Abstract
This article reviews the role of immune competent cells infiltrating the kidney and their association with oxidative stress and renal angiotensin activity in the development of salt-sensitive hypertension.
We discuss the alteration of the pressure-natriuresis relationship resulting from renal inflammation and its improvement resulting from immunosuppressive treatment.
The potential role of T cell-driven reactivity in sustaining the renal inflammation is examined in the light of accumulating evidence of autoimmune mechanisms in experimental and clinical hypertension.
Keywords: Heat Shock proteins, lymphocytes, macrophages, pressure natriuresis, T cells
Introduction
Salt sensitivity is a condition defined by significant variations in blood pressure in direct relation to the sodium content of the diet and is present in 80% of hypertensive individuals older than 60 years (1). A low sodium diet is a well-established measure in the treatment of hypertension and the lack of compliance of this prescription is a recognized cause of worsening or lack of effectiveness of the prescribed drug treatment for hypertension. The mechanisms involved in the pathogenesis of salt-sensitive hypertension have been previously reviewed (2). This article will focus on the impairment of the renal sodium excretion resulting from the tubulointerstitial inflammation that is a common characteristic of salt-driven hypertensive states and on the role played by lymphocytes and macrophages in the pathogenesis of hypertension.
Renal tubulointerstitial inflammation and impairment of pressure natriuresis
Impairment of pressure natriuresis is central to the pathophysiology of sodium balance that results from microvascular damage in the kidney (3, 4). As postulated by Guyton and co-workers (5, 6), in salt-sensitive hypertension the slope of the pressure-natriuresis relationship is less steep and shifted to the right; as a consequence, a higher than normal blood pressure is required to achieve any increment in urinary sodium excretion. Higher blood pressure is therefore an adaptive response to maintain sodium balance.
Renal inflammation, and its constant companions, oxidative stress and activation of the renal renin-angiotensin system play a critical role in the impairment of the pressure-natriuresis relationship (7, 8, 9, 10). Acting in concert, these pathogenic conditions cause microavascular disease of the afferent arterioles and a reduction of the peritubular capillary network with a decrease in the diffusion capacity imposed by the accumulation of cells, edema and eventally fibrosis in tubulointerstitial areas of the kidney. Rarefaction and loss of peritubular capillaries has been demonstrated in experimental (11, 12), and human (13) hypertension. In addition, the inflammation is associated with local angiotensin II (AII) generation which not only favours glomerular vasoconstriction and upregulates the tubuloglomerular feedback but induces intense proximal tubular sodium reabsorption (14). Inflammation and local AII activity, in association with the reduced number of peritubular capillaries, are responsible for the development of oxidative stress that tends to increase as damage progresses and by itself generates a powerful drive for sodium retention (15, 16). In addition, inflammation compromises the function of dopamine D1 receptors that are involved in sodium excretion and have been implicated in the human and rodent models of salt-sensitive hypertension (17).
Impairment of the pressure-natriuresis relationship is a recognized feature of human hypertension (18), but this feature has not been specifically examined in experimental models of salt-driven hypertension characterized by significant tubulointerstitial inflammation. To evaluate the pressure natriuresis relationship in rats with acquired salt-sensitive hypertension, we utilized the model of salt sensitive hypertension induced by transient inhibition of nitric oxide (NO) synthesis (19). To create this model, rats were administered orally Nω-nitro-L-arginine methyl ester (L-NAME), which is a nonspecific inhibitor of NO synthase, for 3 weeks. During this period the hypertension is mediated by L-NAME. Rats are then allowed to recover on a normal salt diet (0.4%) for one week before being placed on a high sodium (4%) diet. Rats rapidly develop salt sensitive hypertension on this regimen and this correlates with the presence of tubulointerstitial inflammation. Control rats receiving vehicle instead of L-NAME do not develop tubulointerstitial inflammation or salt sensitive hypertension. Furthermore, if the rats are given the immunosuppressive drug, mycophenolate mofetil (MMF), during the L-NAME infusion, the MMF does not alter the ability of L-NAME to raise blood pressure acutely but prevents the development of tubulointerstitial inflammation and the development of post LNAME salt sensitive hypertension (20).
