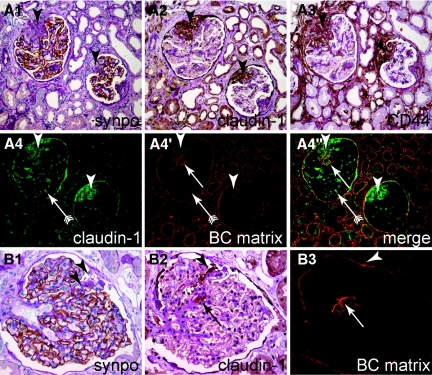
Activated parietal cells are present in glomerulosclerotic lesions in human FSGS. Serial sections of a human biopsy diagnosed as primary FSGS were stained for (A1) synaptopodin, (A2) claudin-1, and (A3) CD44 (PAS counterstain). Two representative glomeruli with sclerotic lesions (arrowheads) with epithelial hyperplasia are shown. Sclerotic lesions are devoid of synaptopodin (podocytes) and contain claudin-1/CD44 positive activated PECs. (A4 through A4″) In an immunofluorescent double staining of the same glomeruli, deposition of BC-type extracellular matrix (arrow) can be observed colocalizing with claudin-1–positive PECs. Erythrocytes within glomerular capillary are visible due to autofluorescence (arrow with tails). (B through B3) Serial staining of a human biopsy diagnosed as nephrosclerosis. An inconspicuous cellular adhesion consisting of claudin-1–positive PECs is shown (B1 and B2, arrowheads). The defect in synaptopodin staining as well as the segmental sclerotic lesion is not obvious (B1, arrowheads). (B3) Staining for BC-type matrix reveals only a thickening of BC at the site of adhesion (arrowhead) and a tangential section of the sclerotic lesion within the tuft, which is also populated by PECs [panels (B2 and B3), arrow].
DISCUSSION
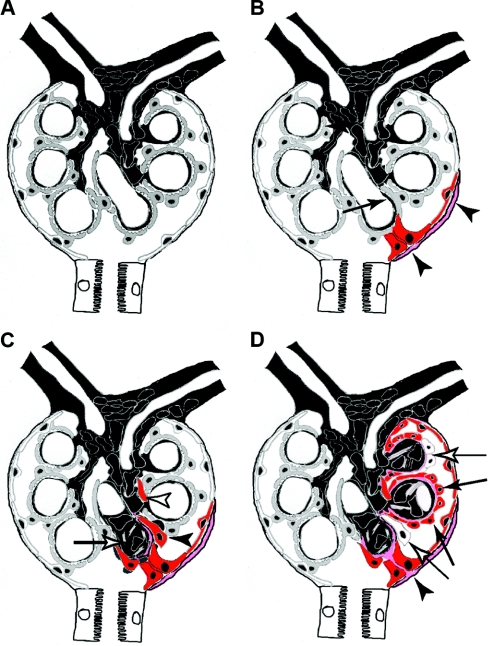
In this study, we provide for the first time definite evidence that PECs participate in the formation of sclerotic lesions, which define focal and segmental glomerulosclerosis (FSGS). The sequence of events leading to glomerulosclerosis observed in three different experimental models of glomerulosclerosis is illustrated in Figure 9.
Figure 9.
Schematic summary of the role of parietal cells in glomerulosclerosis. (A) A normal glomerulus consists of the endocapillary compartment (capillaries and mesangium in black) and epithelial cells: podocytes lining the capillary tuft (gray cells) and PECs lining the inside of BC (white cells). (B) Focal activation of PECs (red) and formation of an adhesion to the capillary are the first events in the formation of a sclerotic lesion. In some cases, focally activated PECs also produced more matrix on BC (pink matrix). Podocytes in the immediate vicinity of an adhesion are often effaced. (C) Activated PECs invade the affected segment of the glomerular tuft (black arrowhead) and deposit BC-type matrix. The invading PECs remain strictly within the extracapillary compartment. Occasionally, they appear disconnected from the adhesion due to tangential sections (white arrowhead). Within the endocapillary compartment, mesangial sclerosis develops within the affected segment (white arrow). Sclerosis of the glomerular tuft progresses from the adhesion. (D) Advanced sclerotic lesion. The former capillary loops are covered by parietal cells (black arrows) and BC-type matrix (pink). In advanced lesions, some invading parietal cells no longer express markers of activation (white arrows). At the site of adhesion, a continuous bridge of BC-type matrix has formed between Bowman's capsule and the glomerular tuft (black arrowhead).
