Abstract
Cathelicidins are a family of antimicrobial peptides acting as multifunctional effector molecules in innate immunity. Cathelicidin-BF has been purified from the snake venoms of Bungarus fasciatus and it is the first identified cathelicidin antimicrobial peptide in reptiles. In this study, cathelicidin-BF was found exerting strong antibacterial activities against Propionibacterium acnes. Its minimal inhibitory concentration against two strains of P. acnes was 4.7 µg/ml. Cathelicidin-BF also effectively killed other microorganisms including Staphylococcus epidermidis, which was possible pathogen for acne vulgaris. Cathelicidin-BF significantly inhibited pro-inflammatory factors secretion in human monocytic cells and P. acnes-induced O2 .− production of human HaCaT keratinocyte cells. Observed by scanning electron microscopy, the surfaces of the treated pathogens underwent obvious morphological changes compared with the untreated controls, suggesting that this antimicrobial peptide exerts its action by disrupting membranes of microorganisms. The efficacy of cathelicidin-BF gel topical administering was evaluated in experimental mice skin colonization model. In vivo anti-inflammatory effects of cathelicidin-BF were confirmed by relieving P. acnes-induced mice ear swelling and granulomatous inflammation. The anti-inflammatory effects combined with potent antimicrobial activities and O2 .− production inhibition activities of cathelicidin-BF indicate its potential as a novel therapeutic option for acne vulgaris.
Introduction
Antimicrobial peptides play important roles in preventing microorganism infections. Most of them are 10–50 residues in length. They can provide an effective and fast acting defense against harmful microorganisms [1], [2]. There are two major vertebrate antimicrobial peptide families including cathelicidins and defensins. Cathelicidins have been found in many mammalians and birds. Recently, a few cathelicidin antimicrobial peptides were identified from snake venoms [3], [4]. They are the first report of reptile cathelicidins.
Acne vulgaris is the most common skin disease. It often occured in areas containing large skin oil glands, such as face, back, and trunk [5]. The pathogenesis of acne is currently attributed to multiple factors such as hormonal factors, hyperkeratinization, resident microbiota, sebum, nutrition, cytokines and toll-like receptors [6]. Propionibacterium acnes act on important roles in acne pathogenesis although they belong to the resident microbiota. P. acnes induce the expression of antimicrobial peptides and pro-inflammatory cytokines/chemokines, which contribute to the inflammatory responses of acne [7]–[10]. Some P. acnes strains may cause an opportunistic infection worsening acne lesions [6]. Antibiotics are employed as therapeutic agents for acne by inhibiting inflammation or killing bacteria. However, antibiotic resistance has been increasing in prevalence within the dermatologic setting [11]. Antimicrobial peptides have been considered as new type of antimicrobial reagents because they have low potential to induce drug resistance of microorganisms. The current work is performed to evaluate the anti-P. acnes abilities of cathelicidin-BF in vitro and in vivo.
Results
Antimicrobial activities of cathelicidin-BF
As listed in Table 1, cathelicidin-BF showed strong antimicrobial abilities against several microorganisms, which are related to acne vulgaris. The MICs value of cathelicidin-BF, LL-37 and clindamycin against two P. acnes strains are 4.7 µg/ml (1.3 µM), 9.4 µg/ml (2.2 µM), and 2.3 µg/ml (5.2 µM), respectively. Cathelicidin-BF showed strong antimicrobial functions (MIC of 1.2–2.3 µg/ml, 0.33–0.65 µM) against two strains of S. epidermidis while LL-37 has no activity against them (no amtimicrobial activity was seen when the concentration of LL-37 was up to 200 µg/ml, 46.8 µM). Clindamycin showed only antimicrobial ability (MIC of 1.2 µg/ml, 2.6 µM) against one (S. epidermidis09B2490) of the two S. epidermidis strains. The MIC values of cathelicidin-BF and LL-37 against S. aureus ATCC2592 are 4.7 µg/ml (1.3 µM for cathelicidin-BF, 1.1 µM for LL-37) while that of clindamycin is 1.2 µg/ml (2.6 µM).
