Abstract
The present study describes the flexor and extensor muscles in Cebus libidinosus' forearm and compares them with those from humans, chimpanzees and baboons. The data is presented in quantitative anatomical indices for similarity. The capuchin forearm muscles showed important similarities with chimpanzees and humans, particularly those that act on thumb motion and allow certain degree of independence from other hand structures, even though their configuration does not enable a true opposable thumb. The characteristics of Cebus' forearm muscles corroborate the evolutionary convergence towards an adaptive behavior (tool use) between Cebus genus and apes.
Introduction
In the last two decades, several behavioral studies have focused on the capuchin's ability to use tools. In its strictest sense, tool use is only found in a handful of old world monkeys (OWM) and apes. The only exceptions among new world monkeys (NWM) are the capuchins, which have been reported to use tools both in the captivity and in the wild [1], [2], [3]. Such studies have reported that the Cebus is capable to handle rocks to open coconuts, to use toothpicks to push food out of a pipe or to extract molasses through the orifices of a box [4], [5], [6]. Recently wild capuchins were observed to fish for termites using twigs, an activity until then only seen in chimpanzees [7]. Such complex behaviors are dependent on versatile grasping ability [8], [9]. Accordingly, Cebus have been reported to display a wide array of grasping strategies and manipulative, comparable to chimpanzees and humans [10], [11].
Dexterous hand ability, and consequently tool use, is associated with the development of primate intelligence and culture [12], [13]. This adaptive behavior therefore denotes an important evolutionary convergence, especially between capuchins and chimpanzees. Capuchin tool use seems also dependent on other neurological, cognitive and morphological convergences [10], [14]. In this sense, capuchins stand as an important model for testing hypotheses regarding the evolution of primate cognition.
Comparative anatomical analysis of primates may yield important knowledge regarding behavior and phylogeny. More specifically, forearm anatomy is crucial to understand manipulative behaviors of the hand. Although a few studies have focused on comparative behavioral assessment of capuchin tool use [8], [9], the literature on their forearm myology is scarce. Early studies have indicated that precision grips were untenable to capuchins due to lack of saddle joint in the hand and therefore tool use ability was not related to thumb mobility [15], [16]. Further behavioral studies, however, have reported that this genus can adduct the thumb towards the index finger, favoring the flexing of the interphalangeal rather than the metacarpophalangeal joint, coined ‘lateral opposability’ [8]. However, there are still no anatomical confirmations of these findings.
In the present study, the flexors muscles of the forearm in the Cebus libidinosus [17] monkey were investigated. Origin, insertion, arterial branching and innervation of each muscle were characterized to provide an anatomical understanding of the manual skills observed in Cebus. The anatomical observations here were then compared to the analogous muscles found in humans [18] and chimpanzees and baboons [19]. The degree of anatomical similarity among the forearm muscles in these species was compared using the Comparative Anatomy Index (CAI) [20].
Materials and Methods
Samples
Eight adult capuchin specimens (Cebus libidinosus) were used (seven males and one female) weighing from one to three kilograms. No animal was killed for the purposes of this study: four of them suffered accidental deaths in their natural habitats and were acquired from anatomical collection of the Neuroscience and Behavior of Primates Laboratory (NECOP) from the Federal University of Goias- Catalão-Goias. The remaining of them belonged to the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) archive and were donated to the University of Goiás in the 1970's. This work was approved by the Institutional Ethical Committee from the Federal University of Goiás (CoEP-UFG 81/2008, authorization from the IBAMA number 15275).
Preparation of the animals for dissection
All procedures involving the animals were done in accordance to the guidelines of the Brazilian Society of Animal Experimentation (COBEA). After the trichotomy with a razor blade, the animals were incubated in water at room temperature for 10–12 hours; and then received perfusion, by the femoral vein, 10% of formaldehyde with 5% of glycerin for fixation. The animals were conserved in 10% of formaldehyde, in covered opaque cubes, to avoid the penetration of light and the evaporation of the formaldehyde.
To undertake the anatomical observations for the present work, after receiving the specimens at the anatomical collection of the Federal University of Goiás each one was processed, by the first author, as follows: (1) it received an injection of latex 601-A (Dupont) stained with Wandalar red diluted in ammonium hydroxide in the abdominal aorta in order to facilitate the visualization of small arteries; (2) it was incubated in water at room temperature for 10–12 hr; and then (3) it received a perfusion of 10% formaldehyde with 5% glycerin through the femoral vein for fixation. The monkeys were preserved in 10% formaldehyde in closed opaque boxes to avoid light penetration and formaldehyde evaporation.
