Abstract
Nephrogenesis is ongoing at the time of birth for the majority of preterm infants, but whether postnatal renal development follows a similar trajectory to normal in utero growth is unknown. Here, we examined tissue collected at autopsy from 28 kidneys from preterm neonates, whose postnatal survival ranged from 2 to 68 days, including 6 that had restricted intrauterine growth. In addition, we examined kidneys from 32 still-born gestational controls. We assessed the width of the nephrogenic zone, number of glomerular generations, cross-sectional area of the renal corpuscle, and glomerular maturity and morphology. Renal maturation accelerated after preterm birth, with an increased number of glomerular generations and a decreased width of the nephrogenic zone in the kidneys of preterm neonates. Of particular concern, compared with gestational controls, preterm kidneys had a greater percentage of morphologically abnormal glomeruli and a significantly larger cross-sectional area of the renal corpuscle, suggestive of renal hyperfiltration. These observations suggest that the preterm kidney may have fewer functional nephrons, thereby increasing vulnerability to impaired renal function in both the early postnatal period and later in life.
The incidence of preterm birth continues to be high in many countries worldwide.1,2 Furthermore, the survival of preterm neonates, particularly those born extremely preterm, has improved substantially over recent decades with those born as early as 25 weeks of gestation reported to have an 82% chance of survival.3 Preterm birth, however, is associated with many postnatal complications during the neonatal period primarily because of the vulnerability of the immature organs.4,5 In this regard, renal dysfunction is commonly observed in preterm neonates.6–8 and this is likely to be associated with the immaturity of the kidney at the time of birth. Nephrogenesis in the human kidney does not reach completion until approximately 36 weeks of gestation, with the majority of nephrons formed in late gestation at a time when preterm infants have already been delivered.9,10
The immature preterm kidney with ongoing nephrogenesis is likely to be vulnerable to the hemodynamic changes associated with preterm birth. Hence, it is important to determine whether kidney development follows the normal growth trajectory of that in utero, after preterm delivery. To date, there have been few studies examining the effect of preterm birth on nephrogenesis.11,12 A human autopsy study by Rodriguez et al.11 found that compared with term-born infants, the number of radial glomerular counts (glomerular generations) in the kidney, an index of renal maturity, was significantly reduced in preterm infants. It is important to note, however, that a large proportion of the neonates in the preterm group in this previous study were intrauterine growth restricted (IUGR), which is a known cause of low nephron endowment.9,13,14 A recent autopsy study by Faa et al.12 also examined autopsied kidneys from 12 premature neonates and found a low number of radial glomerular counts and marked interindividual variability among preterm neonates. It could not be ascertained from the study whether any of the preterm neonates were also IUGR.
Recent nonhuman primate studies, undertaken using a baboon model of preterm birth, have demonstrated that although nephrogenesis continued after preterm delivery, postnatal nephrogenesis was associated with an increased risk of abnormal glomerular development, with the proportion of abnormal glomeruli ranging from 0.2% up to 18%.15,16 The aim of the current study was to examine renal morphology and glomerular maturation in the preterm human neonate and to determine whether preterm birth also leads to the development of abnormal glomeruli in the human kidney.
RESULTS
Cause of Death and Illness
The most common causes of death and illness in the preterm neonates included sepsis (58%), intraventricular/intracranial hemorrhage (42%), necrotizing enterocolitis (37%), and respiratory disease (32%). There was only one neonate in the preterm group that was diagnosed with acute renal failure and one neonate with mild renal failure. Six of the preterm neonates were diagnosed with IUGR (two per postconceptional age grouping). The majority of the gestational controls investigated in this study died acutely in utero with an undetermined cause of death (72%). Other causes included acute asphyxia (16%), feto-maternal hemorrhage (6%), and preterm labor (6%).
Neonatal Age and Growth Characteristics (Table 1)
Table 1.
Age, sex, and body weights of the gestational controls and preterm neonates (non-IUGR and IUGR)
| Control (n = 32) | Preterm (n = 22) | Preterm + IUGR (n = 6) | |
|---|---|---|---|
| Gestational age (weeks) | 31.0 ± 0.8 (24 to 38) | 27.9 ± 0.7* (24 to 35) | 27.0 ± 0.7* (25 to 30) |
| Postnatal age (days) | 18.3 ± 3.4 (2 to 42) | 30.7 ± 12.5 (2 to 68) | |
| Postconceptional age (weeks) | 31.0 ± 0.8 (24 to 38) | 30.0 ± 0.7 (24 to 38) | 31.4 ± 1.8 (25 to 37) |
| Sex ratio (M:F) | 16:16 | 13:9 | 3:3 |
| Birth weight (g) | 1619 ± 147 | 1228 ± 107 | 628 ± 108*# |
| Autopsy weight (g) | 1642 ± 146 | 1468 ± 157 | 1150 ± 330 |
Data presented as mean ± SEM with data range in parentheses.
*P < 0.05 compared with control group;
#P < 0.05 compared with preterm group.
Gestational age at birth in the two preterm groups ranged from 24 to 35 weeks and postnatal age ranged from 2 to 68 days. Gestational age of the stillborn control neonates was in the same range as the postconceptional age of the preterm neonates (24 to 38 weeks). Gestational age of the non-IUGR and IUGR preterm neonates at birth was significantly lower compared with the gestational controls; however, postconceptional age was similar in all groups.
