Abstract
Translating discoveries made in isolated renal cells and tubules to the in vivo situation requires the assessment of cellular function in intact live organs. Multiphoton imaging is a form of fluorescence microscopy that is ideally suited to working with whole tissues and organs, but adequately loading cells with fluorescence dyes in vivo remains a challenge. We found that recirculation of fluorescence dyes in the rat isolated perfused kidney (IPK) resulted in levels of intracellular loading that would be difficult to achieve in vivo. This technique allowed the imaging of tubular cell structure and function with multiphoton microscopy in an intact, functioning organ. We used this approach to follow processes in real time, including (1) relative rates of reactive oxygen species (ROS) production in different tubule types, (2) filtration and tubular uptake of low-molecular-weight dextrans and proteins, and (3) the effects of ischemia-reperfusion injury on mitochondrial function and cell structure. This study demonstrates that multiphoton microscopy of the isolated perfused kidney is a powerful technique for detailed imaging of cell structure and function in an intact organ.
Advanced imaging techniques are central to modern biomedical research. Translating findings from isolated cells into whole organisms requires the development of novel methods to allow measurements to be made in intact functioning tissues. Multiphoton imaging is a form of fluorescence microscopy that permits far greater tissue penetration, and with less phototoxicity, than conventional confocal imaging; thus, it is highly suited to working with whole organs. Multiphoton imaging of the kidney in anesthetized rodents is a powerful technique,1,2 and has made it possible for some aspects of tubular cell physiology to be studied in vivo.3,4 However, as with other organ systems, achieving sufficient intracellular loading and steady state equilibration of fluorescence dyes to make meaningful measurements remains a significant technical challenge because of problems such as intravascular dilution, dye excretion, and the effects of extracellular esterases (which cleave AM-ester dyes before they enter cells).5
We have recently demonstrated that aspects of cellular redox state and mitochondrial function can be measured in live slices of rat kidney using multiphoton microscopy.6 However, ionic microenvironments and the large osmotic gradients developed by the countercurrent mechanism are key determinants of renal function, and both may be lost in a slice preparation. It is, therefore, necessary to make these measurements in an intact functioning organ, as cellular metabolism is closely coupled to solute transport.7 The isolated perfused rat kidney (IPK) preparation has been used extensively for functional studies in renal research, but it has not, to our knowledge, been used for live imaging-based studies. The IPK provides a model in which solute filtration and transport, and gradients, are preserved, and different solutions and reagents can be infused at known concentrations directly into the organ via the renal artery under tightly controlled conditions. We have discovered that with use of a recirculating perfusion system, adequate concentrations of fluorescence dyes can be loaded into tubular cells in the IPK to allow detailed imaging of cell structure and function using multiphoton microscopy. Furthermore, changes in signal can be observed in real time in response to toxic stimuli, such as ischemia-reperfusion (IR) injury. We believe that this is a powerful technique combining the best of both in vitro and in vivo approaches, and it has valuable potential for studies of renal physiology and pathophysiology.
RESULTS
Imaging of Endogenous Fluorophores in the Kidney—NADH
The kidney emits a large amount of autofluorescence (particularly in the proximal tubule [PT]6) because of the existence of a number of endogenous fluorophores, some of which have yet to be identified. NADH functions as the substrate for complex I of the respiratory chain and in a number of cellular redox reactions. NADH is fluorescence in the reduced, but not the oxidized (NAD+), form; thus, the fluorescence signal emitted provides a useful readout of mitochondrial redox state,8,9 which is determined by factors such as substrate supply and respiratory chain complex activity. NADH signal was clearly visible in the IPK at 720-nm excitation and showed a typical mitochondrial location in the basolateral aspect of tubular cells (Figure 1A). The identity of the signal was confirmed by an increase in response to perfusion with a hypoxic solution (Figure 1B). NADH fluorescence can, therefore, also be used as a readout of local tissue oxygenation.
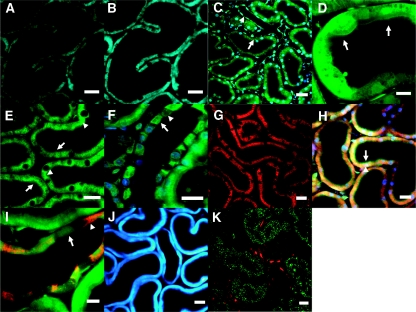
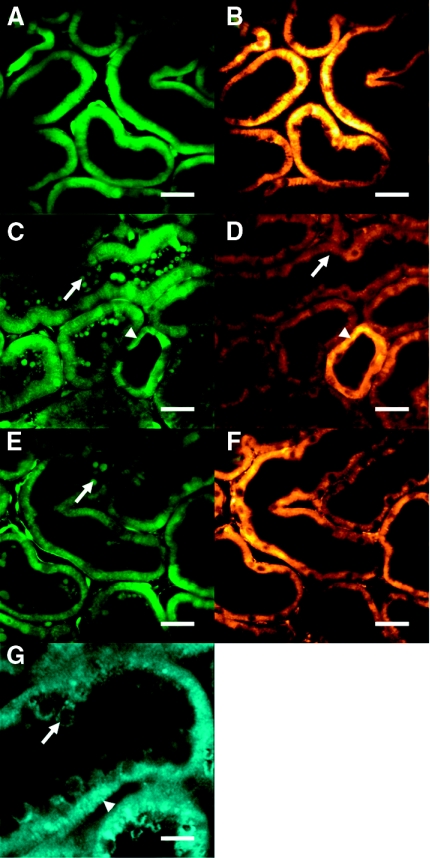
Figure 1.
