Abstract
Preclinical evaluation of antibody-based immunotherapies for the treatment of type 1 diabetes (T1D) in animal models is often hampered by the fact that the human antibody drug does not cross-react with its mouse counterpart. In this issue of Science Translational Medicine, researchers describe a new mouse model that expresses the human isoform of a molecule targeted by T1D antibody therapies that are currently being tested in clinical trials—the human epsilon chain of the CD3 complex expressed on T cells. Anti-CD3 is capable of reducing insulin needs in individuals with recently diagnosed T1D; however, the precise underlying mechanisms of action and the minimal effective dose have been difficult to define. The new humanized mouse model will be instrumental in optimizing anti-CD3–based therapies and accelerating their clinical realization.
Type 1 (juvenile) diabetes (T1D) is one of the most widespread autoimmune disorders in the world. Nearly 20 million people suffer from T1D worldwide, and the incidence has been increasing by 2 to 5% in the past few years (1). The etiology of T1D is multi-faceted and involves a complex interplay of genetic and environmental factors resulting in the destruction of insulin-producing β-cells in the islets of Langerhans of the pancreas. This wreckage causes high blood-sugar concentrations (hyperglycemia), the need for daily insulin injections, and, in the long term if not managed properly, severe vascular side effects.
The gradual loss of pancreatic β-cells over years is the result of an autoimmune response that likely involves a series of dysfunctions in patients’ immune systems that unleash pathogenic autoreactive immune effector T cells (Teffs) specific for β-cell antigens (2). In healthy individuals, Teffs are normally kept in check by various mechanisms. Regulatory T cells (Tregs) play a key role in this process, but in patients who are in the process of developing T1D, their ability to suppress Teffs is inefficient, and this aberration facilitates the destruction of β-cells (3). Antibodies to CD3—a protein complex that is associated with the T cell receptor (TCR) and participates in T-cell activation by antigen— can restore normality to some of this immune dysregulation, because anti-CD3 antibodies (anti-CD3s) both reduce the number of Teffs and foster the development of Tregs (4, 5). However, the precise molecular mechanisms that underlie the effects of anti-CD3s on Teff and Treg functions are not fully understood, and unfortunately, not all anti-CD3s exhibit equal efficacy against T1D or uniformly favorable risk-benefit ratios in patients. These observations imply the need for animal models that can permit the evaluation and predict the behavior of humanized therapeutic anti-CD3s that are currently being tested in the clinic. In this issue of Science Translational Medicine, Kuhn et al. describe such a model (6).
The non-obese diabetic (NOD) mouse model for T1D recapitulates many of the immune imbalances as well as genetic and environmental influences present in T1D patients (7). As a consequence, the NOD mouse has been extensively used for preclinical testing of more than 100 candidate therapeutics for T1D (8). However, very few agents demonstrate a capacity to curb the autoimmune response after clinical onset of T1D. Antibodies that specifically target the human epsilon chain of the CD3 complex (huCD3ε) on T cells have rapidly emerged as potent immune regulators that reduce Teffs and augment Tregs; these functions result in long-term tolerance—a physiological state in which T cells do not respond to a particular antigen—with respect to pancreatic β-cell proteins (9).
On the basis of these promising preclinical data, two clinical trials were launched using two different humanized monoclonal antibodies (mAbs) specific for huCD3ε (teplizumab and otelixizumab). In both of these investigations, preservation of C-peptide (formed when proinsulin is cleaved to produce insulin) was achieved for more than 3 years in patients with recent-onset T1D (10–12); however, cytokine release–related side effects occurred in many patients when the drug was administered and, in the European trial, all Epstein-Barr virus (EBV)–infected patients showed transient reactivation of the virus, which was rapidly controlled by an anti-EBV T cell response (13). Overall, the risk-benefit ratio was acceptable, but there was certainly room for improvement, especially if one considers that anti-CD3 might have to be administered to patients more than once. Other anti-CD3s, among them one called visilizumab (4, 14), exhibited less favorable risk-benefit ratios, and the clinical trials were discontinued.
