Abstract
Paracrine signaling between podocytes and glomerular endothelial cells through vascular endothelial growth factor A (VEGFA) maintains a functional glomerular filtration barrier. Heparan sulfate proteoglycans (HSPGs), located on the cell surface or in the extracellular matrix, bind signaling molecules such as VEGFA and affect their local concentrations, but whether modulation of these moieties promotes normal crosstalk between podocytes and endothelial cells is unknown. Here, we found that the transcription factor Wilms' Tumor 1 (WT1) modulates VEGFA and FGF2 signaling by increasing the expression of the 6-O-endosulfatases Sulf1 and Sulf2, which remodel the heparan sulfate 6-O-sulfation pattern in the extracellular matrix. Mice deficient in both Sulf1 and Sulf2 developed age-dependent proteinuria as a result of ultrastructural abnormalities in podocytes and endothelial cells, a phenotype similar to that observed in children with WT1 mutations and in Wt1+/− mice. These kidney defects associated with a decreased distribution of VEGFA in the glomerular basement membrane and on endothelial cells. Collectively, these data suggest that WT1-dependent sulfatase expression plays a critical role in maintaining the glomerular filtration barrier by modulating the bioavailability of growth factors, thereby promoting normal crosstalk between podocytes and endothelial cells.
Maintenance of an intact glomerular filtration barrier is essential to preserve normal renal function. The barrier consists of fenestrated glomerular endothelial cells, podocytes with their interdigitating foot processes and slit diaphragms, and the glomerular basement membrane (GBM) that separates these two cell types. Defects in any component of the glomerular filtration barrier leads to massive proteinuria, a condition referred to as nephrotic syndrome, that if refractory to treatment, results in end stage renal disease (ESRD).1,2 Thus, a better understanding of how the filtration barrier is maintained is essential to developing improved treatments to prevent ESRD.
Signaling between podocytes and endothelial cells is necessary to maintain the integrity of the glomerular filtration barrier.3–5 The most important signal identified thus far is vascular endothelial growth factor A (VEGFA), which is highly expressed by podocytes, whereas its receptors are primarily found on glomerular endothelial cells.6,7 Podocyte-specific haploinsufficiency for VEGFA during development results in glomerular endothelial lesions similar to pre-eclampsia, including loss of fenestrae and endothelial cell swelling.8 Moreover, the soluble form of VEGF receptor-1 (sFlt-1) that neutralizes VEGF is increased in the plasma of individuals with pre-eclampsia, a condition associated with proteinuria.9 Mice or rats injected with sFlt-1 protein also develop endotheliosis and proteinuria.9,10 Additionally, targeted mutation of Vegfa in podocytes of adult mice leads to profound thrombotic glomerular injury, with widening of the subendothelial space of glomerular capillaries and focal areas of podocyte foot process effacement.11 The requirement for VEGFA for the formation and maintenance of endothelial fenestrae is further supported by studies demonstrating that in conditionally immortalized human glomerular endothelial cells fenestration is induced in response to VEGFA.12 Taken together, these observations indicate that proper paracrine signaling by VEGFA is vital to maintain a functional glomerular filtration barrier.
The Wilms' tumor-1 (WT1) transcription factor regulates VEGFA expression in embryonic kidneys.13,14 In mature kidneys, WT1 expression is restricted to podocytes, which also express high levels of VEGFA. Mutations in the WT1 gene, associated with Denys-Drash syndrome (DDS), cause a severe early-onset nephrotic syndrome in humans.15 Our previous study provides evidence that WT1 mutations may alter glomerular VEGFA signaling by reducing the anti-angiogenic isoform VEGF165b,16 which has been suggested to play a role in glomerular maturation and podocyte protection.17,18
The activity of signaling transduction pathways can be modulated not only by regulating the expression of genes encoding diffusible signaling molecules but also by altering the bioavailability of these signaling molecules. Heparan sulfate proteoglycans (HSPGs) are highly charged proteins located on the cell surface or in the ECM, which are capable of binding signaling molecules such as VEGFA.19 Alterations in the level of 6-O-sulfation of the heparan sulfate (HS) chains within the HSPG can modulate the binding and release of signaling molecules, profoundly affecting their local concentrations.20–22
In this study, we demonstrate that WT1 transcriptionally regulates the expression of heparan sulfate 6-O-endosulfatases, and that these sulfatases are critically involved in the maintenance of the glomerular filtration barrier by modulating the bioavailability of signaling molecules within the kidney glomerulus. Together, our study provides novel insights for WT1's role in regulating cell-cell communication that is required for functional maintenance of the adult kidney glomerulus.
RESULTS
Decreased Expression of Sulf1 in WT1 Heterozygous Mutant Kidneys
To understand molecular mechanisms of WT1-associated glomerular disease, we used gene expression microarrays to identify genes that are misregulated in Wt1 heterozygous mouse glomeruli and in podocytes from humans carrying WT1 mutations, as genes misregulated in both human and mouse are more likely to play a major role in WT1-associated glomerular disease.
Human primary podocyte cultures were established from one child as a control and three children with genetically confirmed DDS (for WT1 mutations see Supplemental Table S1). In addition, a previously established adult human primary podocyte culture was used as a second control.23 All primary cultures expressed podocyte specific markers such as WT1, SYNPO, POD-1, NPHS1, NPHS2 ACTN4, and CD2AP (Supplemental Table S1).
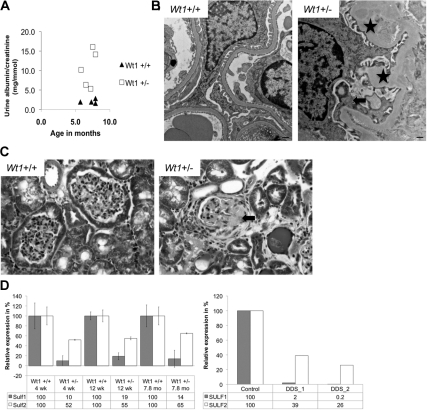
Murine glomeruli were isolated from four 7-month-old wild-type and four Wt1+/− mice at the fourth backcross onto a Friend virus B-type (FVB) background. These Wt1+/− mice exhibit proteinuria at 7 months (Figure 1A), several months earlier than Wt1+/− mice on a pure 129 Sv/Jae background, and demonstrate signs of glomerular disease such as focal areas of podocyte foot process effacement, thickening of the glomerular basement membrane (Figure 1B), and glomerulosclerosis (Figure 1C).