A range of endogenous and exogenous fluorophores can be imaged in the intact kidney using multiphoton microscopy. (A) NADH was excited at 720 nm and displayed a typical mitochondrial pattern (basolateral and striated) in renal tubules. (B) the identity of the signal was confirmed by an increase in fluorescence intensity in response to perfusion with a hypoxic buffer. (C) Hoechst 33342 (blue) was used to label cell nuclei, whereas calcein-AM (green) was used as a marker of viability and loaded well into the cytosol of both tubular (arrowed) and endothelial cells (arrowhead). (D) At higher resolution subcellular structures were visible such as the apical brush border (arrowed) in the proximal tubule. (E) Quinacrine loaded preferentially into the brush border (arrowed) and subapical vesicles (arrowhead) in proximal tubular cells. (F) Quinacrine signal was markedly heterogeneous along the collecting duct because of preferential loading of the dye into principal cells (arrowed) rather than intercalated (arrowhead). (G) TMRM (a ΔΨm-dependent dye) loaded into proximal tubular cells, demonstrating the high density of mitochondria in this nephron segment; (H) TMRM was also coloaded with Hoechst 33342 and calcein-AM to identify mitochondria (red, arrowhead), nuclei (blue), and brush border (green, arrowed), respectively, within proximal tubular cells. (I) TMRM signal (red) varied along the collecting duct, partly reflecting known differences in mitochondrial density, which is higher in intercalated cells (arrowhead) than principal cells (arrowed). (J) Monochlorobimane forms a fluorescence adduct in the presence of glutathione, which was clearly visible in tubular cells in the blue range. (K) Rhod-2-AM, a Ca2+-sensitive dye that generally localizes to mitochondria, loaded well into endothelial cells in capillaries (red) running between proximal tubules (indentified by green autofluorescence). Scale bars = 20 μm, except in (D) where scale bar = 10 μm.
Imaging of Exogenous Fluorescent Dyes in Tubular Cells in the Kidney
We have found that a range of fluorescence dyes can be successfully loaded into tubular cells in the IPK by using a recirculating perfusion system, permitting detailed imaging of cell structure and function. Hoechst 33342 is a widely used label for cell nuclei and was clearly visible in cells throughout the kidney after infusion (Figure 1C). Calcein-AM is an established marker of cell viability, as it enters cells and is cleaved to the fluorescence form by intracellular esterases that are only active in live cells;10 diffuse uptake of the dye was observed in both tubules and capillaries in the IPK (Figure 1C), which allowed visualization of subcellular structures in some detail, such as the PT brush border (Figure 1D).
Quinacrine is a fluorescent dye that has been used to label intracellular vesicles in the kidney11 and other organs.12 After the infusion of quinacrine into the IPK, widespread uptake of the dye was observed into tubular cells, with localization predominantly in the PT brush border and subapical vesicles (Figure 1E). We observed a markedly heterogeneous fluorescence signal along the collecting duct (Figure 1F), most likely because of selective uptake of quinacrine into principal cells (rather than intercalated), which has been described in previous studies using isolated tubules.13
As demonstrated in the NADH images, PTs contain a high density of mitochondria that provide energy in the form of ATP, which is required to perform large amounts of solute transport in this nephron segment. Proton pumping by respiratory chain complexes leads to a potential difference (ΔΨm) across the inner mitochondrial membrane that is central to mitochondrial function, affecting the rate of ATP production, and also other key processes such as Ca2+ uptake and reactive oxygen species (ROS) generation.14 ΔΨm can be measured by the partitioning into mitochondria of lipophilic cationic dyes, such as tetramethyl rhodamine methyl ester (TMRM).15 Perfusion of the IPK with TMRM resulted in a fluorescence signal identical in distribution to that of NADH (Figure 1G), implying mitochondrial uptake of the dye. Coloading of IPKs with Hoechst 33342, calcein-AM, and TMRM permitted simultaneous identification of intracellular nuclei, cytosol, and mitochondria, respectively (Figure 1H). TMRM signal intensity varied along the collecting duct (Figure 1I), most likely reflecting known differences in mitochondrial density, which is higher in intercalated cells than in principal cells.16
Glutathione (GSH) is an important intracellular antioxidant that plays a key role in the maintenance of redox state and metabolism of drugs in the PT (for review see reference 17). GSH depletion has been shown to cause structural and functional abnormalities in the IPK.18 We demonstrated previously that GSH levels can be measured in rat kidney slices using monochlorobimane (MCB), which is conjugated to GSH by glutathione S-transferase to form a fluorescence adduct.6 MCB was successfully loaded into renal tubular cells in the IPK and was clearly visible at 720-nm excitation (Figure 1J).
