Abstract
Proinflammatory cytokines contribute to renal injury, but the downstream effectors within kidney cells are not well understood. One candidate effector is Klotho, a protein expressed by renal cells that has antiaging properties; Klotho-deficient mice have an accelerated aging-like phenotype, including vascular injury and renal injury. Whether proinflammatory cytokines, such as TNF and TNF-like weak inducer of apoptosis (TWEAK), modulate Klotho is unknown. In mice, exogenous administration of TWEAK decreased expression of Klotho in the kidney. In the setting of acute kidney injury induced by folic acid, the blockade or absence of TWEAK abrogated the injury-related decrease in renal and plasma Klotho levels. TWEAK, TNFα, and siRNA-mediated knockdown of IκBα all activated NFκB and reduced Klotho expression in the MCT tubular cell line. Furthermore, inhibition of NFκB with parthenolide prevented TWEAK- or TNFα-induced downregulation of Klotho. Inhibition of histone deacetylase reversed TWEAK-induced downregulation of Klotho, and chromatin immunoprecipitation showed that TWEAK promotes RelA binding to the Klotho promoter, inducing its deacetylation. In conclusion, inflammatory cytokines, such as TWEAK and TNFα, downregulate Klotho expression through an NFκB-dependent mechanism. These results may partially explain the relationship between inflammation and diseases characterized by accelerated aging of organs, including CKD.
Acute kidney injury (AKI) and progressive loss of renal function are associated with interstitial inflammation and tubular injury.1 Members of the TNF superfamily of cytokines are key mediators of renal injury, and the transcription factor nuclear factor-kappaB (NFκB) is a mediator of their biologic activity.2–4 TNF has pleiotropic actions on glomerular and tubular cells that contribute to renal damage.5,6 More recently, TNF-like weak inducer of apoptosis (TWEAK, TNFSF12) was identified as a mediator of kidney tubulointerstitial inflammation through interaction with its receptor Fn14.7,8 During kidney injury TWEAK may have additional actions, not shared with TNF, such as noncanonical activation of NFκB resulting in expression of CCL21.9 Both TWEAK and TNF may promote tubular cell injury and death.8,10 Cell death contributes to tubular cell loss in both AKI and chronic kidney disease (CKD).11 In addition, TNF and TWEAK are mediators of inflammation and atherosclerosis, which are key events in CKD patients exhibiting high mortality rates.12–14 In fact, CKD has features consistent with accelerated aging such as accelerated atherosclerosis. α-Klotho or Klotho is a kidney-secreted hormone with antiaging properties.15,16 Klotho is a pleiotropic protein with glucuronidase activity that modulates calcium transport by TRPV5,1 serves as a necessary coreceptor for fibroblast growth factor 23 (FGF23),17 regulating Pi homeostasis,18 inhibits Wnt signaling,19 has antioxidant properties,20 and represses IGF-1 signaling.16
Klotho is downregulated during kidney injury. Kidney Klotho mRNA is reduced in long-term hypertension, diabetes mellitus, and CKD models21 as well as in experimental ischemia-reperfusion-induced AKI,22 and in uremic hyperplastic parathyroid glands.23 Decreased expression of Klotho mRNA and protein was confirmed in humans with CKD.24 Because renal failure and inflammation are both associated with accelerated aging features, such as vascular injury and calcification, that are also observed in Klotho knock-out (KO) mice, we examined the relationship between inflammation and Klotho expression.
The factors regulating Klotho expression are poorly understood. LPS administration decreased Klotho mRNA, suggesting that the expression of Klotho is modulated by acute inflammatory stress in vivo.25 However, the specific molecules that downregulated Klotho could not be identified in cell culture experiments. Angiotensin II downregulates Klotho mRNA expression in tubular cells both in culture and in vivo, but the intracellular molecular mechanisms were not addressed in detail.26,27 Oxidative stress also downregulates Klotho.28,29 Statins and Rho kinase inhibitors prevented angiotensin-induced downregulation of Klotho in cultured cells.27 Antioxidants, statins, and Rho kinase inhibitors are known to prevent activation of NFκB in response to stimuli such as angiotensin II.30–32
Despite evidence relating inflammation to low Klotho expression and to accelerated aging, there had been a poor understanding of the inflammatory mediators that downregulate Klotho and of the intracellular mechanisms involved. A role for NFκB had not been characterized. We now report that TWEAK and TNF promote the NFκB-dependent downregulation of Klotho expression in cultured tubular cells and in the kidney in vivo.
RESULTS
Klotho mRNA and Protein Are Downregulated in Acute Inflammatory Tubular Injury
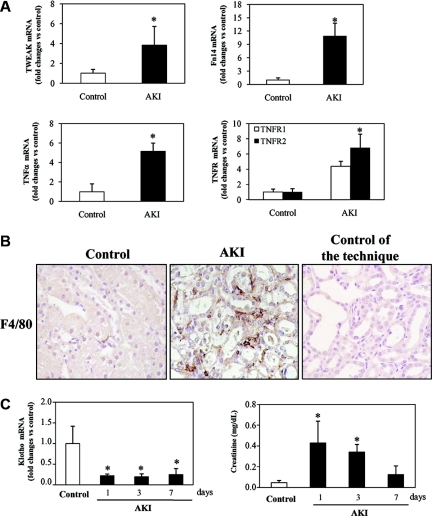
Folic acid nephropathy is a model of AKI characterized by acute renal failure and tubulointerstitial inflammation.7 In this AKI model there is an increased renal expression of the inflammatory cytokines belonging to the TNF superfamily—TWEAK and TNFα—and of their receptors: Fn14 for TWEAK and TNFR1 and TNFR2 for TNFα (Figure 1A).8,33,34 This is associated with tubulointerstitial inflammation characterized by a five-fold (P < 0.002 versus control) increased number of interstitial F4/80-positive macrophages (Figure 1B) and by activation of the inflammation-related transcription factor NFκB in tubular cells.7,35 Klotho mRNA expression was decreased in experimental AKI induced by folic acid (Figure 1C) or cisplatin (Supplemental Figure S1). The time course was studied in folic acid-induced AKI. Interestingly, Klotho downregulation remained significant after recovery of renal function (Figure 1C).
Figure 1.
