Abstract
Objective
To determine if duration of antibiotic exposure is an independent risk factor for necrotizing enterocolitis (NEC)
Study design
A retrospective, 2:1 control-case analysis was conducted comparing neonates with NEC to those without from 2000 through 2008. Controls were matched on gestational age, birth weight, and birth year. In each matched triad, demographic and risk factor data were collected from birth until the diagnosis of NEC in the case subject. Bivariate and multivariate analyses were utilized to assess associations between risk factors and NEC.
Results
124 cases of NEC were matched with 248 controls. Cases were less likely to have respiratory distress syndrome (p=0.018) and more likely to reach full enteral feeding (p=0.028) than controls. Cases were more likely to have culture-proven sepsis (p<0.0001). Given the association between sepsis and antibiotic use, we tested for and found a significant interaction between the two variables (p=0.001). When neonates with sepsis were removed from the cohort, the risk of NEC increased significantly with duration of antibiotic exposure. Exposure for >10 days resulted in a nearly three-fold increase in the risk of developing NEC
Conclusions
Duration of antibiotic exposure is associated with an increased risk of NEC among neonates without prior sepsis.
Keywords: sepsis, antimicrobials, prematurity, neonates
Necrotizing enterocolitis (NEC) is a disease process of the gastrointestinal (GI) tract that occurs primarily in premature neonates. Approximately 1% to 5% of very low birth weight infants (<1500 grams) will develop NEC,1,2 with an associated mortality rate of 25% to 33%.3 NEC is believed to be a multifactorial disease process with risk factors including prematurity,4 low birth weight,5 enteral feeding,6 and alterations in bacterial colonization of the GI tract.7
Healthy term breast fed infants are typically colonized with several microbial species by one week of age, including anaerobic species of Bifidobacterium and Lactobacillus. Normal intestinal flora may provide resistance to colonization by highly pathogenic bacteria and stimulate anti-inflammatory cytokine production.8 In contrast, the hospitalized preterm neonate has less microbial diversity and fewer anaerobes, typically becoming colonized with Escherichia coli, species of Enterococcus and Klebsiella pneumoniae.9–11 Proliferation of these organisms can result in invasion of the intestinal wall, translocation, and inflammatory cytokine production.12
The widespread use of antibiotics in the neonatal intensive care unit (NICU) population may contribute to aberrant gut colonization. Antibiotic exposure may reduce the biodiversity of the fetal microbiotia, delay beneficial colonization with normal GI flora, and/or promote proliferation of pathogenic and antibiotic-resistant organisms.13–15 The increase in potentially pathogenic organisms and decrease in normal gut flora coupled with impairment in the intestinal epithelial barrier may predispose preterm neonates to NEC.7
We hypothesize that an increased duration of antimicrobial exposure in neonates will increase the probability of developing NEC.
METHODS
A retrospective, 2:1 control-case[MH1] analysis was performed to investigate the association between the duration of antimicrobial therapy and subsequent diagnosis of NEC among neonates born from January 1, 2000 to December 31, 2008, and admitted to the Newborn Special Care Unit (NBSCU) at Yale-New Haven Children’s Hospital. The NBSCU is a 54 bed, level IIIc16 NICU for infants with complex medical and surgical conditions.
Cases included neonates diagnosed with NEC modified Bell’s stage ≥IIA.17 Each identified case was matched to two controls. The control population included neonates without NEC with matching performed on age (number of hospital days prior to NEC in matched cases), gestational age (± 1 week), birth weight (± 200 grams) and year of hospital admission (± 1 year). Neonates with major congenital abnormalities (including those of the GI tract) were excluded from the study, as well as neonates transferred from outside hospitals.
Cases and controls were identified by review of an electronic database, which maintains demographic, hospital course, and outcome data for all admissions to the NBSCU. Additional information not available from the database was obtained by chart review. Data collection on all subjects included demographics, potential risk factors for NEC, and outcomes. Risk factor data were collected for each case and its 2 matched controls from birth up to and including the day prior to NEC in the case subject. For example, if the case patient developed NEC on day of life 15, data pertaining to the hospital course in the case and two assigned controls were collected from birth through day of life 14.