Table 1. Antimicrobial activities of cathelicidin-BF.
| MIC (µg/ml) | |||
| Microorganisms | BF | LL-37 | CL |
| P. acnes ATCC6919 | 4.7 (1.3 µM) | 9.4 (2.2 µM) | 2.3 (5.2 µM) |
| P. acnes ATCC11827 | 4.7 (1.3 µM) | 9.4 (2.2 µM) | 2.3 (5.2 µM) |
| S. epidermidis 09A3726 | 2.3 (0.65 µM) | NA | NA |
| S. epidermidis 09B2490 | 1.2 (0.33 µM) | NA | 1.2 (2.6 µM) |
| S. aureus ATCC2592 | 4.7 (1.3 µM) | 4.7 (1.1 µM) | 1.2 (2.6 µM) |
MIC: minimal peptide concentration required for total inhibition of cell growth in liquid medium. These concentrations represent mean values of three independent experiments performed in duplicates.
BF: canthelicidin-BF; CL: clindamycin.
Bacteria killing kinetics
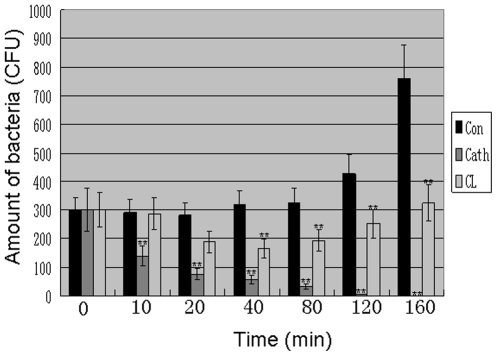
Using clindamycin as a positive control, antibacterial properties of cathelicidin-BF against P. acnes ATCC6919 were tested by the colony counting assay. As illustrated in Fig. 1, cathelicidin-BF could rapidly exert its antibacterial activities. It just took less than 160 minutes to kill all the P. acnes at the concentration of one time of MIC. The antibacterial activity was proved to be lethal for P. acnes. P. acnes were not capable of resuming growth on agar plates after a 6-h treatment with concentrations above the corresponding MICs. In contrast, the antibiotics, clindamycin could not clean the bacteria at the concentration of one time of MIC. Besides, P. acnes treated by one time MIC of clindamycin was capable of resuming growth after 80 min of the treatment (Fig. 1).
Figure 1. Bacterial killing kinetics of cathelicidin-BF against P. acnes.
Amount of bacteria co-cultured with different sample for different time (CFU) was counted according to method described in the “Materials and Methods” section. These CFU represent mean values of three independent experiments. The values for cathelicidin-BF and clindamycin were significant different from the values for the control (*P<0.05 and **p<0.01). Con: control; Cath: cathelicidin-BF; CL: clindamycin.
The Effects on membrane morphology
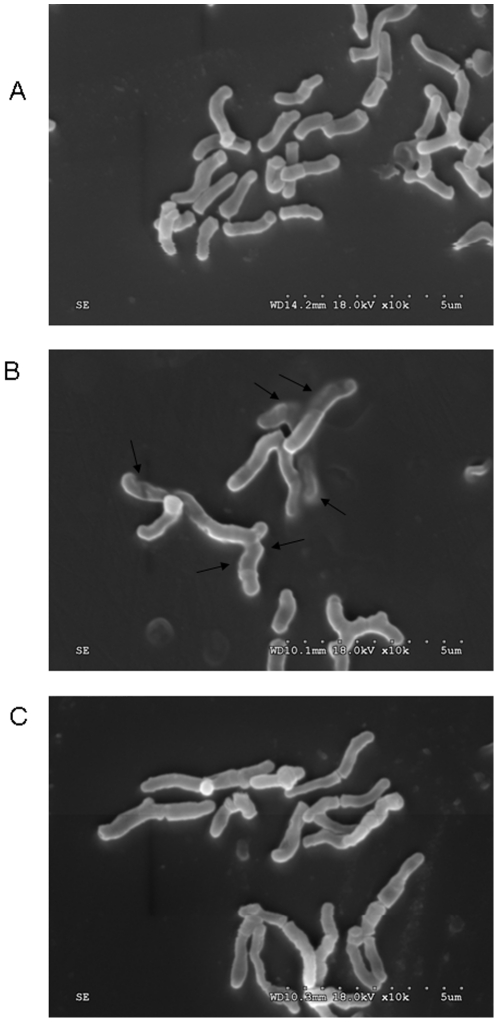
The morphology difference of untreated, clindamycin-treated and cathelicidin-BF-treated P. acnes was studied by SEM as illustrated in Fig. 2. There are clear morphology differences among these P. acnes. The outer membranes of untreated P. acnes were long, spindle-shaped, and smooth (Figs. 2A). But once treated with cathelicidin-BF, the intracellular inclusions were found effluxed extracellularly (the representatives are indicated by arrows), indicating that the breaks might be formed in the plasma membranes of P. acnes. In addition, the cell swell was observed obviously in the cathelicidin-treated P. acnes (Fig. 2B). Clindamycin-treated P. acnes had no significant morphology difference from the untreated bacterium (Fig. 2C), suggesting that it does not act on membranes. In fact, clindamycin kills bacteria by inhibiting protein synthesis.