Cytokine and Klotho expression in murine model of renal tubulointerstitial inflammation. (A) Expression of inflammatory cytokines (TNFα and TWEAK) and TWEAK and TNF receptors (Fn14 and TNFR1/TNFR2, respectively) mRNA were measured by real time RT-PCR in kidneys of mice with AKI induced by a folic acid overdose at 72 hours. Expression of cytokines and their receptors is increased in this model. Mean ± SEM of six animals per group. *P < 0.05 versus control. (B) Representative immunohistochemistry of renal macrophage infiltration at 72 hour AKI mice Macrophages were identified by staining with F4/80 antibody. Increased macrophage-positive cells were noted in AKI mice with respect to healthy control mice. Controls for the technique are stained with nonspecific IgG. (C) Time course of kidney Klotho mRNA expression and plasma creatinine levels in folic acid-induced AKI. Kidney Klotho mRNA is decreased even when renal function has recovered. Mean ± SEM of six animals per group. *P < 0.005 versus control.
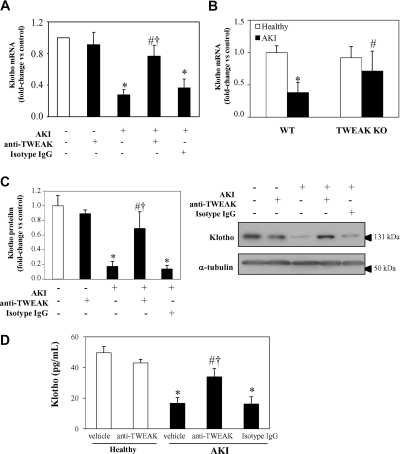
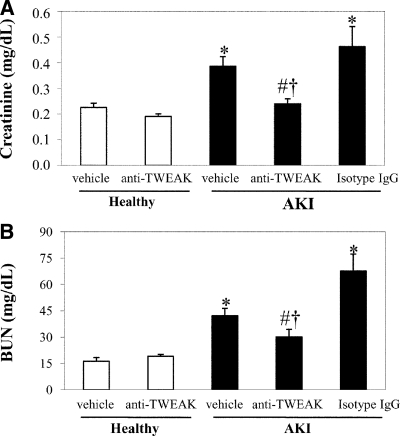
Treatment with neutralizing anti-TWEAK antibody prevented the decrease in Klotho mRNA and protein expression in folic acid-induced AKI, suggesting a contribution of this cytokine to the downregulation of renal Klotho expression (Figure 2, A and C). Absence of TWEAK in TWEAK KO mice also prevented the reduction in renal Klotho mRNA during AKI, compared with wild-type (WT) mice (Figure 2B). However, the basal mRNA expression of Klotho in untreated WT or TWEAK KO kidney was similar. Moreover, Klotho plasma concentration was also reduced in folic acid-induced AKI, and treatment with anti-TWEAK neutralizing antibodies prevented the decrease in Klotho plasma levels (Figure 2D). We confirmed that the decreased renal function during AKI was significantly improved by neutralizing anti-TWEAK antibody pretreatment (Figure 3, A and B). Moreover, in TWEAK KO mice with AKI, renal function and histologic AKI score were preserved compared with WT mice with AKI (Supplemental Figure S2).
Figure 2.
Antagonism or absence of TWEAK prevented downregulation of Klotho in renal tubulointerstitial inflammation. (A and B) The decreased total kidney Klotho mRNA expression observed in folic acid-induced AKI at 72 hours (RT-PCR) is improved by (A) TWEAK antagonism, using a neutralizing anti-TWEAK antibody (mean ± SEM of six animals per group; *P < 0.002 versus healthy mice; #P < 0.02 versus AKI; †P < 0.02 versus AKI+Isotype IgG) or by (B) TWEAK absence as shown by comparing WT and TWEAK KO mice (mean ± SEM of five animals per group; *P < 0.005 versus WT healthy; #P < 0.03 versus WT AKI). (C) Total kidney Klotho protein, measured by Western blot, is also reduced in AKI at 72 h, and this reduction is prevented by TWEAK antagonism. Mean ± SEM of six animals per group. *P < 0.002 versus healthy mice; #P < 0.02 versus AKI; †P < 0.02 versus AKI+Isotype IgG. (D) Plasma levels of Klotho, measured by ELISA, are decreased in mice with AKI at 72 hours, and this is improved by anti-TWEAK antibody pretreatment. Mean ± SEM of six animals per group. *P < 0.005 versus healthy mice; #P < 0.05 versus AKI; †P < 0.05 versus AKI+Isotype IgG.
Figure 3.
TWEAK neutralization improves renal function during AKI. AKI was induced by a folic acid overdose in mice and renal function was assessed at 72 hours measuring plasma creatinine and BUN. (A) Plasma creatinine levels. (B) BUN. Mean ± SEM of six animals per group. *P < 0.004 versus healthy mice; #P < 0.04 versus AKI; †P < 0.03 versus AKI+Isotype IgG.
In Vivo Exogenous TWEAK Decreases Renal Klotho through NFκB Activation
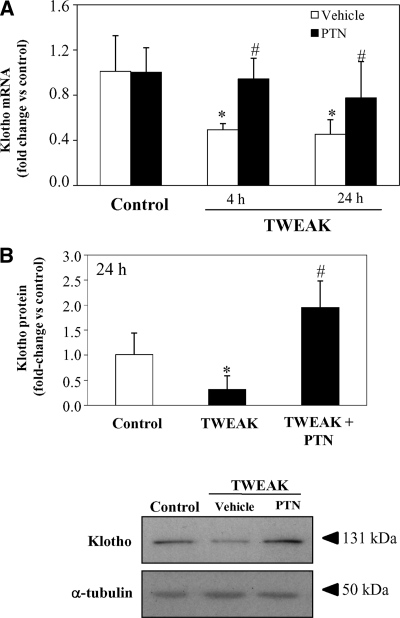
The beneficial effect of TWEAK blockade in preventing downregulation of renal Klotho expression in AKI might be a direct effect or an indirect consequence of ameliorated tissue injury.7 Thus, we explored whether TWEAK directly regulated kidney Klotho expression in vivo. Systemic injection of TWEAK decreased renal Klotho mRNA levels in vivo at 4 and 24 h (Figure 4A). We have previously shown that the kidney proinflammatory action of TWEAK depends on NFκB activation in tubular cells.7 In this regard, Klotho downregulation induced by TWEAK was prevented by treatment with the NFκB inhibitor parthenolide (PTN) (Figure 4A), suggesting that reduction of Klotho expression by TWEAK is mediated by NFκB activation. We confirmed by Western blot that TWEAK reduced expression of Klotho at the protein level at 24 h (Figure 4B).
Figure 4.
