Abstract
There is a significant immune response to ischemia-reperfusion injury (IRI), but the role of immunomodulatory natural killer T (NKT) cell subtypes is not well understood. Here, we compared the severity of IRI in mice deficient in type I/II NKT cells (CD1d−/−) or type I NKT cells (Jα18−/−). The absence of NKT cells, especially type II NKT cells, accentuated the severity of renal injury, whereas repletion of NKT cells attenuated injury. Adoptively transferred NKT cells trafficked into the tubulointerstitium, which is the primary area of injury. Sulfatide-induced activation of type II NKT cells protected kidneys from IRI, but inhibition of NKT cell recruitment enhanced injury. In co-culture experiments, sulfatide-induced activation of NKT cells from either mice or humans attenuated apoptosis of renal tubular cells after transient hypoxia via hypoxia-inducible factor (HIF)-1α and IL-10 pathways. Renal tissue of patients with acute tubular necrosis (ATN) frequently contained NKT cells, and the number of these cells tended to negatively correlate with ATN severity. In summary, sulfatide-reactive type II NKT cells are renoprotective in IRI, suggesting that pharmacologic modulation of NKT cells may protect against ischemic injury.
Acute kidney injury may predispose the high morbidity and mortality of patients and can induce the progressive loss of kidney function.1 Ischemia-reperfusion injury (IRI), a major cause of acute kidney injury, is characterized by the participation of a prominent inflammatory response. Various components of the innate and adaptive immune systems are known to play a role in kidney IRI.2–4 Therefore, immunomodulatory and anti-inflammatory measures for IRI were evaluated.5,6 Recently, the role of Foxp3+CD4+CD25+ regulatory T cells (Tregs) in IRI were exploited, and Tregs revealed the protective activity against IRI by ischemic preconditioning and by promoting tubular repair.7,8
Natural killer T (NKT) cells, expressing both T cell receptors (TCRs) and natural killer cell receptors, have been reported to regulate autoimmune disease and allogeneic immune response.9–11 However, only a few reports have investigated the role of NKT cells in kidney IRI, despite the fact that these cells are known to traffic into postischemic kidneys as early as 3 hours after IRI.12,13 NKT cells have been proposed as a bridge between the innate and adaptive immune systems.14–16 NKT cell recruitment into the site of injury was directed by the interaction between CXCL16 and CXCR6, which is expressed on NKT cells and activated Th1 cells.17 Previously, we have presented that glomerulus-expressed CXCL16 upon the stimuli in experimental crescentic glomerulonephritis and the blocking of CXCL16 decreased NKT cell recruitment.18 Most NKT cells recognize antigens presented by CD1d, and CD1d is expressed on dendritic cells, macrophages, and various other cell types.14,19,20 CD1d-restricted NKT cells can be broadly categorized into type I and type II NKT cells. Type I NKT cells recognizes glycolipid antigen through an invariant TCR that is encoded by the Vα14Jα218 gene in mice and the Vα24Jα18 gene in humans.16 Stimulation of type I NKT cells by α-galactosylceramide results in cytokine secretion, TCR downregulation, and apoptosis. Depending on the surrounding milieu and the antigens involved, the type I NKT cells may demonstrate either the Th1-type or the Th2-type cytokine secretion profile.19 In animal models, NKT cells (mainly type I) have been reported to affect the development and progression of diabetes mellitus, experimental autoimmune encephalitis (EAE), experimental anterior uveitis, and systemic lupus erythematosus.21–24 Type II NKT cells have variable TCR-V gene rearrangements that are distinct from those of type I NKT cells.16 Type II NKT cells recognize the self-glycolipid 3-sulfated galactosylceramide (sulfatide) and can be identified by sulfatide/CD1d-tetramers.25 Although type II NKT cells have not been studied extensively, the activation of type II NKT cells by sulfatide prevents EAE and concanavalin A-induced hepatitis.25,26 Type II NKT cells may regulate type I NKT cells, inhibit effector functions of conventional T cells, and modify dendritic cell functions.26
On the basis of the knowledge of the robust inflammatory process that occurs in postischemic kidneys after IRI and the regulatory functions of sulfatide-reactive type II NKT cell, we hypothesized that NKT cells may modulate the initial injury process in postischemic kidneys. We compared renal functional and structural injury status in a mouse IRI model in which NKT cells were manipulated via in vivo and ex vivo activation, depletion, or blocking. Then we analyzed the effect of NKT cell manipulation on the changes of histology and of molecules that may be involved.