Figure 2. Scanning electron micrographs of control (A), cathelicidin-BF-treated (B), and clindamycin-treated (C) P. acnes.
The arrows indicate damage to the plasma membranes of bacteria or the intracellular inclusions efflux.
Cathelicidin-BF inhibits P. acnes-induced O2 .− production
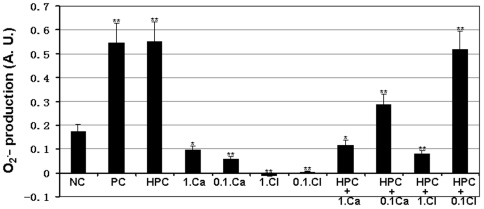
Previous work by Grange et al has indicated that P. acnes significantly induces O2 .− production, which affects IL-8 levels [12]. The effects of cathelicidin-BF on P. acnes-induced O2 .− production was tested in this work. Both live and heat-killed P. acnes significantly induced O2 .− production as the results from Grange et al [16]. As illustrated in Fig. 3, cathelicidin-BF with 1× or 0.1× MIC could significantly inhibited P. acnes-induced O2 .− production. Different from cathelicidin-BF, only 1× MIC clindamycin significantly inhibited P. acnes-induced O2 .− production but 0.1× MIC clindamycin had little effect.
Figure 3. Effects of cathelicidin-BF and clindamycin on ROS production by P. acnes-stimulated keratinocytes.
The human HaCaT keratinocyte cells were incubated with 1×106 bacteria at in the presence of test sample 37°C for 18 h. These represent mean values of three independent experiments. The values for cathelicidin-BF and clindamycin were significant different from the value for the negative control (*P<0.05 and **p<0.01). NC: Negative control; PC: live P. acnes; HPC: heat-killed P. acnes; Ca: cathelicidin-BF; CL: clindamycin; 1. and 0.1.:, 1× MIC and 0.1× MIC.
