Abstract
Loss of podocytes promotes glomerulosclerosis, but whether this results from a continued primary insult or a secondary mechanism triggered by the initial loss of podocytes is unknown. We generated chimeric mice in which only a subpopulation of podocytes expressed hCD25, which is the receptor for the immunotoxin LMB2. In addition, genetic labeling of hCD25-negative cells with human placental alkaline phosphatase allowed the study of these two distinct podocyte populations. Administration of LMB2 did not cause podocyte injury in hCD25-negative control mice. In contrast, LMB2 severely damaged or sloughed off the subpopulation of hCD25-positive podocytes within the chimeric glomeruli. Moreover, hCD25-negative podocytes, which were immune to the initial toxin injury, developed injury as early as 4 d after LMB2 injection, evidenced by foot process effacement, upregulation of desmin, and downregulation of nephrin, podocin, and podocalyxin. Furthermore, the magnitude of secondary injury correlated with the magnitude of primary injury, supporting the concept of an amplified cascade of podocyte injury. In conclusion, podocyte damage can propagate injury by triggering secondary damage of “remnant” intact podocytes, even when the primary insult is short-lived. This transmission of podocyte injury may form a vicious cycle leading to accelerated podocyte deterioration and glomerulosclerosis.
Loss of podocytes leads to glomerular sclerosis, the morphologic hallmark of chronic kidney disease.1–5 A number of factors, including genetic, mechanical, and immunological stresses, as well as toxins, can cause podocyte injury.6–9 As podocyte deterioration is often relentless, the question as to whether the ongoing injury is a result of a continued primary insult or a secondary mechanism triggered by the initial loss of podocytes per se has remained unresolved.
Previously, we established a transgenic mouse line (NEP25), which expresses human (h) CD25 selectively on podocytes.10 By injecting the hCD25-targeted recombinant immunotoxin, anti-Tac(Fv)-PE38 (LMB2),11 podocyte-selective injury can be induced in NEP25 mice in a dose-dependent manner. With high-dose LMB2 (≥1.25 ng/g BW), NEP25 mice develop massive nonselective proteinuria, severe glomerulosclerosis, and renal failure, and die within 14 d. With low-dose LMB2 (0.625 ng/g BW), NEP25 mice develop moderate proteinuria, which peaks 1 to 2 wk after injection and gradually decreases. Although LMB2 is rapidly cleared from the circulation with half life of 35 min,11 podocyte injury progresses over weeks. Thus, 3 wk after injection, mice develop focal segmental glomerulosclerosis. With either dose, the injury is initially confined to podocytes, but later other cells within and outside the glomerulus become affected. These progressive injury phenotypes prompted us to hypothesize that injury of some podocytes secondarily injures other podocytes that escaped the initial injury.12
In this regard, numerous studies in subtotally nephrectomized animals have shown that loss of a large number of nephrons imposes stresses, e.g., glomerular hypertension or secondary glomerular hypertrophy with decrease in podocyte density in the remnant glomeruli, thereby secondarily damaging podocytes.13–18 Thus, this mechanism appears to become critically important at the late phase of renal injury. We were intrigued by the possibility that podocyte damage in and of itself could damage other podocytes within the glomerulus at an early phase, i.e., before maladaptive responses to nephron loss occur.
In both the experimental animal models and clinical nephropathies studied thus far, the entire podocyte population is exposed to the primary insult. These settings, including our NEP25 model, preclude determination of whether continued podocyte damage is caused by a lingering effect of primary insult or by a mechanism secondary to the initial loss of some podocytes. To overcome this problem, we generated chimeric mice in which only a subpopulation of the podocytes carries the hCD25 transgene and, therefore, are selectively targeted by the immunotoxin LMB2. We demonstrate that hCD25-negative podocytes, which are not targeted by LMB2, are damaged by a secondary mechanism triggered by initial damage of hCD25-positive cells. These studies provide evidence of podocyte-to-podocyte transmission of damage that may cause a vicious cycle leading to sclerosis.
RESULTS
Identification of hCD25-Positive versus hCD25-Negative Podocytes
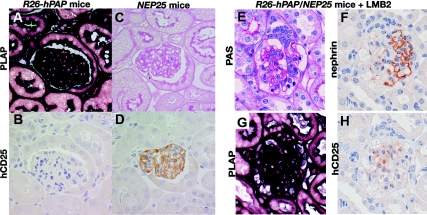
hCD25 is regulated by the nephrin promoter and is, therefore, rapidly downregulated after toxin injury when podocyte nephrin expression is reduced. To identify the genotype of each podocyte in paraffin and electron microscopy tissue sections, we created chimeric mice using R26-hPAP transgenic mice as the source of non-hCD25-carrying cells. The R26-hPAP line is insensitive to LMB2 and ubiquitously expresses human placental alkaline phosphatase (PLAP), which is readily identifiable by histochemical or immunological staining.19 We stained PLAP histochemically and immunologically in paraffin sections of the kidney of heterozygous R26-hPAP transgenic mice. As reported previously, both methods intensely and highly specifically stained all types of cells including podocytes in R26-hPAP mice (Figure 1 A through D and S1).
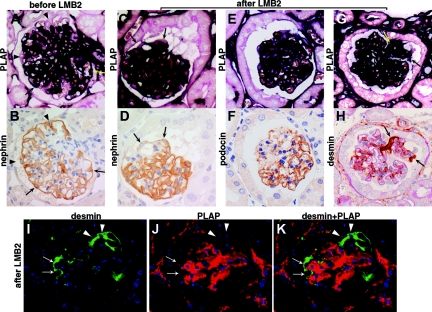
Figure 1.
PLAP and hCD25 staining in R26-hPAP, NEP25, and R26-hPAP/NEP25 dual transgenic mice. (A through D): Kidneys of R26-hPAP and NEP25 mice were embedded in the same paraffin block and stained for PLAP and hCD25. In R26-hPAP mice, all types of cells are intensely stained for PLAP (A), but no cell for hCD25 (B). In NEP25 mice, no cell stain for PLAP (C), but all podocytes stain for hCD25. PLAP staining is most intense along the plasma membrane. (E through H) Podocyte injury was induced in R26-hPAP/NEP25 dual transgenic mice by injecting LMB2 (6 d after 25 ng/g BW of LMB2). The glomerulus shows vacuolar degeneration of epithelial cells, adhesion, and early sclerosis (E), segmental loss of nephrin staining (F), and global loss of hCD25 staining (H). In the same glomerulus, all cells are intensely stained for PLAP (G), thus demonstrating stable labeling of injured podocytes. A and C, B and D are from the same section. A and B, C and D, E through H are from adjacent sections. Magnification, ×400.