We (Franco M, Tapia E, unpublished observations) therefore evaluated the pressure-natriuresis relationship in 3 groups of rats (5–8 rats per group): 1) Normotensive control rats, 2) Hypertensive L-NAME-treated rats and 3) Normotensive L-NAME-treated rats rendered salt-resistant by the administration of MMF (20 mg/kg daily by gavage). Prior to the experiments the rats from these groups had essentially similar and normal blood pressure. Pressure natriuresis was evaluated by standard methods (20, 21) Briefly, male Sprague Dawley rats weighing 400–450g (were anesthetized (pentobarbital sodium 50mg/kg, intraperitoneally) and underwent tracheotomy (for airway) and jugular vein (for fluid and anaesthesia administration), and transfemoral aortic catheterization (for blood pressure control) The left kidney was exposed, a ureteral catheter was inserted for urine collections and an aortic clamp was placed above the left renal artery to regulate renal arterial pressure. Isotonic saline infusion containing inulin (1g/dl at an infusion rate of 30µl/min) was given and after stabilization for a period of 1 hour, the renal arterial pressure (as determined by the infra renal aortic catheter) was modified with the aortic clamp. 30-minute urinary samples were obtained at blood pressures of 150, 130, 110 and 90 mmHg in the hypertensive rats and at 130, 110, and 90 mmHg in the normotensive control and MMF-treated rats. Equilibration of 10–15 minutes was allowed for every blood pressure level. Blood samples were obtained at midpoint in each urinary collection and transfusions of equivalent volumes were given after each blood sampling. Inulin and sodium concentrations were measured in each of the samples.
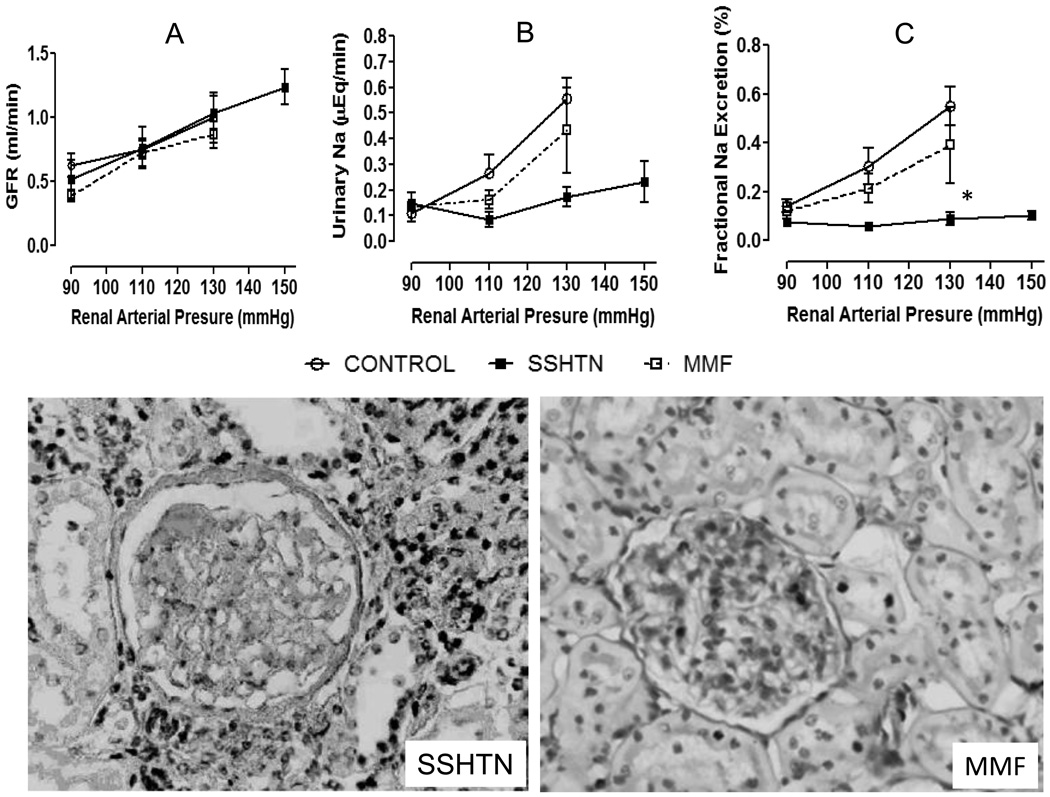
Figure 1 shows the relationship between renal arterial pressure and urinary sodium excretion, fractional excretion of sodium and GFR. There were no significant differences in the GFR among the study groups (Figure 1A) in the range of renal arterial pressures under study. In contrast, severe impairment in the urinary sodium excretion and fractional sodium excretion was found in the rats with salt sensitive hypertension (Figure 1B and 1C) and was associated with severe tubulointerstitial inflammation while the glomeruli are essentially normal (Figure 1, Histology, left side). This defect that was partially corrected by MMF administration (Figures 1B, 1C) which also significantly reduced the tubulointerstitial inflammation (Figure 1, Histology MMF, right side).