Cytokine production inhibition by cathelicidin-BF
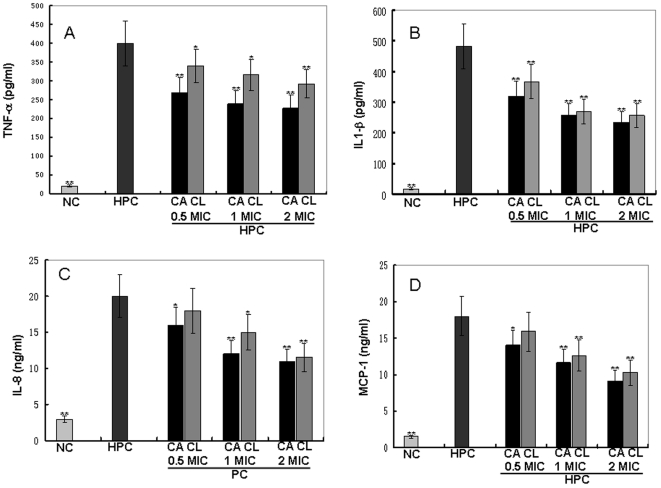
Several pro-inflammatory cytokines including TNF-α, IL-1β, IL-8, and MCP-1 were induced by heat-killed P. acnes as listed in Fig. 4. Both cathelicidin-BF and clindamycin could significantly inhibit cytokines' secretion induced by P. acnes in a dose-dependent manner. For example, TNF-α, one of the most important pro-inflammatory cytokines was induced to a concentration of 400 pg/ml by the heat-killed P. acnes (Fig. 4A). 0.5, 1, and 2 times of MIC of cathelicidin-BF could inhibit 32.5, 40.3, and 43.3% of the induced TNF-α secretion, respectively, while the inhibition rate of clindamycin was 14.7, 20.7, and 27%, respectively. To account for any reduction in pro-inflammatory cytokines resulting from cytotoxic effects of cathelicidin-BF, the cytotoxicity induced by these extracts was determined by MTT assays in THP-1 cells. cathelicidin-BF had little cytotoxic effects with only 0.3, 0.7, and 1.4% cell growth inhibition at concentration of 0.5, 1, and 2 times of MIC, respectively. In addition, after an 18-h incubation, only cathelicidin-BF (no heat-killed P. acnes) did not increase the secretions of either TNF-a, IL-8, IL-1b, or MCP-1 by THP-1 cells.
Figure 4. Effects of cathelicidin-BF and clindamycin on cytokine production by P. acnes-stimulated monocytic cells.
Human monocytic THP-1 cells (1×106 cells/ml) were incubated with heat-killed (incubated at 80°C for 30 min to kill the bacteria) P. acnes (wet weight 100 µg/ml) alone or in combination with different concentrations (0.01, 0.05, and 0.1 mg/ml) of tested sample for 18 h. These represent mean values of three independent experiments. The values for cathelicidin-BF and clindamycin were significant different from the value for the HPC group (*P<0.05 and **p<0.01). NC: Negative control; HPC: heat-killed P. acnes; CA: cathelicidin-BF; CL: clindamycin.
In vivo mice ear colonization inhibition of P. acnes and anti-inflammation by cathelicidin-BF
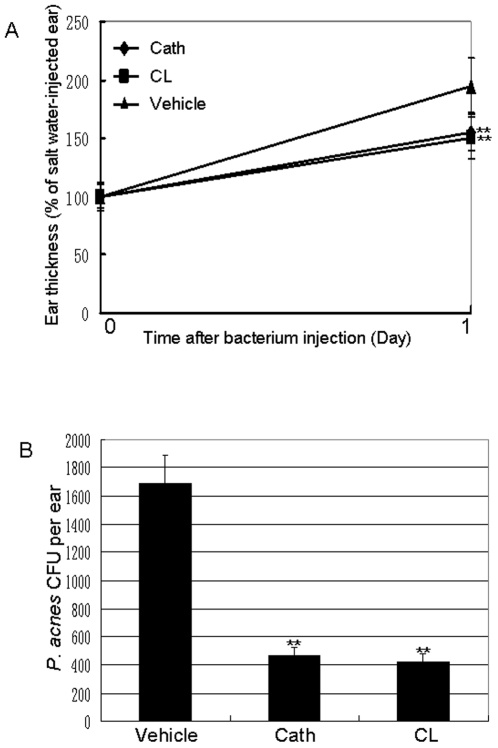
Intradermally injected P. acnes induced severe inflammation in the ears of Kunming mice as illustrated in Fig. 5A. One day after the injection, the ear thickness was about two time of the control. Both cathelicindin-BF and clindamycin 0.2% gels could inhibit the inflammation induced by P. acnes. After one day treatment, 0.2% cathelicindin-BF (425 times of MIC) and clindamycin (950 times of MIC) gel could inhibit 42% and 47% of the ear swelling, respectively.
Figure 5. Effects of 0.2% cathelicidin-BF and clindamycin gel on P. acnes-induced inflammation and P. acnes growth in vivo.