Dissection and documentation
The dissection of the forearm was performed with emphasis on the flexors muscles of the forearm and registered with a digital camera. The first author of the present paper dissected both sides of the eight specimens of Cebus libidinosus. The nomenclature of the forelimb muscles follows, whenever it is possible, that used in human anatomy (The Federative Committee on Anatomical Terminology, 1998). When no such parallel was possible, they were referred to following the patterns of the international nomenclature from the Human Anatomic Nominal. The data collected were analyzed and compared with the patterns described for human, chimpanzee and baboon species.
Statistical analysis
Based on Aversi-Ferreira [20], we used a simple comparative non-parametric method for two different species associated on anatomical concepts of normality and variation as nominal variables. Relative frequency (RF) was defined as: RF = (N−nv)/N; where N is the total number of specimens of the sample and nv is the number of individuals presenting variation of the normal pattern.
When more than one parameter (location, nerve, blood vessel, origin and insertion of a muscle) was necessary, they were associated to a specific pondered value with respect to their degree of relevance in comparative analysis. Parameters with less variation were ascribed a higher value. Therefore, innervation, origin and insertion, and vascularization were ascribed the weighs 3, 2, 1, respectively.
The Pondered Average of Frequencies (PAF) was calculated using the RF values:
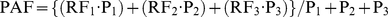 where RF1 is the frequency of the muscle innervation and P1 is 3; RF2 is the frequency of the muscle origin and P2 is 2, RF3 is the frequency of the muscle vascularization and P3 is 1.
where RF1 is the frequency of the muscle innervation and P1 is 3; RF2 is the frequency of the muscle origin and P2 is 2, RF3 is the frequency of the muscle vascularization and P3 is 1.
To consider the proximity between the structures studied, the difference in the relative frequency is calculated, or Comparative Anatomy Index (CAI) between samples from different species:
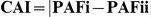 ; where indexes i and ii represent samples 1 and 2.
; where indexes i and ii represent samples 1 and 2.
From the equations above, it follows that the close to zero CAI values represents greater similarity between samples it represents, whereas a CAI closer to 1.0 means higher divergence between samples. More specifically, CAI value of 0 indicates high similarity between the structures analyzed, from 0 to 0.200 as similar structures, from 0.200 to 0.650 as somewhat similar, from 0,650 and 1,000 as dissimilar.
For the purposes of the present work, the Cebus was primarily chosen as the reference for comparison against human, chimpanzee and baboon morphology, although they were also analyzed among themselves.
For example, regarding the muscle flexor carpi radialis, RF was 1 to all parameters in Cebus specimens and RF = 0 was set for the absence of any parameters in the other species.
Then,
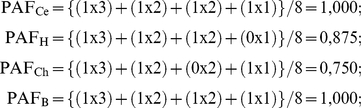 |
Where the indexes Ce, H, Ch and B represents respectively Cebus, humans, chimpanzees and baboons. In order, the first parameter is innervation, the second is origin of muscle, the third is insertion of muscle, and fourth is vascularization (see table 1). Note that vascularization is the only difference between Cebus and humans whereas insertion differs between Cebus and chimpanzees and no differences were observed between Cebus and baboons in this muscle.
Table 1. Comparative analyses of the flexor superficial muscles forearm among Cebus libidinosus (C.l.), human (Homo), chimpanzee (Pan) and baboon (Papio).
| Muscle | Features | Cebus libidinosus | Homo | Pan | Papio |
| Flexor carpi ulnaris | Origin | Medial epicondyle of humerus and olecranon | Highly similar to C.l. CAI = 0.0 | Highly similar to C.l. CAI = 0.0 | Highly similar to C.l. CAI = 0.0 |
| Insertion | Pisiform bone | ||||
| Innervation | Ulnar nerve | ||||
| Vascularization | Ulnar artery | ||||
| Palmaris longus | Origin | Medial epicondyle of humerus | Variable, may be absentSomewhat similar to C.l. CAI = 0.425 | Highly similar to C.l. CAI = 0.0 | Highly similar to C.l. CAI = 0,0 |
| Insertion | Palmar aponeurosis | ||||
| Innervation | Median nerve | ||||
| Vascularization | Ulnar artery | ||||
| Flexor carpi radialis | Origin | Medial epicondyle of humerus | Similar to C.l.Vascularized by radial arteryCAI = 0.125 | Double insertion in metacarpal II and IIISomewhat similar to C.l. CAI = 0.250 | Highly similar to C.l. CAI = 0.0 |
| Insertion | Base of metacarpal II | ||||
| Innervation | Median nerve | ||||
| Vascularization | Ulnar artery | ||||
| Flexor digitorum superficialis | Origin | Humeral head – medial epicondyle of the humerus; Radial head – anterior surface of the radius | Three heads of origin – humeral, radial and ulnar.Somewhat similar to C.l. CAI = 0.375 | Highly similar to HomoSomewhat similar to C.l. CAI = 0.375 | Highly Similar to C.l. CAI = 0.0 |
| Insertion | Middle phalanges of II to V fingers | ||||
| Innervation | Median nerve | ||||
| Vascularization | Ulnar artery and branches of the radial artery | ||||
| Pronator teres | Origin | Medial epicondyle of humerus | Two heads of origin, humeral and ulnarSomewhat similar to C.l. CAI = 0.375 | Highly similar to HomoSomewhat similar to C.l. CAI = 0.375 | Highly similar to C.l. CAI = 0.0 |
| Insertion | Postero-lateral portion of the radius | ||||
| Innervation | Median nerve | ||||
| Vascularization | Ulnar artery | ||||
| Superficial group | Somewhat similarGCAI = 0.26 | Similar GCAI = 0.20 | Highly similar GCAI = 0.0 | ||
From these values, the CAI is calculated,
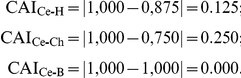 |
The CAI calculated indicate that the muscle flexor carpi radialis of Cebus and baboons are highly similar, Cebus and humans are similar, and Cebus and chimpanzees are somewhat similar structures, according to the parameters adopted here.