To test whether injured podocytes are stably labeled with PLAP, we generated NEP25/R26-hPAP double transgenic mice, in which all cells express both hCD25 (susceptible to toxin) and PLAP. After LMB2 injection (0.625, 1.25, 2.5, and 25 ng/g BW), NEP25/R26-hPAP mice were analyzed at various time points (4 to 42 d). Periodic acid—Schiff (PAS) staining and immunostaining for nephrin and podocalyxin confirmed that glomeruli in these kidneys have various degrees of podocyte injury. All cells clearly stained for PLAP, even glomeruli with severe downregulation of nephrin, podocalyxin, and the transgene product hCD25 (Figure 1 E through H). Thus, PLAP can serve as an hCD25(-) marker, even in injured podocytes.
Chimeric Mice: Assessment of Podocytes at Baseline
We next generated NEP25↔R26-hPAP chimeric mice, in which only a fraction of podocytes expresses hCD25. We then performed renal biopsy in NEP25↔R26-hPAP chimeric mice before LMB2 injection. Although the percentage of hCD25-positive (+) podocytes was variable among glomeruli and among mice, the average percentage of PLAP(+) or hCD25(+) cells within a given chimeric kidney was similar from section to section, as these sections sampled a large number, >50 glomeruli. In addition, in wild-type↔R26-hPAP chimeric mice, we confirmed that distribution of PLAP-positive cells was similar in left and right kidneys within a given mouse. In the subsequent studies, therefore, we assessed hCD25 (+) staining in a single section with >55 glomeruli for each chimeric mouse and calculated the hCD25 index to represent the average percentage of hCD25 podocytes (Table 1).
Table 1.
Profiles of the chimeric mice used in the present study
| Dose of LMB2 (ng/g BW) | Time Interval between LMB2 Study (d) | Code of Each Chimeric Mouse | hCD25 Index before LMB2 (range 0 to 1)a | Nephrin Index after LMB2 (range 0 to 8)b | rs (P)c | Podocin Index after LMB2 (range 0 to 8)b |
|---|---|---|---|---|---|---|
| 25 | 4 | C1 | 0.11 | 7.93 | −0.943 (< 0.01) | 7.88 |
| C2 | 0.16 | 7.76 | 7.42 | |||
| C3 | 0.58 | 5.68 | 6.03 | |||
| C4 | 0.59 | 6.5 | 6.84 | |||
| C5 | 0.67 | 3.82 | 2.82 | |||
| C6 | 0.78 | 3.67 | 3.34 | |||
| 2.5 | 9 | C7 | 0.26 | 4.14 | −0.8 (< 0.05) | 4.33 |
| C8 | 0.63 | 5.29 | 5.81 | |||
| C9 | 0.78 | 0.89 | 0.41 | |||
| C10 | 0.85 | 0.27 | 0.19 | |||
| C11 | 0.99 | 0 | 0.00 | |||
| 25 | 42 | C12 | 0.02 | 7.53 | −0.8 (−0.083) | 7.50 |
| C13 | 0.08 | 7.77 | 7.94 | |||
| C14 | 0.15 | 6.74 | 7.28 | |||
| C15 | 0.19 | 4.64 | 5.23 |
A total of 15 chimeric mice (C1 through C15) were used. Each chimeric mouse showed a unique percentage of hCD25-positive podocytes on biopsy specimens before LMB2 injection, represented as the hCD25 index. After LMB2 injection (dose and time interval indicated), these mice showed variable podocyte injury, represented as the nephrin and podocin indices. Nephrin and podocin indices closely correlated with rs = 0.99 (P < 0.001).
a0: no hCD25 staining, 1: 100% hCD25 staining.
b0: complete loss of nephrin/podocin staining, 8: intact nephrin/podocin staining.
cSpearman's rank correlation coefficient (rs) between hCD25 and nephrin indices with P value for each experiment.
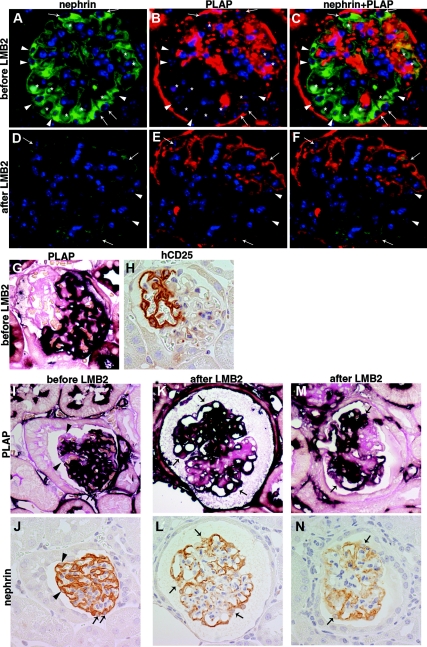
PLAP staining was seen primarily on the cell membranes. It was therefore difficult to determine PLAP positivity for the interior glomerular cells. In contrast, PLAP positivity versus negativity was clearly evident for most surface podocytes (Figure 2 I and J). Immunofluorescent staining (Figure 2 A through C) and immunoelectronmicroscopy (Figure 3A) confirmed that PLAP protein was distributed in a chimeric pattern.
Figure 2.
NEP25↔R26-hPAP chimeric mice at baseline and 4 d after 25 ng/g BW of LMB2. (A through F) Double immunofluorescent staining for nephrin (green) and PLAP (red) in a chimeric mouse before LMB2 (A through C) demonstrates global staining of nephrin and patchy (i.e., chimeric-pattern) staining of PLAP. Asterisks show RBCs with autofluorescence. Arrows depict DAPI nuclear staining (blue) with nephrin and PLAP, i.e., hCD25 (-) podocytes, and arrow heads depict DAPI with nephrin alone, i.e., hCD25 (+) podocytes. After LMB2 (D through F), nephrin staining is diminished globally, both in PLAP (+) podocytes (arrows) and in PLAP (-) podocytes (arrow heads), demonstrating nephrin is downregulated in hCD25 (-) podocytes. These panels and those in Figure S1 were stained in an identical fashion at the same time and photographed under the same conditions. (G and H) Serial sections from a NEP25↔R26-hPAP chimeric mouse at baseline were stained for PLAP and hCD25. PLAP (+) cells are negative for hCD25, and PLAP (-) surface cells are positive for hCD25. (I through N) Serial section analysis for PLAP and nephrin. I and J, K and L, M and N are from adjacent sections. Before LMB2 (I and J), both PLAP (+) (arrows) and PLAP(-)(arrow heads) surface podocytes show normal nephrin staining pattern. After LMB2 (K and L), nephrin staining is downregulated in some PLAP (+) surface podocytes (arrows) in both glomeruli with (M and N) and without (K and L) adhesion. Magnification, ×400.