RESULTS
Sulfatide-reactive NKT Cells Attenuate Ischemia-Reperfusion Injury
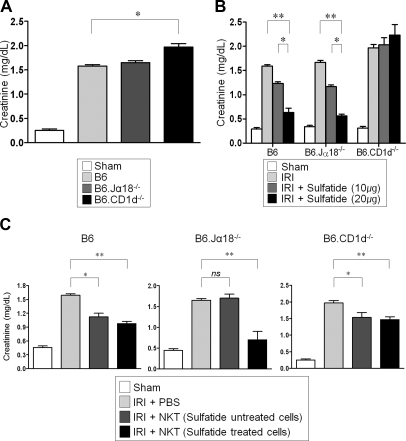
To determine the specific role of sulfatide-reactive NKT cells on IRI in the kidney, renal ischemia was induced. Renal function deteriorated substantially after IRI (sham control versus disease control; Cr mg/dl [μmol/L]; 0.46 ± 0.03 [40.66 ± 2.652] versus 1.59 ± 0.03 [140.56 ± 2.652]; P < 0.05). In B6.Jα18−/− mice, which are deficient in type I NKT cells, the severity of renal dysfunction was similar to that of wild-type B6 mice (Cr 1.67 ± 0.04 mg/dl [147.63 ± 3.536 μmol/L]). However, in B6.CD1d−/− mice (deficient for both type I and type II NKT cells), severe renal dysfunction occurred (Cr 1.98 ± 0.07 mg/dl [175.03 ± 6.188 μmol/L]; P < 0.05 compared with B6 disease control) (Figure 1A). The in vivo activation of type II NKT cells by sulfatide administration lessened the degree of IRI in B6 as well as in B6.Jα18−/− mice in a dose-dependent manner. However, sulfatide was not effective in B6.CD1d−/− mice (Figure 1B). To verify the reno-protective effects of sulfatide-reactive NKT cells, we adoptively transferred NKT cells with ex vivo activation to B6 mice and B6.Jα18−/− mice. The adoptive transfer of NKT cells, especially sulfatide-activated cell, induced significant renal protection against IRI in the both mice (Figure 1C).
Figure 1.
Sulfatide-reactive NKT cells protected kidney from ischemia-reperfusion injury. (A) Renal function was deteriorated substantially after IRI. In B6.Jα18−/− mice, the severity of renal dysfunction was similar to wild-type mice, but B6.CD1d−/− mice revealed severe dysfunction. (B) Type II NKT-cell activation by sulfatide attenuated the degree of IRI in B6 as well as in B6.Jα18−/− mice in a dose-dependent manner. However, it was not effective in B6.CD1d−/− mice. (C) Adoptive transfer of sulfatide-activated NKT cells protected the kidney from IRI, especially when the transferred cells were preactivated by sulfatide (n = 6/group at each experiment; ns, not significant; *P < 0.05; **P < 0.01).
Changes in the Cytokine Milieu and the Effect of NKT Cell Activation
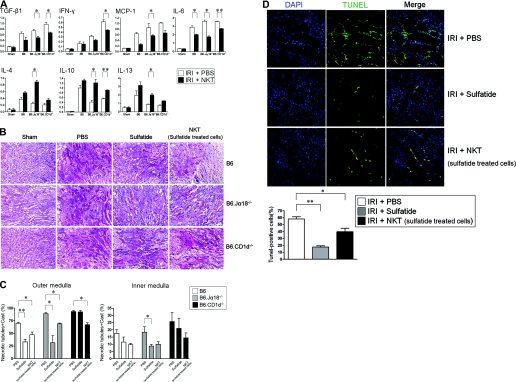
To examine the expression patterns of cytokines that may affect the extent of renal damage by IRI, we quantified mRNA levels using real-time PCR 1 day after IRI. The proinflammatory cytokines, such as TGF-β, IFN-γ, monocyte chemotactic protein-1, and IL-6, were upregulated by IRI in B6 and NKT cell-deficient mice. However, after the adoptive transfer of sulfatide-activated NKT cells before IRI, the upregulation of proinflammatory cytokines was significantly suppressed. Although IRI induced the upregulation of regulatory cytokines (IL-4, IL-10, and IL-13), the sulfatide-activated NKT cell transfer enhanced the expression of these cytokines in mice rendered IRI (Figure 2A).
Figure 2.
Changes in cytokines, renal histology, and apoptosis induced by the activation of natural killer T (NKT) cells. (A) The expression patterns of intrarenal cytokines were significantly altered by the adoptive transfer of sulfatide-activated NKT cells (n = 6/group at each experiment; *P < 0.05; **P < 0.01). (B and C) Ischemia-reperfusion injury (IRI) induced tubular necrosis. The damage was more extensive in B6.Jα18−/− and B6.CD1d−/− mice (second column). Administration of sulfatide reduced the necrotic changes significantly in B6 and B6.Jα18−/− mice but not in B6.CD1d−/− mice (third column). The adoptive transfer of sulfatide-activated NKT cells attenuated the ischemic damage in mice (fourth column) (magnification, ×200). The histologic changes were quantified in blinded way by a renal pathologist. Outer medulla area was the main site that showed significant protection by sulfatide activation (*P < 0.05; **P < 0.01). (D) IRI on B6.Jα18−/− mice revealed prominent apoptosis assessed by TUNEL staining, but administration of sulfatide markedly blocked apoptosis process. For each experimental condition, six visual fields were randomly selected for counting the surviving cells with normal nuclear morphology. (*P < 0.05; **P < 0.01; magnification, ×400).