A special case is the palmaris longus muscle that is absent in 10% of human population [18] and that presents many variations. To calculate the FR, the same purposed pondered values were used, but they were adjusted by a 10% decrease to innervation and vascularization. Adjustment to origin and insertion were set at 50% decrease since Gray [18] observed that variations in this muscle occur mainly found at its origin and insertion.
Other important parameter to be calculated is the ‘Group CAI’ (GCAI) for structures, such as superficial and deep muscles in the forearm, which combines the summation RF of individual muscles summation average of CAI for the species not used as reference (Homo, Pan and Papio). To calculated GCAI we used the following equation:
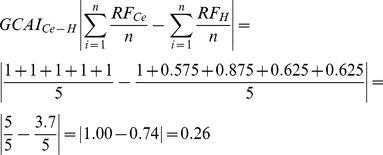 |
or,
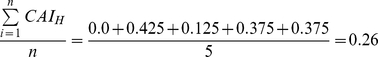 |
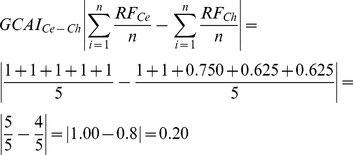 |
or,
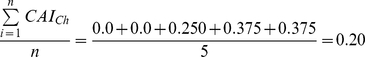 |
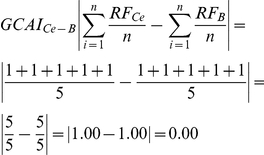 |
or,
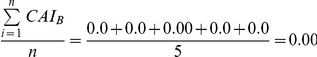 |
The GCAI calculated above shows that the group of superficial muscles of Cebus's forearm is highly similar to baboon, similar to chimpanzees, and somewhat similar to humans.
Results and Discussion
Recently, we have reported a descriptive anatomical comparison of the extensor forearm muscles of Cebus libidinosus [21], especially in comparison with new world monkeys. In the present study, we have expanded on those previous data by applying a non-parametric statistical test (Comparative Anatomy Index) [20] to compare the anatomy of the forearm flexor of muscles of Cebus libidinosus with those of other primates that use tools (humans and chimpanzees) and to baboons, which has not be reported to show this behavior. We also further expanded the findings of the previous report by applying the same statistical test to forearm extensor muscles.
Table 1 summarizes the similarities and differences across the superficial muscles forearm from Cebus, Homo, Pan and Papio.
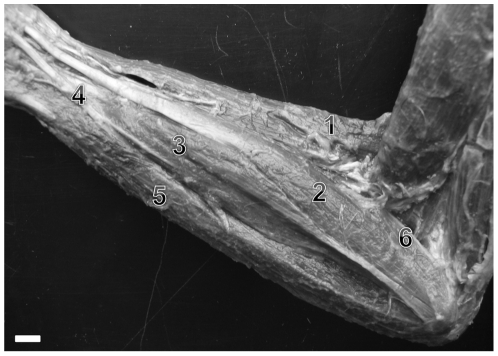
According to Aversi-Ferreira and colleagues [22], the flexor carpi ulnaris muscle and palmaris longus muscle (Figure 1) are similar in all primates species analyzed here with regards to origin, insertion, innervation and vascularization. Flexor carpi radialis, flexor digitorum superficialis and pronator teres muscles, on the other hand, (Figure 1) are more similar between Cebus and Papio. The insertion of the flexor carpi ulnaris muscle on pisiform in Cebus is evident because this bone is well developed in this species.
Figure 1. Photograph of the anterior aspect of forearm right of a Cebus libidinosus (C.l.).