Potential risk factors for NEC included antenatal corticosteroid exposure, 5-minute Apgar score, small for gestational age, respiratory distress syndrome (RDS), the presence of a patent ductus arteriosus (PDA), laboratory confirmed bloodstream infection, feeding practices, antibiotic exposure, and umbilical catheter use. More specifically, data regarding feeding practices included the day of first enteral feeding, type of feeding (breast milk only, formula only, or a combination), maximum enteral feeding volume achieved (milliliters per kilogram per day) and, if applicable, day of life full enteral feeds were achieved (i.e. when total parenteral nutrition (TPN) was discontinued). The duration of antibiotic exposure included the cumulative number of days a neonate was on any antibiotic. These data were initially collected in hours of exposure which was then converted into days. Data regarding the specific antimicrobial regimen utilized were also collected. Standard empiric antimicrobial therapy in the NBSCU during the study period included ampicillin and gentamicin for suspected early-onset sepsis (≤ 72 hours of life), vancomycin and entamicin for suspected late-onset sepsis (>72 hours of life), and ampicillin plus gentamicin and clindamycin for suspected or confirmed NEC.
Outcome data were also collected and included the presence or absence of bronchopulmonary dysplasia (BPD), duration of mechanical ventilation, duration of TPN, length of hospital stay, and death. Unlike the risk factor data, continuous outcome data (i.e. duration of mechanical ventilation and TPN and length of stay) were reflective of the entire NBSCU stay.
Definitions
RDS was defined as respiratory distress and an oxygen requirement necessitating intubation and the administration of surfactant. BPD was defined as the need for supplemental oxygen at 36 weeks post-menstrual age in association with characteristic radiographic changes.18 A PDA was defined by the presence of clinical signs (i.e. continuous murmur, hyperdynamic precordium, bounding pulses, widened pulse pressure, increased pulmonary vascular markings or cardiomegaly on chest x-ray, increased oxygen requirement), echocardiographic confirmation by a pediatric cardiologist, and subsequent treatment with either Indomethacin or Ibuprofen. Sepsis was defined as a laboratory confirmed bloodstream infection according to criteria from the Centers for Disease Control and Prevention that were current during the study period.19 Blood cultures were assessed during this time period using a fluorescent-detection system for the presence of carbon dioxide (Bactec II or 9240®, Becton Dickinson, USA).
Statistical Analysis
Comparisons between cases and controls on demographic and clinical characteristics, as well as established risk factors for NEC, were first made via unadjusted conditional logistic regression analysis. Patient characteristics showing significant trends (p≤0.10) in association with NEC diagnosis were evaluated in a multivariate conditional logistic regression. The interaction between the diagnosis of sepsis and cumulative exposure to antimicrobial therapy was also evaluated. Data are presented as unadjusted and adjusted odds ratios (OR). All data were analyzed by using SAS 9.2 (Cary, NC). Significance in the adjusted and unadjusted analyses was established with alpha of less than 0.05. This study was approved by the Human Investigation Committee of the Yale University School of Medicine.
RESULTS
The 124 cases of NEC were matched with 248 controls. Neonates with NEC were diagnosed, on average, at day of life 22 ± 15, with 51% receiving only medical treatment and 49% undergoing surgical intervention. Cases and controls were well matched with respect to gestational age (28.2 ± 3.4 weeks in cases v. 28.1 ± 3.4 weeks in controls; p=0.849), birth weight (1162 ± 573 grams in cases v. 1169 ± 584 grams in controls; p=0.902), and year of admission to the NBSCU (p=1.000). Matching was also effective in the subgroups of cases with birth weight < 750 grams (647 ± 60 grams in cases v. 660 ± 77 grams in controls; p=0.290) and ≥ 750 g (1319 ± 567 grams in cases v. 1324 ± 583 grams in controls; p=0.479). No significant differences were observed between cases and controls with respect to sex, the proportion of small for gestational age infants, pre-natal corticosteroid exposure, 5-minute Apgar score, umbilical catheter use, the timing of first enteral feeding, BPD, and length of NBSCU stay (Tables I and II).
Table 1.