The histologic changes were very consistent with the functional changes. IRI induced tubular necrosis, which involved disruption and sloughing of tubular epithelial cells. The damage was more extensive in B6.Jα18−/− and B6.CD1d−/− mice than in B6 mice. However, the administration of sulfatide reduced the severity of the pathologic changes that occurred after IRI in B6 and B6.Jα18−/− mice, but not in B6.CD1d−/− mice. Again, the adoptive transfer of sulfatide-activated NKT cells attenuated the ischemic changes (Figure 2B), irrespective of mouse strains. Tubular necrosis was quantified in blinded way. As shown in Figure 2C, the activation of NKT cells significantly protected tubular necrosis especially in the outer medulla area.
When IRI was induced in B6.Jα18−/− mice, the degree of apoptosis was found to be prominent by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining. However, the in vivo administration of sulfatide or adoptive transfer of sulfatide-activated NKT cells markedly inhibited apoptosis after IRI in these mice (Figure 2D).
Active Participation of NKT Cells at the Site of Ischemia
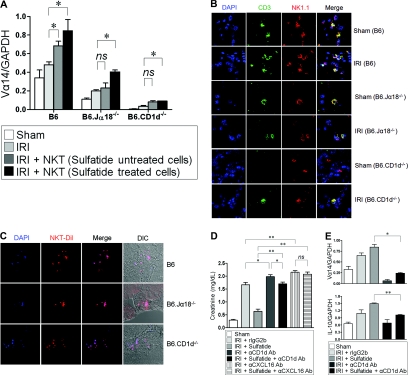
Next, we assessed the notion that NKT cells were involved at the site of inflammation induced by IRI. After IRI, NKT cells were recruited to the kidney, as evidenced by the upregulation of Vα14 mRNA expression. In addition, sulfatide-activated NKT cell transfer enhanced Vα14 mRNA expression in the kidneys (Figure 3A). The number of NK1.1+CD3+ cells increased in all mice after IRI compared with the sham controls as traced by confocal microscopy. However, the number of NK1.1+CD3+ cells was obviously lower in B6.Jα18−/− and in B6.CD1d−/− than in wild-type mice (Figure 3B). NKT cell recruitment after IRI was further confirmed using fluorescently-tagged, sorted NKT cells. When fluorescently-tagged cells were introduced via the tail vein after IRI, the transferred cells were detectable in tubulointerstitial area (Figure 3C).
Figure 3.
Recruitment of natural killer T (NKT) cells to the site of injury. (A) Vα14 mRNA expression was upregulated after ischemia-reperfusion injury (IRI). NKT cell transfer after sulfatide activation enhanced the upregulation of intrarenal Vα14 mRNA expression more (n = 6/each group; ns, not significant; *P < 0.05). (B) Trafficking of NKT cells was traced. The number of NK1.1+CD3+ cells was increased after IRI compared with sham controls (magnification, ×800). (C) CM-DiI-tagged NKT cells were transferred into mice via tail vein after IRI. The transferred NKT cells were detectable at tubulointerstitial area (magnification, ×800). DIC, differential interference contrast. (D) The severity of renal injury was significantly reduced by the administration of sulfatide compared with disease control mice, but the beneficial effect of sulfatide disappeared by injection of anti-CD1d Ab (300 μg/mouse) or anti-CXCL16 Ab (500 μg/mouse) (n = 6/each group; *P < 0.05; **P < 0.01). (E) The blocking of cellular trafficking was assessed by Vα14 mRNA expression. Administration of anti-CD1d Ab significantly suppressed intrarenal expression of Vα14. Vα14 mRNA expression was not recovered by the administration of sulfatide (upper panel). Sulfatide administration enhanced the expression of IL-10 after IRI, but it was downregulated by the blocking of NKT cell recruitment regardless of cellular activation (lower panel) (*P < 0.05). DAPI, 4',6'diamino-2-phenylindole-2HCl; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The degree of IRI was drastically decreased by the administration of sulfatide, but this beneficial effect disappeared when NKT recruitment was blocked. When anti-CD1d Ab or anti-CXCL16 Ab were administered after IRI, the protective effect of NKT-cell activation by sulfatide was marginal or abolished (Figure 3D). In line with the functional derangement described above, ischemic necrosis was worsened when NKT cell recruitment was blocked (Supplemental Figure 1). The blocking of cellular trafficking was further confirmed by Vα14 mRNA expression. By the administration of anti-CD1d Ab, the intrarenal expression of Vα14 was almost completely inhibited and could not be restored by the administration of sulfatide. IL-10 expression was enhanced by sulfatide-induced activation of NKT cells, but it was downregulated by the blocking of NKT cell recruitment, regardless of cellular activation (Figure 3E).
