Abstract
The neuropeptide calcitonin gene-related peptide (CGRP) is implicated in the underlying pathology of migraine. Serum levels of CGRP, which are elevated during a migraine attack, have been reported to return to normal with alleviation of pain. In addition, CGRP administration has been shown to cause a migraine-like headache in susceptible individuals. Importantly, CGRP receptors are found on many cell types within the trigeminovascular system that are thought to play important roles in controlling inflammatory and nociceptive processes. Based on these findings, it was proposed that blockage of CGRP receptor function and, hence, the physiological effects of CGRP would be effective in aborting a migraine attack. This review will summarize key preclinical data that support the therapeutic potential of using CGRP receptor antagonists or molecules that bind CGRP within the context of current neurovascular theories on migraine pathology.
Keywords: calcitonin gene-related peptide, headache, migraine, pain, trigeminal nerve
Migraine is a neurovascular disorder associated with dysfunction of the cerebral nerves and blood vessels that affects 12% of the general population.1,2 Although early theories posited the cerebral blood vessels as the site of origination of migraine attacks, current hypotheses place the primary dysfunction in the brain, specifically in brain stem centers important in regulating vascular tone and pain sensation. It is now thought that migraine-specific triggers cause primary brain dysfunction resulting in dilation of cranial blood vessels that are innervated by sensory fibers of the trigeminal nerve.3,4 The dilated blood vessels mechanically activate perivascular trigeminal sensory nerve fibers that evoke release of neurotransmitters that promote a peripheral inflammatory response within the dura and peripheral sensitization of trigeminal nerve fibers. Release of neurotransmitters within the central nervous system (CNS) also occurs in response to trigeminal nerve activation resulting in increased signaling that can lead to central sensitization, which is characterized by hyperalgesia and allodynia.5 The release of the neurotrasmitter known as α-calcitonin gene-related peptide (CGRP) from trigeminal neurons is thought to play an important role in migraine attacks since many of the pathophysiological events associated with migraine have been shown to involve CGRP.6,7
KEY ROLE OF CGRP IN MIGRAINE
Results from several human studies have demonstrated that serum levels of CGRP obtained from the external jugular vein are elevated in patients during migraine (with and without aura) as well as cluster headaches.8–11 However, in one study, no difference was found between CGRP levels in external jugular or cubital fossa blood during or outside of an attack in a study using an intrapatient comparison design.12 This is in contrast to the findings of several studies in which CGRP levels in cubital venous blood13,14 or saliva15 were elevated in migraineurs outside of an attack. Further confirmation of the involvement of CGRP in migraine was provided by data from clinical trials in which elevated CGRP levels seen during an attack returned to baseline levels coincident with alleviation of headache pain in response to treatment with triptans.16 In addition, data to suggest a causative role for CGRP in migraine were demonstrated when CGRP infusion to migraine patients was shown to cause a delayed headache that fulfilled the International Headache Society criteria for migraine without aura.17 However, the most convincing data that support a critical role of CGRP in migraine come from a clinical study in which infusion of a novel non-peptide CGRP receptor antagonist was shown to effectively treat migraine.18 Based on results from these studies, CGRP is thought to play a central role in migraine pathology since it appears to be involved in both initiating and sustaining migraine attacks.
Calcitonin gene-related peptide is synthesized in the cell bodies of trigeminal ganglion neurons where it is stored in large, dense-core secretory vesicles, and transported axonally to peripheral tissues or to nerve terminals located in the CNS (Fig.). Data obtained from animal studies have shown that CGRP released from activated trigeminal ganglion nerves can cause dilation of cerebral and dural blood vessels, release of inflammatory mediators from mast cells, and transmission of nociceptive information from intracranial blood vessels to the CNS.19–22 Based on findings from recent studies, CGRP is now thought to function in an autocrine manner to stimulate its own synthesis in trigeminal ganglion neurons23 as well as to stimulate the production and release of nitric oxide and other inflammatory cytokines from satellite glial cells found associated with trigeminal neurons in the ganglion.24,25 A better understanding of the role of CGRP in migraine pathology has been more clearly delineated by studies on the cellular mechanisms by which sumatriptan and other triptan drugs function to abort migraine attacks.4,26,27 Triptans exhibit affinity for 5-HT1B, 5-HT1D, and 5-HT1F serotonin receptors,28 which are present on meningeal vessels, perivascular trigeminal nerve terminals, and neurons located in the trigeminal nucleus caudalis.29,30 Triptan-induced activation of 5-HT1 receptors inhibits vasodilation of intracranial vessels and blocks neurogenic inflammation and central transmission of nociceptive stimuli by inhibiting CGRP release from trigeminal nerves.31,32 Results from these biological studies demonstrate that CGRP performs a number of important functions within the trigeminovascular system that contribute to migraine pathology.