A Comparison of Demographic and Outcome Data in Cases and Controls
| Variable | Case (n = 124) | Control (n = 248) | Unadjusted OR (95% CI)* |
|---|---|---|---|
| Male Sex | 74 (59.7%) | 135 (54.4%) | 1.28 (0.80, 2.00) |
| RDS | 70 (56.5%) | 167 (67.3%) | 0.48 (0.27, 0.85) |
| BPD | 53 (42.7%) | 111 (44.8%) | 0.87 (0.49, 1.54) |
| Days on ventilator | 32.2 ± 32.7 | 18.8 ± 27.2 | 1.03 (1.02, 1.05) |
| Days on TPN | 50.9 ± 34.8 | 24.3 ± 18.7 | 1.06 (1.04, 1.07) |
| Hospital days | 38.3 ± 39.7 | 69.0 ± 48.2 | 1.005 (1.00, 1.011) |
| Death | 29 (24.4%) | 10 (4.0%) | 10.17 (3.91, 26.45) |
Conditional Logistic Regression
Abbreviations: OR, odds ratio; CI, confidence interval; RDS, respiratory distress syndrome; BPD, bronchopulmonary dysplasia; TPN, total parenteral nutrition; NEC, necrotizing enterocolitis
Table 2.
A Comparison of Risk factors for NEC in Cases and Controls
| Variable | Case (n = 124) | Control (n = 248) | Unadjusted OR (95% CI) |
|---|---|---|---|
| Prenatal corticosteroids | 110 (88.7%) | 213 (85.9%) | 0.73 (0.34, 1.53) |
| SGA | 16 (12.9%) | 21 (8.5%) | 3.23 (0.98, 10.63) |
| 5-minute Apgar | 7.8 ± 1.6 | 7.5 ± 1.8 | 1.16 (0.98, 1.37) |
| PDA | 15 (12.1%) | 51 (20.6%) | 0.52 (0.28, 0.98) |
| UAC | 13 (10.5%) | 18 (7.3%) | 3.17 (0.80, 12.63) |
| UVC | 9 (7.3%) | 19 (7.7%) | 0.90 (0.30, 2.73) |
| Type of Feed | |||
| Formula | 42 (33.9%) | 61 (24.6%) | Reference |
| Breast milk | 32 (25.8%) | 94 (37.9%) | 0.44 (0.24, 0.81) |
| Both | 40 (32.3%) | 74 (29.8%) | 0.75 (0.42, 1.33) |
| Did not feed | 10 (8.1%) | 19 (7.7%) | 0.79 (0.27, 2.35) |
| Day of first feed | 5.3 ± 3.9 | 5.2 ± 3.0 | 1.02 (0.94, 1.11) |
| Maximum enteral feeding volume (cc/kg/day) | 112.3 ± 41.1 | 96.3 ± 49.7 | 1.01 (1.00, 1.02) |
| Full feeds achieved | 81 (65.3%) | 135 (54.4%) | 2.03 (1.15, 3.59) |
| Day of life achieved | 16.6 ± 8.9 | 15.1 ± 9.4 | 1.05 (0.99, 1.11) |
| Sepsis | 31 (25%) | 28 (11.3%) | 2.62 (1.48, 4.66) |
| DOL sepsis | 20.8 ± 14.5 | 14.0 ± 11.4 | |
| Multiple episodes | 3 (9.7%) | 4 (14.3%) | |
| Type of sepsis organism | |||
| Gram-positive | 12 (9.7%) | 18 (7.3%) | 1.54 (0.72, 3.30) |
| Gram-negative | 14 (11.3%) | 8 (3.2%) | 4.78 (1.78, 12.8) |
| Fungal | 5 (4.0%) | 2 (0.8%) | 5.96 (1.12, 31.82) |
| Days on antibiotics | 6.8 ± 6.8 | 6.4 ± 6.3 | 1.02 (0.98, 1.07) |
| Type of antibiotic | |||
| Ampicillin | 115 (92.7) | 231 (93.2) | 0.93 (0.37, 2.33) |
| Gentamicin | 115 (92.7) | 233 (94.0) | 0.79 (0.31, 2.01) |
| Vancomycin | 33 (26.6) | 77 (31.1) | 0.71 (0.38, 1.31) |
| Cefotaxime | 16 (12.9) | 37 (14.9) | 0.84 (0.44, 1.59) |
| Clindamycin | 11 (8.9) | 8 (3.2) | 4.16 (1.29, 13.44) |
Conditional Logistic Regression
Abbreviations: NEC, necrotizing enterocolitis; OR, odds ratio; CI, confidence interval; SGA, small for gestational age; PDA, patent ductus arteriosus; UAC, umbilical arterial catheter; UVC, umbilical venous catheter; DOL, day of life
A few notable differences, however, were observed between the case and control populations. A significantly higher proportion of controls were diagnosed with RDS and PDA as compared with cases (Tables I and II). Also, neonates with NEC were more likely to obtain a higher enteral feeding volume or reach full enteral feeding as compared with controls (Table II). Neonates diagnosed with NEC required a significantly longer duration of mechanical ventilation and TPN over their entire hospital stay and had a higher rate of death prior to discharge than controls (Table I).