Figure 1.
Wt1 heterozygous mice (Wt1+/−) develop glomerular disease. (A) Urine albumin/creatinine ratio. Each symbol represents one animal. (B) Transmission electron micrograph demonstrating podocyte foot process effacement (arrow) and thickening of the glomerular basement membrane (asterisk) in Wt1+/− mice. Size bars = 500 nm. (C) Trichrome staining reveals sclerotic changes in glomeruli of Wt1+/− mice (arrow). (D) qRT-PCR for Sulf1 and Sulf2 in glomeruli isolated from Wt1+/− mice and in podocytes cultures from children with WT1 mutations. Four-week-old mice (wild type [+/+], n = 2: heterozygotes [+/−], n = 2) and the 12-week-old mice (+/+, n = 4, and +/−, n = 3) were from the ninth backcross 129 onto FVB; 7.8-month-old mice (+/+, n = 2, and +/−, n = 2) were from the fourth backcross 129 onto FVB. Error bars: ±SEM.
A total of 766 genes were differentially expressed at least 1.5-fold in glomeruli from Wt1+/− mice as compared with wild-type mice (P < 0.05) (Supplemental Table S2). Fifty-three of these genes also showed differential expression in the same direction in DDS podocytes as compared with controls (Supplemental Table S2). One hundred seventy-eight differentially expressed genes were not represented on the human microarray. Among the identified genes, Sulf1 showed a 8.5-fold reduction in Wt1+/− mice (also noted by Ratelade et al.24 in another Wt1 mouse model) and a 4.4-fold reduction in DDS podocytes as compared with controls, which was confirmed by quantitative reverse transcription-polymerase chain reaction (RT-PCR) and immunofluorescence (Figure 1D, Supplemental Figure S1). The expression of the closely related Sulf2 gene was less dramatically decreased in both DDS podocytes and glomeruli from Wt1+/− mice (Figure 1D). By immunofluorescence staining no obvious difference in Sulf2 expression was seen between glomeruli from Wt1 wild-type and heterozygous mice (Supplemental Figure S1).
Sulf1 and Sulf2 selectively remove 6-O sulfate groups from trisulfated disaccharides along HS chains on the cell surface and in the extracellular matrix,20–22 and thereby modulate the binding of extracellular factors to HS and receptors such as VEGFA and FGF2.25–29 That these two signaling molecules are involved in glomerulosclerosis3–5,8,11,30–32 prompted us to study the roles of WT1 in regulating gene expression and the function of Sulf1 and Sulf2 in kidney glomeruli.
Sulf1, Sulf2, and Wt1 Expression Partially Overlaps in the Kidney
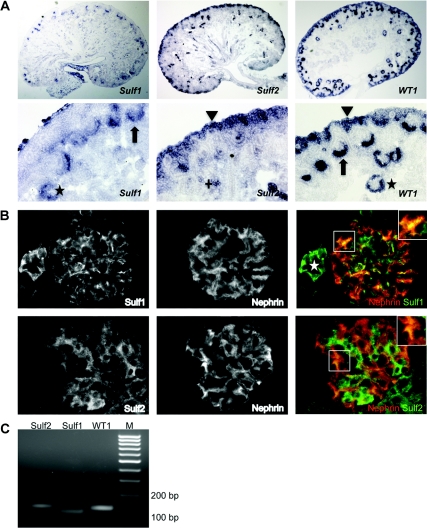
Both in situ hybridization and immunostaining were performed to test whether Sulf1, Sulf2, and Wt1 are coexpressed in the kidney. In embryonic (E17) kidneys Sulf1 and Wt1 mRNAs were coexpressed in the proximal part of the S-shaped body, which ultimately gives rise to podocytes in the glomerulus. In contrast, Sulf2 was not detected in these structures but was present in the nephron progenitor population that also expresses Wt1, as well as in a subset of tubules (Figure 2A).
Figure 2.
Sulf1, Sulf2, and Wt1 partially overlap in the kidney. (A) In situ hybridization on embryonic mouse kidneys (E17). Arrows mark immature podocytes in the proximal part of the S-shaped bodies, asterisks mark podocytes in more mature glomeruli, arrowheads mark the nephron progenitor population, and the cross marks a subset of tubuli. (B) Immunofluorescence for Sulf1, Sulf2, and nephrin on glomeruli from adult mice. Insets in the upper right: higher magnification of the boxed area; nephrin in red, Sulf in green. The asterisk marks a blood vessel. (C) Expression of Wt1, Sulf1, and Sulf2 mRNA in immortalized differentiated murine podocytes detected by RT-PCR.
In adult mouse glomeruli, Sulf1 protein showed overlapping localization with nephrin, indicating that Sulf1 localizes within podocytes to areas adjacent to the GBM (Figure 2B). Compared with Sulf1, Sulf2 exhibited a distinct and more diffuse cytoplasmic staining pattern that also partially overlapped with nephrin, suggesting expression in podocytes (Figure 2B). A centrally located hydrophilic domain in Sulf1 and Sulf2 is required for their localization at the plasma membrane.22 Interestingly, in this domain there is only 60% sequence homology between Sulf1 and Sulf2 that may account for the differences seen in the subcellular localization in glomerular cells. Although the conditions used to stain Sulf1 and Sulf2 did not permit co-staining with endothelial or mesangial cell markers, the incomplete overlap of each with nephrin suggests that their expression is not restricted to podocyte. Consistent with Sulf expression in vivo, Sulf1 and Sulf2 were both expressed in an immortalized murine podocyte cell line (Figure 2C) that expresses podocyte markers including WT1, α-actinin 4, α3-integrin, synaptopodin, and nephrin (Supplemental Figure S2). This was confirmed in two additional independently established podocyte cell lines (Supplemental Figure S3).
WT1 Binds the Sulf1 Promoter In Vivo and Regulates the Expression of Sulf1 In Vitro
At E18.5, Wt1 is expressed in both progenitor cells and developing mouse glomeruli. Sulf2 was previously identified as a potential WT1 target in a chromatin immunoprecipitation (ChIP)/Chip study using chromatin from E18.5 kidneys.14 To also assess direct interaction of WT1 with the Sulf1 promoter, we performed ChIP in embryonic mouse kidneys (E18.5) using primers that flank the transcriptional start site of Sulf1 (Figure 3A) and a cis-element cluster, predicted by the program Cister.33 Sulf1 promoter sequences but not exon 1 sequences were enriched in the WT1 ChIP, demonstrating that WT1 binds specifically the Sulf1 promoter in vivo (Figure 3B). This result is consistent with findings from Ratelade et al., who demonstrated binding of WT1 to the Sulf1 promoter in the mesonephric M15 cell line.24
Figure 3.