The Pathomechanism of the Development of Sclerotic Lesions
The process of FSGS was triggered by an initial insult of different etiologies (e.g., podocytopenia) as shown consistently by others. This resulted in focal activation of PECs, which formed cellular adhesions between BC and the capillary tuft. The adhesion then provided the entry site from where PECs migrated (invaded) onto the capillary tuft. Cell lineage tracing allowed us to determine unequivocally the origin of PECs.
So far, the PEC has not been recognized much—predominantly because of a lack of specific marker molecules. However, a role for PECs in the development of sclerotic lesions has been proposed before in human FSGS with mild to severe hyperplasia,25 and especially in collapsing lesions.9–14 More recently, we proposed an involvement of PECs expressing the progenitor markers CD133 and CD24 in some forms of FSGS in human biopsies.15
In this study, the 5/6 Nx + DOCA-salt model also developed lesions with epithelial hyperplasia in a fraction of the glomeruli and we identified the proliferating cells as PECs. However, an important finding in the present study was that the histology of many glomerulosclerotic lesions in the 5/6 Nx + DOCA-salt model and especially in the models with a slower progression (i.e., chronic Thy-1.1 model and MWF model) did not suggest the presence of additional cells (i.e., activated PECs). Nonetheless, lineage tracing as well as the use of markers for PECs showed unequivocally that also in these “noncellular lesions” PECs were present on the glomerular tuft. In fact, especially in the earliest lesions the loss of podocyte markers was associated with the presence of PECs on the glomerular tuft. Therefore, the loss of podocyte markers could reflect replacement of podocytes by PECs rather than dysregulation or loss of markers by podocytes.
Parietal Cell Deposit Matrix
The second major finding of this study is that deposition of BC-type matrix within sclerotic lesions always colocalized with CD44 and/or claudin-1–positive PECs. The fact that in some early lesions PECs were observed without BC-type matrix deposition may suggest that the PECs invade the tuft first, and that deposition of matrix is a secondary event. The immunostainings for the two types of matrixes showed that the BC-type matrix localizes directly on the still existing GBM. This finding is also consistent with the notion that podocytes were replaced by PECs, which then synthesized new matrix on the GBM.
Cai et al. have observed by immunoelectron microscopy that two different types of matrix deposition occurred in sclerotic lesions.26 First, they detected BC-type matrix (i.e., collagen type IV α1 and α2) on top of the GBM, which was deposited by flat epithelial cells. Because lineage tracing was not available, Cai et al. speculated at that time that these cells were “dedifferentiated podocytes”. However, our study now shows that these cells originated from PECs. Second, Cai et al. described that matrix is also deposited within the affected segment of the mesangium, which differs from the matrix deposited by activated PECs. Our study suggests that segmental mesangial sclerosis is an event secondary to the invading PECs because migrating PECs were observed also in early lesions, which had not yet developed mesangial sclerosis of the affected segment. However, no definite experimental proof for this hypothesis can be provided at this time.
Activation and Migration of PECs
The present paper demonstrates a role of PECs in the pathogenesis of FSGS lesions. According to current knowledge, these are mostly caused by podocytopenia, chronic hypertension, and hyperfiltration. The sclerotic lesions are characterized by no or mild hyperplasia of the epithelial cells. In previous studies, we and others have investigated the role of PECs in collapsing glomerulopathy (CG) and crescentic glomerulonephritis (CrGN) where the lesions are typically associated with proliferative hyperplasia within the extracapillary compartment. It was shown that PECs (and also podocytes) form the cellular or crescentic lesions within Bowman's space.15,17,18,24 In addition, we and others observed that PECs could migrate on top of the glomerular convolute and cover or replace podocytes also in CG and CrGN.14,15,17,27 In summary, it is suggestive that the activation and migration/invasion of PECs might represent a common pathologic phenomenon in very different glomerular diseases that eventually lead to glomerular scarring.