Ca2+ is an intracellular secondary messenger with many important roles in cell signaling; a range of AM-ester dyes are now available to measure changes in intracellular Ca2+ levels. In our experience, the loading of these Ca2+-dependent dyes is generally poor in tubular cells in both kidney slices and the IPK for reasons that are currently unclear. However, we have found that rhod-2-AM (a Ca2+-dependent dye that typically localizes to mitochondria19) does load into endothelial cells in peritubular capillaries (Figure 1K).
In summary, we have found that a range of exogenous fluorescence dyes can be loaded into tubular cells in the IPK, which (along with endogenous fluorophores like NADH) allow various aspects of cell structure and function to be imaged in an intact functioning organ using multiphoton microscopy. In the following sections we will provide some examples of how this approach can be applied to make useful measurements of physiologic and pathophysiologic processes.
Imaging of Reactive Oxygen Species Production in the Kidney
Multiphoton imaging of the IPK facilitates direct side-by-side comparisons to be made in cellular physiology of different tubule segments and cell types. ROS have wide-ranging physiologic and pathophysiologic roles in the kidney, and oxidative stress is implicated as an important underlying mechanism in a variety of conditions affecting the PT (e.g., cystinosis,20 diabetic nephropathy,21 and heavy-metal poisoning22). Potential intracellular sources of ROS production include the mitochondrial respiratory chain (predominantly from complexes I and III23) and NADPH oxidases.24 Most studies to date concerning ROS production in the kidney have relied on isolated cells in vitro, and relatively little is known about ROS production in intact organs.
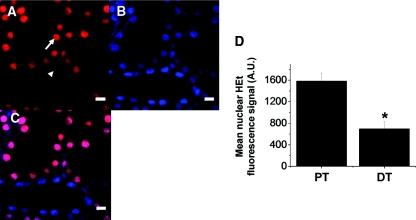
Dihydroethidium (HEt) is an established marker of ROS production that becomes fluorescent in the presence of superoxide.25–27 After infusion of HEt into the IPK, we obtained a clear intracellular fluorescence signal in renal tubules that was predominantly located in cell nuclei (oxidized HEt binds avidly to DNA); this was demonstrated by co-localization of the HEt signal with that of the established nuclear dye Hoechst 33342 (Figure 2, A through C). After 20 minutes of HEt infusion in the IPK, mean nuclear fluorescence signal in the PT (1582.8 ± 150.9 A.U.) was significantly higher than that in the distal tubule (DT: 694.9 ± 133.6 A.U., n = 3, P < 0.05) (Figure 2D), consistent with increased levels of superoxide in the former, which may be important in determining intrinsic vulnerability to oxidative stress.
Figure 2.
Reactive oxygen species production is higher in proximal tubular cells than in distal tubular cells. (A and D) Infusion with the superoxide-sensitive dye dihydroethidium (HEt) caused an increase in fluorescence signal in the cell nuclei of renal tubules, and after 20 minutes the signal was higher in proximal tubules (arrowed) than in distal tubules (arrowhead). The origin of the signal was confirmed by simultaneously loading tubular cells with the nuclear dye Hoechst 33342 (B), and demonstrating colocalization of the signals (C). Scale bars = 10 μm. Values given are mean nuclear HEt fluorescence signal (±SEM) from three separate experiments (*P < 0.05).
Imaging of Solute Filtration/Uptake and Metabolism in the Kidney
A major advantage of the IPK over other in vitro preparations, such as tissue slices or isolated tubules, is that it is an intact functioning organ capable of glomerular filtration and tubular reabsorption of solutes. These processes can be imaged in real time using fluorescently tagged molecules of differing sizes. For example, perfusion of the IPK with a low-molecular-weight (10 kD) rhodamine-labeled dextran led to a rapid increase in fluorescence signal in the lumens of both capillaries and tubules (Figure 3A), consistent with free filtration of the molecule by glomeruli. In contrast, after infusion with a large (100 kD) FITC-labeled dextran, the fluorescence signal was confined to the vasculature, and did not appear in tubular lumens (Figure 3B).