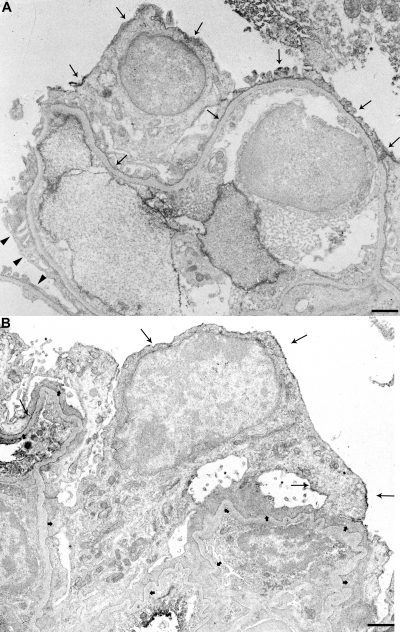
Figure 3.
Immunoelectronmicroscopy for PLAP in NEP25↔ R26-hPAP chimeric glomerulus at baseline (A) and 9 d after injection of LMB2 (B). (A) Plasma membrane of the cell body and foot processes (arrows) and invaginated membrane of some podocytes are clearly stained with PLAP, whereas those of other podocytes (arrow heads) are not stained. (B) Plasma membranes (arrows) of most podocytes are intensely stained for PLAP (long arrows). The invaginated membrane is also stained for PLAP. The podocytes show extensive foot process effacement (short arrows) and microvillous transformation (asterisks). Bars represent 1 μm.
Analysis of serial sections confirmed that each surface podocyte was positive for either PLAP or hCD25 (Figure 2 G and H) with the exception that some areas of foot processes appeared positive for both, possibly reflecting interdigitation of foot processes of PLAP (+) podocytes and hCD25 (+) podocytes. Therefore, in subsequent analyses, we identified the genotype of individual surface podocytes by PLAP positivity on the apical membrane in close proximity to podocyte nuclei. The term “hCD25(-) podocytes” is used to indicate podocytes not carrying hCD25 as determined and validated by PLAP-positive staining.
Early Response of Chimeric Mouse Podocytes
We first performed analyses at a very early phase after high-dose LMB2 (25 ng/g BW). Four days after toxin injection, nonchimeric NEP25 mice with ICR genetic background showed massive proteinuria. Urinary albumin/creatinine ratio (ACR) increased, from baseline 0.10 ± 0.02 to 58.36 ± 4.41 (mg/mg). In most podocytes, nephrin staining was diminished, with nephrin index averaging 3.24 ± 0.32 (scale: 0 to 8). Of note, neither proteinuria nor podocyte injury was observed at any time points in wild-type mice10 or R26-hPAP transgenic mice not carrying hCD25 after toxin injection (data not shown).
In chimeric NEP25↔R26-hPAP mice, there was variable chimerism of podocytes at baseline, with an index of hCD25 ranging from 0.11 to 0.78, where 0.00 is no hCD25 and 1.00 is 100% of podocytes carrying hCD25. Four days after LMB2 injection, urinary ACR ranged from 1.08 to 67.26 (Table S2), and nephrin index ranged from 3.67 to 7.93 (Table 1). The nephrin index was negatively correlated with the degree of chimerism (Table 1). Most hCD25 staining was lost in all chimeric mice except for mouse C2, in which a few podocytes with faint hCD25 staining were observed in several glomeruli.
In all chimeric mice, most hCD25 (+) podocytes showed downregulation of nephrin after LMB2, confirming that LMB2 effectively injured hCD25(+) podocytes (Figures 2 D through F, K through N, and S1). In addition, 9.7 to 58.5% of hCD25(-) surface podocytes (with definitive PLAP positivity), although not targeted by LMB2, also showed downregulation of nephrin (Figures 2 D through F, K through N, and S1). Downregulation of podocin and vascular endothelial growth factor (VEGF) and upregulation of desmin were also observed in PLAP (+) surface podocytes (Figure S3).
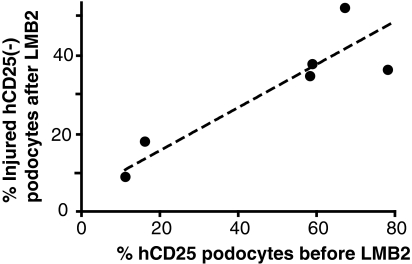
The percentage of hCD25podocytes with downregulated nephrin was variable among mice (Figure S2), but correlated with the percentage of hCD25 (+) cells at baseline (Spearman's rank correlation coefficient (rs), 0.83, P < 0.01; Figure 4). Thus, more podocytes injured by the primary toxin insult resulted in more podocytes injured by secondary, nontoxin-mediated mechanisms.
Figure 4.
Correlation between hCD25 index before LMB2 and the average percentage of injured surface hCD25(-) podocytes after LMB2. hCD25 index, determined semiquantitatively, is the same as that shown in Table 1. The average percentage of injured surface hCD25(-) podocytes was determined as described in the Concise Methods section. Thus, the greater the number of hCD25(+) podocytes, which are the target of the primary injury, the greater the number of injured hCD25(-) podocytes that acquire secondary injury.
Late Secondary Responses of Chimeric Mouse Podocytes
We next studied nonchimeric and chimeric mice at a later stage, 9 d after LMB2 toxin injection. Given that most nonchimeric NEP25 mice died within 7 d after 25 ng/g BW of LMB2,10 the dosage of LMB2 was decreased to 2.5 ng/g BW. Nonchimeric NEP25 mice (n = 3) on ICR genetic background showed massive proteinuria, with ACR averaging 73.69 ± 5.10, and developed severe glomerular damage with nearly complete downregulation of nephrin, podocalyxin, and hCD25. Nephrin indices in these mice were 0.25, 0.32, and 0.75, respectively (scale: 0 to 8).