1).brachioradialis muscle, 2) flexor carpi radialis muscle, 3) flexor digitorum superficialis muscle, 4) palmaris longus muscle, 5) flexor carpi ulnaris muscle and, 6) pronator teres muscle. (bar = 1,2 cm).
In general, all superficial muscles in the Cebus' forearm present similarities to the other primates considered here. However, considering some specific details (shown in table 1), more similarities, based on the statistical analysis used in the present work, are found between superficial muscles in the Cebus and Papio.
Table 2 indicates the similarities and differences among the forearm deep muscles from Cebus, Homo, Pan and Papio, with regards to origin, insertion, innervation and vascularization.
Table 2. Comparative analysis of the flexor deep muscles forearm among C.l., human (Homo), chimpanzee (Pan) and baboon (Papio).
| Muscle | Features | Cebus libidinosus | Homo | Pan | Papio |
| Pronator quadratus | Origin | Internal portion antero-lateral of the distal third of the ulna | Innervated by median nerveSomewhat similar to C.l. CAI = 0.375 | Highly similar to C.l. CAI = 0.0 | Highly similar to C.l. CAI = 0.0 |
| Insertion | Border antero-medial of the distal third of the radius | ||||
| Innervation | Ulnar nerve | ||||
| Vascularization | Ulnar artery | ||||
| Flexor digitorum profundus | Origin | Proximal portion of the anterior surface of the ulna | Highly similar to C.l. CAI = 0.0 | Not included tendon to index finger.Similar to C.l. CAI = 0.0625 | Tendons from radial head to I, II and III fingers; and from ulnar head to III, IV and V fingers; associated with flexor digitorium superficial. To CAI purposes, it was considered inexistent.Dissimilar from C.l. CAI = 1.00 |
| Insertion | Base of the phalanges | ||||
| Innervation | Ulnar nerve | ||||
| Vascularization | Ulnar artery | ||||
| Flexor pollicis longus | Origin | Medial epicondyle of the antero-medial surface of the radius | Also originates from the adjacent part of the interosseous membrane.Similar to C.l. CAI = 0.125 | Highly similar to C.l. CAI = 0.0 | Attached to belly of the flexor digitorum profundus muscle.Somewhat similar to C.l. CAI = 0.250 |
| Insertion | Distal phalange of the thumb and a tendon to index finger | ||||
| Innervation | Median nerve | ||||
| Vascularization | Ulnar artery | ||||
| Deep muscles | Similar GCAI = 0.167 | Similar GCAI = 0.020 | Somewhat similar GCAI = 0.417 | ||
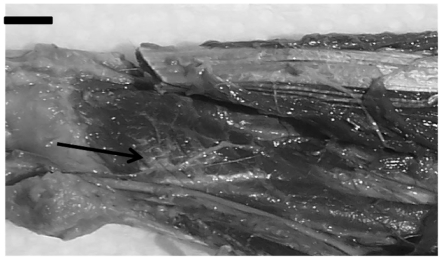
Pronator quadratus muscle (Figure 2) is identical in all non-human primates considered. In humans, however this muscle is innervated by the median nerve [18], not the ulnar nerve as shown in non-human primates.
Figure 2. Photograph of the anterior aspect of forearm right of C.l.
The arrow is indicating the pronator quadratus muscle. (bar = 0,5 cm).
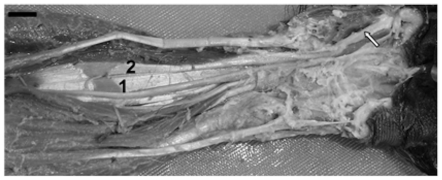
The features observed in table 2 indicate that, in general, Cebus and Pan show great similarity regarding the flexor digitorum profundus and flexor pollicis longus muscles (CAI = 0.0625 and CAI = 0.0, respectively; also Figure 3), closely followed by Homo (CAI = 0.0 and CAI = 0.125, respectively). Interestingly, these muscles are more distinct in baboons, especially the digitorum profundus (CAI = 1.0), which was not the case for any superficial flexor muscles. The flexor digitorum profundus and flexor pollicis longus muscles are involved in finger (including thumb) movement. This anatomical evidence is consistent with the Cebus' manipulation skills.
Figure 3. Photograph of the anterior aspect of forearm left of C.l.
The arrow is indicating the tendon of flexor pollicis longus. 1) flexor digitorum profundus muscle and 2) flexor pollicis longus muscle. (bar = 0,7 cm).
The bellies of the flexor digitorum profundus and flexor pollicis longus muscles are clearly attached to each other, in contrast to humans where bellies are separated and individualized [18]. Indeed, these differences allow for the hand skills required by capuchins' arboreal habits, such as grabbing and holding [23].