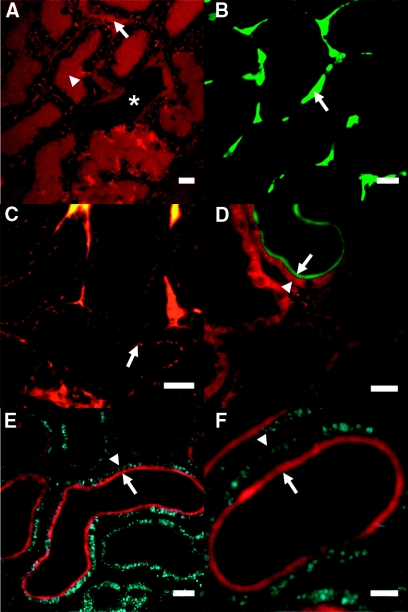
Figure 3.
Multiphoton imaging allows visualization of solute filtration/uptake and metabolism in the intact kidney. (A) After infusion with a small (10 kD) rhodamine-labeled dextran, fluorescence signal increased rapidly in both capillary (arrowed) and proximal tubular (arrowhead) lumens; the example image depicted shows a central distal tubule (starred), where the dextran subsequently appeared at a later time point. (B) After infusion with a large (100 kD) FITC-labeled dextran, the fluorescence signal was confined to capillaries (arrowed) and did not appear in tubular lumens. (C) Over time, the smaller dextran was visible in the apical aspect of proximal tubular cells (arrowed), consistent with uptake in this nephron segment. By infusing kidneys with fluorescent-labeled insulin, we were able to demonstrate simultaneous imaging of insulin uptake and mitochondrial function. For example, Cy2-insulin (green) was coimaged with the ΔΨm-dependent dye TMRM (red) (D), whereas Cy3-insulin (red) was coimaged with NADH (blue) (E and F); the apical brush-border uptake of insulin (arrowed) contrasted with the predominantly basolateral mitochondrial signals (arrow heads). Scale bars = 20 μm in all images [except (F) where scale bar = 10 μm].
The PT is responsible for the reabsorption of small proteins and other molecules from the filtrate to prevent their loss in the urine. Infusion into the IPK of either fluorescent-labeled low-molecular-weight (10 kD) dextran (a marker of fluid-phase endocytosis28) or insulin (a marker of receptor-mediated endocytosis29) led to rapid apical uptake at the PT brush border, and these signals could be coimaged with basolateral mitochondrial signals (Figure 3, C through F), highlighting the potential to make simultaneous measures of both solute transport and cellular metabolism using this approach.
Imaging of Mitochondrial Function in the Kidney during Ischemia and Reperfusion
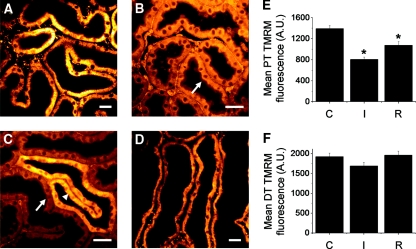
Ischemia-reperfusion (IR) is a major cause of acute kidney injury in both native and transplanted organs. The IPK has been used previously in a series of investigations on the effects of IR, which showed that the PT is particularly vulnerable.30–33 Studies using isolated tubules have shown that ΔΨm dissipates rapidly in PT mitochondria in response to ischemia;34 we demonstrated a similar response in live kidney slices, but we also showed that ΔΨm is maintained to a much greater extent in the DT. After loading tubular cells in the IPK with TMRM, we found that the fluorescence signal in PT mitochondria rapidly decreased in response to cessation of perfusion, and redistribution of the dye was observed from the mitochondrial compartment to the cytosol, consistent with dissipation of ΔΨm (Figure 4, A and B). After 30 minutes of ischemia, the mean mitochondrial TMRM signal in the PT was significantly lower than the control value–obtained preischemia (803.5 ± 48.2 versus 1392.6 ± 57.3 A.U., n = 4, P < 0.01) (Figure 4E). In contrast to the PT, the TMRM signal was maintained in mitochondria in the DT for up to 30 minutes of ischemia without visible redistribution of dye to the cytosol (Figure 4C); the mean mitochondrial signal after 30 minutes was lower than the preischemia value, but the difference was not statistically significant (1689.8 ± 91.7 versus 1921.4 ± 88.8 A.U., n = 4, P = 0.40) (Figure 4F). After 20 minutes of reperfusion postischemia, the mean mitochondrial TMRM signal in the PT (1076.2 ± 68.9) was higher than the ischemic value (P < 0.03), but not as high as the control value (P < 0.02), consistent with only partial repolarization of the organelles.
Figure 4.
Mitochondrial membrane potential is better maintained in distal tubules during ischemia than in proximal tubules. Control TMRM signal in the proximal tubules (PTs) (A) decreased rapidly in response to ischemia (B = 15 minutes, C = 20 minutes); redistribution of the dye from the mitochondrial compartment to the apical cytosol (arrowed) was observed, consistent with dissipation of ΔΨm. (C) In contrast, mitochondria in the distal tubules (DTs) remained comparatively well energized during ischemia (arrow head). After 30 minutes of ischemia the organs were reperfused; after 20 minutes the TMRM signal in PT mitochondria was increased (D), but not back to the control level. Scale bars = 20 μm. Mean mitochondrial TMRM fluorescence signal (±SEM) is depicted for PTs (E) and DTs (F); C = control, I = 30 minutes ischemia, R = 20 minutes reperfusion (n = 4, *P < 0.05 compared with control).