Figure.
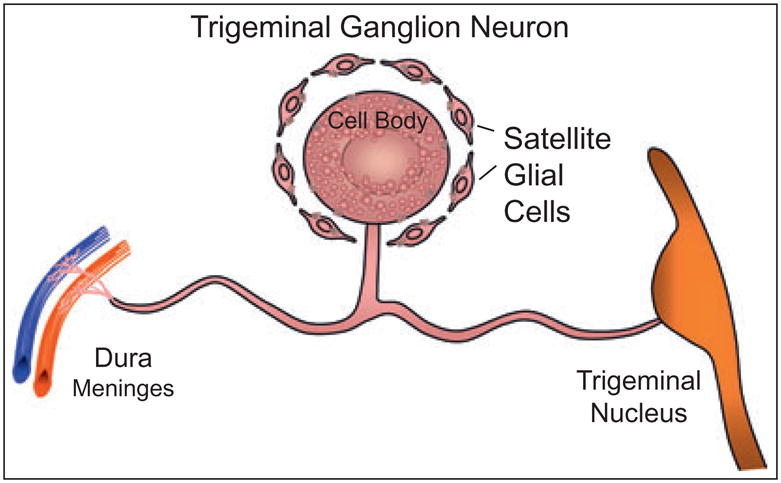
Schematic of a single trigeminal ganglion neuron and associated satellite glial cells. Calcitonin gene-related peptide that is synthesized in the neuronal cell body can be released from the cell body or from peripheral or central terminals following axonal transport.
CGRP: STRUCTURE AND FUNCTION
In humans, CGRP exists in 2 forms that are termed α-CGRP and β-CGRP, which are derived from separate genes and differ by 3 amino acids yet exhibit similar biological functions.33–36 Both α-CGRP and β-CGRP belong to the calcitonin family of peptides that includes calcitonin, adrenomedullin, intermedin (adenomedullin-2), and amylin.34,37 The 37-amino acid neuropeptide α-CGRP arises from alternative splicing of the calcitonin-CGRP gene and is widely distributed in the central and peripheral nervous systems.38 β-CGRP is encoded by a different gene that is highly homologous to the calcitonin-CGRP gene and is primarily found in enteric nerves and in the pituitary gland.39 While both CGRP isoforms are located in nerves, immunohistological studies have demonstrated that α-CGRP is preferentially expressed in sensory neurons and its concentration is 3-to 6-fold higher than that of β-CGRP.40 Importantly, α-CGRP has been reported to be the predominant isoform expressed in trigeminal ganglia neurons33 and dilation of human cerebral arteries has been shown to be mediated largely by α-CGRP.41
Calcitonin gene-related peptide is one of the most abundant peptides in nerve tissue and is widely expressed in both peripheral and central neurons.37 CGRP-containing neurons innervate all major organs and joints of the human body and thus mediate effects important for the normal functioning of the immune, respiratory, endocrine, gastrointestinal, musculoskeletal, and cardiovascular systems.42 For example, physiological studies have shown that CGRP functions in the regulation of neuromuscular junctions, antigen presentation, vascular tone, and neurotransmission. While it is clear that a broad variety of biological functions have been attributed to CGRP, its ability to cause vasodilation and facilitate nociception is most relevant to migraine pathology.31 Results from functional blood vessel studies have demonstrated that exogenous CGRP can cause vasodilation of cerebral arteries and meningeal vessels both in vitro and in situ and that it is the most potent vasodilator among the known perivascular peptides.43–45 CGRP has also been shown to induce degranulation and subsequent release of pro-inflammatory agents from dural mast cells.46 In addition, efferent release of CGRP from trigeminal nerve terminals facilitates transmission of painful stimuli from intracranial vessels to the CNS leading to hyperalgesia and increased allodynia, pathological events characteristic of a migraine attack.5,47 More recently, evidence is accumulating to support an important role for CGRP released from neuronal cell bodies located in the trigeminal ganglion in regulating the activity of neurons and satellite glial cells. For example, it has been shown that CGRP can function in a positive feedback manner to increase its own synthesis within trigeminal neurons.23 In addition, CGRP released within the ganglion is likely to stimulate the release of nitric oxide and several pro-inflammatory cytokines from satellite glial cells.24,25 Thus, CGRP has been shown to mediate key cellular events at multiple sites within the trigeminovascular system that are thought to be involved in the underlying pathology of migraine. Taken together, these data provide evidence that receptor antagonist molecules, which selectively bind to CGRP receptors to prevent their function, should be effective in migraine treatment by blocking the pathophysiological activities of CGRP.