We next studied the extent of injury in chimeric mice (n = 5). The hCD25 index at baseline ranged from 0.26 to 0.99, reiterating variable chimerism. After LMB2 injection, all chimeric mice showed massive proteinuria. Morphologically, injury assessed by nephrin downregulation was less than the nonchimeric NEP25 transgenic mice, ranging from 0.89 to 5.29, and the nephrin index was inversely correlated with the hCD25 index assessed before LMB2 injection (Table 1).
In two mice with low hCD25 index (mice C7, C8), glomerular structure was remarkably preserved, and most surface podocytes could be identified. In these mice, the majority of surface podocytes were positive for PLAP, indicating that most hCD25(+) podocytes had been lost. Nephrin and podocin were downregulated, and desmin was upregulated even in the remaining hCD25(-) podocytes (Figures 5 C through H and S1). Double immunofluorescent staining for desmin and PLAP revealed that the majority of desmin-positive podocytes was also positive for PLAP (Figure 5 I through K). Immuno EM for PLAP demonstrated that PLAP(+), i.e., hCD25(-)-negative podocytes were injured with extensive foot process effacement (short arrows) and microvillous transformation (Figure 3B).
Figure 5.
Injury in hCD25 (-) podocytes of NEP25↔ R26-hPAP chimeric mice 9 d after 2.5 ng/g BW of LMB2. (A through H) Serial section analysis. Upper and lower panels are paired and from adjacent sections. Before LMB2 (A and B), both PLAP (+) (arrows) and PLAP(-)(arrow heads) surface podocytes show normal nephrin staining pattern. After LMB2, nephrin (A and D) and podocin (E and F) are downregulated and desmin (G and H) is upregulated in some PLAP (+) surface podocytes (arrows). (I through K) Double immunofluorescent staining for desmin and PLAP. Desmin is upregulated both in PLAP (+) podocytes (arrows) and in PLAP (-) podocytes (arrow heads), demonstrating damage of hCD25 (-) podocytes. Of note, PLAP (+) cells do not carry the hCD25 transgene and therefore are not targeted by LMB2. Magnification, ×400.
We next investigated the extent of injured podocytes versus extent of podocytes susceptible to toxin. As shown in Figure S4, the intact podocyte area after toxin decreased below the hCD25-negative podocyte area measured before toxin in these chimeric mice, confirming that damage propagated beyond toxin-susceptible cells into hCD25(-) podocytes.
Long-Term Effects of Minimal Initial Injury in Chimeric Mice
We next studied the long-term effects of injury in chimeric mice with low percentage of hCD25(+) podocytes (n = 4). Assessment was made 42 d after injection of high-dose (25 ng/g BW) LMB2. Unlike nonchimeric NEP25 mice, all four chimeric mice survived until sacrifice at day 42. Urinary ACR peaked on day 7 with 5.25 to 51.77, and gradually decreased to 0.65 to 3.81 by day 42 (Table S2). These chimeric mice showed focal segmental glomerular sclerosis (Figure 6). Glomeruli had neither hCD25(+) podocytes nor PLAP(-) surface podocytes, indicating that all hCD25 podocytes were eliminated.
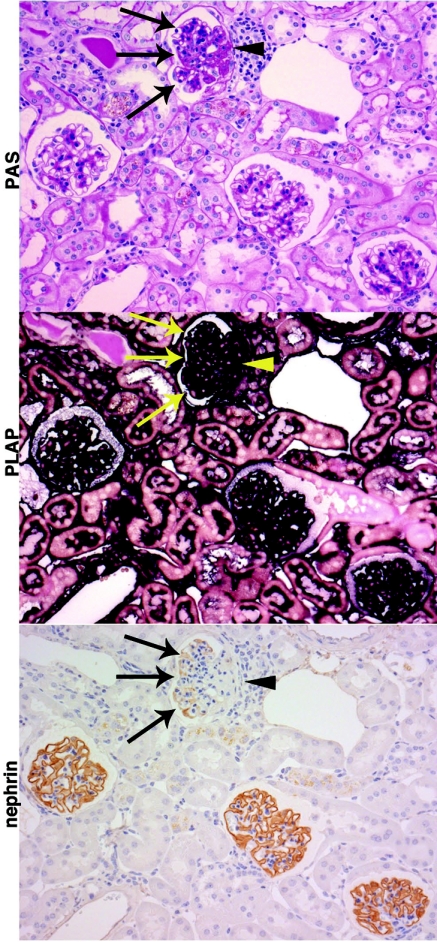
Figure 6.
Focal segmental glomerulosclerosis in chimeric mice 6 wk after 25 ng/g BW of LMB2. Chimeric mice with relatively small baseline hCD25(+) podocyte population were injected with 25 ng/g BW of LMB2 and analyzed 6 wk later (mice C12, 13, 14, 15). Representative PAS, PLAP, and nephrin staining on serial sections from a representative mouse are shown. One glomerulus shows segmental sclerosis on PAS staining. All cells in the sclerotic lesion (arrow head) or in the nonsclerotic portion (arrows) are positive for PLAP, indicating that all hCD25(+) podocytes have been effectively eliminated by LMB2. The nonsclerotic portion shows extensive downregulation of nephrin. The other three glomeruli show normal structure in PAS staining and normal nephrin expression pattern. Magnification, ×200.
Notably, in nonsclerotic portions in sclerotic glomeruli, nephrin was preferentially downregulated in podocytes adjacent to sclerotic lesion, although those podocytes were PLAP (+), i.e., insensitive to LMB2 (Figure 6).
Glomeruli without sclerosis showed normal immunostaining for nephrin (Figure 6), podocin, and desmin (data not shown). In addition, there was no abnormal reabsorption of albumin in proximal tubular cells, confirming that the filtration barrier remained intact in these glomeruli. Thus, a substantial number of glomeruli maintained normal structure after losing a fraction of podocytes (Table 2).
Table 2.