Cellular and Molecular Mechanisms of the Protective Effect of Sulfatide-reactive NKT Cells against IRI
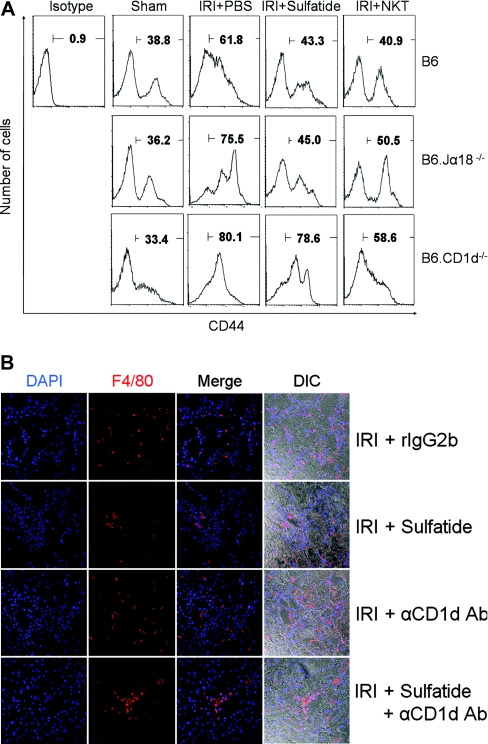
In sham-operated mice (B6), CD44+ T cells constituted 36 to 38% of the intrarenal mononuclear cells, but IRI induced the increment of intrarenal T cell population. In vivo administration of sulfatide significantly reduced the number of T cells in wild-type and B6.Jα18−/− mice but not in B6.CD1d−/− mice. Again, the adoptive transfer of NKT cells reduced the intrarenal T cell population (Figure 4A). The intrarenal infiltration of inflammatory cells showed kinetics similar to that of T cells. Macrophage infiltration, which was prominent after IRI, was markedly inhibited by in vivo administration of sulfatide. However, the blocking of NKT cell recruitment by an anti-CD1d Ab induced a prominent macrophage infiltration (Figure 4B).
Figure 4.
Effect of natural killer T (NKT)-cell activation on cellular recruitment. (A) In sham controls (B6), CD44+T cells were 36 to 38% of intrarenal mononuclear cells. Ischemia-reperfusion injury (IRI) increased the number of intrarenal T cells. In vivo administration of sulfatide reduced the number of CD44+T cells after IRI in wild-type and B6.Jα18−/− mice but not in B6.CD1d−/− mice. The adoptive transfer of NKT cells reduced the intrarenal T cell population. (B) Macrophage (F4/80+) infiltration was prominent after IRI (first row) and was markedly inhibited by in vivo administration of sulfatide (second row). The blocking of NKT cell recruitment by anti-CD1d Ab induced prominent macrophage infiltration regardless of sulfatide administration (third and fourth row) (magnification, ×200). DIC, differential interference contrast.
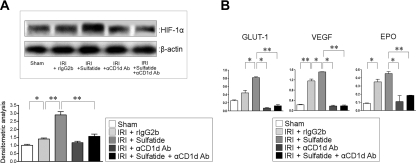
The molecular mechanism of the beneficial effect of sulfatide-reactive NKT cells was probed in the context of HIF pathway. HIF-1α expression was enhanced by IRI, and NKT-cell activation by sulfatide further increased HIF-1α expression. By the administration of anti-CD1d Ab, HIF-1α expression was downregulated irrespective of NKT-cell activation (Figure 5A). The target genes of HIF-1α showed similar patterns of expression. Glucose transporter-1 (GLUT-1), vascular endothelial growth factor (VEGF), and erythropoietin (EPO) were upregulated by IRI and were further enhanced by sulfatide administration, but the inhibition of NKT cell recruitment decreased the expression levels of target genes (Figure 5B).
Figure 5.
Changes of HIF-1 and its target genes in ischemia-reperfusion injury (IRI). (A) HIF-1α expression was enhanced by IRI, and sulfatide administration after IRI increased HIF-1α expression further. Administration of anti-CD1d Ab reduced HIF-1α expression irrespective of sulfatide administration (*P < 0.05; **P < 0.01). (B) GLUT-1, VEGF, and EPO were upregulated by IRI and were further enhanced by sulfatide administration. NKT cell blocking by anti-CD1d Ab diminished the expression of the target genes (*P < 0.05; **P < 0.01).
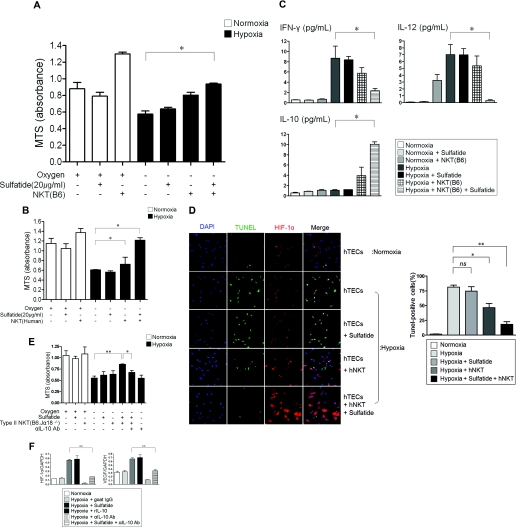
Activation of NKT Cells Prevents Hypoxic Damage in Tubular Epithelial Cells (TECs) via HIF-1 and IL-10 Pathways
The physiologic role of sulfatide-reactive NKT cells was further evaluated using an in vitro cell culture system. Mouse TECs and NKT cells were grown separately using the TranswellTM system. TECs proliferated well under normoxic conditions, and their proliferation was significantly hampered under hypoxic conditions, but sulfatide-activated NKT cells partially restored the proliferation of TECs (Figure 6A). The changes of various cytokine expression were measured using the Bio-Plex method. IFN-γ and IL-12, which were upregulated by hypoxia, were downregulated by sulfatide-activated NKT cells, but the concentration of IL-10 was increased by NKT-cell activation (Figure 6B). Under hypoxia, human TECs showed reduced proliferation. However, sulfatide-induced activation of sorted human NKT cells restored their proliferative capacity to a level similar to that observed under normoxia (Figure 6C). Like in a murine in vitro system, human NKT-cell activation resulted in downregulation of proinflammatory cytokines (such as IL-8 and IL-6) and upregulation of IL-10 (Supplemental Figure 2).