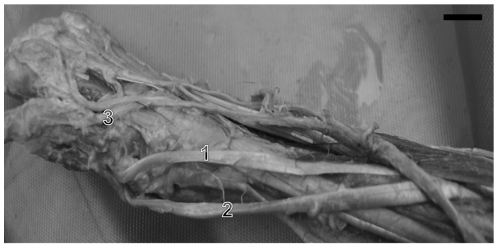
The descriptive analysis of the extensor muscles has been detailed in length elsewhere [21] (shown here in Figure 4). Here we provide a brief assessment of those findings under the light of CAI. In Table 3, we show the CAI and GCAI values for the extensor forearm muscles. High similarity (i.e. CAI = 0) between Cebus, Pan and Papio is evident in almost all extensor muscle, except for the deep dorsal sub-group. In this sub-group, the Cebus' abductor pollicis longus and extensor pollicis brevis show a greater similarity to modern humans and chimpanzees, respectively. It is important to note that the extensor pollicis brevis is not completely differentiated as a distinct muscle from the abductor pollicis longus in Cebus or in any other primate, except for humans and gibbons. The fleshy part, which constitutes this bundle, is deeply blended but it is differentiated into two separate tendons [21]. This configuration is highly similar to that of Pan and it is further differentiated in Homo, but not seen in Papio. Interestingly, the deep dorsal sub-group was pointed by [21] as the major point, in the forearm, which sets capuchins apart from the remaining new world monkeys.
Figure 4. Photograph of the posterior aspect of right forearm of C.l.
1) tendon of the extensor pollicis brevis, 2) tendon of the abductor pollicis longus muscle, 3) tendon of the extensor pollicis longus muscle. (Bar = 1,2).
Table 3. Comparative analyses of the extensor muscles forearm among C.l., human (Homo), chimpanzee (Pan) and baboon (Papio). (Based on Aversi-Ferreira et al., 2010).
| Muscle | Features | Cebus libidinosus | Homo | Pan | Papio |
| Radial Group | |||||
| Brachioradialis muscle | Origin | Latero-distal portion of the humerus on supracondylar ridge | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0. | Highly similar to C.l.CAI = 0.0 |
| Insertion | Styloid process of radius | ||||
| Innervation | Radial nerve | ||||
| Vascularization | Radial artery | ||||
| Extensor carpi radialis longus | Origin | Latero-distal portion of the humerus on the supracondylar ridge | No attached bellies with others muscles.Somewhat similar to C.l.CAI = 0.22 | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0 |
| Insertion | Dorsal surface of the base of the second metacarpal on its radial side | ||||
| Innervation | Radial nerve | ||||
| Vascularization | Radial artery | ||||
| Extensor carpi radialis brevis | Origin | Lateral epicondyle of the humerus | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0 |
| Insertion | Dorsal surface of the base of the second metacarpal | ||||
| Innervation | Radial nerve | ||||
| Vascularization | Radial artery | ||||
| Supinator | Origin | Lateral epicondyle of the humerus | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0 |
| Insertion | Medium portion of the radious | ||||
| Innervation | Radial nerve | ||||
| Vascularization | Radial artery | ||||
| Superficial Dorsal Group | |||||
| Extensor digitorum communis | Origin | Lateral epicondyle of the humerus | Lesser variation regarding the distribution of tendons to fingers.Somewhat similar to C.l.CAI = 0.22 | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0 |
| Insertion | Dorsal aponeurosis in the second to fifth proximal phalanges | ||||
| Innervation | Radial nerve | ||||
| Vascularization | Radial artery | ||||
| Extensor digiti quinti proprius | Origin | Lateral epicondyle of the humerus | Only one insertion tendon to little finger.Somewhat similar to C.l.CAI = 0.22 | Fleshy portion is well detached.Somewhat similar to C.l.CAI = 0.22 | Highly similar to C.l.CAI = 0.0 |
| Insertion | Dorsum of the IV and V fingers | ||||
| Innervation | Radial nerve | ||||
| Vascularization | Radial artery | ||||
| Ulnar Group | |||||
| Extensor carpi ulnaris | Origin | Lateral epicondyle of the humerus | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0 | Highly similar to C.l.CAI = 0.0 |
| Insertion | Metacarpal of the little finger | ||||
| Innervation | Radial nerve | ||||
| Vascularization | Posterior interosseous artery | ||||
| Deep Dorsal Group | |||||
| Extensor pollicis longus | Origin | Posterior surface in the medium third of the ulna and interosseous membrane | Single insertion in distal phalange of the thumb.Somewhat similar to C.l.CAI = 0.250 | Describe as derived from of a common extensor muscle primitive.Somewhat similar to C.l.CAI = 0.22 | Similar to Pan.Somewhat similar to C.l.CAI = 0.22 |
| Insertion | Bases of the proximal and distal phalanges of the thumb | ||||
| Innervation | posterior interosseous nerve | ||||
| Vascularization | posterior interosseous artery | ||||