Substantial number of glomeruli maintained normal structure after losing a minor population of podocytes
| Code of Each Chimeric Mouse | Glomeruli Containing One or More hCD25-Positive Podocytes at Baseline (%) | Sclerotic Glomeruli 42 d after 25 ng/g BW of LMB2 (%) |
|---|---|---|
| C12 | 28.6 | 8.5 |
| C13 | 46.3 | 1.0 |
| C14 | 90.6 | 25.3 |
| C15 | 88.8 | 41.8 |
Percentage of glomeruli containing one or more hCD25-positive podocytes was determined on biopsy specimens obtained before LMB2 injection. Percentage of sclerotic glomeruli was determined on autopsy specimens obtained 42 d after 25 ng/g BW of LMB2. Both values were obtained by three-dimensional analysis using serial sections.
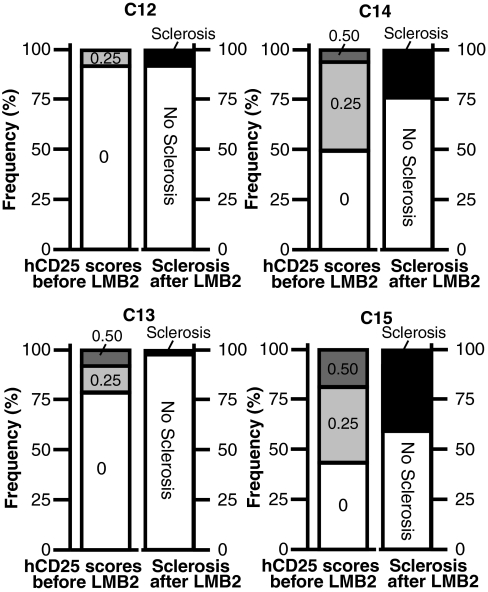
Figure 7 shows the frequency distribution of hCD25 positivity (left columns) among chimeric glomeruli studied at baseline in four chimeric mice compared with the percentage of sclerotic glomeruli (right columns) at 42 d after LMB2 in the same mice. Within a given kidney, glomeruli with a greater population of podocytes with LMB2 susceptibility more readily developed sclerosis. The threshold of hCD25 positivity for a given glomerulus to develop sclerosis was between 0.25 and 0.5. However, scarcity of chimeric mice within this range limited further analysis.
Figure 7.
Frequency distribution of hCD25 scores before LMB2 (left columns) and the percentage of normal and sclerotic glomeruli after LMB2 (right columns). Each panel represents results from an individual mouse. The left columns represent frequency distribution of baseline hCD25 positivity and the right columns represent the percentage of normal and sclerotic glomeruli after LMB2. The former was determined on the basis of >55 glomeruli in a single section, and the latter was determined by serial section analysis on >70 glomeruli. hCD25 scores 0.00, 0.25, and 0.5 represent hCD25 areas of 0, 1 to 25, and 26 to 50%, respectively. None of these mice had even a single glomerulus with hCD25(+) area greater than 50% before LMB2, indicating that chimeric contribution of LMB2-susceptible podocytes was small in these mice. Mice C14 and C15 had greater number of glomeruli with LMB2-susceptible podocytes than mice C12 and C13, and later showed greater number (25.8 to 41.8%) of sclerotic glomeruli than C12 and C13 did (1 to 8.5%). To determine the threshold percentage of LMB2-susceptible podocytes that is sufficient to develop sclerosis, the two columns obtained from independent analyses are compared based on the notions (1) that the percentage of hCD25 (+) podocytes is similar between biopsied and autopsied kidneys of the same chimeric mouse, as demonstrated earlier, and (2) that glomeruli with more hCD25 (+) podocytes are more prone to develop glomerulosclerosis, which was shown at the whole kidney level. According to this limited analysis, threshold hCD25 positivity for sclerosis is variable among mice and is affected by percentage of glomeruli containing hCD25 (+) podocytes. Thus, mice C12 and C13 had the fewest glomeruli containing hCD25(+) podocytes and showed much less sclerosis than mice C14 and C15 with widespread hCD25(+) podocytes.
DISCUSSION
The present study revealed that podocytes that escaped the initial toxin injury later became injured. This indicates that injury of some podocytes per se can cause injury of other podocytes, thus potentially forming a vicious cycle of progressive damage. Notably, this secondary podocyte injury can be observed at the very early stages, as early as 4 d after the primary podocyte injury before development of any sign of nephron loss or establishment of glomerulosclerosis. This observation contrasts the pattern documented in the late phases of renal failure, typically in the subtotal nephrectomy model.13,14,20 The glomerulus-to-glomerulus expansion of sclerosis after subtotal nephrectomy is thought to reflect excessive stress imposed on the remnant glomeruli. Thus, substantial nephron loss causes hemodynamic derangements and hypertrophy in the remnant glomeruli, which lead to sclerosis in these remnant glomeruli.17 This classic vicious cycle can explain the spread of injury from one glomerulus to another in late stages, i.e., after loss of a large population of nephrons.
More recently, Sato et al., using a diphtheria toxin receptor transgenic rat model, a model similar to the nonchimeric NEP25 model, demonstrated persistent podocyte loss over 14 wk,21 duplicating the pattern seen in PAN model.22 Of note, in all these models of glomerulosclerosis using podocyte toxins, all podocytes were exposed to the primary insult; hence, progressive podocyte damage and loss may reflect heterogeneity among podocytes in the rate of response to the toxins. In this regard, the present study clearly demonstrates that expansion of segmental sclerosis involves propagation of damage into podocytes that are not exposed to a primary insult (Figure 6).
Focal segmental glomerulosclerosis may be primary, caused by toxins, gene mutation, or idiopathic,22–24 or secondary, i.e., after loss of nephron number.17,25,26 Theses two forms of focal segmental glomerulosclerosis may have two separate mechanistic paradigms for expansion of podocyte injury.2 The present study indicates that, in either or both forms, the initial primary insult per se, if it occurs in large scale, can be brief in duration for triggering subsequent progressive loss of podocytes through the autonomous propagation of podocyte-to-podocyte damage. A phenomenon similar to this appears to occur in inducible podocin knockout mice, which have mosaic glomeruli.27,28
Kriz and others proposed earlier that synechiae formation and misdirected filtration are key morphologic events leading to glomerulosclerosis.25,29 The synechiae formation may, in turn, create a hostile environment for neighboring podocytes to preserve their structural integrity. In the present study, spread of podocyte damage started at early stages, i.e., the allotted time was too short for synechiae to form.