Systemic TWEAK injection in healthy mice decreases renal Klotho mRNA and protein in vivo in healthy mice. TWEAK or vehicle were injected intraperitoneally. Total kidney Klotho mRNA and protein were decreased in a time-dependent manner in TWEAK-injected mice, compared with vehicle-treated control mice. This effect was reverted by pretreatment with Parthenolide (PTN). (A) Quantitative RT-PCR analyses of renal Klotho mRNA in mice 4 or 24 hours after TWEAK, PTN, or TWEAK/PTN. Mean ± SEM of six animals per group. *P < 0.01 versus control; #P < 0.05 versus TWEAK alone. (B) Total kidney Klotho protein levels in mice treated with TWEAK and PTN for 24 hours measured by Western blot. Mean ± SEM of six animals per group. *P < 0.02 versus control; #P < 0.02 versus TWEAK alone.
TWEAK and TNFα Decreases Klotho Expression in Cultured Renal Tubular Cells
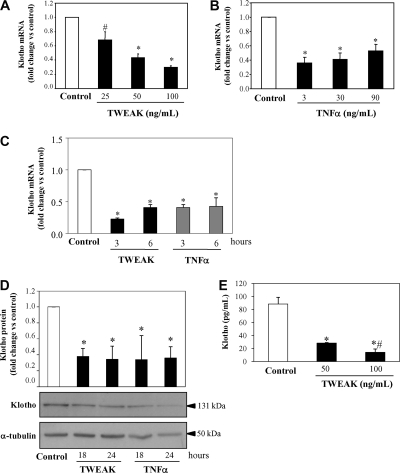
TWEAK administration may have recruited secondary mediators of injury in vivo that lead to the decreased renal Klotho expression. Therefore, we explored whether TWEAK and TNFα, an inflammatory cytokine from the same family that also activates NFκB, directly regulate Klotho expression in cultured tubular cells. We have previously shown that both, TWEAK and TNFα, increase the expression of a variety of genes implicated in inflammation through the activation of NFκB.7 Thus, TWEAK and TNF increased the mRNA expression of bona fide NFκB RelA/p65 target inflammatory genes such as MCP-1 and IL-6 (48- and 28-fold for TWEAK and 26- and 12-fold for TNF at 3 h in renal tubular cells, P < 0.05).7 Here we show that both TWEAK and TNFα decreased Klotho mRNA expression in a dose-dependent manner by 3 h (Figure 5, A through C). Moreover, TWEAK and TNF also decreased Klotho protein (Figure 5D). Finally, Klotho concentration in cell supernatants also decreased after TWEAK treatment (Figure 5E).
Figure 5.
Inflammatory cytokines decrease Klotho expression in cultured tubular cells in an NFκB-dependent manner. TWEAK (A) and TNFα (B) decrease Klotho mRNA expression in a dose-dependent manner at 3 hours in MCT cells. Mean ± SD of three independent experiments. *P < 0.0001 versus control; #P < 0.001 versus control. (C) Time course of TWEAK (100 ng/ml) and TNFα (30 ng/ml) decreased Klotho mRNA expression in tubular cells. Mean ± SD of three independent experiments. *P < 0.0001 versus control. Real time RT-PCR. (D) Representative Western blot and quantification of three independent experiments shows decreased Klotho protein in MCT cells treated with TWEAK (100 ng/ml) or TNFα (30 ng/ml). Mean ± SD of three independent experiments. *P < 0.008 versus control. (E) MCT cells were treated with 100 or 50 ng/ml TWEAK for 24 hours, and Klotho levels were measured in cell supernatants by ELISA. TWEAK decreased soluble Klotho in a dose-dependent manner. Mean ± SD of three experiments. *P < 0.05 versus control; #P < 0.05 versus TWEAK 50 ng/ml.
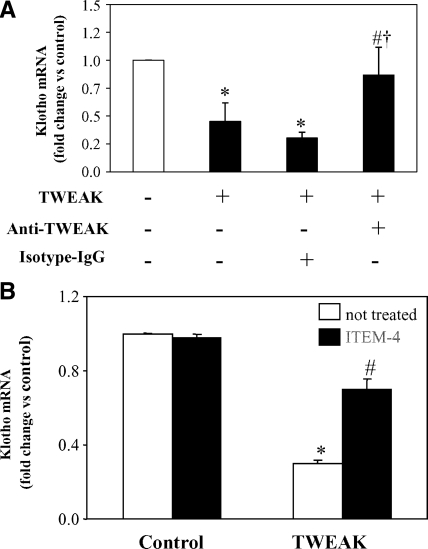
TWEAK blockade with an anti-TWEAK antibody prevented Klotho downregulation induced by TWEAK, confirming the specificity of the interaction (Figure 6A). Anti-TNF neutralizing antibodies prevented TNF-induced Klotho mRNA downregulation (data not shown). TWEAK required binding to Fn14 because the anti-Fn14 neutralizing antibody ITEM-4 prevented TWEAK-induced Klotho mRNA downregulation (Figure 6B).
Figure 6.
Fn14 mediates TWEAK-induced Klotho downregulation in cultured tubular cells. (A) A neutralizing anti-TWEAK antibody (10 μg/ml) rescued Klotho levels in MCT cells treated with 100 ng/ml TWEAK for 3 hours. Mean ± SD of three independent experiments. *P < 0.002 versus control; #P < 0.02 versus TWEAK alone; †P < 0.006 versus TWEAK+Isotype IgG. (B) ITEM-4 (10 μg/ml), a neutralizing anti-Fn14 antibody, prevented Klotho downregulation induced by TWEAK in MCT cells, suggesting that this effect of TWEAK is mediated through Fn14 binding. Mean ± SD of three independent experiments. *P < 0.005 versus control; #P < 0.02 versus TWEAK alone.