Imaging of Cell Structure in the Kidney during Ischemia and Reperfusion
Previous histologic35 and in vivo imaging36 studies of IR-induced acute kidney injury have described marked structural changes in the PT, and micropuncture experiments have demonstrated that accumulation of cell debris in the PT lumen can obstruct flow, causing increases in pressure and back-leak of solutes.37 This may represent an important mechanism by which an ischemic insult to renal tubules can lead to a reduction in GFR.
We investigated changes in structure in tubular cells in the IPK during IR by loading them with either calcein-AM (Figure 5A) or quinacrine. Abnormalities in cell architecture were related to alterations in mitochondrial function (ΔΨm) by coloading tubules with TMRM (Figure 5B). Within 10 minutes of the onset of ischemia, widespread apical membrane blebbing and whole cell shedding was noted in PTs, but not in DTs, leading to accumulation of intraluminal cell debris (Figure 5, C and G). Redistribution of quinacrine was observed to take place from intracellular vesicles in the PT to fragments of cell material being shed from the apical membrane (Supplemental Movie 1). Structural changes in the PT were mirrored by a reduction in ΔΨm, which was better preserved in the DT (Figure 5D). After 30 minutes of ischemia the kidneys were reperfused, after which partial repolarization of PT mitochondria was observed (Figure 5F); however, PT lumens contained large amounts of slow-moving cellular debris (Figure 5E and Supplemental Movie 2). Nuclei stained with Hoechst 33342 were observed within the intraluminal material, confirming that entire cells were shed from the PT epithelium, as well as apical fragments. Examination of PTs at higher magnification revealed evidence of tubular obstruction by intraluminal casts of debris (Supplemental Movie 3).
Figure 5.
Changes in cell structure and mitochondrial function occur in the proximal tubule during ischemia-reperfusion injury. Renal tubular cells were coloaded with calcein-AM to identify structure (A) and TMRM to measure ΔΨm (B). After 10 minutes of ischemia, marked structural changes were observed in proximal tubules (C), including apical membrane blebbing and widespread accumulation of cell debris (arrowed) in tubular lumens. These changes were mirrored by a reduction in mitochondrial TMRM signal and redistribution of the dye into the cytosol (arrowed), consistent with depolarization of mitochondria (D). In contrast, cell structure and ΔΨm were relatively well maintained in distal tubules (arrow heads) (C and D). After 30 minutes of ischemia and 20 minutes of reperfusion, intraluminal cellular debris (arrowed) remained clearly visible (E), but there was evidence of partial re-energization of mitochondria in proximal tubules (F). (G) An example image of NADH autofluorescence during ischemia is depicted, showing bright signal in the basolateral mitochondria (arrow head) and apical shedding of whole cells into the tubular lumen (arrowed).
In summary, the IPK provides a useful model to examine the effects of IR on tubular cell structure and function, in an intact working organ, and make direct comparisons between different tubule types.
DISCUSSION
We have demonstrated for the first time that multiphoton imaging of the IPK allows detailed study of cell structure and physiology in an intact functioning organ. The advantages of this technique (compared with an in vivo approach) include the stability of the preparation, greater control over variables such as perfusion pressure and dye concentration, and superior intracellular dye loading (achieved using a recirculating perfusion system). This experimental model can be applied (1) to make side-by-side comparisons of different cell types, (2) to relate cellular function to overall organ function, and (3) to follow changes in real time in models of renal injury. For example, we have shown that ROS levels in the kidney, measured using HEt, are higher in the PT than in the DT, which may be important in understanding their respective vulnerabilities to oxidative stress. We have also demonstrated that mitochondrial signals in the PT can be co-imaged with uptake of fluorescently labeled low-molecular-weight proteins, highlighting the potential to make simultaneous measures of solute transport and cellular metabolism. Lastly, we have shown that marked changes take place in cell structure and mitochondrial function in the PT during ischemic acute kidney injury.