Occasionally, in the NEP25 model, cells carrying parietal epithelial cell characteristics cover the surface of severely injured glomerular capillaries (Figure S5).30 Among the chimeric mice analyzed 4 d after LMB2 injection, in which the genotype of individual podocytes was determined, only mouse C6 rarely contained such lesions. These cells were positive for claudin-1 and negative for nephrin and podocalyxin (Figure S5). Of note, throughout the analyses of individual podocytes (Figures S2 and 4), when surface cells were completely negative for nephrin, we referred to the adjacent section stained for podocalyxin. We excluded the cells that were negative for podocalyxin from the analysis. This was to avoid the possibility that rare viscerally residing parietal epithelial cells were counted as damaged podocytes.
For initially intact podocytes to be damaged as a result of damage in other podocytes, in all likelihood, the threat to intact podocytes must come from injury in adjacent podocytes. Thus, podocyte injury may cause release of toxic substances by damaged podocytes, including transforming growth factor β, endothelin-1, chemokines, and wingless-related MMTV integration site (Wnt) family members. These factors are increased in injured glomeruli and exacerbate podocyte injury in other models.31–36 Their expressions are increased in glomeruli of NEP25 mice after LMB2 injection (unpublished observation). Conversely, podocyte injury, causing reduction in intact podocyte population, may cause decrease in the ambient concentration of protective cell survival factors, such as VEGF. Secondary podocyte injury could also result from loss of proper cell-to-cell interactions that are essential for podocyte survival. Another potential mechanism is transmission of death signal through the gap junction. Although LMB2 (Mw 63,000 Daltons) itself, or its toxin moiety (Mw 38,000 Daltons), unlikely passes through gap junctions,37,38 small molecules that transmit death signal may pass. Lastly, inflammatory cell infiltration is absent from glomeruli in this model.10
In an attempt to identify the specific substances involved in the podocyte-to-podocyte transmission of damage, we sought an in vitro protocol that simulates the in vivo phenomenon. As described in the supplement and Table S1, however, we could not establish an in vitro protocol that allows podocyte-to-podocyte transmission of damage to occur. In this regard, a recent study in mice undergoing experimental unilateral ureteral obstruction39 showed that podocyte injury is drastically curtailed in the absence of physical forces for filtration, suggesting a certain limitation of an in vitro system to dissect the nature of cell-to-cell interactions occurring in progressive podocyte damage.
If the podocyte-to-podocyte damage transmission is, indeed, uniquely an in vivo phenomenon, the substances or factors involved appear to operate only in the presence of filtration. For example, podocyte injury causes leakage of macromolecules into Bowman's space through filtration, which may not only be a result but possibly a cause of podocyte injury. The well-known predictive value of nonselective proteinuria for the subsequent progression of chronic kidney diseases40–44 is in favor of this notion. Although the notion of toxic effect of leaked serum proteins on podocytes is not new,45 Kriz presented an elegant overview of the potential toxic role of serum proteins in the context of various experimental observations made over the past 30 yr.46
We observed infrequent detached PLAP (+) podocyte debris in Bowman's capsule 4 d after LMB2 injection (Figure S6). This finding and the loss of podocalyxin, the most durable podocyte marker in our hands, observed 9 d after LMB2 injection (Figure S4), suggest that at least some of the podocytes injured secondarily at the early phase were eventually lost from the glomerulus. However, we could not assess the quantity of podocytes that have actually been lost, as dying podocytes inevitably lose podocyte makers. The long-term experiments in the present study indicate that some, if not all, glomeruli can restore normal glomerular structure without developing sclerosis when the primary injury occurs in a minor population of podocytes, i.e., <25% as a whole kidney average. Thus, our data support the notion proposed by Wiggins and others that a certain threshold of primary injury exists for triggering the local vicious cycle of progressive glomerulosclerosis.47 In this regard, the podocyte-to-podocyte damage propagation demonstrated in the present study suggests that this threshold is determined by the magnitudes of increase in toxic substances and/or decrease in survival factors, depending on the extent of initial podocyte injury.
CONCISE METHODS
Generation of NEP25↔R26-hPAP Chimeric Mice
The Animal Experimentation Committee of Tokai University School of Medicine and the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center approved the protocol, in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Nephrin-hCD25 (NEP25) transgenic line maintained in C57BL/6 genetic background was backcrossed with ICR strain more than five times. R26-hPAP transgenic mice were backcrossed with ICR strain more than five times. Heterozygous R26-hPAP mice were mated with each other to obtain homozygous mice. Homozygous R26-hPAP mice were selected on the basis of high plasma PLAP activity, and homozygosity was confirmed by mating with wild-type mice and genotyping their offspring.
Male NEP25 heterozygous transgenic mice and male R26-hPAP homozygous mice were mated with wild-type superovulated ICR females. In a few initial experiments, heterozygous male R26-hPAP mice were also used. Four- to eight-cell stage embryos were harvested from both lines at 2.5 dpc. After removing the zona pellucida by pronase treatment, NEP25 and R26-hPAP embryos were aggregated at 1:1 ratio and cultured for 48 h in M16 medium. The resultant chimeric blastocysts were transferred into the uterus of pseudopregnant ICR females at 2.5 dpc; 574 pairs of embryos were aggregated in 14 experiments, and 112 mice were born. However, the efficiency of integration of both types of embryos was low. Only 18 NEP25↔R26-hPAP chimeric mice were obtained; 14 of these and one NEP25↔ wild-type mouse were used in the present study.
Chimeric mice carrying both NEP25 and R26-hPAP transgene were identified by PCR performed on tail DNA as previously reported.10,19 Renal biopsy was performed before LMB2 injection, and the ratio of hCD25-positive podocytes was assessed by immunostaining. The ratio of hCD25 positivity was semiquantified as described later.
Induction of Podocyte Injury
Recombinant immunotoxin, LMB2 (Anti-Tac (Fv)-PE38), was generated in E. Coli and purified as previously.11,37 More than two weeks after baseline biopsy, 2.5 or 25 ng/g BW of LMB2 was intravenously injected. Four, 9, or 42 d after LMB2 injection, mice were euthanized. Kidneys were perfusion-fixed with 4% buffered paraformaldehyde at mean arterial pressure for some assessments. In addition, nonchimeric NEP25 mice on ICR genetic background were treated and analyzed in an identical fashion as comparisons. Kidney samples were further fixed in 4% buffered paraformaldehyde overnight and embedded in paraffin. Parts of kidney samples were incubated in graded (15 to 30%) sucrose/PBS solution and frozen for immunoelectron microscopy.