CGRP RECEPTORS AND PEPTIDE ANTAGONISTS
Functional CGRP receptors are composed of a G-protein-coupled receptor known as the calcitonin-like receptor (CLR) and a single transmembrane domain protein called receptor activity modifying protein type 1 (RAMP1).42 RAMP1 functions to traffic mature CRL proteins to the surface of the cell membrane.48 Structure-function investigations have demonstrated that an 18 amino acid sequence located at the N-terminus of the CRL is important for CGRP docking but is not involved in the activation of the mature CGRP receptor.49 Binding studies have demonstrated that the first 7 N-terminal amino acids are essential for receptor activation.50 Importantly, while deletion of amino acids 2 and 7 that are involved in the formation of a disulfide bridge does not appear to affect receptor affinity, it does result in a loss of all biological activity of the CGRP receptor.51 Not surprisingly, the first CGRP receptor antagonists were N-terminal truncated fragments of the CGRP peptide.52 CGRP8–37, which includes all but the first 7 amino acids of the normal peptide, functions as an antagonist of CGRP receptors by blocking binding of endogenous full-length CGRP. Although CGRP8–37 has been demonstrated to inhibit vasodilation and neurogenic inflammation in animal models, its clinical effectiveness is severely limited due to its short half-life53 that contributes to its lack of potency in vivo. Other truncated CGRP analogs that exhibit even higher affinities for CGRP receptors than those reported for CGRP8–37 have been developed, but they have also not proven useful in clinical studies because of similar limitations.54 Thus, while these truncated forms of CGRP would not be clinically useful, results from animal studies on CGRP8–37 have provided valuable physiological information to demonstrate why blockage of CGRP receptors by small non-peptide molecules should be beneficial in treating migraine.
Calcitonin gene-related peptide receptors, which are activated upon binding CGRP, are expressed by several different cell types found within the trigeminovascular system and are involved in mediating several key pathophysiological events associated with migraine. Thus, blocking CGRP receptor activation would be expected to affect the function of vascular smooth muscle cells, mast cells, trigeminal ganglion neurons, and second-order neurons within the CNS. For example, human cerebral and meningeal vessels are reported to express functional CLR and RAMP1 proteins.55,56 Blockage of these CGRP receptors on smooth muscle cells would inhibit the dilation of major cerebral vessels as well as meningeal vessels. Dural mast cells have also been reported to express functional CGRP receptors.46 Thus, inhibiting CGRP receptor activity would be expected to prevent mast cell degranulation and the subsequent release of histamine and other pro-inflammatory agents, which are thought to play an important role in dural inflammation and peripheral sensitization.57 Inhibition of CGRP receptors on second-order sensory neurons within trigeminal nuclei in the caudal brain stem and upper cervical spinal cord58 would block pain transmission to the CNS and, thus, prevent the development of central sensitization. In addition, blockage of CGRP receptors expressed by trigeminal ganglion neurons would likely function to decrease its own synthesis.23 Finally, inhibition of CGRP receptors would prevent activation of satellite glial cells associated with trigeminal neuronal cell bodies in the ganglion and the release of inflammatory molecules.24 This is likely to have important implications for migraine pathology since nitric oxide and several cytokines (TNF-α and IL-1β), which are released from activated satellite glial cells, can cause sensitization and activation of trigeminal ganglion neurons leading to further synthesis and release of CGRP.59,60 Thus, blockage of CGRP receptors would be expected to inhibit the inflammatory and nociceptive effects of CGRP that are implicated in the underlying pathology of migraine.