WT1 binds the Sulf1 gene promoter in vivo and regulates its transcription. (A) Structure of the Sulf1 5′UTR and the flanking region upstream of the TSS. Arrows indicate the location of the primers used to amplify chromatin after ChIP. Numbers refer to ENSMUST00000088585 (Ensembl). (B) ChIP on embryonic mouse kidneys (E18.5) and PCR amplification of the promotor region and exon 1 of the Sulf1 gene. WT1-#1 to 3: Samples immunoprecipitated with a WT1 antibody. PolyII: immunoprecipitation with a RNA polymerase II antibody (positive control). Negative controls: immuoprecipitation with a TGF-B antibody or rabbit IgG (RabIgG). Input #1 to 3: nonprecipitated samples. (C) Expression of Wt1, Sulf1, and Sulf2 measured by quantitative RT-PCR 48 hours after transfection of differentiated mouse podocytes with either a scramble siRNA as a control or a siRNA against Wt1. Knockdown experiments were performed independently twice. Columns represent means ± SEM; ***P < 0.001. Abbreviation: TSS, transcriptional start site.
WT1 regulation of Sulf expression specifically in podocytes was further examined by knocking down Wt1 expression in murine immortalized podocytes. After Wt1 siRNA transfection, Wt1 mRNA levels were reduced by 83% (P < 0.001), associated with a 75% reduction of Sulf1 mRNA (P < 0.001) but no statistically significant reduction was found for Sulf2 mRNA (Figure 3C).
Collectively, our data demonstrate that WT1 directly activates Sulf1 expression in podocytes. In contrast, although WT1 binds to the Sulf2 promoter in embryonic kidneys14 and may regulate Sulf2 expression both in nephron progenitor and immature podocytes, Sulf2 expression in differentiated podocytes appears less sensitive to WT1.
Mice Deficient in Sulf1 and Sulf2 Develop Glomerular Disease
To further link Wt1-dependent Sulf expression to the maintenance of intact glomeruli, we investigated kidney phenotypes in Sulf single and double mutant mice. Previous studies had demonstrated that mice genetically deficient in either Sulf1 or Sulf2 alone exhibit only mild phenotypes.34–36 In contrast, most mice deficient in both Sulf1 and Sulf2 show neonatal lethality, suggesting that Sulf1 and Sulf2 play overlapping yet critical roles in mouse development.34,35,37,38 Surviving Sulf1−/−; Sulf2−/− adults have smaller kidneys but neither renal function nor kidney histology appears to be altered by 4 months of age.34 However, in the present study, proteinuria was first detected at 5 months of age. At 5 to 9 months of age, 5 out of 6 Sulf1−/−;Sulf2−/− mice and half of Sulf1+/−;Sulf2−/− mice had proteinuria (Figure 4A; Supplemental Table S3) and developed glomerulosclerosis (Figure 4B), whereas age-matched Sulf1−/−;Sulf2+/−, Sulf1+/−;Sulf2+/−, and Sulf +/−;Sulf2+/+ control mice appeared normal (Figure 4, A and B). Sulf1−/−;Sulf2−/− mice had mild to moderate mesangial matrix expansion that in some glomeruli (approximately 10 to 15%) was also segmental with adhesion of the glomerular tuft to Bowman's capsule (Figure 4B). No intracapillary proliferation or crescents were seen.
Figure 4.
Sulf-deficient mice develop glomerular disease. (A) Urine albumin/creatinine ratio. Each symbol represents one animal. Different symbols represent different genotypes. (B) PAS staining reveals sclerotic changes (arrow) in Sulf1−/−;Sulf2−/− mice (n = 5; GSI: 1.65, 1.84, 1.85, 1.96, 2.28) as compared with controls (n = 5; GSI: 1.15, 1.16, 1.28, 1.48, 1.55). (C) Transmission electron micrograph demonstrating partial foot process effacement (arrow), thickening of the GBM with an expansion of the subendothelial space (asterisk), and loss of endothelial cell fenestration (arrowhead) in Sulf1−/−;Sulf2−/− mice (n = 2) as compared with a control (n = 1). Right-hand panels are magnifications of the left-hand panels. Size bars = 500 nm.
In the tubulointerstitium, no significant changes, that is, tubular atrophy, tubular dilation, inflammatory infiltrates, or fibrosis, were visible. Intrarenal arteries and arterioles showed no pathologic changes. In addition, ultrastructural analysis of Sulf1−/−;Sulf2−/− mouse kidneys revealed thickening of the GBM along with marked widening of the subendothelial space as well as focal areas of loss of endothelial cell fenestration and podocyte foot process effacement (Figure 4C). WT1, nephrin, collagen IVα4, and platelet-endothelial cell adhesion molecule expression were unchanged in mutant mice, indicating that podocytes, endothelial cells, and the GBM were grossly intact (Supplemental Figure S4).
Sulfatase Deficiency Alters VEGFA In Vivo
We postulated that Sulf deficiency may result in an oversulfation of HS chains on the podocyte surface, ultimately decreasing the amount of VEGFA released by podocytes and available to the endothelial cells. To test this hypothesis in vivo, immunogold labeling with an antibody against VEGFA was performed on kidney sections from control and Sulf-deficient mice. The specificity of this antibody is demonstrated by immunohistochemistry in podocytes and by Western blot analysis (Supplemental Figure S5). In a control kidney, VEGFA was detected in podocytes and their foot processes as well as in the GBM and at endothelial cells (Figure 5). Although VEGFA was still detected in podocytes of Sulf1−/−;Sulf2−/− mice, its distribution in the GBM and in endothelial cells was significantly diminished in both affected and less affected areas within glomeruli (Figure 5), suggesting a role for sulfatases in regulating the bioavailability of VEGFA.
Figure 5.