A significantly higher proportion of neonates with NEC were diagnosed with culture-proven sepsis than those without (Table II). Timing of sepsis varied between populations, with neonates in the control group being diagnosed with sepsis, on average, one week earlier than the case group (Table II). The majority of sepsis (65%) in the case population occurred within 24 hours of, or concurrent with, the diagnosis of NEC with 71% of these patients then undergoing surgery for NEC. The type of antibiotic exposure differed only with respect to a significantly higher proportion of clindamycin use in the case population as compared with controls. However, the total duration of exposure to any and all antibiotics prior to our study cut-off point (i.e. the day of life NEC was diagnosed in the case subject) did not differ significantly between cases and controls in the unadjusted analysis (Table II). When duration of exposure to each individual antibiotic was analyzed, no significant differences were noted between cases and controls with respect to ampicillin (5.2 v. 4.5 days; p=0.149), gentamicin (5.9 v. 5.0 days; p=0.238), vancomycin (5.3 v. 4.6 days; p=0.893), clindamycin (5.8 v. 6.0 days; p=0.967), and cefotaxime (5.0 v. 6.6 days, p=0.337).
In the adjusted analysis, attainment of full enteral feeding (p=0.028) remained a significant predictor of NEC, and RDS (p=0.018) was protective. Near significant trends included SGA as a risk factor and breast milk as a protective factor for NEC (Table III). PDA was not significant (p=0.220). Also in the adjusted analysis, we found that the interaction between sepsis and antibiotic use was significant (Table III). Given discrepancies in the proportion of neonates with sepsis in the case and control populations and the strong interaction of sepsis with antibiotic use, the 59 neonates with sepsis were removed from the cohort and separate analyses performed. When the cohort with sepsis was analyzed, we observed a decreased risk of NEC as the cumulative duration of antibiotic therapy lengthened. On day 1–2 of prior antibiotic exposure, neonates with sepsis had a 24.54 fold increased risk of developing NEC. This risk decreased with each day of antibiotics to 15.59 for 3–4 days, 9.90 for 5–6 days, 6.29 for 7–8 days, 4.00 for 9–10 days and 2.54 for >10 days.
Table 3.
Multivariate Regression Analysis of Risk Factors for NEC
| Characteristic | Adjusted OR (95% CI)* | p-value* |
|---|---|---|
| SGA | 3.40 (0.96, 12.05) | 0.058 |
| RDS | 0.46 (0.24, 0.88) | 0.018 |
| Type of feed | ||
| Breast milk | 0.57 (0.30, 1.10) | 0.093 |
| Breast milk & formula | 0.74 (0.39, 1.41) | 0.360 |
| No feeds | 0.90 (0.28, 2.85) | 0.854 |
| Formula | Reference | |
| Full feeds reached | 2.11 (1.08, 4.10) | 0.028 |
| Sepsis | 11.20 (3.44, 36.45) | <0.0001 |
| Antibiotic exposure | 1.10 (1.02, 1.19) | 0.015 |
| Sepsis by Antibiotic Exposure | 0.85 (0.78, 0.94) | 0.001 |
Conditional Logistic Regression
Abbreviations: NEC, necrotizing enterocolitis; OR, odds ratio; CI, confidence interval; SGA, small for gestational age; RDS, respiratory distress syndrome
In those without sepsis, which represented 84% (313 of 373) of the study population, antibiotic exposure was determined to be a significant independent risk factor for NEC (Table III). We also observed a steadily increasing risk of NEC as duration of cumulative antibiotic exposure increased (Figure). After 1–2 days of antibiotic exposure, the risk of developing NEC in this population increased by 1.19 times and continued to increase with each additional day of antibiotic exposure to 1.43 for 3–4 days, 1.71 for 5–6 days, 2.05 for 7–8 days, 2.45 for 9–10 days, and 2.94 for >10 days of exposure (Figure).