NON-PEPTIDE CGRP RECEPTOR ANTAGONISTS
The first potent and selective non-peptide antagonist of the human CGRP receptor was BIBN4096BS,61 which is now referred to as olcegepant. The specific affinity of olcegepant for the CGRP receptor is dependent on residues within the extracellular region of RAMP1 rather than the CLR protein.62 Since this site is required for binding of CGRP, olcegepant functions as a CGRP receptor antagonist by directly competing for the binding site of the endogenous ligand CGRP. Results from a phase IIa clinical trial on olcegepant provided the first evidence to support the use of a non-peptide CGRP receptor antagonist for the acute treatment of migraine.18 Data from that clinical proof-of-concept study demonstrated the effectiveness and safety of olcegepant. The response rate of >60% (pain free at 2 hours) was similar to values reported for oral triptans.63 A somewhat surprising finding from the studies on olcegepant had been the lack of cardiovascular side effects such as changes in basal blood pressure or heart rate.18,64 The lack of vasoconstrictor activity may prove to be a major advantage for using CGRP receptor antagonists to treat migraine.
A large amount of preclinical data on olcegepant (BIBN4096BS) supports the use of CGRP antagonists for the treatment of migraine. This information obtained from studies conducted in animals and humans has been recently summarized in several comprehensive review articles.65,66 Initially, the ability of olcegepant to function as an anti-migraine agent was supported by results that olcegepant could inhibit the vasodilatory effect of CGRP released following stimulation of the trigeminal ganglion.64 In addition, olcegepant was able to reverse the effects of α-CGRP-induced dilation of human middle cerebral and middle meningeal arteries67 as well as human temporal artery.68 An important finding is the reported lack of vasoactive effect of olcegepant.69 In that study, no significant systemic or cerebral blood flow changes were observed following infusion of olcegepant to healthy volunteers. This finding is in agreement with animal studies that provide evidence that CGRP exerts minimal vasodilatory effects under normal resting conditions.64 Finally, olcegepant was recently shown to block the autoactivation of CGRP synthesis in trigeminal ganglion neurons, an event thought to function in an autocrine manner such that CGRP release from neuronal cell bodies stimulates its own further synthesis.23
Although olcegepant has been shown to be effective in treating migraine attacks18 and CGRP-induced headache,70 a severe limitation is that this compound has to be delivered via intravenous injection. It is encouraging that potent oral CGRP receptor antagonists are now under investigation for the treatment of migraine. In particular, the CGRP receptor antagonist, MK-0974, reported to exhibit good oral bioavailability, is a promising candidate. It has recently been shown in a phase II clinical study to be effective and generally well tolerated for treating moderate to severe migraine attacks with a primary endpoint of pain relief at 2 hours.71,72 Generally, the effective MK-0974 doses were comparable to those of rizatriptan. The incidence of the most often reported adverse events for MK-0974, which included nausea, dizziness, and somnolence, was similar to the placebo group. Pharmacological studies have provided evidence that MK-0974 is a highly selective, potent oral antagonist of the human CGRP receptor.73 In the same study, MK-0974 was shown to inhibit capsaicin-induced dermal vasodilation mediated by CGRP in rhesus monkey pharmacodynamic assay. Phase III clinical studies are underway to further evaluate the effectiveness of this novel class of CGRP receptor antagonist molecules. In addition, other CGRP receptor antagonists are in development that reportedly exhibit good potency with respect to binding and function (cAMP assay). One potentially exciting compound referenced as compound 37 was found to exhibit good oral bioavailability in rat and dog with long half-lives and very low clearances.74