In order to pinpoint, earlier in the translation process, which new anti-CD3 therapeutics are likely to display unwanted side effects in patients and to optimize dosing regimens for more promising candidates, researchers require robust preclinical testing systems that allow them to anticipate potential problems in the clinics. Although both mice and humans express the CD3ε protein, their amino acid sequences differ, and the anti-huCD3ε antibodies developed for clinical use do not bind to the mouse CD3ε molecule (mCD3ε), making direct preclinical testing impractical. Now, Kuhn et al. (6) have developed a NOD mouse colony that expresses the huCD3ε molecule on the surface of mouse T cells (NOD-huCD3ε). This study represents a crucial step toward (i) gaining a better understanding of the mechanisms by which humanized anti-huCD3ε antibodies restore tolerance in vivo and (ii) having the ability to evaluate the dose-efficacy responses for safer and more effective clinical use of these biologics.
IMPROVING THE RISK-BENEFIT RATIO
In the mid-1980s, a hamster anti-mCD3ε mAb (clone 145-2C11) was derived by immunizing Armenian hamsters with a murine cytolytic T-cell clone (15). Then in 1994, Chatenoud and colleagues first demonstrated that this antibody could cure overt autoimmune diabetes in NOD mice (16). A 5-day treatment with 145-2C11 (5 μg) just after disease onset was sufficient to cure T1D in 64 to 80% of NOD mice. However, the efficacy of intact 145-2C11 was associated with potent mitogenic activity that results from the ability of the fragment crystallized (Fc) domain of the mAb to interact with Fc receptors (FcR) on monocytes/macrophages, thus inducing a massive systemic cytokine release (17). This activity, shared by all intact anti-CD3ε–specific mAbs, promotes extensive T cell proliferation and cytokine production, making nonmodified anti-CD3s unsuitable as interventions in T1D.
Indeed, the risk:benefit ratio for the development of any potential therapeutic drug for T1D has to be carefully weighed, because T1D frequently affects children and young adults, and life-long insulin therapy can afford a reasonable life expectancy for most patients. However, insulin cannot prevent all life-threatening long-term complications, and overall life expectancy can be reduced by 10 to 15 years as a result of such complications, which include retinopathy, nephropathy, cardiovascular diseases, and neuropathy. Thus, to move CD3-targeted therapies from bench to bedside, it was crucial to develop CD3ε-specific mAbs that do not bind to FcRs. Preclinical studies demonstrated that the FcR-nonbinding F(ab′)2 fragment of 145-2C11 possesses anti-diabetic properties in NOD mice that are identical to the full-length parent mAb but without the dangerous mitogenic activity (18).
These data paved the way for the development, for clinical applications, of non-mitogenic anti-huCD3ε–specific mAbs that resemble true human antibodies. The first attempt at synthesizing such a mAb occurred in 1979 (19). This mAb, called OKT3, was generated in mice, and although it was directed against huCD3ε, it retained the ability to bind FcRs and thus displayed mitogenic properties in vitro (in human cells) and in vivo (inducing cytokine release in patients) (17). Therefore, researchers embarked on the following two-step process to improve the mAb. OKT3 was “humanized” by genetically engineering the mAb’s constant and variable regions to transform the mouse antibody into a human one, so as to avoid a human anti-mouse antibody (HAMA) response in patients. Then, in order to eradicate the mitogenic properties of OKT3, punctual mutations of the Fc domain were made such that it no longer bound to FcRs.