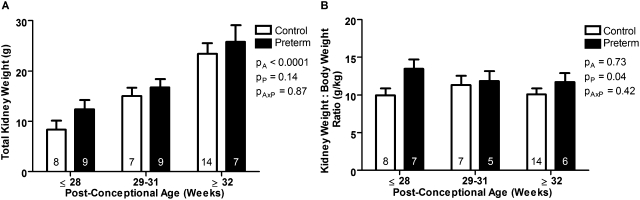
Total combined kidney weights were also similar between groups (Figure 1A). Kidney weight relative to body weight, however, was significantly greater in the preterm group compared with the gestational control group (P < 0.05) (Figure 1B). Within the two preterm groups, only birth weight was significantly smaller in the IUGR preterm neonates (Table 1); however, there was no difference in body weights at autopsy, kidney weight, or kidney-to-body weight ratio.
Figure 1.
Increased kidney-to-body weight ratio in preterm neonates. (A) Total combined kidney weight and (B) kidney weight relative to body weight in gestational controls (white bars) and preterm neonates (black bars), grouped by postconceptional age. The number of neonates in each group is indicated on the bars. Total kidney weight significantly increased with increasing postconceptional age (A). Compared with the gestational controls, the preterm neonates had a significantly increased kidney-to-body weight ratio (B). Bars represent mean ± SEM. PA, postconceptional age; PP, prematurity; PAxP, interaction.
There was a significant positive correlation between body weight at autopsy and postconceptional age in both the preterm neonates and the gestational controls (control: r2 = 0.87, P < 0.0001; preterm: r2 = 0.66, P < 0.0001). Kidney weight also correlated significantly with increasing postconceptional age in both groups (control: r2 = 0.57, P < 0.0001; preterm: r2 = 0.59, P < 0.0001).
Assessment of Nephrogenesis
Nephrogenic Zone Width.
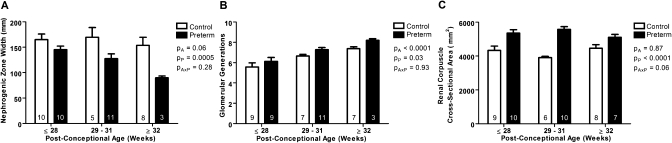
In 10 kidneys (4 preterm and 6 gestational controls with a postconceptional age ≥32-weeks) nephrogenesis was complete before autopsy, with no evidence of a nephrogenic zone. In kidneys with ongoing nephrogenesis the width of the nephrogenic zone tended to decrease with increasing postconceptional age (P = 0.06) (Figure 2A). Overall, nephrogenic zone width was significantly decreased in the preterm group compared with the gestational controls (P < 0.001).
Figure 2.
Decreased nephrogenic zone width, increased number of glomerular generations, and increased renal corpuscle size in kidneys from preterm neonates. (A) Nephrogenic zone width, (B) glomerular generation number, and (C) mean renal corpuscle cross-sectional area in kidneys from gestational controls (white bars; 6 neonates with completed nephrogenesis were not included in A and B) and preterm neonates (black bars; 4 neonates with completed nephrogenesis were not included in A and B), grouped by postconceptional age. The number of neonates in each group is indicated on the bars. Average nephrogenic zone width was significantly less in preterm neonates compared with gestational controls (A). The number of glomerular generations significantly increased with increasing postconceptional age, and was significantly greater in the preterm neonates compared with the gestational controls (B). Mean renal corpuscle cross-sectional area was significantly larger in the preterm neonates compared with the gestational controls (C). Bars represent mean ± SEM. PA, postconceptional age; PP, prematurity; PAxP, interaction.
Glomerular Generations.
Kidneys with ongoing nephrogenesis and those with completed nephrogenesis were analyzed separately. In kidneys that had completed nephrogenesis (6 controls and 4 preterm), there was no significant difference in the average number of glomerular generations between the preterm group compared with the gestational controls (control: 7.8 ± 0.4; preterm: 8.3 ± 0.6; P = 0.55). In kidneys with ongoing nephrogenesis, the number of glomerular generations increased significantly with postconceptional age (Figure 2B). Importantly, the number of glomerular generations formed was significantly greater in the preterm kidneys compared with the gestational controls (P < 0.05).
There was a significant positive correlation between body weight at autopsy and the number of glomerular generations in both the preterm group (r2 = 0.54, P < 0.001) and the gestational controls (r2 = 0.54, P < 0.0001). Similarly, there was a significant positive correlation between kidney weight and the number of glomerular generations within both groups (control: r2 = 0.42, P < 0.001; preterm: r2 = 0.43, P < 0.001).
Assessment of Glomerular Size, Maturity, and Morphology
Renal Corpuscle Cross-sectional Area.
Average renal corpuscle cross-sectional area was significantly greater in the preterm group than in the gestational controls (P < 0.0001) (Figure 2C). Linear regression analyses showed no correlation between mean renal corpuscle area and gestational age, postconceptional age, or postnatal age in either the control or the preterm groups.
Glomerular Maturity.