ALTERNATIVE THERAPEUTIC APPROACH – CGRP BINDING MOLECULES
As an alternative approach to inhibit the pathophysiological functions of CGRP during migraine, agents have recently been developed that can bind directly to CGRP and block its binding to CGRP receptors or inhibit its synthesis. One agent is a CGRP-binding RNA-Spiegelmer (NOX-C89) that is a single-stranded mirror image L-oligonucleotide.75 This RNA molecule recognizes and can bind to a region encompassing approximately 13 amino acids located at the N-terminus of CGRP.76 The NOX-C89 molecule was designed to be resistant to degradation by endogenous enzymes but yet retain its inhibitory function on CGRP. In a closed cranial window model, pretreatment with the NOX-C89 was shown to inhibit CGRP dilation of rat dural arteries but was not effective in preventing vasodilation caused by electrical stimulation.77 Using the same model, the other CGRP inhibitory agent, a monoclonal antibody directed against the human form of CGRP, was also shown to block CGRP-induced dilation of dural vessels.78 Similarly, both agents have been reported to inhibit CGRP-induced relaxation of rat middle cerebral arteries in a concentration-dependent manner.78 Furthermore, based on results from these studies, it is thought that neither agent is able to cross the blood-brain barrier to any appreciable amount. Importantly, the infusion of the RNA-Spiegelmer or the CGRP monoclonal antibody did not significantly change mean arterial pressure or affect basal-resting dural or pial artery diameter.77
CONCLUSION
Based on experimental and clinical studies, CGRP is believed to play an important role in the generation of pain during migraine attacks. CGRP receptor antagonists would be expected to block CGRP-mediated cellular events at the level of cerebral blood vessels, dura, ganglion, and second-order neurons since functional CGRP receptors are expressed by cell types in these regions of the trigeminovascular system. Although the non-peptide CGRP receptor antagonist olcegepant was shown to be effective in treatment of migraine, its formulation requires that it be administered intravenously, which greatly reduces its use as a frontline anti-migraine therapy. However, the therapeutic potential of the oral formulations of non-peptide CGRP receptor antagonists appears quite promising given the recent results of the phase II clinical trial with MK-0974. It was encouraging to see that this compound was effective in treating migraine but only minor side effects were noted with no vasoconstrictor events reported. It will be equally exciting to see how well other oral CGRP receptor antagonists currently in development as well as molecules that bind CGRP and inhibit its function perform in clinical studies. Finally, since CGRP levels are also elevated in cluster headache,8,10,79 it is likely that blocking CGRP receptor function would also be beneficial in treating this severe form of headache.
Abbreviations
- CGRP
calcitonin gene-related peptide
- CLR
calcitonin-like receptor
- CNS
central nervous system
- RAMP1
receptor activity modifying protein type 1
Footnotes
Conflict of Interest: Dr. Durham has received grant support from Capnia, Colucid, Glaxo Smith Kline, MAP Pharmaceuticals, Merck, and Minster Pharmaceuticals.