Activated PECs Are Associated with Profibrotic Effects
The fourth major finding of this study was that activated PECs were associated with profibrotic rather than regenerative effects. This may be surprising because, in a previous study, we showed that in development a small number of (nonactivated) PECs migrate onto the glomerular tuft where they differentiate into podocytes.19 This has spurred the hope that this regenerative mechanism for podocytes can be used also in the adult kidney. However, in the present study, we have analyzed exclusively the fraction of glomeruli where sclerotic lesions have developed and, thus, where a possible regenerative mechanism has failed. Within these lesions, PECs were undoubtedly associated with profibrotic events: All sclerotic lesions contained PECs; PECs deposited matrix and were associated with mesangial sclerosis of the affected segment. No PECs were present on the remaining intact segments of the glomerular tuft outside the sclerotic lesion. These findings were also corroborated by a previous study, where we showed that PECs did not show signs of differentiation into podocytes when localized in a pathologic lesion in crescentic GN, collapsing glomerulopathy, and also some forms of FSGS.15
In this context it is also an interesting question whether additional podocyte loss may also occur secondary to invading PECs. It is clear that podocyte injury can induce the development of FSGS.6,7,28 However, in most studies, a reduced number of podocytes is associated with the presence of a glomerular scar, even if the primary injury was not directed primarily against podocytes. It is likely that podocytes can also be lost secondarily because of the invasion of PECs. In fact, as mentioned above, in previous studies we observed segmental lesions in which podocytes were directly covered by PECs.13,14,17
CONCISE METHODS
Transgenic Mice
The generation of PEC-rtTA, POD-rtTA, LC1, R26R, and Thy1.1 transgenic mice was described previously.19,21,22,29,30,31 To render the transgenic mouse lines susceptible for the development of FSGS, the mice were crossed up to five times into the 129/Sv genetic background. Animals were housed under standard SPF-free conditions. All animal procedures were approved by the LANUV NRW (8.87-50.10.35.08.106; 8.87-50.10.35.08.254 and 50.203.2 – AC 10/06).
Doxycycline Treatment
The PEC-rtTA, POD-rtTA, and PEC-rtTA/Thy-1.1 animals received doxycycline hydrochloride (dox) via the drinking water ad libidum for a total of 7 or 14 days (5% sucrose, 1 mg dox/ml, protected from light), which was exchanged every 2 days. To assure that the mice were free from dox during the experimental procedures, the mice received normal drinking water ad libidum for at least 7 days (washout period).
Animal Experiments
5/6 Nx + DOCA-Salt Model
Six-week-old PEC-rtTA and POD-rtTA mice (n = 10 each) received dox as described above. For the 5/6 Nx, the mice were anesthetized with ketamine-xylazine (100 mg/ml ketanest and 20 mg/ml xylazine in normal saline 0.9%; 0.1 ml/10 g of body wt) and after shaving a laparotomy was made. The vasculature of the right kidney was removed and used as control for the efficiency of the genetic tagging after dox treatment (t = 0). The upper and lower poles of the left kidney (two thirds of the kidney) were excised and Gelastypt (Sanofi-Aventis, Frankfurt, Germany) was used to stop bleeding. After 7 days, a 50-mg pellet (released over 21 days) of deoxycorticosterone acetate (“DOCA”; Innovative Research, FL) was implanted subcutaneously and 0.5 to 0.9% of NaCl was added to the drinking water. During this period, body weight and proteinuria were closely monitored. New 50-mg DOCA pellets were implanted after 3 weeks and animals were sacrificed after the 6th week. The remnant left kidney was recovered and processed for histology.
MWF Rat Model
MWF rats are spontaneausly hypertensive and develop proteinuria and FSGS. Sixteen-month-old male and female MWF rats were sacrificed and the kidneys were collected and processed for histology.