NFκB Mediates TWEAK- and TNFα-induced Klotho Downregulation
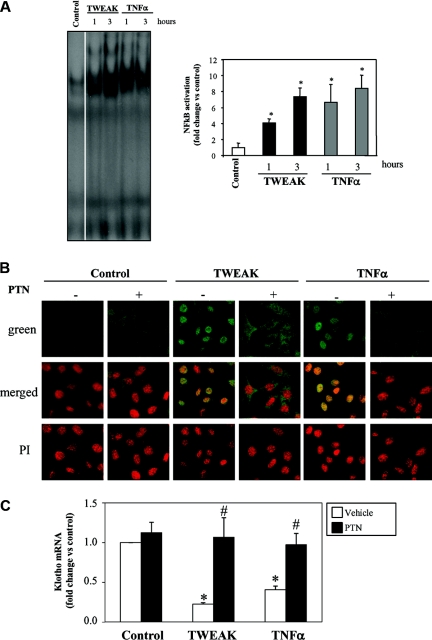
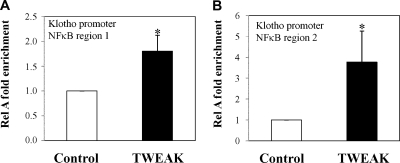
In murine tubular epithelial cells TWEAK and TNFα increased the DNA binding activity of NFκB as assessed by electrophoretic mobility shift assay (EMSA) (Figure 7A) and induced the translocation of the RelA/p65 subunit from the cytoplasm to the nucleus (Figure 7B). Degradation of IκBα is required for RelA translocation to the nucleus. In tubular cells, Klotho mRNA expression was decreased in cells transfected with a siRNA targeting IκBα, indicating that Klotho expression is regulated by NFκB (Supplemental Figure S3). PTN inhibits NFκB activation by preventing the degradation of IκBα.36 PTN prevented RelA translocation and NFκB activation induced by either TWEAK or TNFα (Figure 7B). Although most known actions of NFκB RelA involve induction of gene transcription, it may also actively repress gene expression.37 In this regard, we observed that PTN prevented the downregulation of Klotho mRNA expression induced by TWEAK or by TNF (Figure 7C). Moreover, chromatin immunoprecipitation (ChIP) assays showed RelA binding at the murine Klotho promoter in murine proximal tubular epithelial (MCT) cells upon TWEAK stimulation for 60 minutes (Figure 8A and B). These results suggest that the inflammatory cytokines, TWEAK and TNFα, decreased Klotho expression through NFκB activation.
Figure 7.
PTN prevents Klotho downregulation induced by TNFα or TWEAK in cultured tubular cells. (A) TNFα or TWEAK promoted DNA binding of NFκB complexes as assessed by EMSA. Quantification (mean ± SD) and representative EMSA of three independent experiments. *P < 0.05 versus control. (B) TNFα or TWEAK promote the translocation of the RelA/p65 protein to the nucleus at 60 minutes in tubular cells. This is prevented by PTN. Nuclear translocation of NFκB subunits is required for DNA binding. Images representative of three independent experiments. Confocal microscopy where RelA is green and propidium iodide (nuclei) is orange (magnification, ×320). (C) The NFκB inhibitor PTN prevents TWEAK- and TNFα-induced downregulation of Klotho mRNA in cultured MCT cells at 3 hours. Mean ± SD of three independent experiments. *P < 0.0001 versus control; #P < 0.0001 versus cytokine alone.
Figure 8.
TWEAK induces RelA binding to the Klotho promoter in cultured tubular cells. (A) Region 1; (B) region 2. MCT cells were stimulated with TWEAK for 60 minutes, and ChIP analyses were performed using an anti-RelA antibody. Promoter copy number was quantified by real-time PCR in duplicate using two specific primers that amplify two different NFκB binding sites of the Klotho promoter. Normal rabbit IgG was used as negative control for the specificity of the immunoprecipitation (intraperitoneally). As a positive control, aliquots of chromatin fragments obtained before intraperitoneally were also subjected to RT-PCR analysis (Input). Immunoprecipitated DNA with RelA binding was normalized to a 100-fold dilution of input chromatin. Data are expressed as fold enrichment of RelA binding compared with negative control antibody (normal rabbit IgG). n = 4; *P < 0.04 versus control.
Histone Deacetylase Activity Is Required for TWEAK-/TNFα-decreased Klotho Expression
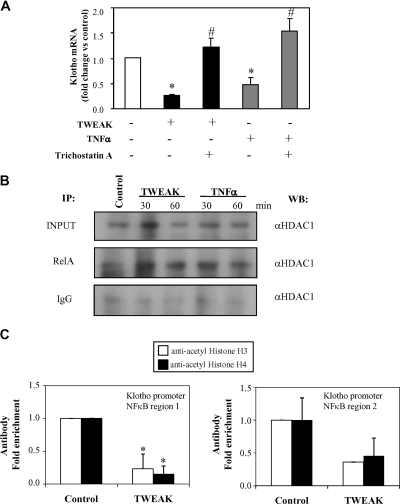
Histone acetylation/deacetylation is an important step in chromatin remodeling that plays a key role in regulation of gene expression. Histone deacetylation mediated by histone deacetylase (HDAC) generally leads to transcriptional repression and may change RelA activity from induction to repression of transcription.37,38 Thus, we tested the modulation of TWEAK-/TNFα-induced downregulation of Klotho expression by the HDAC inhibitors trichostatin A (TSA) or valproic acid. TSA or valproic acid pretreatment prevented Klotho downregulation induced by TWEAK or TNFα (Figure 9A and Supplemental Figure S4, respectively). Moreover, RelA is associated with HDAC1 in nuclei of TWEAK- or TNFα-stimulated cells as assessed by immunoprecipitation (Figure 9B). ChIP assays using antibodies against acetylated histones corroborated that TWEAK promoted histone H3 and H4 deacetylation at the murine Klotho promoter (Figure 9C).
Figure 9.
TWEAK- and TNFα-mediated downregulation of Klotho mRNA in cultured tubular cells requires HDAC activity. (A) The HDAC inhibitor TSA rescues TWEAK- or TNFα-induced repression of Klotho at 3 hours in MCT cells. Cells were prestimulated with TSA (100 ng/ml) 1 hour before addition of cytokines. Mean ± SD of three independent experiments. *P < 0.0001 versus control; #P < 0.001 versus cytokine alone. (B) TWEAK and TNFα induce RelA association with HDAC1 in MCT cells. Cells were stimulated with TWEAK (100 ng/ml) or TNFα (30 ng/ml) for 30 and 60 minutes, to coincide with peak RelA nuclear translocation. Nuclear extracts were immunoprecipitated with 3 μg anti-RelA antibody or control IgG and then Western blotted with anti-HDAC1. Nuclear extract input was 25 μg. (C) TWEAK induces deacetylation at the Klotho promoter in MCT cells at 60 minutes as assessed by ChIP. Anti-acetylated histone H3 and H4 antibodies were used. The presence of acetylated histone H3 and H4 at the Klotho promoter was detected by RT-PCR with specific primers for two different NFκB binding sites of the Klotho promoter. Normal rabbit IgG was used as negative control for the specificity of the immunoprecipitation (intraperitoneally). As a positive control, aliquots of chromatin fragments obtained before intraperitoneally were also subjected to RT-PCR analysis (Input). Immunoprecipitated DNA with histone modification was normalized to a 100-fold dilution of input chromatin. Data are expressed as fold enrichment of RelA binding compared with negative control antibody (normal rabbit IgG). n = 4; *P < 0.04 versus control.