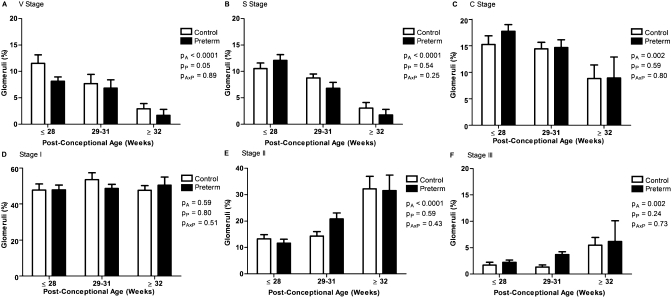
The percentage of glomeruli in the immature stages of glomerular development (stages V [vesicle], S [comma-shaped and S-shaped], and C [capillary loop]) significantly decreased with increasing postconceptional age (P < 0.01) (Figure 3). Conversely, the percentage of glomeruli at stages II and III of development significantly increased with increasing postconceptional age (P < 0.01). The most common stage of development, with approximately 50% of all glomeruli in each kidney, was stage I; there was no change in the percentage of glomeruli at stage I with increasing postconceptional age. Importantly, the percentage of immature V-stage glomeruli was significantly less in the preterm group than in the gestational controls (P ≤ 0.05).
Figure 3.
Percentage of immature V-stage glomeruli is reduced in the kidneys of preterm neonates. The percentage of glomeruli at each stage of maturity, in the gestational control (white bars) and preterm neonates (black bars), grouped by postconceptional age. Control: ≤28 weeks (n = 9), 29 to 31 weeks (n = 5), ≥32 weeks (n = 9); preterm: ≤28 weeks (n = 8), 29 to 31 weeks (n = 12), ≥32 weeks (n = 6). The percentage of glomeruli at stages V (A), S (B), and C (C) significantly decreased with increasing postconceptional age, whereas the percentage of glomeruli at stages II (E) and III (F) significantly increased. There was no change in the percentage of glomeruli at stage I with increasing postconceptional age (D). A significantly lower percentage of V-stage glomeruli were observed in the preterm kidneys compared with the gestational controls (A). Bars represent mean ± SEM. PA, postconceptional age; PP, prematurity; PAxP, interaction.
Glomerular Morphology.
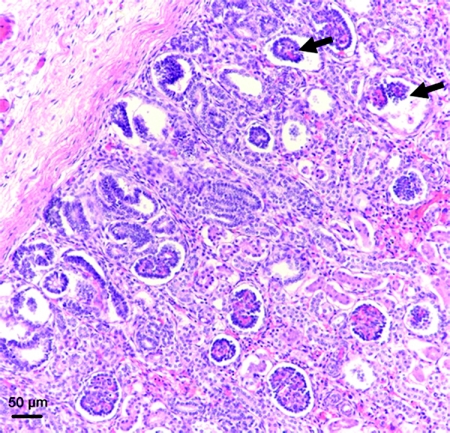
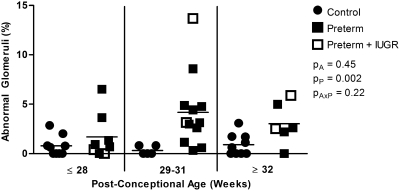
Morphologically abnormal glomeruli, with a dilated Bowman's space and shrunken tuft, were commonly observed in the outer cortex of preterm kidneys (Figure 4). All abnormal glomeruli were noted to be in stage I of development. Overall, the proportion of abnormal glomeruli was significantly greater in the kidneys of the preterm neonates compared with the gestational controls (Figure 5). In preterm kidneys, the proportion of abnormal glomeruli ranged from 0 to 13.7%, with a similar range observed across all postconceptional age groupings. The kidney with the highest proportion of abnormal glomeruli (13.7%) was from a preterm neonate diagnosed with IUGR. The percentage of abnormal glomeruli did not correlate with gestational, postconceptional, or postnatal age.
Figure 4.
Abnormal glomerular morphology in the kidney of a preterm neonate. Representative photomicrograph depicting abnormal glomeruli, with dilated Bowman's space and shrunken tuft (arrows), in the outer cortex of a preterm neonatal kidney.
Figure 5.
Increased percentage of morphologically abnormal glomeruli in kidneys from preterm neonates. The percentage of abnormal glomeruli in the kidneys of gestational controls and preterm neonates, grouped by postconceptional age. Control: ≤28 weeks (n = 9), 29 to 31 weeks (n = 5), ≥32 weeks (n = 9); preterm: ≤28 weeks (n = 8), 29 to 31 weeks (n = 12), ≥32 weeks (n = 6). The percentage of abnormal glomeruli was significantly increased in the preterm group compared with the gestational controls. PA, postconceptional age; PP, prematurity; PAxP, interaction.
Neonatal Medications
The full medical history, including the administration of medications, was available for 20 of the 28 preterm neonates. The most commonly administered medications were antenatal steroids (55%), antibiotics (80%), nonsteroidal anti-inflammatory drugs (indomethacin) (50%), and inotropes (dopamine and dobutamine) (80%). Exposure to prenatal and/or postnatal medications was not associated with an increased percentage of abnormal glomeruli.
Oligonephronia in a Preterm Neonate
One preterm neonate was excluded from all analysis because of significant renal abnormalities. This neonate, a male born at 28 weeks gestation, survived postnatally for 19 days with death attributed to necrotizing enterocolitis. Preterm delivery was associated with a history of maternal pre-eclampsia and histologic evidence of grade 2 (of 3) acute chorioamnionitis. Postnatally, he was exposed to 5 doses of indomethacin and 9 doses of gentamicin.