Figure 1.
Glomerular filtration rate (A), urinary sodium excretion (B) and fractional sodium excretion (C) in relation to renal artery pressure controlled by an aortic clamp at 90, 110, 130 mmHg in control rats (open crcles), in rats with salt-sensitive hypertension induced with transient inhibition of nitric oxide synthase with L-NAME) (SSHTN, closed squares) and in rats treated with L-NAME in whom suppression of inflammation was induced with the administration of mycophenolate mofetil (MMF, open squares). Impaired pressure natriuresis is evident in rats with inflammation-induced SSHTN that is partially corrected with MMF treatment. Methodology described in the text. Representative light microscopy (PAS staining × Original magnification ×200) of a biopsy of a rat in the SSHTN group (left side) showing tubulointerstitial inflammation and well-preserved glomerular structure. Tubulointerstitial inflammation is suppressed by MMF treatment (right side) *p<0.05 (two-tailed) vs. the rest (multigroup ANOVA, Tukey post-tests)
T cells play a role in SSHTN
As discussed in recent editorials (22, 23) accumulating evidence indicates that T cells have a role in the development of hypertension. Renal tubulointerstitial infiltration of immunocompetent cells is practically a universal finding in experimental models of hypertension and has been also found in essential hypertension in humans. In experimental animals (reviewed in 4,7) a number of strategies designed to suppress immune reactivity have resulted in prevention or correction of hypertension. These strategies include lymphocyte depletion by neonatal thymectomy in the Lyon hypertensive rats (24), hypertensive NZB mice (25), DOCA-salt hypertension (26) and the chronic phase of renal infarction (27). Similar antihypertensive effects have been reported in the SHR by lymphocyte depletion using anti-lymphocyte serum (28) and, recently, the elegant studies of Guzik et al have shown that the Rag −/− mice that lacks lymphocytes do not develop angiotensin II-induced hypertension, a capacity that is restored by adoptive transfer of T lymphocytes (29). Antihypertensive effects have also been found with cytokine depletion (interleukin 6-knockout mice) (30). A critical role of inflammation in the pathogenesis of hypertension has also been suggested by studies that show that inhibition of the proinflammatory transcription nuclear factor κ B (NFκB) corrects the hypertension in the spontaneously hypertensive rat (SHR) (31) and reduces the blood pressure levels in the double transgenic dTGF rats alongside with amelioration of AII-induced tissue damage (32). The immunosuppressive drug MMF has been especially effective at lowering blood pressure as shown in the SHR (33), in Dahl salt sensitive rats (34, 35), in rats with salt-sensitive hypertension induced by transient AII infusion (36) and in rats in which nitric oxide synthesis is transiently inhibited (18). MMF also prevents hypertension associated with chronic lead toxicity (37), overload proteinuria (38), cellophane wrapped kidneys (39) and prenatally programmed hypertension (40). Antihypertensive effects have also been demonstrated with the administration of other immunosuppressive drugs such as cyclosporin A (41) and cyclophosphamide (42). Similar beneficial effects have been found with the suppression of renal inflammation resulting from a reduction in oxidative stress induced by antioxidant diets (43, 44) and melatonin administration (45). Recently, Crosswhite and Sun (46) demonstrated that in vivo knockdown of IL-6 by RNA interference decreases renal inflammatory infiltration and attenuates cold-induced elevation of blood pressure.