The IPK represents a useful model for investigating the effects of IR. Shortly after the onset of ischemia, we observed structural changes in PT cells that were associated with dissipation of ΔΨm in mitochondria. PT cells contain an extensive apical cytoskeleton as part of their endocytotic machinery, and it has been suggested that the apical structural changes that take place during ischemia are due to impairment of normal actin polymerization.38 Infusion into the IPK of cytochalasin D (which inhibits actin polymerization) causes apical membrane blebbing analogous to the effects of ischemia, and it also leads to a reduction in tubular function and GFR.39 Actin polymerization is an ATP-dependent process; the PT has limited capacity for anaerobic glycolysis,40 so ischemia is likely to cause rapid falls in intracellular ATP levels. This could explain the early loss of normal cytoskeletal architecture in the PT, and also the inability to maintain ΔΨm (by ATP hydrolysis and reverse activity of the ATP synthase). After 20 minutes of reperfusion, ΔΨm increased in PT mitochondria, but not back to the control level, suggesting ongoing energetic defects in this nephron segment, as described previously in isolated PTs exposed to IR.34 We observed cell nuclei in tubular lumens after IR, suggesting that whole epithelial cells may have been shed; interestingly, previous studies of humans and rabbits with acute kidney injury have suggested that increased numbers of viable epithelial cells are present in excreted urine.41
Although we were able to demonstrate loading of a number of different exogenous fluorophores in tubular cells, we were unable to do so with Ca2+-sensitive dyes for reasons that are currently unclear, but which may include the effects of extracellular esterases that cleave the dyes from their AM-ester forms before they can enter cells5 (although it is notable that calcein-AM seems to load well into PTs). This remains a significant technical challenge, which is not specific to the IPK preparation, as we have encountered similar problems in previous work with kidney slices. Localized loading of rhod-2-AM has been reported in tubular cells in vivo after subcapsular injection.4 In the future, technological advances such as transgenic animals selectively expressing Ca2+-sensitive fluorescence molecules in tissues of interest may render the usage of exogenous dyes obsolete,42 but it is likely to be some time before this newer technology is widely available. Transfection of cDNA encoding fluorescence proteins into kidney cells has been achieved in vivo using micropuncture of the renal tubule with direct infusion of the construct and a viral vector into the tubular lumen,43 but this approach has not been widely adopted. In the meantime, the development of strategies to improve exogenous dye loading remains crucial if the full potential of multiphoton microscopy to visualize intracellular processes is to be realized in intact tissues.
There are some established weaknesses of the IPK model compared with the in vivo situation,44 which also need to be considered. Although large amounts of solute transport occur in the IPK, the fractional excretion of sodium is higher than in vivo, and resistance to arterial flow tends to decrease over time. Furthermore, the IPK is dennervated and it is no longer exposed to circulating hormones and cytokines that regulate kidney function in a whole organism (although this can be an advantage with fewer confounding factors). However, the disadvantages of the IPK as a research model have to be weighed against its advantages, which are essentially that it combines some of the benefits of both in vivo (i.e., preserved organ architecture and function) and in vitro (i.e., superior dye loading) approaches. Perfusate composition and flow can be tightly controlled and rapidly changed, and dyes and reagents can be infused directly into the organ at known concentrations, bypassing the general circulation. Although the useful experimental life of IPKs is limited, partly because the medulla tends to become increasingly ischemic,45 it is worth noting that superficial PT function (the focus of this work) is generally well preserved.44
In summary, multiphoton microscopy of the IPK provides a powerful tool for detailed imaging of cell structure and function in an intact organ. We believe that this complementary approach will be extremely useful for studying mechanisms of kidney disease.
CONCISE METHODS
Materials
Calcein-AM, HEt, MCB, rhod-2-AM, rhodamine-labeled dextran (10 kD), and TMRM were all obtained from Molecular Probes, Invitrogen (Paisley, U.K.). FITC-labeled dextran (100 kD), Hoechst 33342, and quinacrine were obtained from Sigma-Aldrich (Poole, Dorset, U.K.). Cy2- and Cy3-labeled insulins were kindly provided by Dr. N. Carvou (University College London, U.K.).
The Isolated Perfused Kidney
All procedures were carried out in accordance with the animals (Scientific Procedures) Act 1986. Adult male Sprague-Dawley rats (weighing 150 to 350 g) were anesthetized with intraperitoneal pentobarbitone (50 to 100 mg/kg). The right renal artery was cannulated via the superior mesenteric artery using an established technique.46 Briefly, after a midline incision, the aorta, vena cava, superior mesenteric artery, and right renal and adrenal arteries were identified. The right adrenal artery was ligated, and a cannula was inserted into the superior mesenteric artery and advanced across the aorta and into the right renal artery. Perfusion was commenced immediately with a HEPES-buffered solution at 37°C (gassed with 95% oxygen and 5% carbon dioxide) containing (in mM) NaCl (118), NaHCO3 (10), KCl (4.7), MgSO4 (1.44), KH2PO4 (1.2), CaCl2 (1.8), HEPES (10), glucose (5), pyruvate (5), lactate (2.5), and butyrate (2.5). Once the cannula was secured in place, the aorta and vena cava were severed and the preparation was quickly moved to a custom-built imaging chamber. To induce hypoxia in the IPK, the perfusate was gassed with nitrogen instead of oxygen. To model IR, the perfusion pump was stopped for 30 minutes and then restarted.
