Abstract
Whether the rate of kidney function decline before the onset of CKD differs among racial and ethnic groups remains unclear. Here, we evaluated kidney function decline and incident CKD among white, black, Hispanic, and Chinese participants in the Multi-Ethnic Study of Atherosclerosis (MESA) during 5 years of follow-up. We estimated GFR using both cystatin C (eGFRcys) and creatinine (eGFRcreat). The definition of incident CKD required eGFRcys <60 ml/min per 1.73 m2 and a decline in eGFRcys ≥1 ml/min per year. Among participants with eGFRcreat >60 ml/min per 1.73 m2 at baseline, blacks had a significantly higher rate of kidney function decline than whites (0.31 ml/min per 1.73 m2/yr faster on average, P = 0.001), even after adjusting for multiple potential confounders. Among Hispanics, Dominicans and Puerto Ricans had faster rates of decline than whites (0.55 and 0.47 ml/min per 1.73 m2/yr faster, respectively). Mexicans, South Americans, or other Hispanics had similar rates of decline compared to whites. We did not detect significant differences in the rates of kidney function decline among Chinese and white participants. Among those with normal or near-normal kidney function at baseline, blacks and Hispanics had the highest rates of incident CKD during follow-up. Adjustment for comorbidities attenuated some of these differences. In conclusion, the average rate of kidney function decline before the onset of CKD differs among racial and ethnic groups. Traditional risk factors do not explain these differences fully, highlighting the need to explore these disparities.
The burden of end-stage renal disease (ESRD) is disproportionately higher for blacks and Hispanics compared with whites in the United States.1 Race/ethnic differences in progression from chronic kidney disease (CKD), defined as estimated GFR (eGFR) <60 ml/min per 1.73 m2, to ESRD have been well documented.2–4 Because of national data reports of similar or lower prevalence of CKD among blacks and Hispanics in the United States,5 race/ethnic differences in rates of ESRD have been primarily attributed to faster rates of progression from established CKD to ESRD.6 However, no studies have prospectively evaluated early kidney function decline before the onset of CKD in a large, ethnically diverse community-based cohort study across the United States.
A few studies have assessed kidney function decline before the onset of CKD among specific populations,7–9 and these have primarily used creatinine-based estimates of eGFR, which may be biased across racial groups because of differences in muscle mass and body composition. Moreover, creatinine-based estimating equations have not been validated among non-white, non-black persons without CKD.10,11 Cystatin C may have an important role in detecting early changes in kidney function across racial groups because it appears to be less influenced by age, sex, and muscle mass. Cystatin C has been shown to be a better predictor of cardiovascular events and death than creatinine, particularly among persons with eGFR >60 ml/min per 1.73 m2.12,13 It has also been shown to be a more accurate marker of GFR decline than creatinine-based estimates in some studies using direct measures of GFR.14,15
A better understanding of race/ethnic differences in rates of early kidney function decline and incident CKD is needed to fill the evidence gap on rates of disease initiation across race/ethnic groups. In addition, this knowledge is critical for reducing disparities in the burden of CKD and ESRD, and may lead to the development of prevention strategies. Therefore, we designed these analyses to (1) compare rates of kidney function decline among white, black, Hispanic, and Chinese persons without CKD, (2) evaluate rates of incident CKD by race/ethnicity, and (3) investigate risk factors that might explain any observed race/ethnic differences.
RESULTS
Participant Characteristics
Among 5179 MESA participants with eGFRcreat >60 ml/min per 1.73 m2, 2496 (48%) were male, 12% self-identified as Chinese, 27% as black, and 22% as Hispanic. Overall, 590 (11%) had diabetes, 2099 (41%) had hypertension, and 361 (7%) had an albumin-to-creatinine ratio (ACR) ≥30 mg/g. At baseline, mean eGFRcys and eGFRcreat were 95 (±17) and 82 (±13) ml/min per 1.73 m2, respectively. Blacks and Hispanics were less likely to have a college degree and were more likely to be diabetic or hypertensive. Blacks had the highest levels of systolic BP. Chinese had the lowest smoking rates, LDL levels, and BMI (Table 1).
Table 1.