Shao et al (47) reported that spleen T cells from angiotensin II-infused rats show increased production of interferon-γ gamma, a prototype Th1 cytokine, and a reduction in IL-4 production. Similar results were found in mRNA of these cytokines in spleen and kidney.
Conclusive evidence of the role played by T cells in the pathogenesis of angiotensin II-induced hypertension was obtained by Guzik et al (29) who showed that Rag−/− mice that lack lymphocytes do not show the expected increase in blood pressure in response to angiotensin II infusion. Furthermore, responsiveness was restored by the adoptive transfer of T cells. Subsequently, Gratze et al (48) showed similar blood pressure unresponsiveness to angiotensin II infusion in the Id2−/− mice, which are deficient in splenic CD8a dendritic cells and have altered CD8 T-cell memory. Interestingly, they performed bone marrow and kidney transplant experiments between Id2−/− and Id2+/− mice that suggest that alterations in circulating immune cells or Id2 in the kidney are not responsible for Ang II resistance
Studies of regulatory T cells (T regs) have also offered evidence of the role of immune overactivity in the pathogenesis of hypertension. Viel et al (49) studied a consomic strain of rats (SSBN2) that has the genome of hypertensive Dahl salt-sensitive rats and chromosome 2 from normotensive Brown Norway rats to evaluate the genetic influences in inflammatory responses. They found that protein expression of FoxP3b, IL10 and TGF-β were increased in SSBN2 rats and T regs of SSBN2 rats stimulated in vitro produce IL10. These studies demonstrated not only that transfer of chromosome 2 from a normotensive rat improves salt-sensitive hypertension but that these effects were associated with increased production of immunosuppressive mediators. Interestingly, adoptive transfer of T regs improves heart remodelling independently of BP effects (50).
In humans, inflammatory markers linked to the atherosclerotic process are associated with hypertension and prehypertension, independently of other coexisting risk factors (51). Immunosuppressive treatment in humans with uncomplicated essential hypertension are not ethically permissible, and consequently the data in humans has been obtained in hypertensive patients in whom MMF was prescribed for a different pathology and in autopsy studies of patients with essential hypertension. Herrera et al. (52) studied 8 patients with mild hypertension and normal renal function in whom MMF was prescribed by their attending physician for psoriasis or rheumatoid arthritis. Blood pressure improved during the 3 months in which MMF was given and worsened after MMF was stopped. Interestingly, urinary TNF-α cytokine levels also improved during MMF treatment. Hughson et al (53) examined kidney autopsy material from hypertensive and normotensive African Americans and white patients and in their report the renal macrophage infiltration was more severe in hypertensive patients and the intensity of macrophage accumulation correlated with the severity of hypertension. Interestingly, more than half a century ago, in renal biopsies taken in association with sympathectomies used at that time as a treatment of hypertension, Sommers et al (54) documented collections of lymphocytes in kidney biopsies of hypertensive patients with minimal or no arteriolar changes.