Histologic Methods
PAS staining, various immunostaining, and histochemical staining for PLAP were performed in 2-μm thick serial paraffin sections.
For alkaline phosphatase histochemistry, deparaffinated and rehydrated sections were heated in a substrate buffer (0.1M Tris-HCL pH 9.5, 0.1 M NaCl, 5 mM MgCl2) at 65 °C for 2 h, incubated in BCIP-NBT solution (Sigma) at 30 °C for 16 h, and then counterstained lightly by PAS staining and covered with aqueous mounting medium, Crystal Mount (Biomeda Corp., Foster City, CA).
The following primary antibodies were used at indicated dilutions: mouse monoclonal anti-synaptopodin antibody (1:1, Progen, clone G1D4), guinea pig polyclonal anti-mouse nephrin antibody (1:200, Progen, GP-N2), rabbit anti-podocalyxin antibody (1:2000; a generous gift from Dr. Kurihara), mouse monoclonal anti-human desmin antibody (1:50, DAKO, D33), rabbit polyclonal anti-human podocin antibody (1:1000, Sigma), mouse monoclonal anti-human CD25 antibody (1:20, NeoMarkers, 25C04), rabbit monoclonal anti-human PLAP antibody (1:50, NeoMarkers, SP15), and rabbit polyclonal anti-claudin-1 antibody (1:200, abcam). For synaptopodin, nephrin, PLAP, hCD25, and claudin-1, slides were heated in citrate buffer (pH 6.0). For podocalyxin and podocin, slides were digested with 0.1% trypsin for 10 min before staining.
For immunoelectron microscopy, 8-μm thick frozen sections were cut, air dried, rinsed in PBS, incubated in 0.114% periodic acid for 10 min, and then in 0.114 mg/ml NaBH4 for 30 min. After blocking in diluted normal goat serum, the sections were incubated in anti-hPAP antibody (1:4000) overnight, incubated in biotin-anti-rabbit antibody (Vector), washed with PBS, and incubated in avidin-peroxidase. Immunoreactive signals were visualized by diaminobenzidine. The stained samples were processed for electron microscopy.
Semiquantification of Immunostaining
In biopsy samples stained for hCD25, the area of hCD25 staining was semiquantified in all glomeruli (n > 55/sample) using scores 0 (hCD25 area = 0), 0.25 (1≤ hCD25 area ≤ 25%), 0.5 (26 to 50%), 0.75 (51 to 75%), and 1.0 (≥ 76%). For each chimeric mouse, the average hCD25 score was calculated and designated as hCD25 index.
For evaluating podocyte injury after LMB2, nephrin and podocin immunostaining was semiquantified. For this, each quadrant of each glomerulus was scored as 0 (no staining), 1 (diminished), or 2 (normal), with total glomerular score ranging from 0 to 8. Scores from all glomeruli on a section for each mouse (>70) were averaged and defined as the nephrin or podocin index. These indices were used to represent the extent of overall podocyte injury in each mouse and are shown in Table 1.
PLAP positivity and nephrin staining were evaluated in individual surface podocytes of all glomeruli in a section. For this, serial sections after LMB2 were stained for PLAP, nephrin, and podocalyxin, and each glomerulus was photographed by BZ9000 (Keyence). In each surface podocyte of which the nucleus was observed, nephrin staining was evaluated as either normal (continuous) or diminished (discontinuous). If nephrin staining was completely negative, podocalyxin staining on the adjacent sections was assessed to confirm that the surface cell was a podocyte. Rare podocalyxin-negative surface cells (Figure S5) were not included in the analysis. Podocytes involved in adhesion were also excluded from the analysis. The genotype of hCD25 was determined on the other adjacent section stained for PLAP. In each glomerulus, surface podocytes were categorized into four types: hCD25(+) with normal nephrin, hCD25(+) with diminished nephrin, hCD25(-) with normal nephrin, and hCD25(-) with diminished nephrin. Mean numbers for these types of surface podocytes are shown in Figure S2. To represent the extent of secondary podocyte injury in each chimeric mouse, we calculated and designated the following percentage as the average percentage of injured surface hCD25(-) podocytes: [mean number of hCD25(-) podocytes with diminished nephrin]/[mean hCD25(-) with normal nephrin + mean hCD25(-) with diminished nephrin] × 100. Correlation between this percentage versus hCD25 index is shown in Figure 4.
For analysis of the chimeric mice 9 d after LMB2 injection, immunohistochemistry was assessed in kidney sections processed and embedded in single blocks to avoid variable conditions. Baseline hCD25 and nephrin in each glomerulus were photographed by BZ9000. The ratios of hCD25 and nephrin-stained areas to total tuft area were quantified using image analysis software, WinROOF (Mitani Co.). Serial sections of kidneys at sacrifice after toxin were stained for nephrin and podocalyxin. The ratios of nephrin- and podocalyxin-stained areas to total tuft area were quantified in a similar manner. These data are shown in Figure S4.
The hCD25 positivity at baseline and the ratio of glomerulosclerosis 42 d after LMB2 were evaluated by three-dimensional analyses. Serial sections (>70 sections) at baseline were stained for hCD25, and the ratio of glomeruli containing more than one hCD25(+) podocyte was determined. The data are presented in Figure 7 and Table 2. Serial sections (>70 sections) at sacrifice after LMB2 were stained for PAS and the percentage of glomeruli with sclerosis assessed. The data are presented in Table 2.
Statistical Analysis
Comparison of nephrin index between glomeruli with and without adhesion in chimeric mouse was performed using Mann–Whitney U test. Association between extent of hCD25(+) podocytes at baseline and injury of hCD25(-) podocytes after LMB2 (Figure 4) and that between hCD25 index and nephrin indices (Table 1) were evaluated by calculating the Spearman rank correlation coefficient. P < 0.05 was regarded as statistically significant.