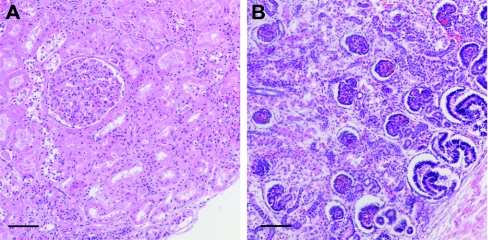
As shown in Figure 6, this neonate's kidney had no evidence of a nephrogenic zone at the time of analysis (30.7-week postconceptional age). Ninety-two percent of glomeruli within the kidney were at stage III of development, and average renal corpuscle area was 34,184 μm2 (approximately sixfold larger than in any other kidney). No abnormal glomeruli were observed in this kidney.
Figure 6.
Larger mean renal corpuscle area in a preterm neonate with oligonephronia. Representative photomicrographs of the outer renal cortex of (A) one preterm neonate with oligonephronia (postconceptional age 30.7 weeks) and (B) one preterm neonate with the appearance of normal renal development (postconceptional age 30.0 weeks). In the neonate with oligonephronia, nephrogenesis is complete, with the single visible glomerulus at stage III of development. In contrast, the kidney of the preterm neonate in (B) exhibits an active nephrogenic zone, and the numerous outer cortical glomeruli are in S-stage, C-stage, and stage I of development. Furthermore, mean renal corpuscle area was significantly larger in the neonate with oligonephronia (A). Bar represents 100 μm.
DISCUSSION
This study comprehensively examines the effects of preterm birth on the normal growth trajectory in autopsied kidneys from both IUGR and non-IUGR preterm neonates compared with stillborn gestational controls. Through this series of structural analyses we have shown that preterm neonates exhibit accelerated postnatal renal maturation with a reduced nephrogenic zone width, reduced percentage of immature V-stage glomeruli, and an increased number of glomerular generations compared with postconceptional age-matched gestational controls. In addition, and of particular concern, preterm kidneys exhibited an enlarged renal corpuscle cross-sectional area and morphologically abnormal glomeruli, with up to 13% of glomeruli in the kidney affected. These findings may have important clinical implications for both the short- and long-term renal health of infants born preterm.
In agreement with previous studies,16,17 the preterm neonates had a significantly increased kidney-to-body weight ratio compared with the gestational controls. Mean renal corpuscle area was also significantly increased in preterm kidneys, averaging over 5000 μm2 compared with approximately 4000 μm2 in controls. These results were anticipated because the neonatal kidneys have undergone a dramatic hemodynamic change after birth whereby the renal system switches from a fetal to a neonatal organ with high blood flow and low vascular resistance.18,19 Whether the glomerular enlargement observed in the preterm kidneys is indicative of glomerular hyperfiltration, however, cannot be determined in the current study. As the full complement of nephrons is not achieved until late in gestation, it is conceivable that the preterm kidney may not be able to cope with the postnatal functional demands, resulting in compensatory glomerular hypertrophy. Previous studies have shown that glomerular hypertrophy and hyperfiltration lead to glomerular injury and later nephron loss; these detrimental changes are strongly linked to the development of long-term renal disease.20,21 Further studies are required to ascertain whether the glomerular hypertrophy is a pathologic, or a normal physiologic, process.
Importantly, we have demonstrated that preterm birth is associated with accelerated renal maturation. Our findings suggest that nephrogenic zone width is significantly reduced in the kidneys of preterm neonates compared with gestational controls at similar postconceptional ages, suggestive of early cessation of nephrogenesis in the postnatal environment and/or accelerated maturation of glomeruli. In support of this finding, the percentage of immature V-stage glomeruli was significantly reduced in the preterm kidney than in the gestational controls. Furthermore, glomerular generation number was significantly increased in the preterm kidneys than in gestational controls at similar postconceptional ages. The mechanisms leading to the accelerated renal maturation observed in the preterm kidney are unknown, but may relate to factors in the care of the neonate which promote organ maturation. Indeed, exposure to antenatal glucocorticoids, which are commonly administered before preterm delivery to aid postnatal respiratory function,22 is associated with renal functional maturation in the preterm neonate.23 Furthermore, the administration of betamethasone is reported to lead to a thinning of the nephrogenic zone in the fetal rhesus monkey24 and an increased number of developed glomeruli were found in the kidneys from preterm baboons after antenatal exposure.16 In this study, many of the preterm neonates were administered antenatal steroids. However, because full medical histories could not be obtained for the whole cohort, we were unable to determine if there were any maturational differences between the neonates that were exposed to steroids compared with the ones that were not.
In contrast, the results of previous autopsy studies by Rodriguez et al.11 and Faa et al.12 indicated a reduced number of glomerular generations in the preterm kidney compared with infants that were born at term, perhaps indicative of a nephron deficit. In a nonhuman primate model of preterm birth, however, we have previously demonstrated that nephron endowment was not affected by preterm birth.16 Similarly, in the current study we found no difference in glomerular generation number between gestational control and preterm neonates in which nephrogenesis was complete (ranging from 7 to 10 generations in each group). Differences in the postnatal clinical course of the preterm neonates examined in the current study and those in previously published studies11,12 may also account for the contrasting findings. Indeed, Faa et al.12 observed marked interindividual variations in radial glomerular counts.