This process yielded the huCD3ε-directed mAb hOKT3γ1 Ala-Ala, also known as teplizumab (20). In 2002, a U.S.-based phase I/II clinical trial was conducted with teplizumab in newly diabetic patients (within the first 6 weeks of diagnosis). A 14-day course of treatment with teplizumab halted progression of the disease in most patients, as shown by the stabilization of serum C-peptide concentrations and the amelioration of the defect in glucose control reflected by lower amounts of glycosylated hemoglobin and exogenous insulin requirements in anti-CD3-treated patients relative to placebo controls (21). Because patients must receive daily insulin injections as a palliative therapy for T1D, insulin itself is not a reliable read out for β-cell function; however, C-peptide concentrations can reveal whether insulin is produced by β-cells endogenously, which is why this measure is used as an endpoint in clinical trials. In 4 of the drug-treated subjects, insulin production was preserved for up to 5 years after the 14-day course of treatment (11), and as intended, this antibody was associated with only mild and transient adverse effects.
A larger, concurrent phase II clinical trial based in Europe was conducted using a different drug, called ChAglyCD3 or otelixizumab, which is an aglycosylated non-mitogenic humanized IgG1 antibody directed against huCD3ε and derived from the parental YTH12.5 mAb (22). A short-term therapy (6 consecutive days) with otelixizumab preserved residual β-cell function in patients with new-onset T1D (23). Importantly, the efficacy was more pronounced in younger individuals and correlated with pancreatic β-cell mass at trial entry. Seventy-five percent of the subset of patients with the highest β-cell mass (C-peptide concentrations in the >50th percentile) drastically reduced their insulin needs for up to 4 years after treatment (10). The main side effect observed in patients in the otelixizumab-treated group was a transient but generalized EBV reactivation (13), which could possibly be attributed to the higher dose of CD3ε-specific mAb in the European-based relative to the U.S.-based trial (~685 μg/kg versus ~500 μg/kg, respectively).
Despite these encouraging early successes with drugs that target the huCD3ε molecule on T cells, there is a general consensus that the risk-benefit ratio could be improved further. This improvement is an important goal, because repeated antibody administration, combination with additional immune modulators, or ideally, antigen-specific tolerance (24) has to be contemplated for the future if we are devise ways to further reduce the risk of long-term side effects associated with any immunosuppressive therapy (25). The new humanized mouse model might be useful for developing dosing regimens that permit a head-to-head comparison of different anti-CD3s. This point is especially timely, because it was reported recently that the U.S.-based phase III trial with teplizumab did not meet its desired endpoint to improve glycemic control and reduce insulin needs (26).
MODEL MECHANISMS OF TOLERANCE MEDIATION
There is no doubt that a precise delineation of the mechanisms by which the various anti-CD3s mediate their antidiabetic activity will be useful in the quest to improve drug tolerability and clinical outcomes. To this end, Kuhn et al. (6) engineered their NOD-huCD3ε mouse model to co-express both the mCD3ε and huCD3ε chains at a 1:1 ratio selectively on the surface of CD4+ and CD8+ T cells. These NOD-huCD3ε mice developed spontaneous T1D with similar kinetics as the parental NOD mice. Using this model, the authors compared the efficacy and mechanisms of action of (i) mitogenic anti-mCD3ε 145-2C11 with anti-huCD3ε YTH12.5 mAbs and (ii) non-mitogenic anti-mCD3ε 145-2C11 F(ab′)2 with anti-huCD3ε otelixizumab. In vitro (using NOD-huCD3ε T cells in culture) and in vivo (using NOD-huCD3ε mice), these mAbs all displayed various similar activities that modulate T cell function and mediate their tolerogenic activity. Similar to the murine 145-2C11 F(ab′)2 (18), a 5-day treatment with otelixizumab cured newly diabetic NOD-huCD3ε mice by lowering their blood glucose levels to normoglycemia and provided long-term tolerance toward pancreatic β-cell antigens. Both mAbs shared the ability to increase systemically the proportion of CD4+CD25+Foxp3+ Tregs (Fig. 1). Otelixizumab-induced Tregs actively suppressed β-cell autoimmunity in the NODhuCD3ε mice in a TGFβ-but not an IL-10–dependent manner, as previously observed with the nonmitogenic 145-2C11 F(ab′)2 in NOD mice (27).