Mechanisms of T cell-induced salt sensitive hypertension
The recognition that T cells play a role in the pathogenesis of salt sensitive hypertension has fuelled research efforts to define the mechanisms involved in this relationship. In 2001, we showed by immunohistological double staining studies that a significant number of infiltrating lymphocytes in renal sections of experimental models of salt sensitive hypertension stained positive for angiotensin II (18, 36). Since at that time it was considered that lymphocytes did not have the capacity to produce angiotensin II, these results were surprising. Subsequent studies by Jurewicz et al (55) demonstrated that lymphocytes had a functional renin-angiotensin system and, more recently, Hotch et al (56) showed that T cells express angiotensinogen, angiotensin converting enzyme and renin and produce physiological levels of AII. Furthermore, they showed that endogenously produced AII modulated T cell function, NADPH oxidase activity and the production of superoxide that regulates production of TNFα. These studies underline the relationship between immune inflammatory reactivity and AII. In experimental models of salt sensitive hypertension in which there is a significant tubulointerstitial accumulation of T cells. The T cells could represent a significant source of local AII activity which would constitute a locally originated humoral stimuli inducing sodium reabsorption. Such may be the case in experimental models salt sensitive hypertension in which the severity of hypertension is correlated with both a reduction of plasma AII levels (likely reflecting plasma volume expansion) and an increment in intrarenal angiotensin II and immune cell infiltration (57).
In recent studies, De Miguel et al (58) have shown that T cells mediate salt-induced hypertension and kidney damage. In their studies, a high salt diet induced infiltration of T lymphocytes in the kidneys of Dahl salt-sensitive rats and the administration of MMF attenuated the immune cell infiltration and reduced by about 50% the renal tissue AII.
In addition to the renal effects of infiltrating T cells, Guzik et al (29) have shown that in AII- induced hypertension lymphocytes accumulate in periaortic tissues and, as will be discussed later, local cytokine production may have a role in altering vascular responsiveness. T cell infiltration may also trigger pro-hypertensive mechanisms in the central nervous system. Lob et al (59) have shown that deletion of superoxide dismutase in the circumventricular organs increase T cell infiltration induced by AII and promotes vascular inflammation by modulating the sympathetic outflow.
Role of Macrophages in SSHTN
The association of renal tubulointerstitial inflammation in salt-driven hypertensive conditions and the improvement of hypertension obtained with immunosuppressive strategies, intuitively suggest that macrophages play a role in the development of salt-sensitive hypertension. However, the elegant studies by Titze and his co-workers (reviewed in 60) have indicated that macrophages in the skin activate an adaptive defence mechanism that minimizes the hypertensive effects of sodium retention. Hypertonic accumulation of sodium proteoglycans in the skin interstitium activates the tonicity-responsive enhancer binding protein (TonEBP) in the skin infiltrating macrophages. TonEBP binds to the promoter of the gene on the vascular endothelial growth factor-C (VEGF-C) which results in VEGF-C secretion by the macrophages and lymphangiogenesis. The increase in lymph capillaries attenuates the blood pressure increase induced by a high salt diet (61). More recent studies by Machnik et al (62) indicate that depletion of the mononuclear phagocyte system induces salt-sensitive hypertension. Their studies demonstrate the significance of the macrophage-induced interstitial lymphangiogenesis to dampen the effects of a high salt diet on blood pressure. While these studies offer compelling evidence in favour of a role played by macrophages in the skin for the adaptation to salt-induced increments in blood pressure, it is not defined if the macrophage infiltration within the kidney is also protective. Hypertonicity is a physiologic feature in the renal medulla and the activation of TonEBP in the renal macrophages has not been examined. Renal inflammation is associated with malfunction of the pressure-natriuresis and, consequently, the achievement of sodium balance mandates an increase in blood pressure (5, 6). Since, as discussed earlier, the reduction of this inflammation improves or prevents salt-sensitive hypertension, it is possible that anti-hypertensive actions of the osmolarity-stimulated macrophages are exerted outside the kidney.
Autoimmunity in SSHTN
The association of immune reactivity and immune impairment with hypertension was reported as early as the 1970s (25–27). The antihypertensive effects of lymphocyte depletion was reported in the athymic “nude” mice (26), with neonatal thymectomy (24, 25, 27), with anti-thymocyte serum (26) and with cyclophosphamide therapy (25, 42, 63) in various experimental models of hypertension In contrast with these reports, thymic grafts and extracts were found to ameliorate hypertension in the SHR by preventing periarteritis (64) and interleukin-2 was reported to ameliorate hypertension (65) which led to the suggestion that that activation of the immune system could be an adaptive response directed to minimize life-threatening increments in blood pressure (28). Investigations on the immune system in hypertensive conditions were essentially stopped subsequently (66).