DISCUSSION
The main finding of our study is that in kidney cells Klotho is negatively regulated by inflammation, and it is downregulated by an NFκB RelA-dependent mechanism. We have identified individual inflammatory cytokines such as TWEAK or TNFα that downregulate Klotho in cell culture and in vivo. Both proinflammatory cytokines signal through NFκB RelA to increase the expression of a variety of inflammatory mediators.7,39 However, in the case of Klotho, NFκB RelA appears to downregulate gene expression in an inflammatory milieu. These findings may have therapeutic implications in kidney injury and inflammation-associated premature aging.
The Klotho gene encodes a single-pass transmembrane protein that binds to multiple FGF receptors and functions as a co-receptor for FGF23, a bone-derived hormone that suppresses phosphate reabsorption and vitamin D biosynthesis in the kidney. In addition, the extracellular domain of Klotho protein is shed and secreted, potentially functioning as a humoral factor. The secreted Klotho protein can regulate multiple growth factor signaling pathways, including insulin/IGF-1 and Wnt, and the activity of multiple ion channels. Klotho also protects cells and tissues from oxidative stress, yet the precise mechanism underlying this activity remains to be determined. As a result, lack of Klotho in mice results in accelerated aging.40 The kidney is the main site of Klotho expression, and kidney injury, as corroborated by our findings, results in reduced renal Klotho expression.25,41 We may speculate that a decreased renal expression of Klotho may have local and systemic adverse consequences. Regarding potential systemic effects, reduced kidney Klotho expression during AKI persisted beyond recovery of renal function and was associated with decreased circulating Klotho. Decreased renal, circulating, and urine Klotho was recently reported in ischemia-reperfusion-induced AKI.42 Patients with kidney disease have progressive injury to multiple organs, among them the cardiovascular system, resulting in a mortality rate which is ten times that of age-matched controls, resulting in effective accelerated aging.14,43 Interestingly, systemic inflammation also promotes cardiovascular morbidity and mortality and has been associated with other age-dependent diseases such as osteoporosis and dementia.44,45 Locally, a reduction of Klotho expression contributed to progression of kidney failure; although its overexpression ameliorated renal injury in a mouse model.46 Ad-Klotho gene transfer improved serum creatinine and reduced renal apoptosis induced by ischemia-reperfusion in rats.22 Klotho transfection reduced H2O2-induced tubular cell apoptosis in culture.29 Klotho is also expressed in extrarenal cells. Thus, Klotho expression is reduced in human CD4 lymphocytes from aged patients.47
Our results show that TWEAK and TNFα induce Klotho downregulation in a dose-dependent fashion in cultured renal cells, that TWEAK decreases kidney Klotho in vivo, and that targeting TWEAK prevents Klotho downregulation during kidney injury. Evidence suggesting that RelA activation is necessary for TWEAK- and TNFα- induced Klotho repression includes the following: (1) IκBα downregulation by siRNA, a maneuver that allows RelA translocation to nuclei, promoted Klotho downregulation, (2) an inhibitor of RelA activation, PTN, prevented TWEAK- and TNFα-induced Klotho downregulation, (3) the temporal pattern of Klotho repression observed is consistent with that of RelA activation induced by TWEAK and TNFα, (4), and RelA is bound to Klotho promoter in MCT cells stimulated with TWEAK.7,36 NFκB-mediated downregulation of gene expression had previously been observed for TNFα, such as downregulation of argininosuccinate synthase, endothelial nitric oxide synthase, and Bmp4 gene expression.48–50 However, it had not been observed for TWEAK. TWEAK activates at 24 h a subset of NFκB2 protein complexes that are different from those observed after TNFα stimulation.9,51 However, both cytokines share the early activation of RelA-containing NFκB complexes.7,9,51 The fact that both TWEAK and TNFα downregulate Klotho and that this is an early event argues against an involvement of NFκB2, which is only activated by TWEAK and whose activation takes place after changes in Klotho mRNA have already occurred.
The main “switch” in NFκB activation is cytoplasmic and leads to the nuclear accumulation of NFκB proteins. In addition, recruitment of NFκB to chromatin is regulated in a promoter-specific manner. This kinetic complexity in NFκB-dependent gene induction is dependent on simultaneously activated pathways and transcription factors.52 In addition to gene-specific factors there are stimulus-specific factors that determine the activator or repressor activity of NFκB on gene transcription.37 Thus, NFκB induced by noncytokine cytotoxic stimuli is functionally distinct from NFκB induced by TNFα: the first is an active repressor of antiapoptotic gene expression, whereas the latter promotes antiapoptotic gene expression.37 There are different mechanisms for p65/RelA-dependent gene repression. Recently, it has become apparent the importance of HDAC activity. HDAC activity is required for p65/RelA-dependent repression of PPARδ in human keratinocytes, of antiapoptotic genes in fibroblasts, and of PDGF in smooth muscle cells.37,53,54 We observed that TWEAK and TNFα increased nuclear RelA association with nuclear HDAC1 at the same time that Klotho expression was decreased in tubular cells. This result suggested that TWEAK- and TNFα-induced Klotho downregulation could be mediated by HDAC activity. In this regard, we observed that HDAC inhibitors, TSA and valproic acid, prevented repression of Klotho induced by TWEAK or TNFα. Furthermore, TWEAK induces histone H3 and H4 deacetylation at the murine Klotho promoter in renal tubular cells.
Although TWEAK targeting by either neutralizing antibodies or in TWEAK KO mice preserved kidney Klotho expression and circulating Klotho levels and also preserved renal function, our studies do not allow us to conclude that Klotho preservation has a role in renal function improvement. However we can speculate on a nephroprotective role of Klotho based on the beneficial effect of Klotho overexpression on progressive renal injury55 and in AKI.42,56
In summary, the inflammatory cytokines TWEAK and TNFα downregulate Klotho in renal tubular cells through an NFκB-dependent mechanism. These results are of interest to kidney injury and to the accelerated aging observed in uremic patients. They identify an additional adverse consequence of NFκB activation in kidney injury. In this regard, our results may be relevant to design therapeutic approaches to regulate Klotho expression.
CONCISE METHODS
Cells and Reagents
MCTs were cultured in RPMI 1640, 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, in 5% CO2 at 37°C.7
RPMI-1640, penicillin, streptomycin, and trypsin-EDTA were from BioWhittaker (Waltham, Massachusetts), FBS from Life Technologies (Carlsbad, California), and recombinant human TWEAK from Chemicon, Millipore (Billerica, Massachusetts). Anti-TWEAK monoclonal antibody (clone P2D10) and specific isotype control (clone P1.17) (Biogen Idec) were used at 10 μg/ml. ITEM-4, neutralizing anti-Fn14 antibody (eBioscience, San Diego, California) was used at 10 μg/ml. Anti-TNF polyclonal antibody was used at 3 μg/ml (BioLegend, San Diego, California). PTN (Sigma, St. Louis, Missouri) at 10 μM inhibits NFκB activation in MCT cells without decreasing cell viability.7 HDAC inhibitors, TSA (Upstate Biotechnology, Millipore) and valproic acid (Sigma), were used at 100 ng/ml and 3 mM, respectively. Klotho concentration in cell supernatants and mice plasma was determined with the E97757Mu ELISA Kit (USCNK, Wuhan, P.R.China).