Characteristics of participants in the multi-ethnic study of atherosclerosis without CKD at baseline by race/ethnicity
| White (n = 1989) | Chinese (n = 617) | Black (n = 1409) | Hispanic (n = 1164) | P | |
|---|---|---|---|---|---|
| Age | 61 (10) | 60 (10) | 60 (10) | 60 (10) | 0.294 |
| Male | 990 (50) | 300 (49) | 637 (45) | 569 (49) | 0.063 |
| Education | <0.001 | ||||
| <HS | 88 (4) | 127 (21) | 145 (10) | 493 (42) | |
| HS + none college level | 854 (43) | 233 (38) | 750 (54) | 555 (48) | |
| completed college or more | 1043 (53) | 256 (42) | 508 (36) | 116 (10) | |
| Income | <0.001 | ||||
| <$20,000 | 183 (9) | 223 (36) | 244 (19) | 417 (37) | |
| $20,000 to $39,999 | 387 (20) | 146 (24) | 394 (30) | 405 (36) | |
| $40,000 to $74,999 | 625 (32) | 125 (20) | 420 (32) | 228 (20) | |
| $≥$75,000 | 750 (39) | 119 (19) | 250 (19) | 91 (8) | |
| Diabetes | 106 (5) | 73 (12) | 219 (16) | 192 (17) | <0.001 |
| Hypertension | 672 (34) | 197 (32) | 789 (56) | 441 (38) | <0.001 |
| Smoking status | <0.001 | ||||
| never | 862 (43) | 467 (76) | 630 (45) | 627 (54) | |
| former | 889 (45) | 115 (19) | 512 (37) | 378 (33) | |
| current | 234 (12) | 34 (6) | 261 (19) | 159 (14) | |
| SBP (mm Hg) | 122 (20) | 122 (20) | 130 (21) | 125 (21) | <0.001 |
| DBP (mm Hg) | 70 (10) | 72 (10) | 75 (10) | 72 (10) | <0.001 |
| HTN medicines | 557 (28) | 150 (24) | 653 (46) | 349 (30) | <0.001 |
| LDL (mg/dl) | 117 (30) | 115 (28) | 117 (33) | 119 (32) | 0.030 |
| HDL (mg/dl) | 52 (16) | 50 (13) | 52 (15) | 48 (13) | <0.001 |
| BMI (kg/m2) | 27.7 (5.1) | 23.9 (3.3) | 30.0 (5.7) | 29.5 (5.2) | <0.001 |
| CRPa (mg/dl) | 1.63 [0.71, 3.82] | 0.88 [0.46, 1.84] | 2.38 [1.04, 4.92] | 2.39 [1.09, 4.97] | <0.001 |
| eGFR CKD-EPI (ml/min per 1.73 m2) | 78 (11) | 84 (12) | 85 (15) | 84 (13) | <0.001 |
| eGFR-cysC (ml/min per 1.73 m2) | 94 (17) | 100 (15) | 96 (17) | 94 (16) | <0.001 |
| UACRa (mg/g) | 4.4 [3.0, 7.7] | 6.0 [3.8, 11.3] | 5.1 [3.0, 11.2] | 5.8 [3.6, 11.0] | <0.001 |
Mean (SD) or N (%).
aMedian [IQR].
Race/Ethnicity and Kidney Function Decline
Among all participants, 4214 (82%) had cystatin C measures at exams 1, 3, and 4, whereas the remaining had cystatin C at baseline and exam 3 or 4. Median follow-up time was 4.64 years.
The age- and sex-adjusted rates of eGFRcys decline in ml/min per 1.73 m2 per year (95% confidence interval [CI]) were fastest for blacks −1.39 (−1.52 to −1.26), followed by Hispanics −1.25 (−1.39 to −1.11), Chinese −1.03 (−1.22 to −0.83), and whites −0.87 (−0.98 to −0.76). Compared with whites, blacks had higher rates of decline and these findings were not attenuated by sociodemographics or traditional risk factors. In contrast, rates of decline among Chinese were not significantly different from whites. Findings did not differ when we used eGFRcreat estimates. (Table 2).
Table 2.
Decline in eGFR by cystatin C and creatinine over 5 years among black, white, and Chinese MESA participants without CKD at baseline
| Race | N | Model 1 Adjusteda β (95% CI) | P | Model 2 Adjustedb β (95% CI) | P | Model 3 Adjustedc β (95% CI) | P |
|---|---|---|---|---|---|---|---|
| eGFRcys ml/min per 1.73 m2 | |||||||
| white | 1989 | Ref | Ref | Ref | |||
| Chinese | 617 | −0.17 (−0.40, 0.06) | 0.14 | −0.16 (−0.39, 0.07) | 0.18 | −0.16 (−0.39, 0.07) | 0.18 |
| black | 1409 | −0.36 (−0.53, −0.19) | <0.001 | −0.31 (−0.49, −0.13) | 0.001 | −0.31 (−0.49, −0.14) | 0.001 |
| eGFR CKD EPI ml/min per 1.73 m2 | |||||||
| white | 1989 | Ref | Ref | Ref | |||
| Chinese | 617 | 0.06 (−0.10, 0.22) | 0.45 | 0.06 (−0.10, 0.22) | 0.45 | 0.05 (−0.11, 0.21) | 0.53 |
| black | 1409 | −0.37 (−0.49, −0.25) | <0.001 | −0.38 (−0.50, −0.26) | <0.001 | −0.38 (−0.50, −0.26) | <0.001 |
aModel 1: adjusted for age, gender, income, and education.