References
- 1.Lipton R, Stewart W, Diamond S, Diamond M, Reed M. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 2.Stewart W, Lipton R, Celentano D, Reed M. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1991;267:64–69. [PubMed] [Google Scholar]
- 3.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 4.Goadsby P, Lipton R, Ferrari M. Migraine – current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 5.Dodick D, Silberstein S. Central sensitization theory of migraine: Clinical implications. Headache. 2006;46:S182–191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 6.Durham P. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46:S3–8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrobon D. Migraine: New molecular mechanisms. Neuroscientist. 2005;11:373–386. doi: 10.1177/1073858405275554. [DOI] [PubMed] [Google Scholar]
- 8.Fanciullacci M, Alessandri M, Figini M, Geppetti P, Michelacci S. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain. 1995;60:119–123. doi: 10.1016/0304-3959(94)00097-X. [DOI] [PubMed] [Google Scholar]
- 9.Goadsby P, Edvinsson L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 10.Goadsby P, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- 11.Goadsby P, Edvinsson L, Elkman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 12.Tvedskov J, Lipka K, Ashina M, Iversen H, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–568. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- 13.Ashina M, Bendtsen L, Jensen R, Schifter S, Olesen J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain. 2000;86:133–138. doi: 10.1016/s0304-3959(00)00232-3. [DOI] [PubMed] [Google Scholar]
- 14.Fusayasu E, Kowa H, Takeshima T, Nakaso K, Nakashima K. Increased plasma substance P and CGRP levels, and high ACE activity in migraineurs during headache-free periods. Pain. 2007;128:209–214. doi: 10.1016/j.pain.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Bellamy J, Cady R, Durham P. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache. 2006;46:24–33. doi: 10.1111/j.1526-4610.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 16.Goadsby P, Edvinsson L. Sumatriptan reverses the changes in calcitonin gene-related peptide seen in the headache phase of migraine. Cephalalgia. 1991;11:3–4. [Google Scholar]
- 17.Lassen L, Haderslev P, Jacobsen V, Iversen H, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 18.Olesen J, Diener H, Husstedt I, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 19.Buzzi M, Moskowitz M. The trigemino-vascular system and migraine. Pathol Biol (Paris) 1992;40:313–317. [PubMed] [Google Scholar]
- 20.Ferrari MD. Migraine. Lancet. 1998;351:1043–1051. doi: 10.1016/S0140-6736(97)11370-8. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez del Rio M, Reuter U, Moskowitz M. Central and peripheral mechanisms of migraine. Funct Neurol. 2000;15:S157–162. [PubMed] [Google Scholar]
- 22.Williamson D, Hargreaves R. Neurogenic inflammation in the context of mirgraine. Microsc Res Tech. 2001;53:167–178. doi: 10.1002/jemt.1081. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Winborn C, Marquez de Prado B, Russo A. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Vause C, Durham P. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thalakoti S, Patil VV, Damodaram S, et al. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longmore J, Dowson A, Hill R. Advances in migraine therapy – 5-HT receptor subtype-specific agonist drugs. Curr Opin CPNS Investig Drugs. 1999;1:39–53. [Google Scholar]
- 27.Mathew N. Pathophysiology, epidemiology, and impact of migraine. Clin Cornerstone. 2001;4:1–17. doi: 10.1016/s1098-3597(01)90035-3. [DOI] [PubMed] [Google Scholar]
- 28.Slassi A, Isaac M, Arora J. Novel serotonergic and non-serotonergic migraine headache therapies. Expert Opin Ther Pat. 2001;11:625–649. [Google Scholar]
- 29.Longmore J, Shaw D, Smith D, et al. Differential distribution of 5HT1D- and 5HT1B-immunoreactivity within the human trigemino-cerebrovascular system: Implications for the discovery of new antimigraine drugs. Cephalalgia. 1997;17:833–842. doi: 10.1046/j.1468-2982.1997.1708833.x. [DOI] [PubMed] [Google Scholar]