Animal Models
Studies were conducted in accord with the NIH Guide for the Care and Use of Laboratory Animals. C57/BL6 mice (12 to 14 weeks old) (IFFA-CREDO, Barcelona, Spain) were treated with 70 μg/mouse PTN or its vehicle (0.05% DMSO) and 0.75 μg/mouse of TWEAK or saline. Treatment and control groups (n = 6) were as follows: 1) PTN followed by TWEAK, 2) vehicle (DMSO) followed by TWEAK, 3) PTN followed by saline, and 4) vehicle (DMSO) followed by saline. PTN was injected 24 h before that TWEAK injection and TWEAK 4 or 24 h before sacrifice. The dose of TWEAK was calculated based on in vitro experiments for an extracellular volume of 7.5 ml/mouse. The dose of PTN was established based on previous experience.7
Folic acid nephropathy is a classical model of AKI.7,57–59 C57/BL6 mice (12 to 14 weeks old) received a single intraperitoneally injection of folic acid (Sigma) 250 mg/kg in 0.3 M sodium bicarbonate or vehicle, and mice were killed 24 or 72 h or 7 d later.7 Mice were dosed intraperitoneally with either 200 μg neutralizing anti-TWEAK mAb (clone P2D10, Biogen Idec)7 or 200 μg isotype IgG (mouse IgG2a, clone P1.17, Biogen Idec) (n = 6 per group). In another set of experiment, TWEAK KO (Biogen Idec)60 mice on the C57Bl/6 background strain received intraperitoneally folic acid injection, and they were killed 72 h later (n = 5 per group).
Cisplatin is a nephrotoxic routinely used in clinical in cancer treatment. C57/BL6 mice (12 to 14 weeks old) received a single intraperitoneally injection of cisplatin (Sigma, 20 mg/kg in saline) or vehicle, and mice were killed 72 h later (n = 5 per group). The dose was derived from preliminary studies in our mice colony. Mean plasma creatinine in cisplatin AKI mice was 0.8 ± 0.1 mg/dl.
The kidneys were perfused in situ with cold saline before removal. One kidney was snap-frozen in liquid nitrogen for RNA and protein studies, and the other was fixed and paraffin-embedded.
Immunohistochemistry
Immunohistochemistry was carried out as described previously in paraffin-embedded tissue sections 5 μm thick.7 Primary antibody was rat polyclonal anti-F4/80 antigen (1:50, Serotec). Sections were counterstained with Carazzi‘s hematoxylin. Negative controls included incubation with a nonspecific Ig of the same isotype as the primary antibody. The total number of F4/80-positive macrophages was quantitated in 20 randomly chosen fields (40×) using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Samples were examined in a blinded manner.
Tubular injury was evaluated in hematoxylin-eosin sections by an outside pathologist (J.B.) blinded to the nature of the samples. Evidence for cell injury (loss of brush border, vacuolization), cell desquamation, focal calcification, tubular dilation, mitosis and signs of regeneration were scored in a semiquantitative scale from 0 to 3, and results from each item were added to yield the histologic AKI score, which had a maximal value of 21.
Quantitative Reverse Transcription-PCR
One microgram RNA isolated with Trizol (Invitrogen, Carlsbad, California) was reverse-transcribed with High Capacity cDNA Archive Kit and real-time PCR was performed on a ABI Prism 7500 PCR system (Applied Biosystems, Foster City, California) using the DeltaDelta Ct method.7 Expression levels are given as ratios to GAPDH. Predeveloped primer and probe assays were obtained for murine GAPDH and Klotho (Applied Biosystems).
Western Blot
Cell samples or tissue were homogenized in lysis buffer (50 mM TrisHCl, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.2% Triton X-100, 0.3% NP-40, 0.1 mM PMSF, and 1 μg/ml pepstatin A) and then separated by 10% SDS-PAGE under reducing conditions. After electrophoresis, samples were transferred to PVDF membranes (Millipore, Bedford, Massachusetts), blocked with 5% skimmed milk in PBS/0.5% vol/vol Tween 20 for 1 h, washed with PBS/Tween, and incubated with rabbit polyclonal anti-Klotho (1:500, Calbiochem, La Jolla, California) or goat polyclonal anti-HDAC1 (1/500, Santa Cruz Biotechnology, Santa Cruz, California). Antibodies were diluted in 5% milk PBS/Tween. Blots were washed with PBS/Tween and incubated with appropriate horseradish peroxidase-conjugated secondary antibody (1:2000, Amersham, Aylesbury, UK). After washing with PBS/Tween the blots were developed with the chemiluminescence method (ECL, Amersham). Blots were then probed with mouse monoclonal anti-α-tubulin antibody (1:2000, Sigma), and levels of expression were corrected for minor differences in loading.
EMSA
Cells were resuspended in buffer A and homogenized.7 Nuclei and cytosolic fractions were separated by centrifugation at 1000 g for 10 minutes. Nuclei (pellet) were washed twice in buffer A and resuspended in the same buffer, with a final concentration of 0.39 mol/L KCl. Nuclei were extracted for 1 hour at 4°C and centrifuged at 100,000 g for 30 minutes. Supernatants were dialyzed in buffer C and then cleared by centrifugation and stored at −80°C. The protein concentration was determined by the bicinchonicic acid method. EMSA was carried out as described previously.7
Immunostaining
Cells plated onto Labtek slides were fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100/PBS, washed in PBS, and incubated with rabbit polyclonal anti-RelA (1:75), followed by FITC secondary antibody (1:200, Sigma). Nuclei were counterstained with propidium iodide.
Immunoprecipitation
Nuclear extracts of MCT cells treated with TWEAK or TNF was obtained as described previously.7 One hundred micrograms of nuclear extract was immunoprecipitated. In brief, each sample was precleared with 90 μl protein A/G–Sepharose beads (Santa Cruz Biotechnology). Precleared nuclear extracts were incubated with 3 μg of rabbit polyclonal anti-RelA (Santa Cruz Biotechnology) overnight at 4°C with gentle rotation. Next, extracts were incubated with 20 μl of bead suspension for 2 h at 4°C with rotating. The complexes were then centrifuged at 4°C for 1 minute, and the beads were washed four times with 100 μl buffer A.7 The beads were then resuspended in SDS sample buffer, boiled for 5 minutes, and analyzed on SDS-10% polyacrylamide gels as described above.