Figure.
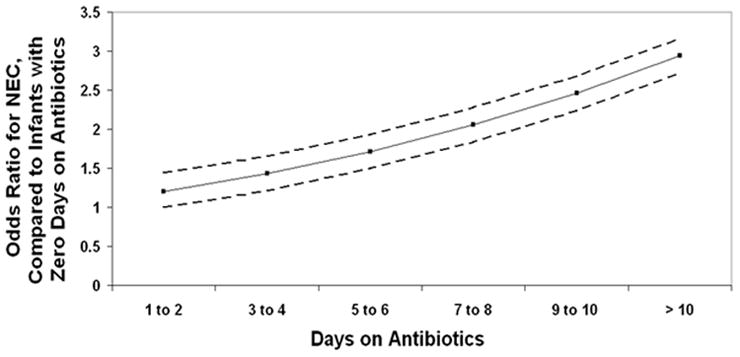
The figure depicts the odds ratio of developing necrotizing enterocolitis (Y-axis) as the cumulative duration of antibiotic exposure increases (X-axis) in neonates without a prior diagnosis of culture-proven sepsis. The comparison group is neonates with zero days of antibiotic exposure. The dotted lines represent 95% confidence intervals. A statistically significant increase is observed.
DISCUSSION
The findings support our hypothesis that increased duration of antibiotic exposure in NICU patients may increase their risk of NEC although, due to the confounding relationship between sepsis and antibiotic use, this was only found in neonates without a prior documented bloodstream infection. Antibiotic use in the neonatal population has been previously described as a potential contributing factor in the development of NEC. Antibiotic administration may result in suppression or eradication of protective anaerobic bacteria which normally colonize the GI tract and result in overgrowth of potentially pathogenic enteric aerobic gram-negative rods.20,21 Previous investigations have determined that overgrowth of pathogenic species is greatly increased after three days of antimicrobial exposure22 as is the risk of NEC.23 Our study suggests that even longer durations of antibiotic exposure to neonates may further increase this risk.
Our findings support the retrospective cohort analysis performed by Cotten et al, which included 4,039 extremely low birth weight (<1,000 grams) neonates born between September 1, 1998 and December 31, 2001.23 Maternal and neonatal data were collected for the first three postnatal days and prolonged (i.e. ≥ 5 days) initial duration of antibiotic exposure analyzed as a potential risk factor for NEC. A multivariate analysis determined that duration of initial antibiotic exposure was associated with an increased risk of NEC (adj. OR: 1.07; 95% CI: 1.04–1.10) and prolonged antibiotic exposure was associated with an increased risk of NEC or death (adj. OR: 1.30; 95% CI: 1.10–1.54).23 Although our sample size was much smaller, we also determined duration of antibiotic exposure to be associated with an increased risk of NEC among infants without sepsis. Unlike the prior investigation, we performed a matched case-control analysis and included the cumulative duration of antimicrobial therapy until the diagnosis of NEC. We also considered other potential risk factors for NEC in our analysis, such as type of enteral feeding and the presence of a PDA.