- 30.Potrebic S, Ahn A, Skinner K, Fields H, Basbaum A. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: Implications for the selective antimigraine action of triptans. J Neurosci. 2003;23:10988–10997. doi: 10.1523/JNEUROSCI.23-34-10988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hargreaves R. New migraine and pain research. Headache. 2007;47:S26–43. doi: 10.1111/j.1526-4610.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- 32.Hargreaves R, Shepheard S. Pathophysiology of migraine – new insights. Can J Neurol Sci. 1999;26:S12–19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 33.Amara S, Arriza J, Leff S, Swanson L, Evans R, Rosenfeld M. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–1097. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- 34.Juaneda C, Dumont Y, Quirion R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol Sci. 2000;21:432–438. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 35.Steenbergh P, Hoppener J, Zandberg J, Lips J, Jansz H. A second human calcitonin/CGRP gene. FEBS Lett. 1985;183:403–407. doi: 10.1016/0014-5793(85)80820-6. [DOI] [PubMed] [Google Scholar]
- 36.Van Rossum D, Hanisch U, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 37.van Rossum D, Hanisch U, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld M, Mermod J-J, Amara S, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- 39.Sternini C. Enteric and visceral afferent CGRP neurons. Targets of innervation and differential expression patterns. Ann N Y Acad Sci. 1992;657:170–186. doi: 10.1111/j.1749-6632.1992.tb22766.x. [DOI] [PubMed] [Google Scholar]
- 40.Mulderry P, Ghatei M, Spokes R, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25:195–205. doi: 10.1016/0306-4522(88)90018-8. [DOI] [PubMed] [Google Scholar]
- 41.Jansen-Olesen I, Mortensen A, Edvinsson L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- 42.Poyner D, Sexton P, Marshall I, et al. Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 43.Allen G, Barbick B, Esser M. Trigeminal-parabrachial connections: Possible pathway for nociception-induced cardiovascular reflex responses. Brain Res. 1996;715:125–135. doi: 10.1016/0006-8993(95)01580-9. [DOI] [PubMed] [Google Scholar]
- 44.Brain S, Williams T, Tippins J, Morris H, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 45.Messlinger K, Hanesch U, Kurosawa M, Pawlak M, Schmidt R. Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can J Physiol Pharmacol. 1995;73:1020–1024. doi: 10.1139/y95-143. [DOI] [PubMed] [Google Scholar]
- 46.Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–174. doi: 10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]
- 47.Malick A, Burstein R. Peripheral and central sensitization during migraine. Funct Neurol. 2000;15:S28–35. [PubMed] [Google Scholar]
- 48.McLatchie L, Fraser N, Main M, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee S, Evanson J, Harris E, Lowe S, Thomasson K, Porter J. Identification of specific calcitonin-like receptor residues important for calcitonin gene-related peptide high affinity binding. BMC Pharmacol. 2006;15:6–9. doi: 10.1186/1471-2210-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maggi C, Rovero P, Giuliani S, Evangelista S, Regoli D, Meli A. Biological activity of N-terminal fragments of calcitonin gene-related peptide. Eur J Pharmacol. 1990;179:217–219. doi: 10.1016/0014-2999(90)90422-3. [DOI] [PubMed] [Google Scholar]
- 51.Zaidi M, Brain S, Tippins J, et al. Structure-activity relationship of human calcitonin-gene-related peptide. Biochem J. 1990;269:775–780. doi: 10.1042/bj2690775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiba T, Yamaguchi A, Yamatani T, et al. Calcitonin gene-related peptide receptor antagonist human CGRP (8–37) Am J Physiol. 1989;256:E331–335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- 53.Mentlein R, Roos T. Proteases involved in the metabolism of angiotensin II, bradykinin, calcitonin gene-related peptide (CGRP), and neuropeptide Y by vascular smooth muscle cells. Peptides. 1996;17:709–720. doi: 10.1016/0196-9781(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 54.Rist B, Lacroix J, Entzeroth M, Doods H, Beck-Sickinger A. CGRP 27–37 analogues with high affinity to the CGRP1 receptor show antagonistic properties in a rat blood flow assay. Regul Pept. 1999;79:153–158. doi: 10.1016/s0167-0115(98)00159-1. [DOI] [PubMed] [Google Scholar]
- 55.Moreno M, Cohen Z, Stanimirovic D, Hamel E. Functional calcitonin gene-related peptide type 1 and adrenomedullin receptors in human trigeminal ganglia, brain vessels, and cerebromicrovascular or astroglial cells in culture. J Cereb Blood Flow Metab. 1999;19:1270–1278. doi: 10.1097/00004647-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Oliver K, Wainwright A, Edvinsson L, Pickard J, Hill R. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J Cereb Blood Flow Metab. 2002;22:620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Levy D, Burstein R, Strassman A. Mast cell involvement in the pathophysiology of migraine headache: A hypothesis. Headache. 2006;46:S13–18. doi: 10.1111/j.1526-4610.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 58.