ChIP assay
ChIP assays using 0.5 to 1 × 106 cells per sample were performed as described previously61 using 10 μg/ml anti-AcH3, anti-AcH4, and anti-RelA (Upstate Biotechnology) antibodies. Normal IgG was used as negative control. In brief, cells fixed with 1% formaldehyde were lysed in SDS-lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1) and sonicated. Chromatin was diluted into ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl) and incubated with the antibodies overnight at 4°C. Antibody-chromatin complexes were precipitated with salmon sperm DNA/Protein A–Agarose beads (Upstate Biotechnology), washed, and eluted from the beads using elution buffer (1% SDS, 0.1 M NaHCO3). After crosslink reversal and proteinase K treatment, DNA was extracted with phenol-chloroform, and ethanol was precipitated. DNA immunoprecipitated from 1 μl eluted DNA was analyzed in duplicate by real-time PCR. Primers were designed to amplify specific NFκB regions (regions 1 and 2) at promoter of the Klotho gene, identified by TFSEARCH: Searching Transcription Factor Binding Sites (http://molsun1.cbrc.aist.go.jp/research/db/TFSEARCH.html). Primers for each promoter are listed in Supplemental Table S1. Aliquots of chromatin obtained before immunoprecipitation were analyzed as input control. Results are presented as fold enrichment of precipitated DNA associated with a given histone modification or RelA binding, relative to a 1/100 dilution of input chromatin.
siRNA Transfection
Cells were seeded in six-well plates and transfected on the following day with 20 nM siRNA using Lipofectamine 2000 reagent (Invitrogen).62 After 48 h, the transfected cells were harvested for Western blot or PCR analysis. siRNA were synthesized by Santa Cruz Biotechnology.
Statistical Analysis
Statistical analysis was performed using SPSS 11.0 statistical software. Results are expressed as mean ± SD. Significance at the P < 0.05 level was assessed by t test for two groups of data and ANOVA for three or more groups.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
Grant support: ISCII and FEDER funds CP04/00060, PS09/00447, 06/0046, SAF2005–03378, EU QLG1-CT-2002-01215, Sociedad Española de Nefrologia, ISCIII-RETIC REDinREN/RD06/0016, Comunidad de Madrid/CIFRA/S-BIO0283/2006, Ministerio de Ciencia y Tecnología (SAF2007/63648, PI10/00072), CAM (S2006/ GEN-0247), RECAVA (RD06/0014/0035;) cvREMOD (091100), IRSIN/FRIAT, FIS PI08/0566. Salary support: FIS to J.A.M. (CD05/00083 and CP10/00479), A.S., and M.C.I., MEC to M.D.S.N., Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CM) to A.O. We thank Mar Gonzalez García-Parreño for help with confocal microscopy, Susana Carrasco for technical help, and Linda Burkly for her critical review and suggestions.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Remuzzi G, Ruggenenti P, Benigni A: Understanding the nature of renal disease progression. Kidney Int 51: 2–15, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Grande MT, Perez-Barriocanal F, Lopez-Novoa JM: Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J Inflamm 7: 19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Locksley RM, Killeen N, Lenardo MJ: The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 104: 487–501, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A: NF-kappaB in renal inflammation. J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Ortiz A, Bustos C, Alonso J, Alcazar R, Lopez-Armada MJ, Plaza JJ, Gonzalez E, Egido J: Involvement of tumor necrosis factor-alpha in the pathogenesis of experimental and human glomerulonephritis. Adv Nephrol Necker Hosp 24: 53–77, 1995 [PubMed] [Google Scholar]
- 6. Justo P, Sanz A, Lorz C, Gomez-Garre D, Mezzano S, Egido J, Ortiz A: Expression of Smac/Diablo in tubular epithelial cells and during acute renal failure. Kidney Int Suppl S52–S56, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A: The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19: 695–703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Justo P, Sanz AB, Sanchez-Nino MD, Winkles JA, Lorz C, Egido J, Ortiz A: Cytokine cooperation in renal tubular cell injury: The role of TWEAK. Kidney Int 70: 1750–1758, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Sanz AB, Sanchez-Nino MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Selgas R, Egido J, Ortiz A: TWEAK activates the non-canonical NFkappaB pathway in murine renal tubular cells: modulation of CCL21. PLoS One 5: e8955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortiz A, Lorz C, Catalan MP, Danoff TM, Yamasaki Y, Egido J, Neilson EG: Expression of apoptosis regulatory proteins in tubular epithelium stressed in culture or following acute renal failure. Kidney Int 57: 969–981, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Sanz AB, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A: Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol 19: 1634–1642, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Munoz-Garcia B, Martin-Ventura JL, Martinez E, Sanchez S, Hernandez G, Ortega L, Ortiz A, Egido J, Blanco-Colio LM: Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: Modulation by atorvastatin. Stroke 37: 2044–2053, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Munoz-Garcia B, Moreno JA, Lopez-Franco O, Sanz AB, Martin-Ventura JL, Blanco J, Jakubowski A, Burkly LC, Ortiz A, Egido J, Blanco-Colio LM: Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) enhances vascular and renal damage induced by hyperlipidemic diet in ApoE-knockout mice. Arterioscler Thromb Vasc Biol 29: 2061–2068, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Prie D, Beck L, Urena P, Friedlander G: Recent findings in phosphate homeostasis. Curr Opin Nephrol Hypertens 14: 318–324, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T: Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317: 803–806, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M: Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280: 38029–38034, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, Kimura K, Hasegawa A, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R: Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun 249: 865–871, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H: Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 20: 2636–2645, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M: FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol 21: 1125–1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y: Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima Y, Nagail R: Molecular cloning of rat klotho cDNA: Markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun 251: 920–925, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Narumiya H, Sasaki S, Kuwahara N, Irie H, Kusaba T, Kameyama H, Tamagaki K, Hatta T, Takeda K, Matsubara H: HMG-CoA reductase inhibitors up-regulate anti-aging klotho mRNA via RhoA inactivation in IMCD3 cells. Cardiovasc Res 64: 331–336, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Saito K, Ishizaka N, Mitani H, Ohno M, Nagai R: Iron chelation and a free radical scavenger suppress angiotensin II-induced downregulation of klotho, an anti-aging gene, in rat. FEBS Lett 551: 58–62, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H: Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 101: e67–e74, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Ruiz-Ortega M, Lorenzo