| Extensor indicis propius | Origin | Posterior surface of the ulna and interosseous membrane | Tendon isolated to index finger.Somewhat similar to C.l.CAI = 0.22 | Highly similar to C.l.CAI = 0.0. | Highly similar to C.l.CAI = 0.0 |
| Insertion | Proximal phalanges of the II, III and IV fingers | ||||
| Innervation | Posterior interosseous nerve | ||||
| Vascularization | Posterior interosseous artery | ||||
| Abductor pollicis longus | Origin | Posterior surface of the ulna, radius and interosseous membrane | Highly similar to C.l.CAI = 0.0 | Double insertion into the trapezoid and base of the first metacarpal.Somewhat similar to C.l.CAI = 0.250 | Similar to Pan.Somewhat similar to C.l.CAI = 0.250 |
| Insertion | Base of the first metacarpal | ||||
| Innervation | Posterior interosseous nerve | ||||
| Vascularization | Posterior interosseous artery | ||||
| Extensor pollicis brevis | Origin | Proximal third of the radius and interosseous membrane | Single insertion in distal phalange of the thumb,Somewhat similar to C.l.CAI = 0.250 | Highly similar to C.l.CAI = 0.0 | Absent. Dissimilar from C.l.CAI = 1.0 |
| Insertion | articular capsule of the trapezoid-metarcapal I articulation and the base of this last bone | ||||
| Innervation | posterior interosseous nerve | ||||
| Vascularization | posterior interosseous artery | ||||
| Extensor group | Similar GCAI = 0.125 | SimilarGCAI = 0.063 | SimilarGCAI = 0.134 | ||
Since the majority of forearm muscles acts on the hand and fingers, the present study described the anatomy of the capuchin forearm muscles and compared them with those of humans, chimpanzees and baboons. The superficial group of flexor muscles was more similar to those of baboons (GCAI = 0.00) than chimpanzees and humans (GCAI = 0.20 and GCAI = 0.26, respectively). On the other hand, the deep flexor muscles were more similar to those of chimpanzees (GCAI = 0.02). They were even found more similar to those of humans (GCAI = 0.167) than those of baboons (GCAI = 0.417). The same pattern was found for extensor muscles, where capuchins were overall more similar to chimpanzees and humans (GCAI = 0.063 and GCAI = 0.125, respectively) than to baboons (GCAI = 0.134).
Baboons are mainly terrestrial monkeys with no reported use of tools either in captivity or in the wild [24]. Capuchins and chimpanzees are both arboreal and terrestrial, and even show an occasional bipedalism [25], [26]. Higher similarities in deep flexor muscles and extensor muscles between capuchin and chimpanzee, as opposed to baboon, suggest a possible link between lifestyle and forearm morphology. For instance, baboons do not show a clear separation among the extensor indicis propius, the extensor digiti quinti proprius and extensor pollicis brevis muscles, as seen in capuchin and chimpanzee. The only exception among extensor muscles is the extensor pollicis longus muscle. Also, the insertions of the extensor muscles in chimpanzees and capuchins are similar between both species but distinct from those in humans. They reflect the predominance of muscular strength over fine hand skills, which is associated with arboreal habits [21]. Nevertheless, Aversi-Ferreira et al., [27] noted that the capuchin shoulder and arm muscles, which aid in locomotion with thoracic members, are more similar to baboons than chimpanzees.
Another important factor regarding complex tool use is thumb opposability. Contrary to apes and macaques, capuchins present only lateral opposability [8]. This concept incorporates thumb prehensive grips observed in this genus. This finding was later corroborated by [21] which pointed to 3 thumb related movements that distance Cebus from NWM, namely: the extensor pollicis longus inserts in digit 1 only, abductor pollicis longus' anterior part is separated into 2 tendons, and extensor pollicis longus is not completely blended with extensor indicis. These features allow the uncoupling of the movements of the thumb from other digits. These are important differences that set capuchins away from closely related NWM. In the case of capuchin abductor pollicis longus, there is even higher similarity to humans than chimpanzees and baboons (CAI = 0.0; CAI = 0.250; and CAI = 0.250, respectively). The conjunct rotation that occurs at the capuchin carpo-metacarpal joint, which also allow this relative opposability, is more similar to OWM than NWM [28]. These findings confirm the evolutionary convergence of hand and forearm anatomy between capuchins and OWM, particularly chimpanzees and humans. They also support the high proximity in grasping and manipulative tasks among these species [9], [10], [11].