Figure 6.
Effect of NKT-cell activation on hypoxia. (A) Mouse TECs (3×104/well) and NKT cells (6×104/well) were grown separately in a TranswellTM system. Hypoxia hampered cell proliferation measured by MTS assay, but sulfatide-induced activation of natural killer T (NKT) cells significantly rescued cell proliferation in hypoxic condition (*P < 0.05). (B) Sulfatide-induced activation of NKT cells differentially regulated the expression of various cytokines measured using the Bio-Plex method. IFN-γ and IL-12, which were upregulated by hypoxia, were downregulated by sulfatide-activated NKT cells, but the concentration of IL-10 was increased by NKT-cell activation. (C) Sulfatide-induced activation of human NKT cells rescued human TEC proliferation under hypoxic condition (*P < 0.05). (D) Apoptosis of human TECs was assessed by TUNEL method. Hypoxia induced the apoptosis of human TECs, but the activation of NKT cells by sulfatide reduced the apoptosis with the enhancement of HIF-1α (magnification, ×600; *P < 0.05; **P < 0.01). (E) Activated type II NKT cells rescued cell proliferation of mouse TECs under hypoxic conditions, but the rescued cell proliferation was hampered by the blocking of IL-10 signaling (*P < 0.05; **P < 0.01). (F) DN32.D3, murine NKT cell line, were activated by sulfatide in the presence and absence of IL-10 signals. Enhanced expression of HIF-1α and VEGF was suppressed by the blocking of IL-10 signaling (*P < 0.05; **P < 0.01). ns, not significant.
The crosstalk between NKT cells and HIF-1α was further delineated. When human TECs were exposed to hypoxia, the apoptotic process progressed with little change in HIF-1α expression. The sulfatide-induced activation of human NKT cells, however, prevented hypoxic TECs from undergoing apoptosis with an increase in the expression level of HIF-1α (Figure 6D). The role of IL-10 pathway in NKT cell was also further investigated. Sulfatide-activated type II NKT cells (sorted from B6.Jα18−/− mice) rescued TEC proliferation under hypoxia, but with the use of blocking anti-IL-10 antibody (Ab), the protective capacity of NKT cells was abolished (Figure 6E). The relationship between HIF and IL-10 was further examined using a mouse cell line. DN32.D3, a murine NKT cell line, showed the significant upregulation of HIF-1α after sulfatide activation or by rIL-10 under hypoxic conditions. Again, IL-10 blocking using an anti-IL-10 Ab abolished the upregulation of HIF-1α that was induced by sulfatide activation (Figure 6F, left panel). VEGF, one of the target genes of HIF-1α, showed expression patterns similar to those of HIF-1α (Figure 6F, right panel).
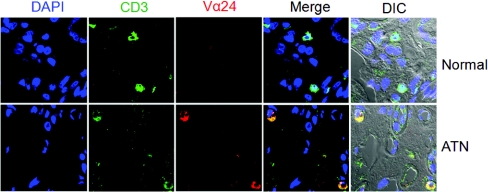
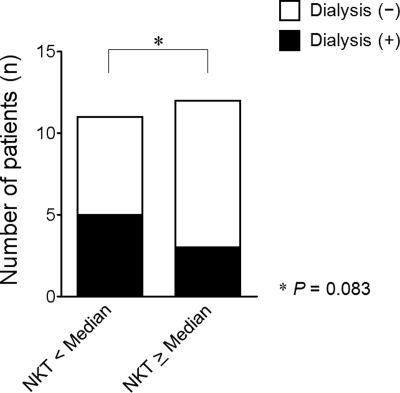
Finally, we investigated the role of NKT cells in human disease. Very few NKT cells (CD3+Vα24+) were found in normal human kidney specimens, but in kidney biopsy tissues from patients with acute tubular necrosis (ATN), NKT cells were observed with an increased frequency (Figure 7). The number of NKT cells observed in human renal tissue varied according to the severity of ATN. In patients with severe renal dysfunction, for whom renal replacement therapy was instituted, NKT cells were found less frequently than in patients for whom renal replacement therapy was not necessary (Table 1 and Figure 8).
Figure 7.
Presence of NKT cells in human renal tissues of acute tubular necrosis (ATN). CD3 and Vα24 were traced by confocal microscopy (magnification ×800). CD3+Vα24+ cell were more frequently found in patients with ATN. DIC, differential interference contrast.
Table 1.