O, Ruperez M, Konig S, Wittig B, Egido J: Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: Molecular mechanisms. Circ Res 86: 1266–1272, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Zhang W, Li Q, Wang L, Yang X: Simvastatin ameliorates glomerulosclerosis in Adriamycin-induced-nephropathy rats. Pediatr Nephrol 23: 2185–2194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyer-Schwesinger C, Dehde S, von RC, Gatzemeier S, Klug P, Wenzel UO, Stahl RA, Thaiss F, Meyer TN: Rho kinase inhibition attenuates LPS-induced renal failure in mice in part by attenuation of NF-kappaB p65 signaling. Am J Physiol Renal Physiol 296: F1088–F1099, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Hoffmann U, Bergler T, Rihm M, Pace C, Kruger B, Rummele P, Stoelcker B, Banas B, Mannel DN, Kramer BK: Upregulation of TNF receptor type 2 in human and experimental renal allograft rejection. Am J Transplant 9: 675–686, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Misaki T, Yamamoto T, Suzuki S, Fukasawa H, Togawa A, Ohashi N, Suzuki H, Fujigaki Y, Oda T, Uchida C, Kitagawa K, Hattori T, Kitagawa M, Hishida A: Decrease in tumor necrosis factor-alpha receptor-associated death domain results from ubiquitin-dependent degradation in obstructive renal injury in rats. Am J Pathol 175: 74–83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanz AB, Sanchez-Nino MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM, Ruiz-Ortega M, Egido J, Ortiz A: Tweak induces proliferation in renal tubular epithelium: A role in uninephrectomy induced renal hyperplasia. J Cell Mol Med 13: 3329–3342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hehner SP, Hofmann TG, Droge W, Schmitz ML: The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol 163: 5617–5623, 1999 [PubMed] [Google Scholar]
- 37. Campbell KJ, Rocha S, Perkins ND: Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Mol Cell 13: 853–865, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Ashburner BP, Westerheide SD, Baldwin AS, Jr.: The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol 21: 7065–7077, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS: TWEAKing tissue remodeling by a multifunctional cytokine: Role of TWEAK/Fn14 pathway in health and disease. Cytokine 40: 1–16, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Carrero JJ, Ortiz A, Qureshi AR, Martin-Ventura JL, Barany P, Heimburger O, Marron B, Metry G, Snaedal S, Lindholm B, Egido J, Stenvinkel P, Blanco-Colio LM: Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin J Am Soc Nephrol 4: 110–118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y: Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW: Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stevens LA, Viswanathan G, Weiner DE: Chronic kidney disease and end-stage renal disease in the elderly population: Current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis 17: 293–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perry VH: Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol 2010 [DOI] [PubMed] [Google Scholar]
- 45. Roux C: Osteoporosis in inflammatory joint diseases. Osteoporos Int 22:421–433, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS: Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A 104: 2331–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Witkowski JM, Soroczynska-Cybula M, Bryl E, Smolenska Z, Jozwik A: Klotho—a common link in physiological and rheumatoid arthritis-related aging of human CD4+ lymphocytes. J Immunol 178: 771–777, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Goodwin BL, Pendleton LC, Levy MM, Solomonson LP, Eichler DC: Tumor necrosis factor-alpha reduces argininosuccinate synthase expression and nitric oxide production in aortic endothelial cells. Am J Physiol Heart Circ Physiol 293: H1115–H1121, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Anderson HD, Rahmutula D, Gardner DG: Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem 279: 963–969, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Zhu NL, Li C, Huang HH, Sebald M, Londhe VA, Heisterkamp N, Warburton D, Bellusci S, Minoo P: TNF-alpha represses transcription of human bone morphogenetic protein-4 in lung epithelial cells. Gene 393: 70–80, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saitoh T, Nakayama M, Nakano H, Yagita H, Yamamoto N, Yamaoka S: TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem 278: 36005–36012, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Natoli G, Saccani S, Bosisio D, Marazzi I: Interactions of NF-kappaB with chromatin: The art of being at the right place at the right time. Nat Immunol 6: 439–445, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Aarenstrup L, Flindt EN, Otkjaer K, Kirkegaard M, Andersen JS, Kristiansen K: HDAC activity is required for p65/RelA-dependent repression of PPARdelta-mediated transactivation in human keratinocytes. J Invest Dermatol 128: 1095–1106, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Liu MY, Khachigian LM: Histone deacetylase-1 is enriched at the platelet-derived growth factor-D promoter in response to interleukin-1beta and forms a cytokine-inducible gene-silencing complex with NF-kappab p65 and interferon regulatory factor-1. J Biol Chem 284: 35101–35112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55. Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, Fujimori T, Xie P, Kanwar YS: Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A 104: 2331–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sugiura H, Yoshida T, Tsuchiya K, Mitobe M, Nishimura S, Shirota S, Akiba T, Nihei H: Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 20: 2636–2645, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Ortega A, Ramila D, Ardura JA, Esteban V, Ruiz-Ortega M, Barat A, Gazapo R, Bosch RJ, Esbrit P: Role of parathyroid hormone-related protein in tubulointerstitial apoptosis and fibrosis after folic acid-induced nephrotoxicity. J Am Soc Nephrol 17: 1594–1603, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Fang TC, Alison MR, Cook HT, Jeffery R, Wright NA, Poulsom R: Proliferation of bone marrow-derived cells contributes to regeneration after folic acid-induced acute tubular injury. J Am Soc Nephrol 16: 1723–1732, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Doi K, Okamoto K, Negishi K, Suzuki Y, Nakao A, Fujita T, Toda A, Yokomizo T, Kita Y, Kihara Y, Ishii S, Shimizu T, Noiri E: Attenuation of folic acid-induced renal inflammatory injury in platelet-activating factor receptor-deficient mice. Am J Pathol 168: 1413–1424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Campbell S, Michaelson J, Burkly L, Putterman C: The role of TWEAK/Fn14 in the pathogenesis of inflammation and systemic autoimmunity. Front Biosci 9: 2273–2284, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Suarez-Alvarez B, Rodriguez RM, Calvanese V, Blanco-Gelaz MA, Suhr ST, Ortega F, Otero J, Cibelli JB, Moore H, Fraga MF, Lopez-Larrea C: Epigenetic mechanisms regulate MHC and antigen processing molecules in human embryonic and induced pluripotent stem cells. PLoS One 5: e10192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sanchez-Nino MD, Sanz AB, Lorz C, Gnirke A, Rastaldi MP, Nair V, Egido J, Ruiz-Ortega M, Kretzler M, Ortiz A: BASP1 promotes apoptosis in diabetic nephropathy. J Am Soc Nephrol 21: 610–621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.