bModel 2: further adjusted for LDL, HDL, BMI, smoking, and CRP.
cModel 3: further adjusted for diabetes, hypertension, and systolic blood pressure.
The rate of kidney function decline was also faster for Hispanics compared with whites, but differences varied by country of origin. Compared with whites, Dominicans had the fastest rates of decline, followed by Puerto Ricans. These differences were not attenuated after adjustment for sociodemographics or comorbidities. Mexican/Central American Hispanics had slightly faster rates of decline compared with whites, but these were not statistically significant. As with the findings among blacks, these findings did not significantly differ when using eGFRcys or eGFRcreat (Table 3).
Table 3.
Decline in eGFR by cystatin C and creatinine over 5 years among MESA participants without CKD by Hispanic ethnicity and country of origin
| Ethnicity | N | Model 1 Adjusteda β (95% CI) | P | Model 2 Adjustedb β (95% CI) | P | Model 3 Adjustedc β (95% CI) | P |
|---|---|---|---|---|---|---|---|
| eGFRcys ml/min per 1.73 m2 | |||||||
| white | 1989 | Ref | Ref | Ref | |||
| all Hispanics | 1164 | −0.29 (−0.47, −0.11) | 0.002 | −0.27 (−0.45, −0.09) | 0.004 | −0.28 (−0.46, −0.09) | 0.003 |
| Mexican/Central American | 503 | −0.23 (−0.48, 0.01) | 0.06 | −0.20 (−0.45, 0.05) | 0.110 | −0.22 (−0.47, 0.03) | 0.08 |
| Dominican | 146 | −0.55 (−0.97, −0.13) | 0.01 | −0.55 (−0.97, −0.13) | 0.01 | −0.54 (−0.97, −0.12) | 0.01 |
| Puerto Rican | 153 | −0.48 (−0.88, −0.07) | 0.02 | −0.46 (−0.87, −0.05) | 0.03 | −0.47 (−0.87, −0.06) | 0.02 |
| South American | 94 | −0.22 (−0.74, 0.31) | 0.42 | −0.20 (−0.72, 0.32) | 0.45 | −0.17 (−0.70, 0.35) | 0.51 |
| other Hispanic | 268 | −0.16 (−0.48, 0.16) | 0.33 | −0.15 (−0.47, 0.17) | 0.35 | −0.14 (−0.47, 0.18) | 0.38 |
| eGFR CKD EPI ml/min per 1.73 m2 | |||||||
| white | 1989 | Ref | Ref | Ref | |||
| all Hispanics | 1164 | −0.15 (−0.28, −0.03) | 0.02 | −0.15 (−0.28, −0.03) | 0.02 | −0.14 (−0.27, −0.02) | 0.03 |
| Mexican/Central American | 503 | −0.11 (−0.28, 0.07) | 0.23 | −0.09 (−0.27, 0.08) | 0.29 | −0.11 (−0.28, 0.07) | 0.24 |
| Dominican | 146 | −0.52 (−0.81, −0.23) | <0.001 | −0.54 (−0.83, −0.25) | <0.001 | −0.55 (−0.84, −0.26) | <0.001 |
| Puerto Rican | 153 | −0.33 (−0.61, −0.04) | 0.03 | −0.33 (−0.62, −0.05) | 0.02 | −0.33 (−0.62, −0.05) | 0.02 |
| South American | 94 | −0.11 (−0.47, 0.26) | 0.56 | −0.11 (−0.47, 0.25) | 0.56 | −0.02 (−0.38, 0.34) | 0.91 |
| other Hispanic | 268 | 0.05 (−0.17, 0.27) | 0.66 | 0.04 (−0.18, 0.26) | 0.74 | 0.08 (−0.14, 0.31) | 0.48 |
aModel 1: adjusted for age, gender, income, and education.
bModel 2: further adjusted for LDL, HDL, BMI, smoking, and CRP.
cModel 3: further adjusted for diabetes, hypertension, and systolic blood pressure.