Left ears of mice were intradermally injected with P. acnes (1×107 CFU per 20 µl in PBS) to induce inflammation. Right ears of the same mice were injected with 20 µl of 0.9% salt water (vehicle). Subsequently, 0.2% cathelicidin-BF gel, 0.2% clindamycin gel or vehicle was applied on the ear skin surface of mice. (A) The increase in ear thickness was measured using a micro caliper before and 24 hours after the bacterial injection. (B) 24 hours after P. acnes injection, CFUs of P. acnes in the ear were enumerated as described in the Materials and methods” section. Data represent mean ± SE of five individual experiments. Cath: cathelicidin-BF; CL: clindamycin. The values for cathelicidin-BF and clindamycin were significant different from the value for the vehicle (*P<0.05 and **p<0.01).
The numbers of P. acnes colonized within the ears were illustrated in Fig. 5B. The number of P. acnes colonized within the ear treated by the vehicle is about 1700. The number of P. acnes in the cathelicindin-BF and clindamycin group is about 470 and 430, respectively. Furthermore, data from MTT assays showed that at concentration of 1, 10, and 30 times of MIC, cathelicindin-BF inhibited 0.2, 1.1, and 1.9% cell growth, respectively, indicating almost no cytotoxicity on human HaCaT keratinocyte cells. These data suggest that dermal application of cathelicindin-BF can effectively relieve P. acnes-induced inflammation without detrimental effects on skin cells.
Discussion
Over the past years, natural antimicrobial peptides (AMPs) have attracted considerable interests as a new type of antimicrobial agents for several reasons including their relative selectivity towards targets (microbial membranes), their rapid mechanism of action and, above all, the low frequency in selecting resistant strains [13]–[15]. Cathelicidin-BF is an antimicrobial peptide identified from the snake venoms of B. fasciatus. Our previous work has indicated that cathelicidin-BF exerted strong and rapid antimicrobial activities against many microorganisms including Gram-negative, Gram-positive bacteria and fungi, especially some clinically isolated drug-resistance microorganisms. Besides, cathelicidin-BF has no hemolytic and cytotoxic activity on human cells [3]. However, its effect toward P. acnes has not been studied.
Several antimicrobial peptides including epinecidin- and granulysin-derived peptides, and frog skin peptides have been found to exert anti-P. acnes functions [16]–[18]. Recently, sebocytes are found to express functional cathelicidin antimicrobial peptides with activity to kill P. acnes [12]. Considering anti-inflammatory activities of some antimicrobial peptides, they are suggested to be potent agents for acne vulgaris treatment [13]. The current work indicated that cathelicidin-BF contained potential antimicrobial activity against P. acnes in vitro (Table 1, Fig. 1). Its MIC against two P. acnes strains is 4.7 µg/ml (1.3 µM), which is comparable to the anti-P. acnes potential antibiotics of clindamycin (2.3 µg/ml, 5.2 µM) (Table 1). SEM study indicated that cathelicidin-BF acted on the membrane of P. acnes (Fig. 2).
Previous work by Grange et al has indicated that the whole P. acnes bacteria or the extract of its surface proteins had the same effects to induce O2 .− production in keratinocytes. In addition, their results also indicted that the toxicity of reactive oxygen species on P. acnes-stimulated keratinocytes is mainly caused by the O2 .− overproduction [12]. In this study, cathelicidin-BF was found to obviously inhibit O2 .− production induced by P. acnes in the HaCaT keratinocyte cells (Fig. 3). By O2 .− production inhibition, cathelicidin-BF may inhibit inflammation because O2 .− can exert a positive effect on IL-8 production [12].
Some factors of P. acnes, such as heat shock protein HSP60, can stimulate the production of pro-inflammatory cytokines IL-1b and TNF-a [19]. In turn, these released cytokines lead to the inflammatory reactions [20]. The anti-inflammatory function of cathelicidin-BF was evaluated by measuring its effects on pro-inflammatory cytokine secretion. It could significantly inhibit P. acnes-induced secretion of several pro-inflammatory factors including TNF-a, IL-8, IL-1b, and MCP-1 In vitro (Fig. 4). In vivo anti-inflammatory effect of cathelicidin-BF was confirmed by relieving P. acnes-induced ear swelling and granulomatous inflammation (Fig. 5). The anti-inflammatory effects combined with potent antimicrobial activities and O2 .− production inhibition activities of cathelicidin-BF indicate its pontential as a novel therapeutic option for acne vulgaris.