Spontaneous (“Chronic”) Murine Thy-1.1 Model
Thy-1.1 transgenic mice ectopically express the Thy-1.1 protein on podocytes, which mediates development of proteinuria within 8 to 14 weeks after birth and FSGS lesions within the first 2 to 4 months.21,22 Four- to 5-week-old nonproteinuric quadruple transgenic PEC-rtTA/LC1/R26R/Thy-1.1 crossbreeds (n = 10) received dox for 7 days (see above) to permanently tag PECs. To verify specific labeling of PECs, a uninephrectomy (Unx, see 5/6 Nx + DOCA-salt model above) was performed after the dox washout period. Two (n = 5) and 4 (n = 5) months after the Unx, the remaining right kidney was perfusion fixed and harvested for further analysis.
Antibody Mediated (“Accelerated”) Thy-1.1 Model
Injection of anti–Thy-1.1 antibodies accelerates the development of proteinuria and FSGS in Thy-1.1 transgenic mice, resulting in an acute and massive proteinuria and dose-dependent development of collapsing FSGS lesions within 7 days.21,22,32,33 Thy-1.1, the triple transgenic PEC-rtTA reporter mouse, and the histone-2B-eGFP reporter mouse34 were intercrossed. The resulting mice (Thy-1.1/PEC-rtTA/LC1/H2B-eGFP/R26R) received dox for 7 days at 4 weeks of age (n = 5). After the washout period, mice received an intravenous injection with 1 mg of anti–Thy-1.1 mAb (19XE5) in 0.1 ml of 0.9% saline solution. Kidney samples were collected 5 days after the injection.
Perfusion Fixation
Mice were anesthetized (ketamine-rompun). The kidney(s) were perfused via the left heart ventricle with 3% paraformaldehyde in PBS (pH 7.6) for 3 minutes. Pieces of the kidney(s) were snap-frozen in liquid nitrogen or (post-)fixed in 3% buffered formalin and embedded in paraffin.
Light Microscopy
For light microscopy, the 3% buffered formalin fixed kidney fragment were dehydrated, and embedded in paraffin. Four-micrometer paraffin sections were stained with periodic acid–Schiff (PAS). To obtain the percentage of glomeruli-containing lesions, at least 60 glomeruli per mouse were evaluated for the presence of hypertrophy and hyperplasia of the glomerular epithelium, adhesions, sclerosis, or hyalinosis.
β-Galactosidase Detection
β-gal activity was detected using enzymatic X-gal staining as described.17 Samples were either counterstained with eosin, washed in tap water, and mounted (Immu-Mount; Thermo Scientific, Waltham, MA) or further processed for immunohistochemistry (see below).
Immunofluorescence
The immunofluorescence stainings were performed on 2-μm paraffin sections (for a list of primary antibodies see Table 1). The collagen IValpha3/LKIV69 and agrin/LKIV69 double stainings were performed on 2-μm acetone fixed cryosections. The following secondary antibodies were used: donkey anti-rabbit, -mouse, or -rat Dylight 488 or Dylight 549 (1:200; Dianova, Hamburg, Germany). The single-chain primary antibody (LKIV69, Table 1) was detected using anti–VSV-Cy3 (Sigma-Aldrich, St. Louis). When staining mouse sections, all secondary antibodies (except the anti-mouse antibody) were immunoadsorbed with 4% normal mouse serum. The nuclei were stained using Hoechst 33342 (Sigma-Aldrich). Sections were evaluated with an Olympus BX 41 microscope using AnalySIS software (Soft Imaging System, Muenster, Germany).
Table 1.