Sulf-deficient mice demonstrate altered VEGFA distribution. Left panel: Immunogold staining on control (n = 3) and Sulf1−/−;Sulf2−/− kidneys (n = 3). Arrows mark VEGFA staining. The lower panels are magnifications of the upper panels. Size bars = 500 nm. Right panel: Quantification of gold particles on two control and two Sulf double mutant kidney samples. For the controls a total of 27 pictures were taken with a surface area of 3 μm2 and for the mutants 20 pictures were taken from less affected areas and 15 pictures from more affected areas. Three different glomeruli were counted and per glomerulus three different capillary loops. Error bars: ±SEM. t test (two-tailed two-sample). Abbreviations: PFP, podocyte foot process; GEC, glomerular endothelial cell.
Knockdown of Sulf1 and Sulf2 In Vitro Affects VEGFA and FGF2 Signaling
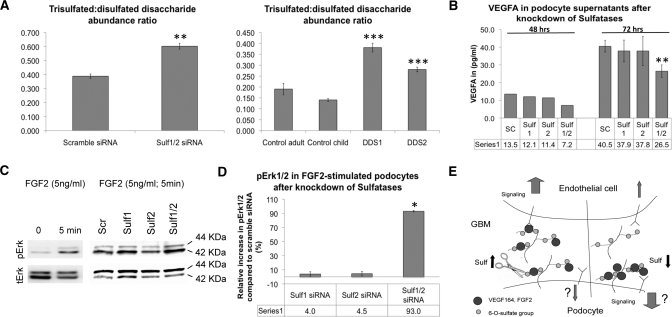
To further support our in vivo findings, we tested whether siRNA-mediated knockdown of Sulf1 and Sulf2 affects podocyte HS sulfation and whether knockdown impairs the release of VEGFA from podocytes in culture. Immortalized podocytes expressed predominantly the HS-binding VEGF164 isoform19,39–41 and lower levels of mRNAs encoding VEGF120 and VEGF188 (Supplemental Figure S6A). siRNAs against Sulf1 and Sulf2, when added simultaneously to the cells, reduced the Sulf1 mRNA level by 60% and Sulf2 by 63%, compared with scrambled siRNA controls (Supplemental Figure S6B). Reduced Sulf1 and Sulf2 expression significantly increased the abundance of trisulfated disaccharides (the substrate of sulfatases) and reciprocally decreased disulfated disaccharides (the product of sulfatases), as measured by disaccharide analysis using liquid chromatography/tandem mass spectrometry on HS isolated from podocyte cultures (Figure 6A, left panel; Supplemental Figure S7). Similar increases in trisulfated HS disaccharides were also detected in primary podocytes from DDS patients (Figure 6A, right panel), further demonstrating that the reduced levels of Sulf1 and Sulf2 in patients with WT1 mutations and in Wt1+/− mice (Figure 1D) are functionally relevant. We next compared the levels of secreted VEGFA in the supernatants of scrambled siRNA- and Sulf siRNA-treated podocytes. Knockdown of either Sulf1 or Sulf2 alone had no significant effect on VEGFA secretion (Figure 6B). In contrast, simultaneous knockdown of both Sulf1 and Sulf2 resulted in a 35% reduction of VEGFA secretion (P < 0.01) (Figure 6B). To exclude the possibility that reduced VEGFA secretion was caused by decreased Vegfa expression, mRNA and protein levels were determined in Sulf knockdown cells. As demonstrated in Supplemental Figure S8, mRNA levels remained unaltered upon Sulf knockdown. However, protein levels were 60% higher in cells in which Sulf1 and Sulf 2 were knocked down. As transcription was not altered, enhanced protein levels may reflect decreased secretion rather than increased expression. Consistent with our immunogold staining results, these findings indicate that Sulf1 and Sulf2 regulate VEGFA binding and release from podocytes, and consequently alter the availability of VEGFA to glomerular endothelial cells (Figure 6E).
Figure 6.
Knockdown of Sulf1 and Sulf2 in vitro modulates VEGFA and FGF2 signaling. (A) Relative abundance of trisulfated disaccharides within HS chains represented by a ratio between trisulfated and disulfated disaccharides in Sulf siRNA knockdown podocytes (left panel) and primary podocytes from DDS patients (right panel), assayed by SEC LC/MS after heparin lyase depolymerization of HS. Samples were analyzed in triplicate. Error bars: ±SEM. **P < 0.01; ***P < 0.001. (B) Secreted VEGFA measured by ELISA in cell supernatants of differentiated podocytes after using a scramble siRNA (Sc) as a control or after knockdown of either Sulf1 or Sulf2 or both simultaneously. VEGFA concentrations were normalized to the total protein in the seeded cells. Error bars: ±SEM from three independent experiments. **P < 0.01. (C, D) Western blot and quantification demonstrating percentage increase in phospho-Erk1/2 (normalized to total Erk1/2), after knockdown of Sulf1 or Sulf2 or both and induction with 5 ng/ml FGF2 for 5 minutes. Columns represent the differences between a scramble siRNA and the Sulf siRNAs. Error bars for FGF2: ±SEM from two independent experiments. *P < 0.05. (E) A model of Sulf-regulated VEGF165 and FGF2 signaling. Left side, physiologic condition; right side, decreased Sulf levels.
In addition to changes in VEGFA-mediated communication between podocytes and endothelial cells, augmented FGF2 signaling in podocytes has also been suggested to result in glomerular disease.31,32 We tested whether knockdown of Sulf1 and Sulf2 would result in increased intracellular FGF2 signaling in podocytes. Upon stimulation with FGF2, serum-starved podocytes responded by increasing phosphorylation of Erk (Figure 6C, left panel). Compared with scrambled siRNA-treated controls, knockdown of either Sulf1 or Sulf2 alone led to no increased pErk1/2, respectively (Figure 6, C[right panel] and D). In contrast, knockdown of both Sulf1 and Sulf2 resulted in 93% increase in pErk1/2 at 72 hours after transfection (Figure 6D). Therefore, in children with DDS, both reduced paracrine VEGFA signaling in endothelial cells and enhanced FGF2 signaling in podocytes may mediate the glomerular phenotype.