Materials and Methods
Peptides synthesis
Two cathelicidins (snake cathelicidin-BF, KFFRKLKKSVKKRAKEFFKKPRVIGVSIPF, and human cathelicidin, LL-37, LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) were synthesized by GL Biochem (Shanghai) Ltd. (Shanghai, China) and analyzed by HPLC and mass spectrometry to confirm their purity higher than 98%.
Microorganism strains and growth conditions
Propionibacterium acnes (ATCC6919 and ATCC 11827), Staphylococcus epidermidis (09A3726 and 09B2490) and Staphylococcus aureus (ATCC 2592) were obtained from Kunming Medical College. P. acnes were cultured in brain heart infusion (BHI) broth (HKM,Guangzhou, China) with 1% glucose at 37°C for 3 days to exponential-phase and for 5 days to stationary phase. The bacteria were cultured in an anaerobic atmosphere using MGC Anaeropack systems (Mitsubishi Gas Chemical Co., Inc, Japan); S. epidermidis and S. aureus were grown in LB (Luria-Bertani) broth as our previous report [21].
Susceptibility testing
MIC (minimal inhibitory concentration) of antimicrobial peptides against microorganisms was determined using broth dilution determination as our previous methods [22]. Peptides were prepared as a stock solution in H2O at a series of concentration. 890 µl special broth (BHI broth for P. acnes, LB broth for S epidermidis and S. aureus), 100 µl bacterial suspension (108 CFU/ml) and 10 µl test peptides were put together in the test tube and shaken at 37°C for 24 h. A tube with corresponding volume of H2O was used as control. The MIC was defined as the lowest concentration of test peptides inhibiting microorganism's growth.
Bacteria killing kinetics
The bacterial effect of cathelicidin-BF against P. acnes (ATCC6919) was tested using clindamycin as positive control. P. acnes was grown to log phase in BHI broth and centrifuged at 5000 rpm for 5 min. The collected bacterium pellet was washed twice by BHI broth and diluted to 1×106 CFU/ml with BHI broth. Cathelicidin-BF or clindamycin with one time of MIC was added into the BHI broth containing P. acnes and cultured at 37°C with shaking. The colony counting was performed at different times as described by Mygind et al [23].
Scanning electron microscopy (SEM)
SEM was performed to study the possible mechanisms of action of cathelicidin-BF on bacteria according to the methods described by Lu et al [15] with minor modification. Propionibacterium acnes ATCC6919 was cultured in BHI liquid medium to exponential-phase. After washing with 0.15 M sodium chloride solution for two times, the bacteria were resuspended and incubated with cathelicidin-BF (1× MIC) at 37°C for 30 min. The pellets after centrifuging at 1000 rpm for 10 min were fixed with 2.5% buffered glutaraldehyde at 4°C for 2 h. The bacteria were then postfixed in 1% buffered osmium tetroxide for 2 h, dehydrated in a graded series of ethanol, frozen in liquid nitrogen cooled tertbutyl alcohol and vacuum dried overnight. After mounting onto aluminum stubs and vacuum sputter-coating with gold, the samples were analyzed with a Hitachi S-3000N SEM under standard operating conditions.