Acute tubular necrosis and natural killer T (NKT) cell infiltration in human kidney
| Case | Age | Gender | Comorbid Condition | RRT | Number of NKT Cellsa |
|---|---|---|---|---|---|
| 1 | 48 | Male | Diabetic nephropathy | No | 2 |
| 2 | 63 | Female | Minimal change disease | Yes | 3 |
| 3 | 80 | Male | Minimal change disease | No | 3 |
| 4 | 72 | Male | Diabetes mellitus | Yes | 3 |
| 5 | 46 | Female | Sepsis | Yes | 4 |
| 6 | 41 | Female | Immune mediated glomerulonephritis | No | 5 |
| 7 | 76 | Female | Hypertension | Yes | 6 |
| 8 | 37 | Female | Thrombotic microangiopathy | No | 7 |
| 9 | 46 | Female | Hepatitis A | Yes | 8 |
| 10 | 31 | Female | Hypercalcemia | No | 9 |
| 11 | 18 | Female | Focal segmental glomerulosclerosis | No | 9 |
| Calcineurin inhibitor toxicity | |||||
| 12 | 25 | Female | None | No | 11 |
| 13 | 37 | Male | Henoch Schönlein nephritis | No | 12 |
| 14 | 69 | Female | Minimal change disease | No | 12 |
| 15 | 67 | Female | Minimal change disease | No | 14 |
| 16 | 53 | Female | Diabetes mellitus | Yes | 15 |
| 17 | 28 | Female | Hepatitis A | Yes | 16 |
| 18 | 45 | Male | Focal segmental glomerulosclerosis | No | 16 |
| 19 | 81 | Female | Focal segmental glomerulosclerosis | Yes | 16 |
| 20 | 78 | Female | IgA nephropathy | No | 17 |
| 21 | 24 | Female | Hepatitis A | No | 31 |
| 22 | 60 | Female | Bronchiectasis | No | 40 |
| 23 | 62 | Male | Diabetes Mellitus | No | 77 |
ATN, acute tubular necrosis; RRT, renal replacement therapy.
aNumber of total NKT cells counted in five randomly selected fields.
Figure 8.
Requirement of dialysis therapy in patients with ATN compared according to the number of total NKT cells in kidney tissues. Dialysis was instituted more frequently when fewer NKT cells were present in kidney.
DISCUSSION
In this study, we demonstrated the protective effects of NKT cells, especially sulfatide-reactive type II NKT cells against IRI. The trafficking of NKT cells into the site of injury occurred, and the activation of type II NKT cells modulated the cytokine secretion and inflammatory cell infiltration. The protective capacity of sulfatide-reactive type II NKT cells was associated with their ability to enhance the HIF-1α and IL-10 pathways. Finally, we demonstrated that NKT cells were present and actively participated in the process of ATN in human disease. These findings suggest that NKT cells, especially type II NKT cells, are feasible therapeutic targets for human disease.
Ischemia-reperfusion injury is the result of multi-step reactions, including nonimmune and immune responses. Accumulation of neutrophils and macrophages is a hallmark of acute kidney injury.27 Also, the adaptive immunity contributes to renal IRI,3,28 and skewing to Th1 or Th2 environment dictated the degree of severity by IRI.6 Also the regulation of injury by certain cell types such as Foxp3+CD4+CD25+ Tregs is highlighted recently.7,8
We assumed that the distinctive role of NKT cell subsets might be critical for determining the magnitude of inflammation caused by IRI. Although NKT cells have been shown to be important regulators of the immune response, there are several functionally distinct types of cells present. Type II NKT cells have not been investigated as thoroughly as type I NKT cells, but several studies have shown that these cells are protective against the development of autoimmune diseases.26,29 The activation of type II NKT cells by sulfatide prevented the development of EAE and hepatitis in mice.25 Plasma concentration of sulfatide was increased rapidly after IRI and decreased thereafter. Therefore ischemic insult may provide the chance of NKT-cell activation (Supplemental Figure 3).