We had also initially hypothesized that the findings of a reduced number of glomerular generations in the preterm kidney by Rodriguez et al.11 may have been confounded by the inclusion of preterm neonates that were also IUGR since this possibility was not explored by the authors. In the present study, although the included IUGR preterm neonates were significantly smaller than the non-IUGR preterm neonates at birth, there was no significant difference in kidney or body weights at autopsy. Hence, it is not surprising that no differences were found in the indices of renal development. This may be due to catch-up growth of the kidney postnatally after preterm birth in the IUGR neonates and/or the severity of the IUGR. Furthermore, a limitation of the current study is that only a small number of preterm IUGR neonates were assessed, which may have reduced the potential to observe statistical differences.
An important finding from this study is that many glomeruli in the outer cortex of the preterm kidney were morphologically abnormal, exhibiting an enlarged Bowman's space and shrunken glomerular tuft, and therefore unlikely to be functional. The abnormal glomeruli (previously observed in human11 and baboon15,16 preterm neonates) were only present in the outer cortex, suggesting that it is those glomeruli newly formed in the extrauterine environment that are “at risk”. Certainly, kidneys with a large number of abnormal glomeruli are likely to suffer a significant deficit of functional nephrons, which is strongly linked to an increased susceptibility to hypertension and renal disease later in life.25 In this regard, a number of recent studies have linked preterm birth with both the development of hypertension26–36 and renal dysfunction;37 perhaps one factor underlying these associations is a reduced nephron endowment in infants born preterm. Further research is needed, however, before this can be fully elucidated.
The large variation in the percentage of abnormal glomeruli between neonates in this study, as well as in our previous studies,15,16 suggests that these abnormalities have not occurred as a result of preterm birth per se, but may be related to differences in the postnatal clinical course of the neonates. No clear link between postnatal medication exposure and the extent of glomerular abnormalities was found in this study or in previous studies,15 possibly because of the low sample size. Certainly, however, the oligonephronia observed in one of the preterm neonates, who had been exposed to a large number of doses of both antibiotics (gentamicin) and nonsteroidal anti-inflammatory drugs (indomethacin) supports previous in vivo and in vitro studies in experimental models that have demonstrated adverse effects on nephrogenesis38–40 and renal morphology.41 In future, it is essential to identify potentially modifiable factors in the postnatal care of the preterm neonate (such as postnatal administration of antibiotics and nonsteroidal anti-inflammatory drugs) that may be associated with impaired nephrogenesis and abnormal glomerular development.
In conclusion, this study comprehensively examines postnatal nephrogenesis in the human preterm kidney. We found a decreased nephrogenic zone width, decreased percentage of immature V-stage glomeruli, and an increased number of glomerular generations, which suggests accelerated postnatal renal maturation. Of concern, there was a significant increase in renal corpuscle cross-sectional area and up to 13% of glomeruli were morphologically abnormal. Together, these detrimental changes in the immature glomeruli may ultimately result in a nephron deficit, which is linked to the development of renal disease and hypertension later in life. These findings, therefore, have significant implications for both the short- and long-term renal health of infants born preterm.
CONCISE METHODS
In this retrospective study, archived neonatal kidneys were obtained from the Women's and Children's Hospital in North Adelaide, South Australia, and The Canberra Hospital in Woden, Australian Capital Territory. Autopsies were performed in the range of years 1996 through 2009. Ethics approval for autopsy was obtained from the Children, Youth and Women's Health Service Research Ethics Committee of South Australia and the Australian Capital Territory Human Research Ethics Committee. Only infants with written informed parental consent for autopsy were included in the study.
Inclusion and Exclusion Criteria
Preterm neonates with postnatal survival >2 days were included in this study. Any neonate with evidence of a congenital abnormality was excluded. Neonates were also excluded if the kidneys were severely macerated and/or the estimated time between fetal death and delivery was >48 hours. One preterm infant was subsequently excluded because of the significant renal abnormalities observed.
Both IUGR and non-IUGR preterm neonates were included in this study; however, data were analyzed separately to identify if there was any effect of IUGR on the preterm kidney. Gestational controls diagnosed with IUGR were excluded.
Neonatal Characteristics
Twenty-eight preterm neonates with postnatal survival of 2 or more days, and 32 gestational controls were examined in this study. Six of the preterm neonates had been diagnosed with IUGR (based on birth weight, growth parameters, and brain weight-to-liver weight ratios). Gestational age at birth was estimated using both the mother's last menstrual cycle and the Dubowitz clinical characteristics.42 Gestational controls were stillborn neonates that died acutely in utero.
Neonates were further divided according to postconceptional age (sum of gestational age and postnatal age in weeks): ≤28 weeks (extremely preterm; control, n = 9; preterm, n = 10), 29 to 31 weeks (very preterm; control, n = 8; preterm, n = 11), and ≥32 weeks (moderately preterm; control, n = 15; preterm, n = 7). There were two preterm IUGR neonates included in each of the postconceptional age groupings.
Clinical History
Maternal and neonatal clinical histories were obtained from the autopsy reports and hospital medical records where available; we had access to the full medical records from 20 preterm neonates. Maternal clinical history included the administration of antenatal steroids. Neonatal clinical history included gestational age at birth, postnatal age at death, birth weight, autopsy weight, kidney weight, neonatal illnesses, medications administered, and cause of death.