DISCUSSION
The data presented here provide evidence that WT1 regulated Sulf expression in podocytes contributes to the maintenance of the glomerular filtration barrier. We identified Sulf1 as potential transcriptional target of WT1. Our data demonstrate that compared with controls, Sulf1 expression is reduced in glomeruli from Wt1+/− mice and in primary podocyte cultures from children with WT1 mutations. In addition, WT1 binds the Sulf1 promoter in embryonic kidneys and knockdown of Wt1 in differentiated murine podocyte cultures results in decreased levels of Sulf1. Sulf2 expression was also decreased in DDS podocytes as well as in isolated glomeruli from Wt1+/− mice, but its expression was not restricted to podocytes and appeared less dependent on WT1.
Interestingly, three other groups have recently also identified Sulf1 and Sulf2 as a WT1 target. Langsdorf et al. demonstrate regulation of Sulf1 and Sulf2 expression by WT1 in murine Sertoli cells.42 Ratelade et al. report decreased Sulf1 expression in glomeruli from mice heterozygous for the most common WT1 mutation found in individuals with DDS (p. Arg394Trp), and demonstrate binding of WT1 to the Sulf1 promoter in a metanephric mesenchymal cell line.24 Additionally, Hartwig et al. identified Sulf2 as a potential WT1 target in embryonic kidneys using a ChIP/chip approach.14 Together with our data, these findings provide evidence that in the kidney WT1 regulates Sulf expression in a developmental manner. Thus, Sulf1 expression appears dependent on WT1 in both undifferentiated progenitor cells and in differentiated podocytes, whereas Sulf2 expression appears to have lost its dependency on WT1 as cells differentiated from progenitors to podocytes. This may possibly be explained by Sulf2 expression coming under the control of other transcription factors in differentiated podocytes, or because expression of Sulf2 becomes epigenetically fixed during podocyte differentiation, such that it is no longer dependent on the presence of WT1.
To investigate whether decreased Sulf expression could directly be involved in glomerular disease, we studied kidney phenotypes in Sulf single and double mutant mice. Sulf1−/−;Sulf2−/− mice presented the most dramatic kidney phenotype including thickening of the GBM along with marked widening of the subendothelial space as well as focal areas of loss of endothelial cell fenestration and podocyte foot process effacement. Together with the finding that Sulf1 and Sulf2 are both expressed in podocytes, this phenotype indicates that there may be functional redundancy for Sulf1 and Sulf2 in podocytes. In addition, half of the Sulf1+/−;Sulf2−/− mice but none of the Sulf1−/−;Sulf2+/− mice presented with proteinuria. This may be related to the higher expression of Sulf2 than Sulf1 in mature podocytes, such that a deficiency in Sulf2 causes a greater overall decrease in sulfatase activity at the GBM. Alternatively, as Sulf2 has a broader expression domain in glomeruli, Sulf2 deficiency in other cell types of the glomerulus may also contribute to the phenotype. Interestingly, the glomerular changes found in Sulf1−/−;Sulf2−/− mice were reminiscent of those we described previously in children with DDS,16 suggesting a causal link between WT1-regulated Sulf expression in podocytes and WT1-associated glomerular disease.
The observation that Sulf-deficient mice exhibited a similar glomerular phenotype to humans and mice with reduced levels of glomerular VEGFA,11 and the fact that sulfatases are involved in VEGF signaling,25,29 prompted us to investigate whether in Sulf-deficient mice VEGFA signaling is affected. Mechanistically, our data provide evidence that sulfatases reduce the binding of VEGF to HS to promote paracrine VEGF signaling in glomerular endothelial cells. Decreased bioavailability of VEGFA at endothelial cells may thus ultimately result in widening of the subendothelial space and loss of fenestration, as has previously been shown in VEGFA conditional knockout mice.11 It is interesting to note that sulfatases may be involved in the release of not only the pro-angiogenic isoform VEGF165a but also the anti-angiogenic isoform VEGF165b. Krilleke et al. have identified the amino acid residues that are critical for binding the mouse VEGF164 to the extracellular matrix: Arg-13, Arg-14, and Arg-49.43 These three amino acids are also present in the human VEGF165b isoform.44 It is therefore likely that, in humans, VEGF165b binds to the same HS chains as VEGF165. Consequently, Sulf-regulated sulfation of HS chains would affect the release of both VEGF165 and VEGF165b. In patients with DDS, VEGF165b signaling may thus be impaired in two ways: (1) because of decreased levels of the VEGF165b isoforms (as published earlier by us16); (2) because of a decreased release of the VEGF165b isoform from the ECM because of Sulf downregulation.
Apart from promoting paracrine VEGFA signaling, our data further suggest a role for sulfatases in repressing FGF2 signaling pathways in podocytes. Decreased expression of sulfatases in podocytes could result in enhanced FGF2 signaling, and may in turn cause podocyte damage as proposed previously.30–32
In conclusion, our results support the novel concept that WT1-regulated sulfatases are necessary for the maintenance of the glomerular filtration barrier, functioning to modulate the bioavailability of critical growth factors involved in the intraglomerular cross-talk between podocytes and endothelial cells.
CONCISE METHODS
Mice
Wt1+/− mice were from the fourth backcross of 129/SvJae onto a FVB background. Sulf1 and Sulf2 single and Sulf1/2 double mutant mice were backcrossed for 6 generations onto a C57BL/6 background.37 All animal studies were approved by the Institutional Animal Care and Use Committees.
Urine Albumin/Creatinine
The ratio of albumin/creatinine in urine samples from mice was determined using the DCA 2000 Urinanalyzer (Bayer Corporation) according to the manufacturer's instructions.
Periodic Acid–Schiff Staining and Glomerulosclerosis Index
Kidneys were fixed in 4% paraformaldehyde and embedded in paraffin. Four-micrometer sections were stained with periodic acid–Schiff (PAS) stain to determine the glomerulosclerosis index (GSI) according to reference 45. Briefly, the extent of mesangial matrix expansion and sclerosis is quantified using a semiquantitative scoring system proposed by el Nahas et al. and Goumenos et al. 46, 47 With use of light microscopy at a magnification of ×400, the score was obtained as a mean of 100 glomeruli in each animal. The severity of matrix expansion was expressed on an arbitrary scale from 0 to 4 with an individual glomeruli score as follows: grade 0, normal glomerulus; grade 1, presence of mesangial expansion/thickening of the basement membrane; grade 2, mild to moderate segmental hyalinosis/sclerosis involving less than 50% of the glomerular tuft; grade 3, diffuse glomerular hyalinosis/sclerosis >50% of the tuft; and grade 4, diffuse glomerulosclerosis with total tuft obliteration and/or collapse.