Finally, the use of fine, independent hand movement and thumb opposability for a wide variety of grasping and manipulation in capuchins is further supported by abundant corticospinal terminations [29]. These terminations are very dense at the ventral horn of cervical segments of the spine, from where motorneurons originate to innervate hand muscles. Rilling and Insel [30] also suggested that the increased number of sensorimotor fibers in Cebus brain may contribute to the wide variety of grasping strategies and manipulation skills. Capuchins also show high level of encephalization indices, in some cases, rivaling those of chimpanzees [30], [31], [32]. The highly developed cognitive skills shown by Cebus [33], [34] are also critical to solving tasks that requires tools.
Overall, capuchins' muscle and neural organization as well as behavioral habits and lifestyle point to an evolutionary convergence with chimpanzees, and even humans, despite a phylogenetic branching of around 30 million years ago [12]. The role phylogenetic constraints on the evolution of the forearm muscles of NWM cannot be underplayed: most of these muscles are remarkably similar across this very diverse group of primates [21]. The myological uncoupling of thumb movement and independent finger movements found capuchins, unique among NWM, is an important adaptive. The acquisition of such features may have allowed for the neurological and cognitive developments of tool use behavior.
In conclusion, the forearm anatomical data amassed from the present study as well as previous ones [21], [22] support the behavioral grasping and manipulation abilities observed in this genus. Forearm muscle shape and differentiation in capuchins is in keeping with capuchin's high encephalization indices and cognitive skills. These findings further corroborate the evolutionary convergence towards an adaptive behavior (tool use) between Cebus genus and apes.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Japan, Japan Society for the Promotion of Science Asian Core Program, and the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research (A) (22240051); and by National Council of Technology and Development - Brazil. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parker ST, Gibson KR. Object manipulation, tool use and sensorimotor intelligence as feeding adaptations in Cebus monkeys and great apes. Journal of Human Evolution. 1977;6:623–641. [Google Scholar]
- 2.Waga IC, Dacier AK, Pinha PS, Tavares MCH. Spontaneous tool use by wild Capuchin monkeys (Cebus libidinosus) in the cerrado. Folia Primatologica. 2006;77:337–344. doi: 10.1159/000093698. [DOI] [PubMed] [Google Scholar]
- 3.Ottoni EB, Izar P. Capuchin monkey tool use: Overview and implications. Evolutionary Anthropology. 2008;17:171–178. [Google Scholar]
- 4.Westergaard GC, Fragaszy DM. The manufacture and use of tools by capuchin monkeys (Cebus apella). Journal of Comparative Psychology. 1987;101:159–168. [Google Scholar]
- 5.Visalberghi E, Fragaszy DM, Savagerumbaugh S. Performance in a tool-using task by common chimpanzees (Pan troglodytes), bonobos (Pan paniscus), an orangutan (Pongo pygmaeus), and capuchin monkeys (Cebsu apella). Journal of Comparative Psychology. 1995;109:52–60. doi: 10.1037/0735-7036.109.1.52. [DOI] [PubMed] [Google Scholar]
- 6.Fragaszy D, Izar P, Visalberghi E, Ottoni EB, De Oliveira MG. Wild capuchin monkeys (Cebus libidinosus) use anvils and stone pounding tools. American Journal of Primatology. 2004;64:359–366. doi: 10.1002/ajp.20085. [DOI] [PubMed] [Google Scholar]
- 7.Souto A, Bione CB, Bastos M, Bezerra BM, Fragaszy DM, et al. Critically endangered blonde capuchins fish for termites and use new techniques to accomplish the task. Biology Letters. 2011 doi: 10.1098/rsbl.2011.0034. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christel MI, Fragaszy D. Manual function in Cebus apella. Digital mobility, preshaping, and endurance in repetitive grasping. International Journal of Primatology. 2000;21:697–719. [Google Scholar]
- 9.Spinozzi G, Truppa V, Lagana T. Grasping behavior in tufted capuchin monkeys (Cebus apella): Grip types and manual laterality for picking up a small food item. American Journal of Physical Anthropology. 2004;125:30–41. doi: 10.1002/ajpa.10362. [DOI] [PubMed] [Google Scholar]