Collection of Kidneys at Autopsy and Tissue Sectioning and Staining
Kidneys were excised, weighed, and cut in half in the longitudinal plane. Larger kidneys were further transversely cut. Portions of the kidney were embedded in paraffin blocks, sectioned at 5 μm, and collected onto glass slides. Complete kidney sections with clear evidence of cortex and medulla were selected and stained with hematoxylin and eosin and used for the assessment of nephrogenesis, glomerular size, maturity, and morphology. During all analyses, researchers were blinded to the gestational age and study grouping.
Assessment of Nephrogenesis
Nephrogenic Zone Width.
The width of the nephrogenic zone was measured using image analysis software (Image Pro Plus v. 6.0 for Windows, Media Cybernetics, Silver Spring, Maryland). This method has previously been utilized to assess renal maturity in both human43 and baboon16 fetal and neonatal kidneys. Kidney sections were viewed at ×200 magnification and the width of the nephrogenic zone was measured in three to four separate regions. The nephrogenic zone was defined as the area in the outer renal cortex exhibiting developing nephrons in the form of comma and S-shaped bodies. An average nephrogenic zone width was determined for each kidney.
Glomerular Generation Number.
The medullary ray glomerular generation counting method was utilized to estimate the number of glomerular generations formed within the kidney. This method counts the number of glomeruli formed along a medullary ray from the corticomedullary junction to the outer renal cortex, including the glomeruli that form after ureteric branching is complete. This method has been validated by Hinchliffe and colleagues,44 and also utilized in previous studies to assess renal maturity in preterm human11–12,45 and baboon neonates.15,16 In one complete paraffin section from each kidney, approximately five clearly distinguishable medullary rays from separate regions of the kidney section were identified and the number of mature glomeruli along one side of the medullary ray was counted. An average number of glomerular generations per kidney was obtained from the five regions. In circumstances when clear medullary rays were not observed, a straight line was drawn from the corticomedullary junction to the outer renal cortex and all mature glomeruli along the line were counted according to the protocol of Rodriguez and colleagues.11
Assessment of Glomerular Size, Maturity, and Morphology
Renal Corpuscle Cross-sectional Area.
Renal corpuscle cross-sectional area was measured using image analysis software (Image Pro Plus v. 6.0 for Windows, Media Cybernetics, Silver Spring, Maryland). A complete section from each kidney was systematically sampled at ×400 magnification with a step length of 1 mm × 1 mm. At each field of view, two glomeruli were chosen for assessment (approximately 200 per kidney). If more than two glomeruli were observed, each glomerulus was assigned a number from 1 to n. With use of a random number table, the first glomerulus (G1) was selected for analysis. The second glomerulus (G2) was selected according to the criteria of Nyengaard and Marcussen,46 where n is the total number of glomeruli per field of view:
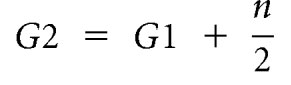 |
If the above equation resulted in G2 > n, then the following equation was utilized:
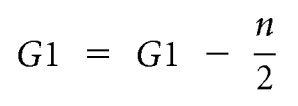 |
The cross-sectional area of each selected renal corpuscle was then determined by tracing the Bowman's capsule, and the average area was calculated for each kidney. Abnormal glomeruli, exhibiting an enlarged Bowman's space and shrunken glomerular tuft, were excluded from the analysis.
Glomerular Maturity and Morphology.
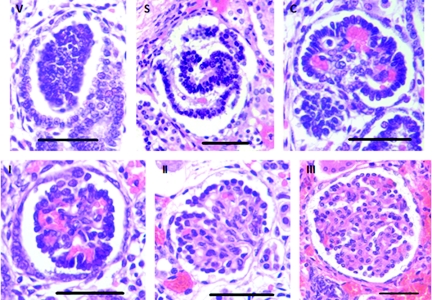
With use of the same sampling method as described above, the maturational stage of all glomeruli present in each field of view was recorded (approximately 300 glomeruli per kidney). The criterion for grading each stage of glomerular maturity is detailed in Figure 7. Immature glomeruli in the early stages of development (stages V, C, and S) were graded according to the criteria of Naruse et al.47 More mature glomeruli (stages I, II, and III) were graded according to the criteria of Thony et al.48 The percentage of abnormal glomeruli at each stage of development per kidney was determined. Glomeruli were classified as abnormal if they exhibited a grossly enlarged Bowman's space and a shrunken glomerular tuft. At each field of view, the numbers of normal and abnormal glomeruli were recorded, and the percentage of abnormal glomeruli per kidney was determined.
Figure 7.
Stages of glomerular maturity in the developing human kidney. The following criteria for the assessment of glomerular maturation are based on Naruse et al.47 and Thony et al.48 (V) Vesicle: Condensate of mesenchymal cells formed adjacent to a ureteric branch tip in the outer nephrogenic zone. (S) Comma-shape and S-shape: Elongated vesicle develops proximal and distal clefts to form a comma-shaped followed by an S-shaped body. (C) Capillary loop: Cells of the lower limb of the S-shaped body differentiate to form an immature, crescent-shaped glomerulus. (I) Stage I: Fully formed glomeruli with at least half of the glomerular tuft lined with dark-staining epithelial cells (podocytes). The inner tuft is a dense collection of cells. (II) Stage II: Less than half the circumference of the tuft is lined with podocytes, with at least five adjoining. The inner tuft may exhibit some lobulation. (III) Stage III: Glomeruli with no podocyte layer surrounding the tuft, and an inner tuft showing lobulation and open capillary loops. There is also evidence of flattening of the parietal epithelial cells lining Bowman's capsule. Bar represents 50 μm.