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT1B/1D receptor agonists. Proc Natl Acad Sci USA. 2004;101:4274–4279. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellamy J, Bowen E, Russo A, Durham P. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci. 2006;23:2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bowen E, Schmidt T, Firm C, Russo A, Durham P. Tumor necrosis factor-α stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96:65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doods H, Hallermayer G, Wu D, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mallee J, Salvatore C, LeBourdelles B, et al. Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J Biol Chem. 2002;277:14294–14298. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- 63.Ferrari M, Roon K, Lipton R, Goadsby P. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: A meta-analysis of 53 trials. Lancet. 2001;358:1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- 64.Kapoor K, Arulmani U, Heiligers J, et al. Effects of BIBN4096BS on cardiac output distribution and on CGRP-induced carotid haemodynamic responses in the pig. Eur J Pharmacol. 2003;475:69–77. doi: 10.1016/s0014-2999(03)02082-x. [DOI] [PubMed] [Google Scholar]
- 65.Edvinsson L, Petersen K. CGRP-receptor antagonism in migraine treatment. CNS Neurol Disord Drug Targets. 2007;6:240–246. doi: 10.2174/187152707781387314. [DOI] [PubMed] [Google Scholar]
- 66.Recober A, Russo A. Olcegepant, a non-peptide CGRP1 antagonist for migraine treatment. IDrugs. 2007;10:566–574. [PubMed] [Google Scholar]
- 67.Salmon A, Damaj M, Marubio L, Epping-Jordan M, Merlo-Pich E, Changeux J. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat Neurosci. 2001;4:357–358. doi: 10.1038/86001. [DOI] [PubMed] [Google Scholar]
- 68.Verheggen R, Bumann K, Kaumann A. BIBN4096BS is a potent competitive antagonist of the relaxant effects of alpha-CGRP on human temporal artery: Comparison with CGRP(8–37) Br J Pharmacol. 2002;136:120–126. doi: 10.1038/sj.bjp.0704682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen K, Birk S, Lassen L, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–147. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- 70.Petersen K, Lassen L, Birk S, Lesko L, Olesen J. BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther. 2005;77:202–213. doi: 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Ho T, Mannix L, Fan X, et al. Randomized controlled trial of an oral CGRP antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 72.Paone D, Shaw A, Nguyen D, et al. Potent, orally bioavailable calcitonin gene-related peptide receptor antagonists for the treatment of migraine: Discovery of N-[(3R,6S)-6-(2,3-difluorophenyl) -2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide (MK-0974) J Med Chem. 2007;50:5564–5567. doi: 10.1021/jm070668p. [DOI] [PubMed] [Google Scholar]
- 73.Salvatore C, Hershey J, Corcoran H, et al. Pharmacological characterization of MK-0974 [N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl) azepan - 3 - yl]-4-(2-oxo-2,3-dihydro-1H - imidazo [ 4,5 - b ] pyridin - 1 - yl) piperidine - 1-carboxamide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther. 2008;324:416–421. doi: 10.1124/jpet.107.130344. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen D, Paone D, Shaw A, et al. Calcitonin gene-related peptide (CGRP) receptor antagonists: Investigations of a pyridinone template. Bioorg Med Chem Lett. 2008;18:755–758. doi: 10.1016/j.bmcl.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 75.Vater A, Jarosch F, Buchner K, Klussmann S. Short bioactive Spiegelmers to migraine-associated calcitonin gene-related peptide rapidly identified by a novel approach: Tailored-SELEX. Nucleic Acids Res. 2003;31:e130. doi: 10.1093/nar/gng130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denekas T, Tröltzsch M, Vater A, Klussmann S, Messlinger K. Inhibition of stimulated meningeal blood flow by a calcitonin gene-related peptide binding mirror-image RNA oligonucleotide. Br J Pharmacol. 2006;148:536–543. doi: 10.1038/sj.bjp.0706742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Juhl L, Edvinsson L, Olesen J, Jansen-Olesen I. Effect of two novel CGRP-binding compounds in a closed cranial window rat model. Eur J Pharmacol. 2007;567:117–124. doi: 10.1016/j.ejphar.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8–37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol. 2007;150:633–640. doi: 10.1038/sj.bjp.0707134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edvinsson L, Goadsby P. Neuropeptides in migraine and cluster headache. Cephalalgia. 1994;14:320–327. doi: 10.1046/j.1468-2982.1994.1405320.x. [DOI] [PubMed] [Google Scholar]