We conducted two sensitivity analyses. First, when we studied rates of decline after excluding persons with ACR >30 mg/g at baseline, race/ethnic differences persisted. Among persons with ACR <30 mg/g, blacks (β −0.22 ml/min per 1.73 m2/yr [−0.40 to −0.04]) and Hispanics (β −0.23 ml/min per 1.73 m2/yr, 95% CI −0.42 to −0.05) declined significantly faster than whites after full adjustment. There were no statistically significant differences between Chinese and whites (β −0.16 [−0.39 to 0.07]). In a second sensitivity analysis, we included only persons with eGFRcreat >90 ml/min per 1.73 m2 and ACR <30 mg/g at baseline. Approximately 25% of the cohort fit this definition (n = 1279), including 300 whites, 442 blacks, 197 Chinese, and 340 Hispanics. Although not statistically significant, blacks declined faster than whites. In age- and sex-adjusted models, blacks had an eGFRcys decline of −0.22, 95% CI: −0.59 to 0.11 ml/min per 1.73 m2 per year faster than whites. Adjustment for covariates did not attenuate the effect size (−0.24, 95% CI −0.59 to 0.12). Among Hispanics of Dominican and Puerto Rican origin, the effect size was similar to that of blacks. Dominican and Puerto Rican Hispanics had an eGFRcys decline of −0.20 (−0.91 to 0.52) and −0.23 (−1.01 to 0.56) ml/min per 1.73 m2/yr, respectively, faster decline compared with whites. Mexicans declined by only −0.08 (−0.55 to 0.38) ml/min per 1.73 m2 per year faster compared with whites.
Race/Ethnicity and Incident CKD
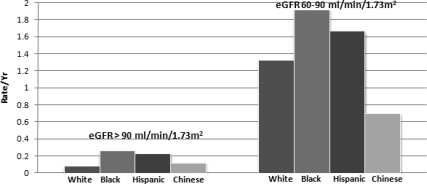
There were 217 cases of incident CKD, defined as reaching an eGFRcys <60 ml/min per 1.73 m2 and having an eGFRcys decline ≥1 ml/min per year. Blacks had the highest incidence rate of CKD in age- and sex-adjusted models among persons with baseline eGFR >90 ml/min per 1.73 m2 and 60 to 90 ml/min per 1.73 m2, followed by Hispanics. Chinese had the lowest rate of incident CKD among those with eGFR 60 to 90 ml/min per 1.73 m2 at baseline only (Figure 1).
Figure 1.
Age- and sex-adjusted rates of incident chronic kidney disease are higher among blacks and Hispanics in MESA.
We studied the role of potential risk factors in explaining the differences in incident CKD by race/ethnicity. Among persons with baseline eGFR >90 ml/min per 1.73 m2, blacks had more than threefold incident CKD compared with whites, and this was not attenuated by adjustment for age, sex, income, education, baseline eGFRcys, lipids, BMI, or inflammation. The association was somewhat attenuated by adjustment for diabetes and hypertension. Hispanics had more than twofold incidence rate compared with whites. The difference was not statistically significant, but the point estimate remained almost the same in all models. Chinese had no significant difference in incident CKD compared with whites. Among persons with eGFR 60 to 90 ml/min per 1.73 m2 at baseline, blacks and Hispanics also had higher rates of incident CKD, but these differences were smaller in magnitude and were attenuated by adjustment for sociodemographics and comorbidities (Table 4).
Table 4.
Exploration of risk factors as intermediaries in the association of race/ethnicity and incident CKD in MESA, stratified by baseline eGFRcys
| Race | N | No. Event | Rate per Year | Model 1a RR (95% CI) | P | Model 2b RR (95% CI) | P | Model 3c RR (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| eGFR-cysC >90 ml/min per 1.73 m2 at baseline | |||||||||
| white | 1139 | 5 | 0.09 | Ref | Ref | Ref | |||
| Chinese | 439 | 3 | 0.14 | 1.36 (0.28, 6.52) | 0.70 | 2.23 (0.40, 12.40) | 0.36 | 2.13 (0.36, 12.53) | 0.41 |
| black | 897 | 14 | 0.34 | 3.40 (1.15, 10.06) | 0.03 | 3.59 (1.20, 10.78) | 0.02 | 2.42 (0.78, 7.48) | 0.12 |
| Hispanic | 690 | 10 | 0.30 | 2.27 (0.62, 8.34) | 0.22 | 2.77 (0.69, 11.18) | 0.15 | 2.57 (0.60, 10.99) | 0.20 |
| eGFR-cysC 60 to 90 ml/min per 1.73 m2 at baseline | |||||||||
| white | 801 | 69 | 1.85 | Ref | Ref | Ref | |||
| Chinese | 178 | 9 | 1.09 | 0.59 (0.31, 1.14) | 0.12 | 0.62 (0.32, 1.21) | 0.16 | 0.52 (0.27, 1.00) | 0.05 |
| black | 490 | 58 | 2.63 | 1.33 (0.96, 1.85) | 0.08 | 1.29 (0.92, 1.82) | 0.14 | 1.08 (0.77, 1.52) | 0.64 |
| Hispanic | 456 | 49 | 2.32 | 1.08 (0.75, 1.55) | 0.67 | 1.09 (0.76, 1.56) | 0.64 | 0.91 (0.63, 1.33) | 0.64 |
aModel 1: adjusted for age, gender, income, education, and baseline eGFR-cysC.