Statistical Analysis
All statistical analyses were undertaken using GraphPad Prism v5.03 for windows (GraphPad Software, San Diego). An unpaired t test was used to determine statistically significant differences in age and growth characteristics between groups. Linear regression analyses were undertaken to determine correlations between the indices of fetal/neonatal growth and renal morphology (birth weight, kidney weight, nephrogenic zone width, glomerular generation number, renal corpuscle cross-sectional area, glomerular maturity, and the percentage of abnormal glomeruli) versus gestational, postnatal, and postconceptional ages. This was followed by an analysis of covariance (ANCOVA) to determine whether there were any significant differences in the slope and y-intercept of the linear regression lines between the gestational control, preterm, and preterm + IUGR groups. No differences were found between the preterm group and the preterm + IUGR group in any indices of renal development (kidney weight, kidney weight-to-body weight ratio, nephrogenic zone width, glomerular generation number, renal corpuscle cross-sectional area, and glomerular maturity); therefore, all data were pooled.
A two-way ANOVA was utilized in the assessment of renal development (nephrogenic zone width, glomerular generation number, renal corpuscle-cross-sectional area, and glomerular maturity) with the factors postconceptional age (A), prematurity (P), and the interaction (AxP). This was followed by a Bonferroni post hoc test to determine the differences between groups at each age point. Sex differences within groups was also analyzed using a two-way ANOVA and no statistically significant differences were found. The level of significance was accepted at P < 0.05.
DISCLOSURES
None.
Acknowledgments
The authors acknowledge Ms. Laura Stamp for her technical assistance. This research was supported by the National Health and Medical Research Council of Australia. Authors M.R. Sutherland and L. Gubhaju were also recipients of an Australian Postgraduate Award while undertaking this work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Tracy S, Tracy M, Dean J, Laws P, Sullivan E: Spontaneous preterm birth of liveborn infants in women at low risk in Australia over 10 years: A population-based study. BJOG 114: 731–735, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Martin J, Hamilton B, Sutton P, Ventura S, Menacher F, Kirmeyer S: Births: Final data for 2004. Natl Vital Stat Rep 55: 1–101, 2006 [PubMed] [Google Scholar]
- 3. Fellman V, Hellstrom-Westas L, Norman M, Westgren M, Kallen K, Lagercrantz H, Marsal K, Serenius F, Wennergren M: One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 301: 2225–2233, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Goldenberg R, Culhane J, Iams J, Romero R: Epidemiology and causes of preterm birth. Lancet 371: 75–84, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCormick MC: The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med 312: 82–90, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Stapleton F, Jones D, Green R: Acute renal failure in neonates: Incidence, etiology and outcome. Pediatr Nephrol 1: 314–320, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Drukker A, Guignard J: Renal aspects of the term and preterm infant: A selective update. Curr Opin Pediatr 14: 175–182, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Choker G, Gouyon JB: Diagnosis of acute renal failure in very preterm infants. Biol Neonate 86: 212–216, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D: The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 99: 296–301, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D: Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777–784, 1991 [PubMed] [Google Scholar]
- 11. Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE: Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7: 17–25, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Faa G, Gerosa C, Fanni D, Nemolato S, Locci A, Cabras T, Marinelli V, Puddu M, Zaffanello M, Monga G, Fanos V: Marked interindividual variability in renal maturation of preterm infants: Lessons from autopsy. J Matern Fetal Neonatal Med 23 [Suppl 3]: 129–133, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Manalich R, Reyes L, Herrera M, Melendi C, Fundora I: Relationship between weight at birth and the number and size of renal glomeruli in humans: A histomorphometric study. Kidney Int 58: 770–773, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Bagby SP: Maternal nutrition, low nephron number, and hypertension in later life: Pathways of nutritional programming. J Nutr 137: 1066–1072, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Sutherland MR, Gubhaju L, Yoder BA, Stahlman MT, Black MJ: The effects of postnatal retinoic acid administration on nephron endowment in the preterm baboon kidney. Pediatr Res 65: 397–402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ: Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Renal Physiol 297: F1668–F1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang HP, Tsai IJ, Lai YC, Cheng CH, Tsau YK: Early postnatal renal growth in premature infants. Nephrology 12: 572–575, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Veille JC, McNeil S, Hanson R, Smith N: Renal hemodynamics: Longitudinal study from the late fetal life to one year of age. J Matern Fetal Investig 8: 6–10, 1998 [PubMed] [Google Scholar]
- 19. Satlin LM, Woda CB, Schwartz GJ: Development of function in the metanephric kidney. In: The Kidney: From Normal Development to Congenital Disease, edited by Vize PD, Woolf A, Bard JBL. New York, Academic Press, 2003, pp 267–325 [Google Scholar]
- 20. Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF: Reduced nephron number and glomerulomegaly in Australian Aborigines: A group at high risk for renal disease and hypertension. Kidney Int 70: 104–110, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Brenner B, Garcia D, Anderson S: Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Roberts D, Dalziel S: Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 3: CD004454, 2006 [DOI] [PubMed] [Google Scholar]