Activated NKT cells have been shown to secrete soluble factors in situ and to migrate directly to and act at the site of injury.10,30 We further confirmed the notion that NKT cells might suppress inflammation at the site of injury. Usually, IRI induces tubular necrosis in outer medullary area. As shown in our work, the transferred NKT cells were localized at the tubulointerstitial area where the ischemic insult was most prominent. In addition, we observed that the ischemic injury was worsened by blocking of NKT cell recruitment, and the degree of renal infiltration of NKT cells in ATN patients seemed to be inversely correlated with the severity of ATN. These results suggest that NKT cells recruited in the injured tissues might reduce the extent of injury. Although the results we have acquired appear to differ from those obtained by Li et al.,13 this study do not produce contradictory outcomes. They aimed to prove the hypothesis that the early activation of type I NKT cell increases IFN-γ production, which plays a role in the kidney damage caused by IRI. In this study, on the other hand, we tried to evaluate the functional importance of type I and II NKT cells, and we investigated the effect of the specific activation of NKT cells on postischemic kidney and scrutinized differential roles of type I and type II NKT cells in the early injury phase of IRI. Another important point is that the severity of disease is quite different between two works, i.e. in previous work, the peak serum creatinine at 24 hours was 0.7 mg/dl, whereas our study yielded a higher level of damage at 1.59 mg/dl with wild-type mice. Also, we did not find significant protective effects by anti-CD1d mAb, although the recruitment of NKT cells was significantly blocked (Figure 3, D and E). The same cells may have exerted the differential effect according to the degree of stimuli.10
Key factors in the cellular adaptation to hypoxia include HIF-1 and HIF-2, which are heterodimeric transcription factors that consist of an oxygen-sensitive α-subunit and a constitutively expressed β-subunit.31 Renal oxygen concentration may vary by site, which offers more a vulnerable environment for renal medulla in hypoxia. HIF-1 and HIF target genes such as EPO, VEGF, and GLUT-1 are upregulated as an adaptive response to hypoxia.32–34 Previous studies have demonstrated that chemical preactivation of HIF protected renal IRI and induced upregulation of HIF-target genes.35,36 Although HIF-1α expression was marginally enhanced by IRI or hypoxia only in our study, NKT activation by sulfatide increased HIF-1α expression significantly. Also, the target genes showed similar patterns of expression. This result is consistent with the results of earlier studies. IRI contributes to the innate and adaptive immune responses that occur during transplantation. IRI may induce acute allograft dysfunction via dendritic cell maturation that is influenced by HIF regulation. Previous work showed that rapamycin attenuated dendritic cell differentiation, HIF-1α expression, and its target gene expression in a dose-dependent manner.37 The link between hypoxia and immune response was recently evaluated.38 Hypoxia induced an increase of HIF-1α in T cells and increased the potency of regulatory T cells. Furthermore, HIF-1α itself induced an increase in Foxp3 expression as well as in the number of functionally active Tregs. In our study, the sulfatide-induced activation of type II NKT cells enhanced HIF-1α and IL-10 expression. Clearly, blocking of NKT cell recruitment and IL-10 signaling abolished the beneficial effects of activated type II NKT cells with the downregulation of HIF-1α expression. IL-10 is also indispensable for exerting the protective property of Tregs.39
Finally, we demonstrated the significance of the presence of NKT cells in human renal tissue with ATN. We performed kidney biopsy in these ATN patients because the patients showed atypical clinical manifestations or had unknown etiology of ATN. NKT cells (CD3+Vα24+) were found rarely in normal human kidney tissue but frequently found in kidney tissue from patients suffering from ATN. The number of NKT cells varied according to the severity of ATN and the timing of kidney biopsy. Although the causes and severity of ATN were diverse in the tissue samples we studied, NKT cells were clearly present in human renal tissues after ischemic damage occurred. In patients with severe renal dysfunction, for whom the renal replacement therapy was instituted, NKT cells were found less frequently than in patients with less severe renal dysfunction for whom renal replacement therapy was not necessary. The result supports our observations that NKT cells play a beneficial role in the renal IRI.
In conclusion, our data demonstrate a significant protective role for NKT cells, especially sulfatide-reactive type II NKT cells, in acute kidney injury caused by ischemia-reperfusion. Modulation of cellular infiltration and cytokine expressions along with molecular changes such as HIF-1 and IL-10 are the main mechanisms for the protection. In the future, large-scale prospective investigations should be undertaken to provide a more comprehensive understanding of the role of NKT cells in human disease.
CONCISE METHODS
All of the experiments were performed under the approval of the Institutional Animal Care and Use Committee of Clinical Research Institute at Seoul National University Hospital and in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Research Council. All of the experiments dealing with human products were also approved by the internal review board of our institution.
Experimental Animals
Male C57BL/6 (B6; 7- to 8-week-old) mice were purchased from Orient Company (Seoul, Korea). B6.CD1d-deficient (B6.CD1d−/−) and B6.Jα18-deficient (B6.Jα18−/−) mice were originally generated by Van Ker and Taniguchi.40,41 All of the mice were raised in a specific pathogen-free animal facility.
Induction of Renal IRI
The mice were anesthetized with ketamine (100 mg/kg of body weight) and pentobarbital sodium (50 mg/kg of body weight Nembutal; Abbott, Wiesbaden, Germany). After bilateral flank incisions, both renal pedicles were crossclamped for 30 minutes with microaneurysm clamps (Roboz Surgical Instrument Co., Gaithersburg, MD).42 During the procedure, the animals were placed on heating pad (37°C). Prewarmed (37°C) PBS (400 μl) was administered subcutaneously for optimal fluid balance. The mice were sacrificed 24 hours after reperfusion (n = 6 to 8/group). Sham-operated mice received the same surgical procedure except for the clamping of renal pedicles. In another set of experiments, anti-CD1d mAb (clone 1B1; 300 μg/mouse per day for −1 and 0 days, purified from hybridomas) or anti-CXCL16 mAb (500 μg/mouse for −1 and 0 days; R & D, Minneapolis, MN) was used to inhibit CD1d-restricted NKT cell recruitment (controls were treated with rIgG2b) or neutralize CXCL16 bioactivity. Renal function was evaluated by serum creatinine levels. Creatinine concentration (μmol/L [mg/dl]) was measured with the modified Jaffé rate reaction using autoanalyzer (Hitachi Chemical Industries, Ltd., Osaka, Japan).