bModel 2: further adjusted for HDL, LDL, CRP, and BMI.
cModel 3: further adjusted for DM and HTN.
We conducted three sensitivity analyses for incident CKD. When we defined incident CKD as reaching an eGFRcys <60 ml/min per 1.73 m2 AND CKD-EPI <60 ml/min per 1.73 m2, we identified 193 cases of incident CKD. Overall, the rate ratio (RR) for blacks was 1.82 (1.20 to 2.75), 1.44 (0.91 to 2.26) for Hispanics, and 0.53 (0.28 to 0.98) for Chinese compared with whites in age- and sex-adjusted models. We also used a definition of “persistent CKD.” For these analyses, we included only persons with three measures of kidney function and defined incident CKD as having eGFRcys <60 ml/min per 1.73 m2 AND having an eGFRcys decline ≥1 ml/min per year at both exams. With use of this third definition, there were 83 cases of incident CKD; age- and sex-adjusted incident rate ratio (IRR) were 1.27 (0.77 to 2.09) for blacks, 1.13 (0.66 to 1.95) for Hispanics, and 0.44 (0.16 to 1.23) for Chinese compared with whites. In our third sensitivity analysis, we excluded persons with ACR >30 mg/g at baseline and stratified by baseline eGFRcys as in our main analyses. After adjustment for age, gender, income, education, and baseline eGFRcys, blacks and Hispanics had higher rates of incident CKD compared with whites among those with eGFRcys >90 ml/min per 1.73 m2. Point estimates were similar to those in our main analyses, but these were not statistically significant: RR 3.33 (0.86 to 12.94) for blacks and 4.12 (0.76 to 22.23) for Hispanics. Among persons with eGFRcys 60 to 90 ml/min per 1.73 m2, Chinese had lower incidence rates of CKD (IRR 0.61, 0.31 to 1.20). Rates of incident CKD did not significantly differ for blacks and Hispanics in this GFR range (IRR 1.0, 0.68 to 1.48 for blacks and IRR 0.98, 0.65 to 1.47 for Hispanics).
DISCUSSION
In a large, multiethnic cohort free of cardiovascular disease and with eGFRcreat >60 ml/min per 1.73 m2 at baseline, we found that kidney function decline varied significantly by race/ethnicity, and that these differences were present even among those without albuminuria at baseline. Blacks and Hispanics had higher rates of decline compared with whites, whereas Chinese and whites had similar rates of kidney function decline. Among Hispanics, we found that rates of decline varied significantly by country of origin, and were highest among Dominicans and Puerto Ricans. These differences were not explained by traditional risk factors. Findings were similar whether we used cystatin C- or creatinine-based estimates of GFR. In addition, we found that blacks had the highest rates of incident CKD, followed by Hispanics, and these differences were most pronounced among those with eGFRcys >90 ml/min per 1.73 m2.
Scant data exist on kidney function decline before the onset of CKD. Estimates from the National Health and Nutrition Survey (NHANES) show a lower prevalence of CKD among blacks and Mexican Americans compared with whites, despite higher rates of incident ESRD.5 These findings have led to the suggestion that race/ethnic differences in kidney function decline occur only once CKD is established (eGFR ≤60 ml/min per 1.73 m2).6,16 Our findings challenge this paradigm and suggest that, in contrast, kidney function decline differs by race/ethnicity starting at very early stages of disease. Previous reports may be explained by their extrapolation of cross-sectional estimates of CKD prevalence to make inferences on early decline.17 In addition, using creatinine-based equations may bias estimates by race. In fact, recalculation of eGFR in NHANES using the new CKD-EPI equation found similar CKD prevalence among blacks and whites in NHANES.11 Emerging literature shows that “preclinical” stages of kidney disease are associated with adverse events and higher cardiovascular risk.13,18 Thus, our findings suggest that interventions to reduce race/ethnic disparities in the burden of CKD and ESRD must be targeted to identify those at highest risk and implemented much earlier in the course of disease than previously thought.