Primary antibodies
| Antibody/Clone | Host Species | Reactivity | Dilution | Supplier/Reference |
|---|---|---|---|---|
| CD44/IM7 | Rat mAb | Mouse | 1:200 | BD Biosciences, San Diego |
| CD44/OX49 | Mouse mAb | Rat | 1:100 | AbD Serotec, Düsseldorf, Germany |
| CD44/156–3C11 | Mouse mAb | Human | 1:100 | Abcam, Cambridge, UK |
| Claudin-1 | Rabbit pAb | m,r,h | 1:50 | Abcam, Cambridge, UK |
| Synaptopodin/G1D4 | Mouse mAb | m,r,h | 1:100 | Progen, Heidelberg, Germany |
| Nestin | Chicken pAb | m,r,h | 1:100 | Abcam, Cambridge, UK |
| Ki-67/SP6 | Rabbit mAb | Mouse | 1:300 | Dianova, Hamburg, Germany |
| Ki-67/MIB-1 | Mouse mAb | Human | 1:10 | Dako, Glostrup, Denmark |
| GFP/JL8 | Mouse mAb | Mouse | 1:200 | Clontech, Mountain View, CA |
| GFP | Rabbit pAb | Mouse | 1:200 | Clontech, Mountain View, CA |
| LKIV69 | Single-chain Ab | m,r,h | 1:50 | Wijnhoven et al.26 |
| Collagen IVA3 | Mouse mAb | h | 1:20 | Wieslab AB, Ideon, Sweden |
| Agrin/JM72 | Mouse mAb | h | 1:200 | Kind gift of Dr. J van der Vlag |
m, mouse; r, rat; h, human; mAb, monoclonal antibody; pAb, polyclonal antibody.
Immunohistochemistry
Immunohistochemistry was performed on 4-μm paraffin- or 3% paraformaldehyde fixed cryo-sections. CD44, claudin-1, nestin, and synaptopodin immunostainings were combined with a PAS staining. Sections were blocked with avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) and 3% H2O2. The sections were subjected to microwave antigen retrieval in Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) followed by incubation with the primary (Table 1) and secondary antibodies. As secondary antibodies, we used biotinylated goat anti-mouse, goat anti-rabbit, goat anti-rat, and goat anti-chicken (Vector Laboratorie). Detection was carried out with vectastain ABC kit (Vector Laboratories) with the use of peroxidase as label and 3,3′-diaminobenzidine as substrate and nickel chloride enhancement. Subsequent to the immunostaining, a PAS staining was performed.
Patients
Eighteen kidney specimens were examined. Sixteen biopsies of native kidneys, and two biopsies of transplanted kidneys containing FSGS lesions, as diagnosed by a renal pathologist, were randomly selected in a blinded fashion if sclerotic lesions were observed on a PAS staining. FSGS lesions resulted from primary FSGS (n = 6), hypertensive nephrosclerosis (n = 10), or recurrent primary FSGS (n = 2). All biopsies were stained for claudin-1, synaptopodin, CD44, and BC-type extracellular matrix.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by TP17 SFB/Transregio 57 of the German Research Foundation (DFG to M.J.M.), the German Ministry for Science and Education (BMBF, 01 GN 0804 to K.E. and M.J.M.). Furthermore, this work received support from the NephCure Foundation (F001 to B.S. and M.J.M.), the Genzyme Renal Innovation Program (GRIP, to B.S.), and The Netherlands Organization for Scientific Research (NWO) (2007/09196/ALW, to B.S.). M.J.M. is a member of the SFB/Transregio 57 DFG consortium “Mechanisms of organ fibrosis”. We apologize for not having cited all relevant papers due to space limitations. We thank Dr. J. van der Vlag (RUNMC, Nijmegen, The Netherlands) for providing the anti-agrin antibody.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Fahr T: Handbuch der speziellen pathologischen Anatomie und Histologie, Vol. VI/1, Berlin, Springer, 1925 [Google Scholar]
- 2. Jennette JC, Olson JL, Schwartz MM, Silva FG: Focal Segmental Glomerulosclerosis. In: Heptinstall's Pathology of the Kidney, Philadelphia, Lippincott Williams & Wilkins, 2007, pp 159–175 [Google Scholar]
- 3. Yang HC, Fogo AB: ‘Idiopathic’ FSGS: An increasingly obsolete diagnosis? Nephrol Dial Transplant 25: 654–656, 2010 [DOI] [PubMed] [Google Scholar]
- 4. D'Agati V: Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol 23: 117–134, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Laurens WE, Vanrenterghem YF, Steels PS, Van Damme BJ: A new single nephron model of focal and segmental glomerulosclerosis in the Munich-Wistar rat. Kidney Int 45: 143–149, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Elger M, Kriz W: Podocytes and the development of segmental glomerulosclerosis. Nephrol Dial Transplant 13: 1368–1373, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Kihara I, Yaoita E, Kawasaki K, Yamamoto T, Hara M, Yanagihara T: Origin of hyperplastic epithelial cells in idiopathic collapsing glomerulopathy. Histopathology 34: 537–547, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Kihara I, Tsuchida S, Yaoita E, Yamamoto T, Hara M, Yanagihara T, Takada T: Podocyte detachment and epithelial cell reaction in focal segmental glomerulosclerosis with cellular variants. Kidney Int Suppl 63: S171–S176, 1997 [PubMed] [Google Scholar]