Dye Loading and Microscope Settings
Dyes were loaded for 20 to 30 minutes at 37°C, using a recirculating perfusion system. Kidneys were imaged using a Zeiss LSM 510 NLO axiovert microscope, coupled to a tunable Coherent Chameleon laser, and light emitted from the specimen was detected using either internal or external (nondescanned) detectors. In a minority of experiments, single-photon excitation was used in addition to the multiphoton laser to allow simultaneous imaging of two dyes with widely differing multiphoton excitation wavelengths. Calcein-AM was used at a concentration of 2.5 μg/ml (with 0.002% pluronic) and was excited at 800 nm. Cy2-insulin was used at 100 nM and excited at either 488 nm (single photon) or 850 to 900 nm (multiphoton), whereas Cy3-insulin was used at 250 nM and was excited at either 514 nm (single photon) or 940 to 1000 nm (multiphoton). FITC-labeled dextran (100 kD) was used at 1 mg/ml and excited at 800 to 850 nm. HEt and Hoechst 33342 were both used at a concentration of 5 μM and MCB was used at a concentration of 50 μM; fluorescence from all three dyes was excited at 700 to 720 nm, along with mitochondrial NADH. Quinacrine was loaded at 1 mM and excited at either 700 to 720 nm or 840 to 860 nm. Rhodamine-labeled dextran (10 kD) was used at 0.03 mg/ml and excited at 850 nm. Rhod-2-AM was loaded at a concentration of 2.5 μg/ml (with 0.002% pluronic) and was excited at 850 to 900 nm. TMRM was used at a concentration of 50 nM and excited at 800 to 860 nm; 10 μM verapamil was used to inhibit dye export from the cells by the multidrug resistance transporter (p-glycoprotein). Emitted light was collected with the following filters: band pass 435 to 485 nm (Hoechst 33342, MCB, and NADH), band pass 500 to 550 nm (calcein-AM, Cy2-insulin, FITC, and quinacrine), and band pass 575 to 640 nm (Cy3-insulin, HEt, rhodamine, rhod-2-AM, and TMRM).
Image Analysis and Statistics
Image processing was performed using Zeiss LSM software (Carl Zeiss Ltd., Welwyn Garden City, Hertfordshire, U.K.) and Image J (National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/). To quantify fluorescence signals, regions of interest were drawn around tubules in a minimum of three different fields (imaged using a ×40 objective), and experiments were repeated in at least three separate kidneys. All values given are the mean signal (±SEM), per image pixel, within the relevant region of interest. For experiments involving HEt, regions of interest were drawn around the nuclei, where the bulk of the signal change was observed. For experiments involving TMRM, the mitochondrial signal was isolated by using a lower threshold limit to remove background cytosolic signal. One-way ANOVA was used to investigate statistical differences among study groups; when differences were found, individual groups were compared with each other using the two-sample t test.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
Funding for this work was provided by grants awarded by Kidney Research UK and The UK Medical Research Council to A.M.H. and C.M.P., respectively. The authors are grateful to Dr. John Haylor, Sheffield Kidney Institute, U.K., for advice on the IPK model.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Molitoris BA, Sandoval RM: Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Renal Physiol 288: F1084–F1089, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Sipos A, Toma I, Kang JJ, Rosivall L, Peti-Peterdi J: Advances in renal (patho)physiology using multiphoton microscopy. Kidney Int 72: 1188–1191, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Molitoris BA, Sandoval RM: Techniques to study nephron function: Microscopy and imaging. Pflugers Arch 458: 203–209, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Peti-Peterdi J, Toma I, Sipos A, Vargas SL: Multiphoton imaging of renal regulatory mechanisms. Physiology (Bethesda) 24: 88–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jobsis PD, Rothstein EC, Balaban RS: Limited utility of acetoxymethyl (AM)-based intracellular delivery systems, in vivo: Interference by extracellular esterases. J Microsc 226: 74–81, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall AM, Unwin RJ, Parker N, Duchen MR: Multiphoton imaging reveals differences in mitochondrial function between nephron segments. J Am Soc Nephrol 20: 1293–1302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blantz RC, Deng A, Miracle CM, Thomson SC: Regulation of kidney function and metabolism: A question of supply and demand. Trans Am Clin Climatol Assoc 118: 23–43, 2007 [PMC free article] [PubMed] [Google Scholar]
- 8. Mayevsky A, Chance B: Oxidation-reduction states of NADH in vivo: From animals to clinical use. Mitochondrion 7: 330–339, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Piston DW, Knobel SM: Real-time analysis of glucose metabolism by microscopy. Trends Endocrinol Metab 10: 413–417, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Bratosin D, Mitrofan L, Palii C, Estaquier J, Montreuil J: Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. Cytometry A 66: 78–84, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH: Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol 287: F329–F335, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R: Exocytotic release of ATP from cultured astrocytes. J Biol Chem 282: 28749–28758, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J: The collecting duct is the major source of prorenin in diabetes. Hypertension 51: 1597–1604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicholls DG: Mitochondrial membrane potential