Our findings that race/ethnic differences in early kidney function decline are not explained by traditional risk factors or the presence of albuminuria are of great interest. One possible explanation is that genetic factors may have an important role in explaining these differences. Studies among persons with nondiabetic ESRD have shown an association of polymorphisms in the MYH9 gene and ESRD among persons of African ancestry.19 Most recently, variants in the APOL1 gene have been found to be strongly associated with ESRD secondary to focal segmental nephrosclerosis and hypertension in blacks.20
Other biologic and environmental risk factors have been proposed to explain faster rates of progression from CKD to ESRD in blacks, including differences in social and demographic factors as well as biologic factors. Prior reports suggest that sociodemographic differences may have important influences on chronic disease through mediators such as chronic stress, psychosocial factors (i.e., pessimism and low self-esteem),21 acculturation,22 environmental pollution,23 or differences in access to care.24,25 Whether or not these factors and their interaction with genetic predisposition may also explain race/ethnic differences in early kidney function decline will be an important area for future study.
Much less is known about Hispanics or Chinese. We found that rates of decline among Hispanics varied by country of origin, with Dominicans and Puerto Ricans having faster declines compared with whites, but not Mexican/Central Americans. It is noteworthy that Dominicans and Puerto Ricans have much higher degrees of African ancestry than other Hispanics26 because genetic risk for ESRD among Hispanics of higher African ancestry has been recently reported.27 Our findings highlight the heterogeneity of Hispanics in the United States. Future studies should consider country of origin when studying kidney disease epidemiology and treatment in Hispanic populations.
We also found that blacks and Hispanics have higher rates of incident CKD, whereas Chinese may have lower rates than whites. These differences were of greatest magnitude among those with eGFRcys >90 ml/min per 1.73 m2 at baseline. These findings suggest that recognition of kidney damage and reduced or declining kidney function is paramount even when the eGFR is “normal”. To that end, the role of traditional and novel biomarkers of kidney injury in identifying those at highest risk for kidney function decline and incident CKD in these populations should be investigated.
Our study is the first to report differences in kidney function decline among persons without CKD in four major race/ethnic groups. Study strengths include a large, well-characterized, diverse population, a longitudinal design with 5 years of follow up, and the use of two markers of renal function to estimate GFR. We are also the first to report differences within Hispanic subgroups. As with all epidemiologic studies, there may be residual confounders that were not included or measured. For example, we used individual income and education to characterize socioeconomic status, whereas studies suggest that these measures may only partially capture associations between sociodemographic factors and health.28
We could not account for duration of risk factors such as diabetes or hypertension. Although we did not have direct measures of GFR, our findings are robust with the use of state of the art equations using both cystatin C and creatinine. We were limited in studying the effect of race/ethnicity on kidney function decline at the highest GFR levels (>90 ml/min per 1.73 m2) because of power and the fact that equations have not been validated at this range of GFR. We also may have been limited by power because of the number of incident CKD cases that may have biased our results toward the null. Future studies with longer follow-up and repeated kidney function measures should be conducted.
In summary, we found that race/ethnic differences in kidney function decline exist among persons without CKD, and are present even in the absence of microalbuminuria. Reasons for these differences remain unclear. Future studies should focus on understanding mechanisms to explain these observations. Moreover, development of biomarkers to detect individuals at highest risk early in the course of disease is needed to design efficient and targeted primary prevention programs for CKD.
CONCISE METHODS
Patients
We included participants from the Multi-Ethnic Study of Atherosclerosis (MESA), a large NHLBI sponsored study designed to understand subclinical cardiovascular disease and its progression in a multi-ethnic cohort. Details on recruitment and design have been previously published.29 Briefly, MESA recruited 6814 men and women who were between 45 and 84 years old, were free of cardiovascular disease, and self-identified as white, black, Hispanic, or Chinese. Patients were recruited from the following areas between July 2000 and August 2002: Baltimore City and Baltimore County, MD; Chicago; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan and the Bronx, NY; and St. Paul, MN. Participants returned for three visits, at years 2002 through 2004 (exam 2), years 2004 through 2005 (exam 3), and years 2005 through 2007 (exam 4). Repeat measures of kidney function were done at visits 3 and 4. The institutional review boards at all participating centers approved the study, and all participants gave informed consent.