DISCLOSURES
Iekuni Ichikawa received research funds from Daiichi Sankyo Co., Ltd. Taiji Matsusaka received a research fund from Chugai Pharmaceutical Co., Ltd.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants DK37868 and DK44757, the Research for the Future Program and Grant-in-Aid for Scientific Research of Japan Society for the Promotion of Science, MEXT, HAITEKU, and in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. Parts of this study were presented in abstract form at the annual meetings of the American Society of Nephrology in 2003 and 2004. We thank Mr. Yutaka Ishikawa, Mr. Akira Akatsuka, Ms. Shiho Imai, Ms. Naoko Sasaoka, Ms. Suguri Niwa, and Ms. Chie Sakurai for technical assistance, and Dr. Hidetake Kurihara for providing anti-podocalyxin antibody and rat podocyte cell line.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Podocyte Injury Can Be Catching,” on pages 1181–1183.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. LeHir M, Kriz W: New insights into structural patterns encountered in glomerulosclerosis. Curr Opin Nephrol Hypertens 16: 184–191, 2007 [DOI] [PubMed] [Google Scholar]
- 2. D'Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Zenker M, Machuca E, Antignac C: Genetics of nephrotic syndrome: New insights into molecules acting at the glomerular filtration barrier. J Mol Med 87: 849–857, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Shah SN, He CJ, Klotman P: Update on HIV-associated nephropathy. Curr Opin Nephrol Hypertens 15: 450–455, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Stitt-Cavanagh E, MacLeod L, Kennedy C: The podocyte in diabetic kidney disease. ScientificWorldJournal 9: 1127–1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Kreitman RJ, Pastan I: Accumulation of a recombinant immunotoxin in a tumor in vivo: Fewer than 1000 molecules per cell are sufficient for complete responses. Cancer Res 58: 968–975, 1998 [PubMed] [Google Scholar]
- 12. Ichikawa I, Ma J, Motojima M, Matsusaka T: Podocyte damage damages podocytes: Autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens 14: 205–210, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Brenner BM, Meyer TW, Hostetter TH: Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307: 652–659, 1982 [DOI] [PubMed] [Google Scholar]
- 14. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM: Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. Am J Physiol 241: F85–F93, 1981 [DOI] [PubMed] [Google Scholar]
- 15. Miller PL, Scholey JW, Rennke HG, Meyer TW: Glomerular hypertrophy aggravates epithelial cell injury in nephrotic rats. J Clin Invest 85: 1119–1126, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fogo A, Ichikawa I: Evidence for a pathogenic linkage between glomerular hypertrophy and sclerosis. Am J Kidney Dis 17: 666–669, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Nagata M, Kriz W: Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int 42: 148–160, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP: Ubiquitous expression of marker transgenes in mice and rats. Dev Biol 214: 128–138, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Hostetter TH: Hyperfiltration and glomerulosclerosis. Semin Nephrol 23: 194–199, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC: Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20: 1041–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Shirato I, Hosser H, Kimura K, Sakai T, Tomino Y, Kriz W: The development of focal segmental glomerulosclerosis in Masugi nephritis is based on progressive podocyte damage. Virchows Arch 429: 255–273, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Shankland SJ: The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Schwartz MM, Bidani AK, Lewis EJ: Glomerular epithelial cell function and pathology following extreme ablation of renal mass. Am J Pathol 126: 315–324, 1987 [PMC free article] [PubMed] [Google Scholar]
- 26. Olson JL, Hostetter TH, Rennke HG, Brenner BM, Venkatachalam MA: Altered glomerular permselectivity and progressive sclerosis following extreme ablation of renal mass. Kidney Int 22: 112–126, 1982 [DOI] [PubMed] [Google Scholar]
- 27. Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, Gubler MC, Antignac C, Esquivel EL: Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol 20: 2181–2189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pollak MR: Surprising results following conditional podocyte inactivation. J Am Soc Nephrol 20: 2086–2088, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Kriz W: The pathogenesis of “classic” focal segmental glomerulosclerosis—Lessons from rat models. Nephrol Dial Transplant 18[Suppl 6]: vi39–vi44, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Asano T, Niimura F, Pastan I, Fogo AB, Ichikawa I, Matsusaka T: Permanent genetic tagging of podocytes: Fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol 16: 2257–2262, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Abbate M, Zoja C, Morigi M, Rottoli D, Angioletti S, Tomasoni S, Zanchi C, Longaretti L, Donadelli R, Remuzzi G: Transforming growth factor-beta1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: A central pathway in progressive glomerulosclerosis. Am J Pathol 161: 2179–2193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu DT, Bitzer M, Ju W, Mundel P, Bottinger EP: TGF-beta concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol 16: 3211–3221, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A: In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: Implication for permselective dysfunction of chronic nephropathies. Am J Pathol 166: 1309–1320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C, Camussi G: Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol 294: F1185–F1194, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Sayyed SG, Hagele H, Kulkarni OP, Endlich K, Segerer S, Eulberg D, Klussmann S, Anders HJ: Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia 52: 2445–2454, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Onda M, Vincent JJ, Lee B, Pastan I: Mutants of immunotoxin anti-Tac(dsFv)-PE38 with variable number of lysine residues as candidates for site-specific chemical modification. 1. Properties of mutant molecules. Bioconjug Chem 14: 480–487, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Yaoita E, Yao J, Yoshida Y, Morioka T, Nameta M, Takata T, Kamiie J, Fujinaka H, Oite T, Yamamoto T: Up-regulation of connexin43 in glomerular podocytes in response to injury. Am J Pathol 161: 1597–1606, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsusaka T, Kobayashi K, Kon V, Pastan I, Fogo AB, Ichikawa I: Glomerular sclerosis is prevented during urinary tract obstruction due to podocyte protection. Am J Physiol Renal Physiol 300: F792–F800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Remuzzi G: Nephropathic nature of proteinuria. Curr Opin Nephrol Hypertens 8: 655–663, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Bazzi C, Petrini C, Rizza V, Arrigo G, D'Amico G: A modern approach to selectivity of proteinuria and tubulointerstitial damage in nephrotic syndrome. Kidney Int 58: 1732–1741, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Bakoush O, Grubb A, Rippe B, Tencer J: Urine excretion of protein HC in proteinuric glomerular diseases correlates to urine IgG but not to albuminuria. Kidney Int 60: 1904–1909, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Remuzzi G, Ruggenenti P, Perico N: Chronic renal diseases: Renoprotective benefits of renin-angiotensin system inhibition. Ann Intern Med 136: 604–615, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R: The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL study. Kidney Int 63: 1499–1507, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Farquhar MG, Palade GE: Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med 114: 699–716, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kriz W: Progression of chronic renal failure in focal segmental glomerulosclerosis: Consequence of podocyte damage or of tubulointerstitial fibrosis? Pediatr Nephrol 18: 617–622, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.