- 23. al-Dahan J, Stimmler L, Chantler C, Haycock GB: The effect of antenatal dexamethasone administration on glomerular filtration rate and renal sodium excretion in premature infants. Pediatr Nephrol 1: 131–135, 1987 [DOI] [PubMed] [Google Scholar]
- 24. Epstein MF, Farrell PM, Sparks JW, Pepe G, Driscoll SG, Chez RA: Maternal betamethasone and fetal growth and development in the monkey. Am J Obstet Gynecol 127: 261–263, 1977 [DOI] [PubMed] [Google Scholar]
- 25. Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K: Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol 16: 2557–2564, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Siewert-Delle A, Ljungman S: The impact of birth weight and gestational age on blood pressure in adult life. A population-based study of 49-year-old men. AJH 11: 946–953, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Keijzer-Veen MG, Dulger A, Dekker FW, Nauta J, van der Heijden BJ: Very preterm birth is a risk factor for increased systolic blood pressure at a young adult age. Pediatr Nephrol 25: 509–516, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S: Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation 112: 3430–3436, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Kistner A, Celsi G, Vanpee M, Jacobson SH: Increased blood pressure but normal renal function in adult women born preterm. Pediatr Nephrol 15: 215–220, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Doyle LW, Faber B, Callanan C, Morley R: Blood pressure in late adolescence and very low birth weight. Pediatrics 111: 252–257, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Bonamy A, Bendito A, Martin H, Andolf E, Sedin G, Norman M: Preterm birth contributes to increased vascular resistance and higher blood pressure in adolescent girls. Pediatr Res 58: 845–849, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Hack M, Schluchter M, Cartar L, Rahman M: Blood pressure among very low birth weight (<1.5 kg) young adults. Pediatr Res 58: 677–684, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Dalziel S, Parag V, Rodgers A, Harding J: Cardiovascular risk factors at age 30 following pre-term birth. Int J Epidemiol 36: 907–915, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Kistner A, Jacobson L, Jacobson S, Svensson E, Hellstrom A: Low gestational age associated with abnormal retinal vascularization and increased blood pressure in adult women. Pediatr Res 51: 675–680, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, Kylintireas I, Contractor H, Singhal A, Lucas A, Neubauer S, Kharbanda R, Alp N, Kelly B, Leeson P: Elevated blood pressure in offspring born premature to hypertensive pregnancy: Is endothelial dysfunction the underlying vascular mechanism? Hypertension 56: 159–165, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Cooper R, Atherton K, Power C: Gestational age and risk factors for cardiovascular disease: Evidence from the 1958 British birth cohort followed to mid-life. Int J Epidemiol 38: 235–244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodriguez-Soriano J, Aguirre M, Oliveros R, Vallo A: Long-term renal follow-up of extremely low birth weight infants. Pediatr Nephrol 20: 579–584, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Cullen L, Young R, Bertram J: Studies on the effects of gentamicin on rat metanephric development in vitro. Nephrology 5: 115–123, 2000 [Google Scholar]
- 39. Gilbert T, Lelievre-Pegorier M, Malienou R, Meulemans A, Merlet-Benichou C: Effects of prenatal and postnatal exposure to gentamicin on renal differentiation in the rat. Toxicology 43: 301–313, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Nathanson S, Moreau E, Merlet-Benichou C, Gilbert T: In utero and in vitro exposure to beta-lactams impair kidney development in the rat. J Am Soc Nephrol 11: 874–884, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Kent AL, Maxwell LE, Koina ME, Falk MC, Willenborg D, Dahlstrom JE: Renal glomeruli and tubular injury following indomethacin, ibuprofen, and gentamicin exposure in a neonatal rat model. Pediatr Res 62: 307–312, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Dubowitz L, Dubowitz V, Goldberg C: Clinical assessment of gestational age in the newborn infant. J Pediatr 77: 1–10, 1970 [DOI] [PubMed] [Google Scholar]
- 43. dos Santos AM, Fonseca Ferraz ML, Pinto Rodriguez ML, Dos Reis MA, Miranda Correa RR, de Paula Antunes Teixeira V, da Cunha Castro EC: Assessment of renal maturity by assisted morphometry in autopsied fetuses. Early Hum Dev 82: 709–713, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Hinchliffe SA, Sargent PH, Chan YF, van Velzen D, Howard CV, Hutton JL, Rushton DI: “Medullary ray glomerular counting” as a method of assessment of human nephrogenesis. Pathol Res Practice 188: 775–782, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Rodriguez MM, Gomez A, Abitbol C, Chandar J, Montane B, Zilleruelo G: Comparative renal histomorphometry: A case study of oligonephropathy of prematurity. Pediatr Nephrol 20: 945–949, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Nyengaard JR, Marcussen N: The number of glomerular capillaries estimated by an unbiased and efficient stereological method. J Microsc 171: 27–37, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Naruse K, Fujieda M, Miyazaki E, Hayashi Y, Toi M, Fukui T, Kuroda N, Hiroi M, Kurashige T, Enzan H: An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int 57: 1836–1846, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Thony HC, Luethy CM, Zimmermann A, Laux-End R, Oetliker OH, Bianchetti MG: Histological features of glomerular immaturity in infants and small children with normal or altered tubular function. Eur J Pediatr 154: S65–S68, 1995 [DOI] [PubMed] [Google Scholar]