For these analyses, we excluded persons with no measure of albuminuria, creatinine, or cystatin at baseline (n = 94), persons who did not have any follow-up measures of creatinine or cystatin C (n = 799), and persons who had CKD at baseline (defined as eGFRcreat <60 ml/min per 1.73 m2) for a total sample size of 5179 persons.
Primary Predictor
Self-reported race/ethnicity and self-reported country of origin (for Hispanics) were assessed at baseline by questionnaire.
Outcomes
Kidney function was measured by creatinine and cystatin C. All assays were performed in frozen serum specimens that were stored at −70°C. Serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY) at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis) and calibrated to Cleveland Clinic. Cystatin C was measured by means of a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring) with a nephelometer (BNII, Dade Behring) and corrected for assay drift. We estimated the GFR with the use of the newly developed CKD-Epi equation11 (GFR = 141 × min(Scr/κ,1)α × max(Scr/κ, 1) − 1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black]) and the cystatin C equation: 76.7 × cys C −1.19. This formula was developed from the pooling of several cohorts with GFR measured from iothalamate.30
Our first outcome of interest was kidney function decline, assessed by both equations in separate models using repeated measures of eGFR. A second outcome, incident CKD (stage 3), was defined as eGFRcys <60 ml/min per 1.73 m2 AND eGFRcys decline ≥1 ml/min per year at any follow-up exam. We chose this definition to reduce misclassification due to eGFR changes close to the threshold. For the incident CKD outcome, we conducted three sensitivity analyses: (1) defining CKD as eGFRcys <60 ml/min per 1.73 m2 AND eGFRcreat <60 ml/min per 1.73 m2; (2) defining CKD as “persistent CKD”, defined as having CKD at both exam 3 and exam 4, defined as eGFRcys <60 ml/min per 1.73 m2, AND having an eGFRcys decline ≥1 ml/min per year at both visits; and (3) excluding persons with ACR >30 mg/g at baseline.
Covariates of Interest
Information on age, self-reported race/ethnicity, level of education, annual household income, and smoking history was obtained using standardized questionnaires. We categorized levels of education as less than high school, high school graduate, and college graduate or above. Income was categorized as annual household income: <$20,000; 20,000 to 39,999; 40,000 to 74,999; and ≥$75,000. BP measurements were obtained using the Dinamap automated BP device (Dinamap Monitor Pro 100). Three sequential measures were obtained and the average of the second and third measurements was recorded. Hypertension was defined as systolic pressure ≥140 mm Hg, diastolic pressure ≥90 mm Hg, or current use of antihypertensive medication. Diabetes was defined as either a fasting glucose ≥126 mg/dl or use of oral hypoglycemic medication or insulin. Cigarette smoking was defined as current, former, or never. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Fasting blood was collected and stored at −70°F until needed for the appropriate assays. HDL cholesterol was measured using the cholesterol oxidase cholesterol method (Roche Diagnostics). LDL cholesterol was calculated using the Friedewald equation. Urine albumin and creatinine were measured in a single morning urine sample by nephelometry and the rate Jaffe reaction, respectively, and expressed as albumin-to-creatinine ratio (ACR) in mg/g.
Statistical Analysis
We compared baseline characteristics by race/ethnicity among MESA participants without CKD at baseline using χ2 or ANOVA where appropriate. To evaluate the association between race/ethnicity and kidney function decline, we used linear mixed models with random intercepts and slopes to estimate and compare linear trends in mean eGFR.31 This approach takes into account the correlation of observations by patient. We truncated at eGFRcreat >120 ml/min per 1.73 m2. Hispanics are presented as overall and by country of origin, given data suggesting that Hispanics may differ genetically, culturally, and in risk factor profile by nationality.26 We used nested models with serial adjustment to understand the role of CKD risk factors chosen a priori that might explain any observed differences by race/ethnicity. Model 1 adjusted for age, sex, income, and education. Model 2 adjusted for model 1 plus HDL, LDL, BMI, smoking, and CRP. Model 3 further adjusted for diabetes, hypertension, and systolic BP. We performed two sensitivity analyses for the outcome kidney function decline. In our first sensitivity analysis, we excluded persons with ACR >30 mg/g because this may represent early stages of kidney damage. To determine whether differences in race/ethnicity may be present at the highest eGFR levels, we conducted a second sensitivity analysis including only persons with eGFRcreat >90 ml/min per 1.73 m2 and ACR <30 mg/g. We studied decline in eGFRcys adjusted for age and gender, followed by covariates in the primary analyses.