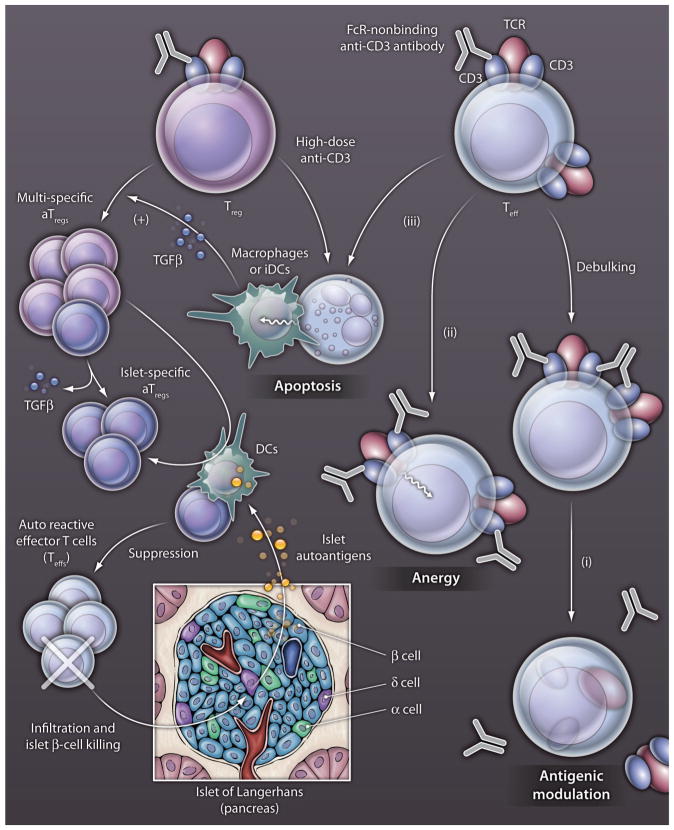
Fig. 1. mAb mechanisms.
Illustrated are the various mechanisms of FcR-nonbinding anti-CD3–specific mAbs in the restoration of immune tolerance in T1D. Efficacy of these anti-CD3–specific mAbs in reversing new-onset T1D relies on several nonmutually exclusive mechanisms that lead to Treg expansion and immune tolerance induction. Two phases are commonly described. The first phase results directly from the presence of the anti-CD3–specific mAbs in the circulation and provides a short-term blockade of the autoreative Teffs, called debulking (5, 9). A second phase begins when the mAbs have been cleared from the circulation, with a series of mechanisms involved in active immune tolerance that maintains the long-term efficacy of anti-CD3–specific mAbs. By binding to the TCT-CD3 complex on Teff, anti-CD3 mAbs induce a series of events that prevent Teffs from attacking pancreatice β-cells in the islets of Langerhans: (i) antigenic modulation of the CD3-TCR complex after its shedding or internalization, (ii) anergy of Teffs and (iii) apoptosis of Teffs and perhaps some Tregs with high-dose anti-CD3, anti-CD3 mAbs delivering a suboptimal activation signal, so that the cells die rather than expand. The apoptotic T cells can be endocytosed and degraded by macrophages or immature dendritic cells (iDCs), which in turn secrete large amounts of TGFβ. In the presence of TGFβ, stimulation with low amounts of anti-CD3 mAbs that remain in the circulation is sufficient to expand multi-specific aTregs. If this expansion occurs in close proximity to mature DCs that present islet-specific autoantigens, the pool of aTregs specific for the islet autoantigens increases, and these aTregs suppress β-cell–specific autorective Teffs.