- 11. Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P: Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int 58: 137–143, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Nagata M, Hattori M, Hamano Y, Ito K, Saitoh K, Watanabe T: Origin and phenotypic features of hyperplastic epithelial cells in collapsing glomerulopathy. Am J Kidney Dis 32: 962–969, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Dijkman H, Smeets B, van der Laak J, Steenbergen E, Wetzels J: The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int 68: 1562–1572, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Dijkman HB, Weening JJ, Smeets B, Verrijp KC, van Kuppevelt TH, Assmann KK, Steenbergen EJ, Wetzels JF: Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int 70: 338–344, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P: Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Hir M, Kriz W: New insights into structural patterns encountered in glomerulosclerosis. Curr Opin Nephrol Hypertens 16: 184–191, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ: Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wijnhoven TJ, Lensen JF, Rops AL, van der Vlag J, Kolset SO, Bangstad HJ, Pfeffer P, van den Hoven MJ, Berden JH, van den Heuvel LP, van Kuppevelt TH: Aberrant heparan sulfate profile in the human diabetic kidney offers new clues for therapeutic glycomimetics. Am J Kidney Dis 48: 250–261, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kollias G, Evans DJ, Ritter M, Beech J, Morris R, Grosveld F: Ectopic expression of Thy-1 in the kidneys of transgenic mice induces functional and proliferative abnormalities. Cell 51: 21–31, 1987 [DOI] [PubMed] [Google Scholar]
- 22. Assmann KJ, van Son JP, Dijkman HB, Mentzel S, Wetzels JF: Antibody-induced albuminuria and accelerated focal glomerulosclerosis in the Thy-1.1 transgenic mouse. Kidney Int 62: 116–126, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G: Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int 34: 481–486, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Thorner PS, Ho M, Eremina V, Sado Y, Quaggin S: Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol 19: 495–502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagata M, Horita S, Shu Y, Shibata S, Hattori M, Ito K, Watanabe T: Phenotypic characteristics and cyclin-dependent kinase inhibitors repression in hyperplastic epithelial pathology in idiopathic focal segmental glomerulosclerosis. Lab Invest 80: 869–880, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Cai YI, Sich M, Beziau A, Kleppel MM, Gubler MC: Collagen distribution in focal and segmental glomerulosclerosis: An immunofluorescence and ultrastructural immunogold study. J Pathol 179: 188–196, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Suzuki T, Matsusaka T, Nakayama M, Asano T, Watanabe T, Ichikawa I, Nagata M: Genetic podocyte lineage reveals progressive podocytopenia with parietal cell hyperplasia in a murine model of cellular/collapsing focal segmental glomerulosclerosis. Am J Pathol 174: 1675–1682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB: Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14: 1998–2003, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Schonig K, Schwenk F, Rajewsky K, Bujard H: Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res 30: e134, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Smeets B, Te Loeke NA, Dijkman HB, Steenbergen ML, Lensen JF, Begieneman MP, van Kuppevelt TH, Wetzels JF, Steenbergen EJ: The parietal epithelial cell: A key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. J Am Soc Nephrol 15: 928–939, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Smeets B, Dijkman HB, te Loeke NA, van Son JP, Steenbergen EJ, Assmann KJ, Wetzels JF, Groenen PJ: Podocyte changes upon induction of albuminuria in Thy-1.1 transgenic mice. Nephrol Dial Transplant 18: 2524–2533, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E: Defining the epithelial stem cell niche in skin. Science 303: 359–363, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.