and aging. Aging Cell 3: 35–40, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Duchen MR, Surin A, Jacobson J: Imaging mitochondrial function in intact cells. Methods Enzymol 361: 353–389, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Madsen KM, Tisher CC: Structural-functional relationship along the distal nephron. Am J Physiol 250: F1–F15, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Lash LH: Role of glutathione transport processes in kidney function. Toxicol Appl Pharmacol 204: 329–342, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Brezis M, Rosen S, Silva P, Epstein FH: Selective glutathione depletion on function and structure of the isolated perfused rat kidney. Kidney Int 24: 178–184, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Trollinger DR, Cascio WE, Lemasters JJ: Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem Biophys Res Commun 236: 738–742, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Laube GF, Shah V, Stewart VC, Hargreaves IP, Haq MR, Heales SJ, Van't Hoff WG: Glutathione depletion and increased apoptosis rate in human cystinotic proximal tubular cells. Pediatr Nephrol 21: 503–509, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Forbes JM, Coughlan MT, Cooper ME: Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Gobe G, Crane D: Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol Lett 2010 [DOI] [PubMed] [Google Scholar]
- 23. Murphy MP: How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gill PS, Wilcox CS: NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Bindokas VP, Jordan J, Lee CC, Miller RJ: Superoxide production in rat hippocampal neurons: Selective imaging with hydroethidine. J Neurosci 16: 1324–1336, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP: Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Zou AP, Li N, Cowley AW, Jr.: Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Gekle M, Mildenberger S, Freudinger R, Silbernagl S: Endosomal alkalinization reduces Jmax and Km of albumin receptor-mediated endocytosis in OK cells. Am J Physiol 268: F899–F906, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Rabkin R, Yagil C, Frank B: Basolateral and apical binding, internalization, and degradation of insulin by cultured kidney epithelial cells. Am J Physiol 257: E895–E902, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Shanley PF, Rosen MD, Brezis M, Silva P, Epstein FH, Rosen S: Topography of focal proximal tubular necrosis after ischemia with reflow in the rat kidney. Am J Pathol 122: 462–468, 1986 [PMC free article] [PubMed] [Google Scholar]
- 31. Shanley PF, Brezis M, Spokes K, Silva P, Epstein FH, Rosen S: Hypoxic injury in the proximal tubule of the isolated perfused rat kidney. Kidney Int 29: 1021–1032, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Shanley PF, Brezis M, Spokes K, Silva P, Epstein FH, Rosen S: Transport-dependent cell injury in the S3 segment of the proximal tubule. Kidney Int 29: 1033–1037, 1986 [DOI] [PubMed] [Google Scholar]
- 33. Heyman SN, Rosen S, Epstein FH, Spokes K, Brezis ML: Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int 45: 981–985, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I: Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A 97: 2826–2831, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donohoe JF, Venkatachalam MA, Bernard DB, Levinsky NG: Tubular leakage and obstruction after renal ischemia: structural-functional correlations. Kidney Int 13: 208–222, 1978 [DOI] [PubMed] [Google Scholar]
- 36. Ashworth SL, Sandoval RM, Tanner GA, Molitoris BA: Two-photon microscopy: Visualization of kidney dynamics. Kidney Int 72: 416–421, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Tanner GA, Sophasan S: Kidney pressures after temporary renal artery occlusion in the rat. Am J Physiol 230: 1173–1181, 1976 [DOI] [PubMed] [Google Scholar]
- 38. Molitoris BA: Actin cytoskeleton in ischemic acute renal failure. Kidney Int 66: 871–883, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Kellerman PS, Clark RA, Hoilien CA, Linas SL, Molitoris BA: Role of microfilaments in maintenance of proximal tubule structural and functional integrity. Am J Physiol 259: F279–F285, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Bagnasco S, Good D, Balaban R, Burg M: Lactate production in isolated segments of the rat nephron. Am J Physiol 248: F522–F526, 1985 [DOI] [PubMed] [Google Scholar]
- 41. Racusen LC: Epithelial cell shedding in acute renal injury. Clin Exp Pharmacol Physiol 25: 273–275, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, Sanbe A, Gulick J, Mathai J, Robbins J, Salama G, Nakai J, Kotlikoff MI: Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci U S A 103: 4753–4758, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanner GA, Sandoval RM, Molitoris BA, Bamburg JR, Ashworth SL: Micropuncture gene delivery and intravital two-photon visualization of protein expression in rat kidney. Am J Physiol Renal Physiol 289: F638–F643, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Maack T: Physiological evaluation of the isolated perfused rat kidney. Am J Physiol 238: F71–F78, 1980 [DOI] [PubMed] [Google Scholar]
- 45. Brezis M, Rosen S, Silva P, Epstein FH: Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J Clin Invest 73: 182–190, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Douthwaite JA, Johnson TS, Haylor JL, Watson P, El Nahas AM: Effects of transforming growth factor-beta1 on renal extracellular matrix components and their regulating proteins. J Am Soc Nephrol 10: 2109–2119, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.