However, when comparing the activity of otelixizumab used in the clinic to the other mAbs, some subtle but noteworthy differences were observed. Although it was known that anti-CD3 mAbs can modulate the TCR-CD3 complex (by internalization or shedding of the complex), rendering cells ‘blind’ to antigen (a process also referred to as antigenic modulation) (9), the otelixizumab-treated NOD-huCD3ε mice showed very specific kinetics for this TCR modulation. In contrast to other anti-CD3ε mAbs that exhibited peak antigenic modulation by 2 to 4 hours after treatment, the effect of otelixizumab was more prolonged and strongest 24 hours after administration. Interestingly, this differential antigenic modulation could not be observed in NOD-huCD3ε mouse–derived T cells cultured in vitro, a finding that emphasizes the necessity of using humanized animal models to more accurately predict the potential human response to drugs. In addition, otelixizumab treatment induced less overall T cell activation (based on CD69 staining) than did the other mAbs. It is conceivable that any variation in the signal strength through the TCR-CD3 complex could lead to significant differences in T cell behavior and function in vivo. Indeed, one example described in the manuscript by Kuhn et al. (6) is the distinct cytokine expression profile found in the serum of otelixizumab-treated NOD-huCD3ε mice, which was characterized by lower IL-6 but sustained IFNγ and TNF concentrations. Because IL-6 is known to have deleterious effects in T1D, these findings suggest that the risk:benefit ratio of otelixizumab might be more favorable than those of the other mAbs. Thus, future efforts should focus on identifying the key molecular and cellular mechanisms specific to individual anti-CD3ε mAbs as well as those shared by multiple therapeutic antibodies.
A CURE ON THE HORIZON?
Several mechanistic aspects should be comparatively addressed using the novel humanized CD3 mouse model. Such studies could allow for rational prioritization of individual anti-CD3s and aid in the development of new biomarkers that could monitor the intermediate success (or lack thereof) of the intervention.
For example, the U.S.-based intervention trial using teplizumab revealed the induction of Foxp3-, CD25-, and CTLA4-positive suppressor CD8+ T cells (28). Such suppressor populations were never described in NOD mice treated with the 145-2C11 mAb. Therefore, it remains unclear whether induction of CD8+ suppressor T cells is a common feature of all humanized anti-huCD3ε mAbs in humans or whether this population is induced only by a specific signal delivered by teplizumab through the TCR-CD3 complex. The use of NOD-huCD3ε mice will be essential to address this issue by direct comparison of teplizumab and otelixizumab activities in vivo.
One can assume that slight changes in epitope recognition between the various anti-huCD3ε mAbs can result in drastic modifications in the signal they deliver to T cells as is frequently observed with naturally occurring autoantibodies (29). The crystal structure of huCD3εγ in complex with the antigen binding fragment (Fab) of OKT3 has been solved (30). The structure shows that OKT3 (teplizumab) interacts exclusively with a conformational epitope on the huCD3ε subunit and has a low affinity for its antigen. Identification of the epitope recognized by otelixizumab is warranted to perform a side-by-side comparison with teplizumab in NOD-huCD3ε mice and could reveal the relation between the selected epitopes recognized by different anti-huCD3ε mAbs and their distinct modulation of T cell functions.
Treatment with FcR-non-binding humanized anti-CD3ε mAbs can promote the generation of anti-idiotypic antibodies that bind to the variable regions of anti-CD3ε mAbs, mimicking antigen binding and, therefore, blocking the activity of anti-CD3 mAbs (21, 23, 31). These antibodies that can appear a few weeks after mAb treatment and represent a potential therapeutic drawback if repeated treatments are needed to establish permanent tolerance. To overcome this problem, it is instrumental to develop a series of humanized FcR-nonbinding CD3ε-specific mAbs with diverse variable heavy chain–variable light chain (VH-VL) pairings and sequences. One attractive approach is the use of random phage display antibody libraries to select the VH-VL pairings that show strong affinity for the antigen of interest by biopanning— successive rounds of selection and amplification— on huCD3ε (32, 33). These antibody fragments could then be cloned, sequenced, and expressed to obtain a panel of humanized anti-CD3ε mAbs. The NOD-huCD3ε mice provide an excellent model to evaluate such a panel of anti-CD3ε mAbs with respect to their tolerogenic properties and their tendencies to diminish anti-idiotypic activity when injected sequentially.