Measurement of O2 .− production
Measurement of O2 .− production was carried out following the protocol described by Grange et al [24]. The human HaCaT keratinocyte cells (1×105 cells/ml, obtained from Cell Bank of Kunming Institute of Zoology, Chinese Academy of Sciences) were cultured in 96-well plates with Dulbecco's modified Eagle's medium (DMEM, Gibco) containing 10% fetal calf serum and penicillin (100 u/ml)–streptomycin (100 µg/ml) at 37°C in a humidified 5% CO2 atmosphere. The cell line was routinely tested to assess the absence of Mycoplasma infection. The monolayer cells were washed three times with PBS (1.5 mM KH2PO4, 2.7 mM Na2HPO4.7H2O, 0.15 M NaCl, pH 7.4), and cultured with serum-free and antibiotic-free DMEM medium, incubating with heat-killed P. acnes (1×106) in the presence of test samples. After co-culturing for 18 h, the cells were washed with PBS and incubated with 100 µl per wells of 5 µM dihydroethidium solution (DHE, a fluorescent superoxide anion indicator) for 30 min. The level of intracellular O2 .− was assessed by spectrofluorimetry (excitation/emission maxima: 480/610 nm) on a spectrofluorimeter (FlexStation 3, Molecular Devices, USA).
Measurement of cytokine production in human monocytic cells
Human monocytic THP-1 cells (1×106 cells/ml, Shanghai Caoyan Biotechnology Co. Ltd, China) were cultured in 24-well plates containing serum-free medium (RPMI 1640 medium, Gibco Life Technologies). They were added with heat-killed (incubated at 80°C for 30 min to kill the bacteria) P. acnes (wet weight 100 µg/ml) alone or in combination with different concentrations (0.01, 0.05, and 0.1 mg/ml) of tested samples for an 18-h incubation. Cell-free supernatants were collected, and concentrations of MCP-1, TNFa, IL-1b, and IL-8 were measured using corresponding enzyme immunoassay kits (Adlitteram Diagnostic Laboratories, Inc, USA).
In vitro cytotoxicity of cathelicidin-BF on human skin and monocytic cells
THP-1 and HaCaT cells were cultured in 96-well plates as described above. Cell viability was evaluated by conventional 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reduction assays. After a 24-h treatment by the test samples, 0.1 ml of MTT (5 mg/ml) was added to each well. The supernatant was removed after 2 h of incubation, and 100 µl acidic isopropanol was mixed with the precipitate. The absorbance at 540 nm of the resulting solution was measured. The experiments were performed in triplicate.
In vivo mice ear colonization of P. acnes
P. acnes (ATCC6919) was grown to the exponential-phase in BHI broth. The bacterium was centrifuged at1000 rpm for 10 min. The bacterium pellet was washed twice with 0.15 M sodium chloride solution, and re-suspended in 0.15 M sodium chloride solution (5×108 CFU/ml). P. acnes (1×107 CFU per 20 µl) was intradermally injected into left ears of Kunming mice (20±2 g). Right ears received the same volume of 0.15 M sodium chloride solution. Placebo gel, cathelicidin-BF or clindamycin 0.2% gel (Polyethylene Glycol (PEG) 400∶ PEG 4000, 1∶1) were administered (nine mice per group) on the skin surfaces of ears and the sites were covered with OpSite dressings and occlusively sealed with adhesive tape. The increase of ear thickness after 24 h bacterial injection was measured using a micro caliper. The increase in ear thickness of the left ear was calculated as percentage of the right ear.
To determine P. acnes number in the ear, the left ear was cut off after 24 h bacterial injection and wiped to remove gel. The ear was homogenized in 0.15 M sodium chloride solution (1 ml per ear) withith a hand tissue grinder. CFUs of P. acnes in the ear were enumerated by plating serial dilutions of the homogenate on BHI plates. The plates were anaerobically incubated at 37°C for 72 hours and the bacterial numbers were counted. All the experimental protocols to use animals were approved by the Animal Care and Use Committee at Kunming Institute of Zoology, Chinese Academy of Sciences. The approval ID for this study was syxk2009-0026.