- 10.Pouydebat E, Gorce P, Coppens Y, Bels V. Biomechanical study of grasping according to the volume of the object: Human versus non-human primates. Journal of Biomechanics. 2009;42:266–272. doi: 10.1016/j.jbiomech.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Pouydebat E, Reghem E, Borel A, Gorce P. Diversity of grip in adults and young humans and chimpanzees (Pan troglodytes). Behavioural Brain Research. 2011;218:21–28. doi: 10.1016/j.bbr.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Byrne RW. Evolution of primate cognition. Cognitive Science. 2000;24:543–570. [Google Scholar]
- 13.Roth G, Dicke U. Evolution of the brain and intelligence. Trends in Cognitive Sciences. 2005;9:250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Perry S. Social traditions and social learning in capuchin monkeys (Cebus). Philosophical Transactions of the Royal Society B-Biological Sciences. 2011;366:988–996. doi: 10.1098/rstb.2010.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Napier JR. The evolution of the hand. Scientific American. 1962;12:49–55. doi: 10.1038/scientificamerican1262-56. [DOI] [PubMed] [Google Scholar]
- 16.Napier JR. Hands. Princeton: Princeton University Press; 1980. [Google Scholar]
- 17.Rylands AB, Schneider H, Langguth A, Mittermeier R, Groves CP, et al. An assessment of the diversity of new word primates. Neotropical Primates. 2000;8:61–93. [Google Scholar]
- 18.Standring S. Forearm, wrist and hand. Gray's anatomy: The anatomical basis of clinical practice. London: Churchill Livingstone; 2008. pp. 839–898. [Google Scholar]
- 19.Swindler DR, Wood CD. Superior member. An Atlas of Primates Gross Anatomy. Washington: University of Washington Press; 1973. pp. 148–155. [Google Scholar]
- 20.Aversi-Ferreira TA. A New Statistical Method for Comparative Anatomy. International Journal of Morphology. 2009;27:1052–1059. [Google Scholar]
- 21.Aversi-Ferreira TA, Diogo R, Potau JM, Bello G, Pastor JF, et al. Comparative Anatomical Study of the Forearm Extensor Muscles of Cebus libidinosus (Rylands et al., 2000; Primates, Cebidae), Modern Humans, and Other Primates, With Comments on Primate Evolution, Phylogeny, and Manipulatory Behavior. Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology. 2010;293:2056–2070. doi: 10.1002/ar.21275. [DOI] [PubMed] [Google Scholar]
- 22.Aversi-Ferreira TA, Aversi-Ferreira RAGMF, Silva Z, Gouvêa-e-Silva LF, Penha-Silva M. Anatomical study of the deep muscles of the forearm of the Cebus apella (Linnaeus, 1766). Acta Scientiarum Biological Sciences. 2005;27:297–301. [Google Scholar]
- 23.Aversi-Ferreira TA, Vieira LG, Pires RM, Silva Z, Penha-Silva N. Comparative study of the superficial muscles of the forearm of the Cebus and Homo. Bioscience Journal. 2006;22:139–144. [Google Scholar]
- 24.Rose MD. Quadrupedalism in primates. Primates. 1973;14:337–357. [Google Scholar]
- 25.Fragaszy DM, Visalberghi E, Fedigan LM. The Complete Capuchin: The biology of the genus Cebus. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- 26.Stanford CB. Arboreal bipedalism in wild chimpanzees: Implications for the evolution of hominid posture and locomotion. American Journal of Physical Anthropology. 2006;129:225–231. doi: 10.1002/ajpa.20284. [DOI] [PubMed] [Google Scholar]
- 27.Aversi-Ferreira TA, Pereira-de-Paula J, Prado YCL, LIma-e-Silva MS, Mata JR. Anatomy of the shoulder and arm muscles of Cebus libidinosus. Brazilian Journal of Morphological Sciences. 2007;24:3–14. [Google Scholar]
- 28.Rose MD. Kinematics of the trapezium-1st metacarpal joint in extant anthropoids and miocene mominoids. Journal of Human Evolution. 1992;22:255–266. [Google Scholar]
- 29.Bortoff GA, Strick PL. Corticospinal terminations in 2 New-monkeys - Further evidence that corticomotoneuronal conections provide part of the neural substrate for manual dexterity. Journal of Neuroscience. 1993;13:5105–5118. doi: 10.1523/JNEUROSCI.13-12-05105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- 31.Stephan H, Andy OJ. Quantitative comparative neuroanatomy of primates - an attempt at a phylogenetic interpretation. Annals of the New York Academy of Sciences. 1969;167:370–&. [Google Scholar]
- 32.Kudo H, Dunbar RIM. Neocortex size and social network size in primates. Animal Behaviour. 2001;62:711–722. [Google Scholar]
- 33.Tavares MCH, Tomaz C. Working memory in capuchin monkeys (Cebus apella). Behavioural Brain Research. 2002;131:131–137. doi: 10.1016/s0166-4328(01)00368-0. [DOI] [PubMed] [Google Scholar]
- 34.Resende MC, Tavares MCH, Tomaz C. Ontogenetic dissociation between habit learning and recognition memory in capuchin monkeys (Cebus apella). Neurobiology of Learning and Memory. 2003;79:19–24. doi: 10.1016/s1074-7427(02)00015-1. [DOI] [PubMed] [Google Scholar]