Last, there is now a growing consensus that combination therapies will be needed to achieve both immune tolerance and β-cell regeneration in patients suffering from T1D. Because of their tolerability, therapeutic efficacy, and ability to induce or expand adaptive Treg (a Treg) functions, anti-CD3ε mAbs are well poised for being a crucial component of combination therapies for T1D. The current vision (34) is that an ideal combination therapy prescription targeted to recent-onset T1D patients or prediabetic individuals at high-risk (for example, those patients who express multiple islet autoantibodies) will consist of an islet β-cell-derived antigen vaccine to induce and expand autoreactive a Tregs (35, 36); a suitable immune modulator, such as anti-CD3s, that reduces Teff function immediately while fostering expansion of a Tregs (24, 37); an anti-inflammatory drug (such as antibodies that neutralize IL-1, IL-6, or IL-12); and a compound that protects β-cells or ideally, supports their regeneration (Fig. 2). The novel humanized mouse model is an ideal system in which to rapidly and rationally optimize such combination therapies.
Fig. 2. Prescription for combinatorial curing of T1D.
Several combination therapies have been proposed to induce, simultaneously or sequentially, immune tolerance and β-cell regeneration in patients suffering from T1D. Efficient combination therapies might consist of (i) an islet β-cell antigen vaccination to induce and expand autoreactive aTregs by using either direct immunization with islet autoantigen or tolerogenic dendritic cells loaded ex vivo with islet autoantigen peptide(s) or protein(s); (ii) a suitable immune modulator that rapidly reduces Teff function while fostering expansion of aTregs. Because of their acceptable tolerability, therapeutic efficacy, and ability to induce or expand aTreg functions, anti-CD3ε mAbs are well poised for being this crucial immune modulator; (iii) a compound that protects pancreatic β-cells and ideally supports their regeneration, such as glucagon-like peptide-1/gastrin, exenatide, or alpha-1-antitrypsin; and (iv) an anti-inflammatory modulator (for example, antibodies to IL-1, IL-6, IL-12, or TNF) to reduce general inflammation. Quelling of inflammation might be required to enhance the expansion of aTregs and to restore β–cell secretory function.
Acknowledgments
Funding: Supported by the NIH, the Juvenile Diabetes Research Foundation (JDRF) and the Brehm coalition. JDRF grant 36-2008-921 to D.B.
Footnotes
Competing interests: The authors declare no competing interests.
References and Notes
- 1.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 3.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman A, Herold KC. Anti-CD3 mAbs for treatment of type 1 diabetes. Diabetes Metab Res Rev. 2009;25:302–306. doi: 10.1002/dmrr.933. [DOI] [PubMed] [Google Scholar]
- 5.Chatenoud L. Immune therapy for type 1 diabetes mellitus—what is unique about anti-CD3 antibodies? Nat Rev Endocrinol. 2010;6:149–157. doi: 10.1038/nrendo.2009.275. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn C, You S, Valette F, Hale G, van Endert P, Bach JF, Waldmann H, Chatenoud L. Human CD3 transgenic mice: Preclinical testing of antibodies promoting immune tolerance. Sci Transl Med. 2011;3:68ra10. doi: 10.1126/scitranslmed.3001830. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 8.Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, Kahn R, Kreuwel HT. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Chatenoud L, Bluestone JA. CD3-specific antibodies: A portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 10.Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, Vandemeulebroucke E, Van de Velde U, Crenier L, De Block C, Candon S, Waldmann H, Ziegler AG, Chatenoud L, Pipeleers D. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 11.Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA Immune Tolerance Network IT N007AI Study Group. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132:166–173. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The environmental determinants of diabetes in the young (TE DDY) study. Ann N Y Acad Sci. 2008;1150:1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keymeulen B, Candon S, Fafi-Kremer S, Ziegler A, Leruez-Ville M, Mathieu C, Vandemeulebroucke E, Walter M, Crenier L, Thervet E, Legendre C, Pierard D, Hale G, Waldmann H, Bach JF, Seigneurin JM, Pipeleers D, Chatenoud L. Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood. 2010;115:1145–1155. doi: 10.1182/blood-2009-02-204875. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter PA, Appelbaum FR, Corey L, Deeg HJ, Doney K, Gooley T, Krueger J, Martin P, Pavlovic S, Sanders J, Slattery J, Levitt D, Storb R, Woolfrey A, Anasetti C. A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graftversus-host disease. Blood. 2002;99:2712–2719. doi: 10.1182/blood.v99.8.2712. [DOI] [PubMed] [Google Scholar]