Statistics
Data were analyzed by X 2 and by t test or repeated measure analysis of variance (ANOVA) comparison of means.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Chinese National Natural Science Foundation (30830021, 30800185, 31025025) and the Ministry of Science and Technology (2010CB529800). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria. Nat Rev Microbio. 2005;l3:238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Hong J, Liu X, Yang H, Liu R, et al. Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotics. PLoS One. 2008;16:e3217. doi: 10.1371/journal.pone.0003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao H, Gan TX, Liu XD, Jin Y, Lee WH, et al. Identification and characterization of novel reptile cathelicidins from elapid snakes. Peptides. 2008;29:1685–91. doi: 10.1016/j.peptides.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Lee J, Jung E, Park Y, Kim K, et al. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur J Pharmacol. 2004;496:189–95. doi: 10.1016/j.ejphar.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 6.Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–32. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 7.Leeming JP, Holland KT, Cunliffe WJ. The pathological and ecological significance of microorganisms colonizing acne vulgaris comedones. J Med Microbiol. 1985;20:11–6. doi: 10.1099/00222615-20-1-11. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Koga T, Uchi H, Hara H, Terao H, et al. Propionibacterium acnes-induced IL-8 production may be mediated by NF-kappaB activation in human monocytes. J Dermatol Sci. 2002;29:97–103. doi: 10.1016/s0923-1811(02)00013-0. [DOI] [PubMed] [Google Scholar]
- 9.Basal E, Jain A, Kaushal GP. Antibody response to crude cell lysate of propionibacterium acnes and induction of pro-inflammatory cytokines in patients with acne and normal healthy subjects. J Microbiol. 2004;42:117–25. [PubMed] [Google Scholar]
- 10.Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158–65. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson JK. Antibiotic resistance of Propionibacterium acnes in acne vulgaris. Dermatol Nurs. 2003;15:359–62. [PubMed] [Google Scholar]
- 12.Grange PA, Chéreau C, Raingeaud J, Nicco C, Weill B, et al. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009;5:e1000527. doi: 10.1371/journal.ppat.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marta Guarna M, Coulson R, Rubinchik E. Anti-inflammatory activity of cationic peptides: application to the treatment of acne vulgaris. FEMS Microbiol Lett. 2006;257:1–6. doi: 10.1111/j.1574-6968.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Falla TJ, Liu H, Hurst MA, et al. Development of protegrins for the treatment and prevention of oral mucositis: structure–activity relationships of synthetic protegrin analogues. Biopolymers. 2000;55:88–98. doi: 10.1002/1097-0282(2000)55:1<88::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Scott MG, Hancock RE. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit Rev Immunol. 2000;20:407–431. [PubMed] [Google Scholar]
- 16.Pan CY, Chen JY, Lin TL, Lin CH. In vitro activities of three synthetic peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor against Propionibacterium acnes, Candida albicans, and Trichomonas vaginalis. Peptides. 2009;30:1058–68. doi: 10.1016/j.peptides.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 17.McInturff JE, Wang SJ, Machleidt T, Lin TR, Oren A, et al. Granulysin-derived peptides demonstrate antimicrobial and anti-inflammatory effects against Propionibacterium acnes. J Invest Dermatol. 2005;125:256–63. doi: 10.1111/j.0022-202X.2005.23805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbán E, Nagy E, Pál T, Sonnevend A, Conlon JM. Activities of four frog skin-derived antimicrobial peptides (temporin-1DRa, temporin-1Va and the melittin-related peptides AR-23 and RV-23) against anaerobic bacteria. Int J Antimicrob Agents. 2007;29:317–21. doi: 10.1016/j.ijantimicag.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol. 2004;150:421–428. doi: 10.1046/j.1365-2133.2004.05762.x. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello C. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Xu X, Xu C, Zhou W, Zhang K, et al. Anti-infection peptidomics of amphibian skin. Mol Cell Proteomics. 2007;6:882–94. doi: 10.1074/mcp.M600334-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Song Y, Li J, Liu H, Xu X, et al. A new family of antimicrobial peptides from skin secretions of Rana pleuraden. Peptides. 2007;28:2069–74. doi: 10.1016/j.peptides.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Mygind PH, Fischer RL, Schnorr KM, Hansen MT, Sönksen CP, et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Zhai L, Wang H, Che Q, Wang D, et al. Novel families of antimicrobial peptides with multiple functions from skin of Xizang plateau frog, Nanorana parkeri. Biochimie. 2010;92:475–81. doi: 10.1016/j.biochi.2010.01.025. [DOI] [PubMed] [Google Scholar]