In our initial bivariate analysis comparing cases and controls, no differences were observed with respect to duration of antibiotic exposure. However, the associations between NEC, sepsis, and antibiotic use presented a challenge to this analysis. An infectious etiology for NEC has been previously suggested given the periodic occurrence of NEC in clusters24 and associations between NEC and infection caused by species of bacteria including the Enterobacteriacae, Clostridia, and coagulase-negative staphylococci25–27 have been described. The more common accepted belief is that infection in patients with NEC is not causative but instead results from impairment in the intestinal barrier leading to translocation of intestinal flora and bacteremia.28 In our cohort, the majority of sepsis occurred in the case population and concurrently with the diagnosis of NEC. As a result, we chose to separate these infants from the majority of the cohort. When we analyzed the effect of antibiotic exposure on the risk of NEC in infants with sepsis, we determined that their risk of NEC was greatest at antibiotic exposure day 1–2 and decreased as duration of exposure increased. This supports the idea that sepsis and NEC are closely associated and, in many cases, may be diagnosed simultaneously.
When we eliminated the potential confounding effects of sepsis from our analysis, we determined that antibiotic duration increased the probability of NEC by approximately 20% per day of exposure (OR=1.2). Furthermore, exposure to more than 10 days of antimicrobial therapy resulted in nearly a three-fold increase in the risk of developing NEC. Approximately 93% of our entire cohort received at least 5 days of empiric antimicrobial therapy which, given our findings and those presented by Cotten et al23 is concerning. Given the widespread use of antibiotics in our and other NICUs and their potential negative effects, antibiotic stewardship programs for the NICU have been suggested. A recent retrospective observational study applied the Center for Disease Control and Prevention’s 12-Step guidelines for assessing antimicrobial prescribing in 4 NICUs.29 Approximately 25% of antibiotic courses and antibiotic days, based on these guidelines, were determined to be “inappropriate.” It was noted that most inappropriate antibiotic use occurred with continuation, not initiation, of antibiotics.29 Implementation of an antibiotic control program in the NICU may help avoid excess antibiotic use and potentially decrease the risk of associated morbidities.
There are several limitations with our investigation which mainly stem from its retrospective nature. Although several potential confounding variables for NEC were collected, additional maternal risk factors may have been helpful (if available) including mode of delivery, chorioamnionitis, maternal antibiotic therapy, hypertension and placental abruption. We did not collect data on the use of H2-blockers in our cohort, a known independent risk factor for NEC.30 Also, it is possible that duration of antibiotic exposure is merely a marker of illness severity and the inclusion of an illness severity score may have been helpful in differentiating this from the true effect of antimicrobial exposure. Lastly, inclusion of a larger control population and more precise criteria for matching, although not possible given the sample size, would have likely improved the validity of our results.
Increased antibiotic exposure in infants in the NICU is associated with an increased risk of developing NEC. A cautious approach to the initiation and continuation of antibiotics, especially in neonates with sterile cultures, should be considered given this association.
Acknowledgments
Supported by the National Center for Research Resources (NCRR) (CTSA grant UL1 RR024139), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
ABBREVIATIONS
- NEC
necrotizing enterocolitis
- GI
gastrointestinal
- NICU
newborn intensive care unit
- NBSCU
newborn special care unit
- RDS
respiratory distress syndrome
- PDA
patent ductus arteriosus
- BPD
bronchopulmonary dysplasia
- TPN
total parenteral nutrition
- OR
odds ratio
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 2.Gibbs K, Lin J, Holzman IR. Necrotising enterocolitis: the state of the science. Indian J Pediatr. 2007;74:67–72. doi: 10.1007/s12098-007-0031-0. [DOI] [PubMed] [Google Scholar]
- 3.Henry M, Moss RL. Current issues in the management of necrotizing enterocolitis. Sem Perinatol. 2004;28:221–33. doi: 10.1053/j.semperi.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003;8:449–59. doi: 10.1016/S1084-2756(03)00123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:278–85. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 6.Henderson G, Craig S, Brocklehurst P, McGuire W. Enteral feeding regimens and necrotizing enterocolitis in preterm infants: multi-center case-control study. Arch Dis Child Fetal Neonatal Ed. doi: 10.1136/adc.2007.119560. Epub 2007 Sept 3. [DOI] [PubMed] [Google Scholar]