Cell Cultures
Normal control kidneys from children and adults were obtained from tumor nephrectomy specimens and from genetically confirmed DDS at the time of nephrectomy, either because of the presence of a Wilms' tumor or before kidney transplantation at ESRD. The parents of individuals with DDS gave informed consent regarding the study, and kidney tissue was used following the guidelines of the local ethics committee. Primary cultures of human podocytes were established using the sieve method as described previously by Pavenstaedt et al.23 A conditionally immortalized podocyte cell line was generated from a temperature-sensitive SV40 large T antigen transgenic mouse (Charles River, St. Louis) according to the protocol used by Schiwek et al.48 Minor changes included the use of the magnetic bead perfusion method as described by Takemoto et al. to isolate glomeruli.49 A conditionally immortalized metanephric mesenchyme cell line (LB22) was generated from a temperature-sensitive SV40 large T antigen transgenic mouse (Charles River, St. Louis).
Microarray Analyses
Glomeruli from 7-month-old Wt1+/+ (n = 4) and proteinuric Wt1+/− (n = 4) mice were isolated using the magnetic bead perfusion method.49 Total RNA was prepared with the PicoPure Kit (Arcturus). cDNA was amplified and labeled using an isothermal linear amplification (Ovation Biotin RNA amplification and labeling system, NuGene Technologies) and applied to Affymetrix Mouse Genome 430 2.0 microarrays. CEL files were normalized by the “robust multichip average” algorithm in GenePattern (Broad Institute of Harvard University and MIT, Boston). Differential expression was assessed using t test statistics (P < 0.05) and a 1.95-fold change cutoff was applied. For human samples, biotin-labeled cRNA was generated using the Enzo BioArray high-yield RNA transcript labeling kit (Enzo Biochem) and hybridized onto Affymetrix Human Genome U133A 2.0 microarrays. Data analysis was performed in the same manner as for the mouse microarrays.
Chromatin Immunoprecipitation
ChIP followed by site-specific PCR was performed on mouse embryonic kidneys (E18.5) according to a published protocol.14 Protein/chromatin complexes were pulled down using anti-WT1 (WT-C19, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-RNA polymerase II (Upstate Biotech), and as a negative control anti-rabbit IgG (Santa Cruz Biotechnology, Inc.). For amplification of the mSulf1 promoter region, the forward primer 5′-TGCTCCTCCTCTTCTTGGAA-3′ and reverse primer 5′-GATAAAACTGCCCGACCTGA-3′ were used. For mSulf1 exon 1, the forward primer 5′-CCAATGCCTTTGTGACCACG-3′ and reverse primer 5′-AGGGAGACGAGCAGTTCTCAT-3′ were used.
RT-PCR
cDNA was synthesized with random hexamers and Superscript III (Invitrogen). Conventional RT-PCR was performed as described previously.50 Quantitative RT-PCR reactions were carried in duplicate using SYBR green and the results normalized to GAPDH as described previously.51 Relative gene expression was calculated by the 2−ΔΔCT method. Primer sequences are listed in the Supplemental Material.
Gene Knockdown in Cultured Cells
Cells were transfected with either Lipofectamine 2000 (Invitrogen Life Technology, Inc.) or Hiperfect (Qiagen) according to the manufacturer's instructions. Sequences of siRNAs and StealthTM RNAi duplexes are listed in the Supplemental Material. RNA was isolated 48 or 72 hours after transfection using the RNeasy kit (Qiagen).
In Situ Hybridization and Immunofluorescence Staining
Tissue in situ hybridization and immunofluorescence staining on frozen or paraffin embedded sections or cultured podocytes were performed as described previously.51–54 A riboprobe was generated from the 3′UTR of the Sulf1 mouse gene by PCR amplification using a forward 5′-ACCCTGCATCTGAACAGACC-3′ and reverse 5′-GCTCAGAATGTTGGCAGTCA-3′ primer and subsequently cloning into the pCRII-TOPO vector (Invitrogen). Riboprobes for mouse Sulf2 were obtained from Dr. Xingbin Ai (Boston University) and for mouse Wt1 from Dr. Jordan Kreidberg.13 Negative controls included hybridization of a sense probe. Immunofluorescence staining was performed with the antibodies listed in the Supplemental Material. F-actin was stained with Alexa Fluor 488 phalloidin (Molecular Probes). Negative controls included omitting the primary antibody.
Immunoelectron Microscopy
Postembedding immunogold labeling was performed as published previously55 using anti-VEGFA (A-20; Santa Cruz Biotechnology) diluted 1:10 in TBS-ovalbumin overnight at 4°C, and 10-nm gold-conjugated anti-rabbit antibodies (BioCell, Cardiff, Wales, UK). In negative control samples, the primary antibody was replaced by PBS or equimolar concentrations of non-immune rabbit IgG.
HS Disaccharide Analysis
Glucosaminoglycans (GAGs) were released from podocytes, purified, depolymerized using bacterial polysaccharide lyases, and analyzed using size exclusion chromatography/mass spectrometry (SEC LC/Ms) as described previously.56,57 Briefly, GAGs were released using 0.5 M NaOH, followed by purification using weak anion exchange chromatography. Samples were then digested into disaccharides by heparin lyases I, II, and III from Flavobacterium heparinum (Ibex, Montreal, Canada) at 37°C. The resultant disaccharides were analyzed directly using SEC LC/MS.
ELISA
Mouse VEGFA immunoassay (R&D Systems) was performed as previously published.9 The protein levels were calculated using a standard curve derived from known concentrations of VEGFA recombinant protein and normalized to the total protein amount in the seeded cells as determined by Bradford assay (Bio-Rad Laboratories).
FGF2 Stimulation Experiments
Forty-two hours after transfection with scrambled siRNA or Sulf siRNA, cells were placed in serum-free media for 6 hours and then induced with 5 ng/ml FGF2 (Cell Signaling) for 5 minutes at 37°C before lysis in 50 mM Tris-HCl (pH 7.4), 0.1% SDS, 150 mM NaCl, 1% NP40, 0.5% Na deoxycholate, 50 mM NaF, 1 mM Na3VO4, and protease inhibitor mixture (Roche Applied Science). Equal protein amounts were analyzed for phosphorylated (pErk1/2) and total Erk1/2 (p44/42 MAPK) amounts on Western blot.