- 15.Leo O, Foo M, Sachs DH, Samelson LE, Bluestone JA. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1987;84:1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Wauwe JP, De Mey JR, Goossens JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–2713. [PubMed] [Google Scholar]
- 18.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 19.Kung P, Goldstein G, Reinherz EL, Schlossman SF. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206:347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- 20.Woodle ES, Xu D, Zivin RA, Auger J, Charette J, O’Laughlin R, Peace D, Jollife LK, Haverty T, Bluestone JA, Thistlethwaite JR., Jr Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3gamma1(Ala-Ala) in the treatment of acute renal allograft rejection. Transplantation. 1999;68:608–616. doi: 10.1097/00007890-199909150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 22.Bolt S, Routledge E, Lloyd I, Chatenoud L, Pope H, Gorman SD, Clark M, Waldmann H. The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur J Immunol. 1993;23:403–411. doi: 10.1002/eji.1830230216. [DOI] [PubMed] [Google Scholar]
- 23.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 24.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M. Anti-CD3 and nasal pro-insulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing T regs. J Clin Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352:1371–1373. doi: 10.1056/NEJMe058018. [DOI] [PubMed] [Google Scholar]
- 26.Protege Phase 3 clinical trial of teplizumab: http://www.pharmpro.com/News/Feeds/2010/10/pharmaceutical-companies-eli-lilly-macrogenics-and-lilly-announcepivotal-clinical-tr/.
- 27.Belghith M, Bluestone JA, Barriot S, Mégret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 28.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michalek K, Morshed SA, Latif R, Davies TF. TSH receptor autoantibodies. Autoimmun Rev. 2009;9:113–116. doi: 10.1016/j.autrev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kjer-Nielsen L, Dunstone MA, Kostenko L, Ely LK, Beddoe T, Mifsud NA, Purcell AW, Brooks AG, Mc-Cluskey J, Rossjohn J. Crystal structure of the human T cell receptor CD3 epsilon gamma heterodimer complexed to the therapeutic mAb OKT3. Proc Natl Acad Sci USA. 2004;101:7675–7680. doi: 10.1073/pnas.0402295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clackson T, Hoogenboom HR, Griffiths AD, Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 33.Chapal N, Chardès T, Bresson D, Pugnière M, Mani JC, Pau B, Bouanani M, Péraldi-Roux S. Thyroid peroxidase autoantibodies obtained from random single chain FV libraries contain the same heavy/light chain combinations as occur in vivo. Endocrinology. 2001;142:4740–4750. doi: 10.1210/endo.142.11.8473. [DOI] [PubMed] [Google Scholar]
- 34.Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Developing combination immunotherapies for type 1 diabetes: Recommendations from the IT N-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol. 2010;160:176–184. doi: 10.1111/j.1365-2249.2010.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bresson D, von Herrath M. Moving towards efficient therapies in type 1 diabetes: To combine or not to combine? Autoimmun Rev. 2007;6:315–322. doi: 10.1016/j.autrev.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Herrath MG. Vaccination to prevent type 1 diabetes. Expert Rev Vaccines. 2002;1:25–28. doi: 10.1586/14760584.1.1.25. [DOI] [PubMed] [Google Scholar]
- 37.Bresson D, Fradkin M, Manenkova Y, Rottembourg D, von Herrath M. Genetic-induced variations in the GAD65 T-cell repertoire governs efficacy of anti-CD3/GAD65 combination therapy in new-onset type 1 diabetes. Mol Ther. 2010;18:307–316. doi: 10.1038/mt.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]