- 7.Claude EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15:1398–403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 8.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, et al. Prokaryotic regulation of epithelial responses by inhibition of IkapptaB-alpha. Science. 2000;289:1560–63. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 9.Millar M, Wilks M, Costletoe K. Probiotics for preterm infants? Arch Dis Child Fetal Neonatal Ed. 2003;88:354–8. doi: 10.1136/fn.88.5.F354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendrop AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–7. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Schwiertz A, Gruhl B, Lobnitz M, Michel P, Radke M, Blaut M. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Ped Research. 2003;54:393–99. doi: 10.1203/01.PDR.0000078274.74607.7A. [DOI] [PubMed] [Google Scholar]
- 12.Panigrahi P. Necrotizing enterocolitis: a practical guide to its prevention and management. Pediatr Drugs. 2006;8:151–65. doi: 10.2165/00148581-200608030-00002. [DOI] [PubMed] [Google Scholar]
- 13.Gewolb I, Schwalbe R, Taciak V, Harrison T, Panigrahi P. Stool microflora in extremely low birth weight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F167–F173. doi: 10.1136/fn.80.3.f167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnemaison E, Lanotte P, Cantagrel S, Thionois S, Quentin R, Chamboux C, et al. Comparison of fecal flora following administration of two antibiotic protocols for suspected maternofetal infection. Biol Neonate. 2003;84:304–10. doi: 10.1159/000073639. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Hoenig J, Malin K, Qamar S, Petrof E, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiotia from preterm infant with and without necrotizing enterocolitis. ISME J. 2009;3:944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stark AR. American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics. 2004;114:1341–7. doi: 10.1542/peds.2004-1697. [DOI] [PubMed] [Google Scholar]
- 17.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:213–88. doi: 10.1016/0045-9380(87)90031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–32. [PubMed] [Google Scholar]
- 19.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 20.Adlerberth I. Establishment of a normal intestinal microflora in the newborn infant. In: Hanson LA, Yolken RH, editors. Probiotics, other nutritional factors and intestinal microflora. Nestle Nutrition Workshop Series. 42. Philadelphia: Lippincott-Raven; 1999. pp. 63–78. [Google Scholar]
- 21.Bennet R, Eriksson M, Nord CE. The fecal microflora of 1–3-month old infants during treatment with eight oral antibiotics. Infection. 2002;30:158–60. doi: 10.1007/s15010-002-2140-z. [DOI] [PubMed] [Google Scholar]
- 22.Goldmann DA, Leclair J, Macone A. Bacterial colonization of neonates admitted to an intensive care environment. J Pediatr. 1978;93:288–93. doi: 10.1016/s0022-3476(78)80523-x. [DOI] [PubMed] [Google Scholar]
- 23.Cotten CM, Taylor S, Stoll B, Goldberg R, Hansen N, Sanchez P, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson PJ, Greenough A, Hird MF, Philpott-Howard J, Gamsu HR. Nosocomial bacterial infections in very low birth weight infants. Eur J Pediatr. 1992;151:451–4. doi: 10.1007/BF01959362. [DOI] [PubMed] [Google Scholar]
- 25.Kosloske AM, Ball WS, Umland E, Skipper B. Clostridial necrotizing enterocolitis. J Pediatr Surg. 1985;50:155–9. doi: 10.1016/s0022-3468(85)80290-6. [DOI] [PubMed] [Google Scholar]
- 26.Scheifele DW. Role of bacterial toxins in neonatal necrotizing enterocolitis. J Pediatr. 1990;117:S44–S46. doi: 10.1016/s0022-3476(05)81129-1. [DOI] [PubMed] [Google Scholar]
- 27.Millar MR, MacKay P, Levene M, Langdale V, Martin C. Enterobacteriaceae and neonatal necrotizing enterocolitis. Arch Dis Child. 1992;67:53–6. doi: 10.1136/adc.67.1_spec_no.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. The Role of the Intestinal Barrier in the Pathogenesis of Necrotizing Enterocolitis. Shock. 2007;27:124–33. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 29.Patel S, Oshodi A, Prasad P, Delamora P, Larson E, Zaoutis T, et al. Antibiotic use in neonatal intensive care units and adherence with centers for disease control and prevention 12 step campaign to prevent antimicrobial resistance. Ped Infect Dis. 2009;28:1047–51. doi: 10.1097/INF.0b013e3181b12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. National Institute of Child Health and Human Development Neonatal Research Network. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–42. doi: 10.1542/peds.2005-1543. [DOI] [PubMed] [Google Scholar]