Protein Extraction and Western Blot
Cells or kidney tissue were lysed in buffer (500 mM NaCl, 50 mM Tris-HCl pH 7.4, 5 mM EDTA, 1 mM EGTA, 25 mM Na pyrophosphate pH 7.4, 1 mM Na3VO4, 50 mM NaF, 0.1% SDS, 1% NP-40, 0.5% Na deoxycholate, and protease inhibitor mixture [Roche Applied Science]). Twenty micrograms of total protein was resolved on a 10 or 12% SDS-PAGE and transferred to a PVDF-membrane (Immun Blot, Bio-Rad Laboratories). Detection of protein bands was performed using horseradish peroxidase–labeled secondary antibodies and enhanced chemiluminescence reagents (ECL Advanced Western Blotting Detection Kit, GE Healthcare) and quantification using the Molecular Imager ChemiDoc XRS system (Bio-Rad Laboratories) and the Quantity One software (Bio-Rad Laboratories).
Statistical Analysis
Data are presented as mean ± SEM. If not otherwise indicated, two-tailed paired t test was performed to determine statistically significant differences between two groups. P values were adjusted for multiple testing correction by the Holm method. A P value of less than 0.05 was considered significant.
Additional materials and methods are described in the Supplemental Material.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Miriam Reutelshöfer for technical assistance and Dr. Helmut Rennke, Dr. Jan Becker, and Dr. Rainer Engers for evaluating the electron micrographs. We thank Drs. Peter Mundel, Moin Saleem, and Johannes Schlondorff for providing immortalized podocytes; Drs. Lawrence Holzman, Jeffrey Miner, Peter Mundel, and Martin Pollak for primary antibodies and Frank Eitner for the human mesangial cell line CC-2259. We thank Joshua Gould from the Broad Institute of MIT and Harvard, Cambridge, MA, Priyanka Pandey from Children's Hospital Boston, and Manfred Beier, Institute of Human Genetics, University of Düsseldorf, Düsseldorf, Germany, for assistance with statistical analyses. V.A.S. acknowledges support from the Fritz Thyssen Stiftung, Forschungskommission Düsseldorf, and the Heinrich Hertz Foundation. X.A. acknowledges start-up funds from BUSM and a NIH grant (R01AG034939). X.S. and J.Z. acknowledge support from NIH grants P41RR10888 and R01HL098950. K.A. acknowledges support from the Deutsche Forschungsgemeinschaft (SFB423, Z2) and the IZKF Erlangen (A11).
Part of this material was presented in abstract form at Renal Week 2009, the annual meeting of the American Society of Nephrology; October 27 through November 1, 2009; San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Zenker M, Machuca E, Antignac C: Genetics of nephrotic syndrome: New insights into molecules acting at the glomerular filtration barrier. J Mol Med 87: 849–857, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Hildebrandt F: Genetic kidney diseases. Lancet 375: 1287–1295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schrijvers BF, Flyvbjerg A, De Vriese AS: The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65: 2003–2017, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Mathieson PW: How much VEGF do you need? J Am Soc Nephrol 17: 602–603, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Foster RR: The importance of cellular VEGF bioactivity in the development of glomerular disease. Nephron Exp Nephrol 113: e8–e15, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Brown LF, Berse B, Tognazzi K, Manseau EJ, Van de Water L, Senger DR, Dvorak HF, Rosen S: Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int 42: 1457–1461, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Robert B, Zhao X, Abrahamson DR: Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am J Physiol Renal Physiol 279: F275–F282, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R: Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605–12608, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O'Hare MJ, Saleem MA, van den Heuvel LP, Mathieson PW: Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int 69: 1633–1640, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gao X, Chen X, Taglienti M, Rumballe B, Little MH, Kreidberg JA: Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development 132: 5437–5449, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Hartwig S, Ho J, Pandey P, Macisaac K, Taglienti M, Xiang M, Alterovitz G, Ramoni M, Fraenkel E, Kreidberg JA: Genomic characterization of Wilms' tumor suppressor 1 targets in nephron progenitor cells during kidney development. Development 137: 1189–1203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pelletier J, Bruening W, Kashtan CE, Mauer SM, Manivel JC, Striegel JE, Houghton DC, Junien C, Habib R, Fouser L, Fine RN, Silverman BL, Haber DA, Housman D: Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 67: 437–447, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Schumacher VA, Jeruschke S, Eitner F, Becker JU, Pitschke G, Ince Y, Miner JH, Leuschner I, Engers R, Everding AS, Bulla M, Royer-Pokora B: Impaired glomerular maturation and lack of VEGF165b in Denys-Drash syndrome. J Am Soc Nephrol 18: 719–729, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Cui TG, Foster RR, Saleem M, Mathieson PW, Gillatt DA, Bates DO, Harper SJ: Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF165b) mRNA and protein. Am J Physiol Renal Physiol 286: F767–F773, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Bevan HS, van den Akker NM, Qiu Y, Polman JA, Foster RR, Yem J, Nishikawa A, Satchell SC, Harper SJ, Gittenberger-de Groot AC, Bates DO: The alternatively spliced anti-angiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol 110: 57–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson CJ, Mulloy B, Gallagher JT, Stringer SE: VEGF165-binding sites within heparan sulfate encompass two highly sulfated domains and can be liberated by K5 lyase. J Biol Chem 281: 1731–1740, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP, Jr.: Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science 293: 1663–1666, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD: Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem 277: 49175–49185, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ai X, Do AT, Kusche-Gullberg M, Lindahl U, Lu K, Emerson CP, Jr.: Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. J Biol Chem 281: 4969–4976, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Pavenstaedt H, Spath M, Schlunck G, Nauck M, Fischer R, Wanner C, Schollmeyer P: Effect of nucleotides on the cytosolic free calcium activity and inositol phosphate formation in human glomerular epithelial cells. Br J Pharmacol 107: 189–195, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ratelade J, Arrondel C, Hamard G, Garbay S, Harvey S, Biebuyck N, Schulz H, Hastie N, Pontoglio M, Gubler MC, Antignac C, Heidet L: A murine model of Denys-Drash syndrome reveals novel transcriptional targets of WT1 in podocytes. Hum Mol Genet 19: 1–15, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Uchimura K, Morimoto-Tomita M, Bistrup A, Li J, Lyon M, Gallagher