For a detailed explanation of methods relative to in vitro and in vivo manipulations, immunohistochemistry, Western blot, flow cytometry, quantitative real-time PCR, confocal microscopy, hypoxia-reoxygenation procedure, human and mouse tubular epithelial cell isolation, and culture, please see the online supplemental data.
Statistical Analyses
The results are expressed as the means ± SD or the means ± SEM where appropriate. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA). To compare more than two groups, one- or two-way ANOVA statistical analysis using Tukey test was performed. Statistical significance was determined at P < 0.05.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Korea Healthcare Technology R & D Project, Ministry for Health and Welfare, Republic of Korea Grant A080656.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelly KJ, Williams WW, Jr., Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV: Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, Rabb H: Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest, 108: 1283–1290, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jo SK, Sung SA, Cho WY, Go KJ, Kim HK: Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant, 21: 1231–1239, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Takada M, Chandraker A, Nadeau KC, Sayegh MH, Tilney NL: The role of the B7 costimulatory pathway in experimental cold ischemia/reperfusion injury. J Clin Invest, 100: 1199–1203, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yokota N, Burne-Taney M, Racusen L, Rabb H: Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 285: F319–F325, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD: Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int 77: 771–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H: Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int 76: 717–729, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Taniguchi M, Seino K, Nakayama T: The NKT cell system: Bridging innate and acquired immunity. Nat Immunol 4: 1164–1165, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Oh K, Kim S, Park SH, Gu H, Roopenian D, Chung DH, Kim YS, Lee DS: Direct regulatory role of NKT cells in allogeneic graft survival is dependent on the quantitative strength of antigenicity. J Immunol 174: 2030–2036, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Yang SH, Jin JZ, Lee SH, Park H, Kim CH, Lee DS, Kim S, Chung NH, Kim YS: Role of NKT cells in allogeneic islet graft survival. Clin Immunol 124: 258–266, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H: Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380–3387, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A: In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med 191: 1895–1903, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stein-Streilein J: Invariant NKT cells as initiators, licensors, and facilitators of the adaptive immune response. J Exp Med, 198: 1779–1783, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arrenberg P, Halder R, Kumar V: Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol, 218: 246–250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang X, Shimaoka T, Kojo S, Harada M, Watarai H, Wakao H, Ohkohchi N, Yonehara S, Taniguchi M, Seino K: Cutting edge: Critical role of CXCL16/CXCR6 in NKT cell trafficking in allograft tolerance. J Immunol 175: 2051–2055, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Yang SH, Kim SJ, Kim N, Oh JE, Lee JG, Chung NH, Kim S, Kim YS: NKT cells inhibit the development of experimental crescentic glomerulonephritis. J Am Soc Nephrol 19: 1663–1671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bendelac A: Selection and adaptation of cells expressing major histocompatibility complex class I-specific receptors of the natural killer complex. J Exp Med 186: 349–351, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porcelli SA, Modlin RL: The CD1 system: Antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol 17: 297–329, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L: The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med 7: 1052–1056, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L: Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med 194: 1801–1811, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh K, Byoun O, Ham DI, Kim YS, Lee DS: Invariant NKT cells regulate experimental autoimmune uveitis through inhibition of Th17 differentiation. Eur J Immunol 41: 392–402, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Yang JQ, Singh AK, Wilson MT, Satoh M, Stanic AK, Park JJ, Hong S, Gadola SD, Mizutani A, Kakumanu SR, Reeves WH, Cerundolo V, Joyce S, Van Kaer L, Singh RR: Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J Immunol 171: 2142–2153, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V: Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med 199: 947–957, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Halder RC, Aguilera C, Maricic I, Kumar V: Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest 117: 2302–2312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Day YJ, Huang L, Ye H, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: Role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H: B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 171: 3210–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Duarte N, Stenstrom M, Campino S, Bergman ML, Lundholm M, Holmberg D, Cardell SL: Prevention of diabetes in nonobese diabetic mice mediated by CD1d-restricted nonclassical NKT cells. J Immunol 173: 3112–3118, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Campos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, Askenase PW: Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med 198: 1785–1796, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haase VH: Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol 291: F271–F281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU: Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Rosenberger C, Rosen S, Shina A, Bernhardt W, Wiesener MS, Frei U, Eckardt KU, Heyman SN: Hypoxia-inducible factors and tubular cell survival in isolated perfused kidneys. Kidney Int 70: 60–70, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Nangaku M, Eckardt KU: Hypoxia and the HIF system in kidney disease. J Mol Med 85: 1325–1330, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M: Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol 14: 1825–1832, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Ma D, Lim T, Xu J, Tang H, Wan Y, Zhao H, Hossain M, Maxwell PH, Maze M: Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1alpha activation. J Am Soc Nephrol 20: 713–720, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rama I, Bruene B, Torras J, Koehl R, Cruzado JM, Bestard O, Franquesa M, Lloberas N, Weigert A, Herrero-Fresneda I, Gulias O, Grinyo JM: Hypoxia stimulus: An adaptive immune response during dendritic cell maturation. Kidney Int 73: 816–825, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J: Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol 38: 2412–2418, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M: Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science 278: 1623–1626, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M: CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278: 1626–1629, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Okusa MD, Linden J, Macdonald T, Huang L: Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol 277: F404–F412, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.