We used Poisson (log-link) regression with robust variance estimation and an offset for follow-up time to study the association of race/ethnicity and incident CKD. We stratified by baseline eGFRcys (>90 ml/min per 1.73 m2 and 60 to 90 ml/min per 1.73 m2) to account for race/ethnic differences in baseline kidney function. For these analyses, we excluded 89 persons with eGFRcys <60 ml/min per 1.73 m2 due to the fact that they may already have kidney disease. We estimated age- and sex-adjusted rates of incident CKD per year by race/ethnicity within each stratum. We then constructed nested models to study the role of risk factors on these associations as above.
DISCLOSURES
None.
Acknowledgments
This work was supported by contracts [N01-HC-95159 through N01-HC-95165] and [N01-HC-95169] from the National Heart, Lung, and Blood Institute for MESA. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This work was also funded by the NIDDK (1K23DK082793-01 to C.P.)
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Declining Renal Function in Persons of Different Race without Chronic Kidney Disease,” on pages 1183–1184.
REFERENCES
- 1. USRDS: Renal Data System (USRDS) Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 2. Peralta CA, Shlipak MG, Fan D, Ordonez J, Lash JP, Chertow GM, Go AS: Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol 17: 2892–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Hall YN, Hsu CY, Iribarren C, Darbinian J, McCulloch CE, Go AS: The conundrum of increased burden of end-stage renal disease in Asians. Kidney Int 68: 2310–2316, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Powe NR: Reverse race and ethnic disparities in survival increase with severity of chronic kidney disease: What does this mean? Clin J Am Soc Nephrol 1: 905–906, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Hanratty R, Chonchol M, Miriam Dickinson L, Beaty BL, Estacio RO, Mackenzie TD, Hurley LP, Linas SL, Steiner JF, Havranek EP: Incident chronic kidney disease and the rate of kidney function decline in individuals with hypertension. Nephrol Dial Transplant 25: 801–807, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vupputuri S, Batuman V, Muntner P, Bazzano LA, Lefante JJ, Whelton PK, He J: Effect of blood pressure on early decline in kidney function among hypertensive men. Hypertension 42: 1144–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shlipak MG, Sarnak M, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen CO: Cystatin-C and risk for mortality and cardiovascular disease in elderly adults. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: Results of a 4-year follow-up study. J Am Soc Nephrol 16: 1404–1412, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G: Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care 31: 971–973, 2008 [DOI] [PubMed] [Google Scholar]
- 16. McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, Cushman M, Howard G: Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol 17: 1710–1715, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Trivedi HS, Pang MM: Discrepancy in the epidemiology of nondiabetic chronic renal insufficiency and end-stage renal disease in black and white Americans: The third National Health and Nutrition Examination Survey and United States Renal Data System. Am J Nephrol 23: 448–457, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Curhan GC: Prediabetes, prehypertension … is it time for pre-CKD? Clin J Am Soc Nephrol 5: 557–559, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS: MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Knob AU, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roy B, Diez-Roux AV, Seeman T, Ranjit N, Shea S, Cushman M: Association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA). Psychosom Med 72: 134–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Day EC, Li Y, Diez Roux AV, Kandula N, Moran A, Rosas S, Shlipak MG, Peralta CA: Associations of acculturation and kidney dysfunction among Hispanics and Chinese from the Multi-Ethnic Study of Atherosclerosis (MESA). Nephrol Dial Transplant 2010, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, Goff DC, Kaufman JD, O'Neill MS: Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 116: 486–491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norris K, Nissenson A: Racial disparities in chronic kidney disease: Tragedy, opportunity, or both? Clin J Am Soc Nephrol 3: 314–316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prakash S, Rodriguez RA, Austin PC, Saskin R, Fernandez A, Moist LM, O'Hare AM: Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol 21: 1192–1199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peralta CA, Li Y, Wassel C, Choudhry S, Palmas W, Seldin MF, Risch N, Siscovick D, Arnett D, Psaty B, Shlipak MG: Differences in albuminuria between Hispanics and whites: An evaluation by genetic ancestry and country of origin: The Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Genet 3: 240–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Behar DM, Rosset S, Tzur S, Selig S, Yudkovsky G, Bercovici S, Kopp JB, Winkler CA, Nelson GW, Wasser WG, Skorecki K: African ancestry allelic variation at the MYH9 gene contributes to increased susceptibility to non-diabetic end-stage kidney disease in Hispanic Americans. Hum Mol Genet 19: 1816–1827, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S: Socioeconomic status in health research: One size does not fit all. JAMA 294: 2879–2888, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Bild D, Bluemke DA, Burke G, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Jr., Kronmal RA, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP: Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol 156: 871–881, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laird NM, Ware JH: Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982 [PubMed] [Google Scholar]