J, Werb Z, Rosen SD: HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: Effects on VEGF, FGF-1, and SDF-1. BMC Biochem 7: 2, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang S, Ai X, Freeman SD, Pownall ME, Lu Q, Kessler DS, Emerson CP, Jr.: QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc Natl Acad Sci U S A 101: 4833–4838, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamanna WC, Frese MA, Balleininger M, Dierks T: Sulf loss influences N-, 2-O-, and 6-O-sulfation of multiple heparan sulfate proteoglycans and modulates fibroblast growth factor signaling. J Biol Chem 283: 27724–27735, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Lai J, Chien J, Staub J, Avula R, Greene EL, Matthews TA, Smith DI, Kaufmann SH, Roberts LR, Shridhar V: Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. J Biol Chem 278: 23107–23117, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Fujita K, Takechi E, Sakamoto N, Sumiyoshi N, Izumi S, Miyamoto T, Matsuura S, Tsurugaya T, Akasaka K, Yamamoto T: HpSulf, a heparan sulfate 6-O-endosulfatase, is involved in the regulation of VEGF signaling during sea urchin development. Mech Dev 127: 235–245, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Mazue G, Bertolero F, Garofano L, Brughera M, Carminati P: Experience with the preclinical assessment of basic fibroblast growth factor (bFGF). Toxicol Lett 64–65 Spec No: 329–338, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Floege J, Kriz W, Schulze M, Susani M, Kerjaschki D, Mooney A, Couser WG, Koch KM: Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephropathy. J Clin Invest 96: 2809–2819, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kriz W, Hahnel B, Rosener S, Elger M: Long-term treatment of rats with FGF-2 results in focal segmental glomerulosclerosis. Kidney Int 48: 1435–1450, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Frith MC, Hansen U, Weng Z: Detection of cis-element clusters in higher eukaryotic DNA. Bioinformatics 17: 878–889, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Holst CR, Bou-Reslan H, Gore BB, Wong K, Grant D, Chalasani S, Carano RA, Frantz GD, Tessier-Lavigne M, Bolon B, French DM, Ashkenazi A: Secreted sulfatases Sulf1 and Sulf2 have overlapping yet essential roles in mouse neonatal survival. PLoS One 2: e575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lamanna WC, Baldwin RJ, Padva M, Kalus I, Ten Dam G, van Kuppevelt TH, Gallagher JT, von Figura K, Dierks T, Merry CL: Heparan sulfate 6-O-endosulfatases: Discrete in vivo activities and functional co-operativity. Biochem J 400: 63–73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lum DH, Tan J, Rosen SD, Werb Z: Gene trap disruption of the mouse heparan sulfate 6-O-endosulfatase gene, Sulf2. Mol Cell Biol 27: 678–688, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ai X, Kitazawa T, Do AT, Kusche-Gullberg M, Labosky PA, Emerson CP, Jr.: SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development 134: 3327–3338, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Ratzka A, Kalus I, Moser M, Dierks T, Mundlos S, Vortkamp A: Redundant function of the heparan sulfate 6-O-endosulfatases Sulf1 and Sulf2 during skeletal development. Dev Dyn 237: 339–353, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Park JE, Keller GA, Ferrara N: The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 4: 1317–1326, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robinson CJ, Stringer SE: The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci 114: 853–865, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Kretzler M, Schroppel B, Merkle M, Huber S, Mundel P, Horster M, Schlondorff D: Detection of multiple vascular endothelial growth factor splice isoforms in single glomerular podocytes. Kidney Int Suppl 67: S159–S161, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Langsdorf A, Schumacher V, Shi X, Tran T, Zaia J, Jain S, Taglienti M, Kreidberg JA, Fine A, Ai X: Expression regulation and function of heparan sulfate 6-O-endosulfatases in the spermatogonial stem cell niche. Glycobiology 21: 152–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krilleke D, DeErkenez A, Schubert W, Giri I, Robinson GS, Ng YS, Shima DT: Molecular mapping and functional characterization of the VEGF164 heparin-binding domain. J Biol Chem 282: 28045–28056, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ: VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 62: 4123–4131, 2002 [PubMed] [Google Scholar]
- 45. Wolf G, Schanze A, Stahl RA, Shankland SJ, Amann K: p27(Kip1) knockout mice are protected from diabetic nephropathy: evidence for p27(Kip1) haplotype insufficiency. Kidney Int 68: 1583–1589, 2005 [DOI] [PubMed] [Google Scholar]
- 46. el Nahas AM, Bassett AH, Cope GH, Le Carpentier JE: Role of growth hormone in the development of experimental renal scarring. Kidney Int 40: 29–34, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Goumenos DS, Brown CB, Shortland J, el Nahas AM: Myofibroblasts, predictors of progression of mesangial IgA nephropathy? Nephrol Dial Transplant 9: 1418–1425, 1994 [PubMed] [Google Scholar]
- 48. Schiwek D, Endlich N, Holzman L, Holthofer H, Kriz W, Endlich K: Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int 66: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schumacher V, Schuhen S, Sonner S, Weirich A, Leuschner I, Harms D, Licht J, Roberts S, Royer-Pokora B: Two molecular subgroups of Wilms' tumors with or without WT1 mutations. Clin Cancer Res 9: 2005–2014, 2003 [PubMed] [Google Scholar]
- 51. Liu Y, Chattopadhyay N, Qin S, Szekeres C, Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH, Kreidberg JA: Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development 136: 843–853, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA: Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol 19: 2069–2075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mundel P, Reiser J, Kriz W: Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8: 697–705, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Haas CS, Campean V, Kuhlmann A, Dimmler A, Reulbach U, Forster C, Aigner T, Acker T, Plate K, Amann K: Analysis of glomerular VEGF mRNA and protein expression in murine mesangioproliferative glomerulonephritis. Virchows Arch 450: 81–92, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Tiedemann K, Sasaki T, Gustafsson E, Gohring W, Batge B, Notbohm H, Timpl R, Wedel T, Schlotzer-Schrehardt U, Reinhardt DP: Microfibrils at basement membrane zones interact with perlecan via fibrillin-1. J Biol Chem 280: 11404–11412, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Shi X, Zaia J: Organ-specific heparan sulfate structural phenotypes. J Biol Chem 284: 11806–11814, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Staples GO, Shi X, Zaia J: Extended N-sulfated domains reside at the nonreducing end of heparan sulfate chains. J Biol Chem 285: 18336–18343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.