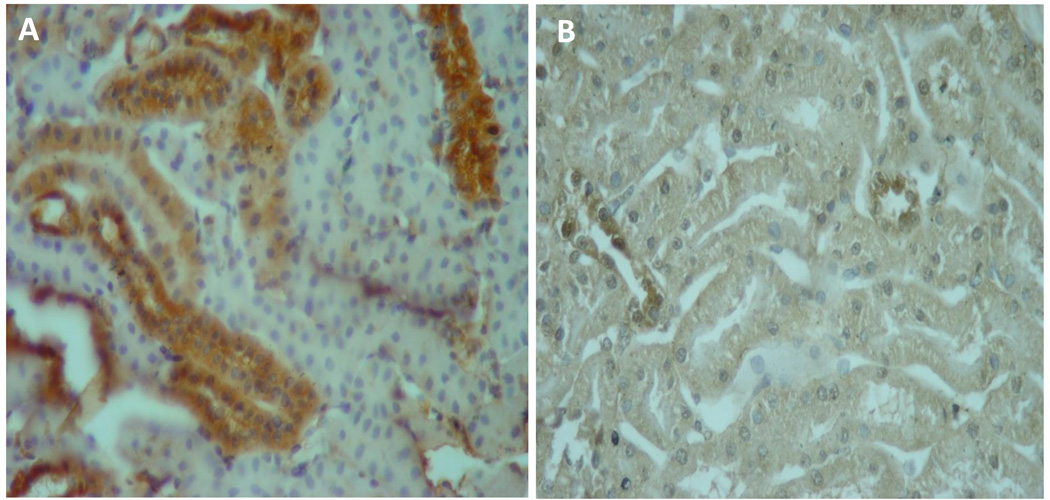
The finding of autoimmune reactivity was first reported by Kristensen et al (67) who found that hypertensive patients have increased serum autoantibodies and delayed-type hypersensitivity reactions against vascular antigens. In 2004, we suggested that autoimmune reactivity to Heat Shock Proteins (HSP) expressed in the kidney could be a mechanism that would sustain a low grade tubulointerstitial inflammation and thereby, play a significant role in the pathogenesis of salt-sensitive hypertension (68). HSP are molecular chaperones that have the potential to trigger autoimmune reactivity (69, 70) and Ishizaka et al (71), as well as our group (72) have shown overexpression of HSP70 in the kidneys of several models of salt-sensitive hypertension (Figure 2). SHR have renal over expression of HSP 70 and plasma anti-HSP70 antibodies (4) and experimental induction of salt-sensitive hypertension is associated with a proliferative response of splenocytes to HSP70 (73). Interestingly, increased serum antibody levels to HSP70 and HSP65 have also been reported in patients with hypertension (74)
Figure 2.
HSP70 overexpression is present in proximal tubular cells of rats with salt-sensitive hypertension induced by transient exposure to L-NAME (A) and absent in rats in which suppression of inflammation has been achieved with mofetil mycophenolate (B). (Immunoperoxidase staining, original magnification ×400)
Recently, the participation of autoimmune reactivity has been suggested by studies showing a relationship between AII and IL-17. IL-17 is a key player in autoimmune responses (75) and Madhur et al. (76) showed that AII infusion increased IL-17 production from T cells and IL-17 protein in the aortic media. They reported that the initial hypertensive response to AII infusion was similar in IL-17−/− and C57BL/6J mice. However, hypertension was not sustained in IL-17 knockout mice, reaching levels 30-mm Hg lower than in wild-type mice by 4 weeks of AII infusion. Furthermore, IL-17, in conjunction with TNF-α, modulated the expression of 30 genes, including a number of inflammatory cytokines/chemokines. Interestingly, IL-17 levels in diabetic humans were found to be significantly increased in those with hypertension compared with the levels in normotensive subjects (76)
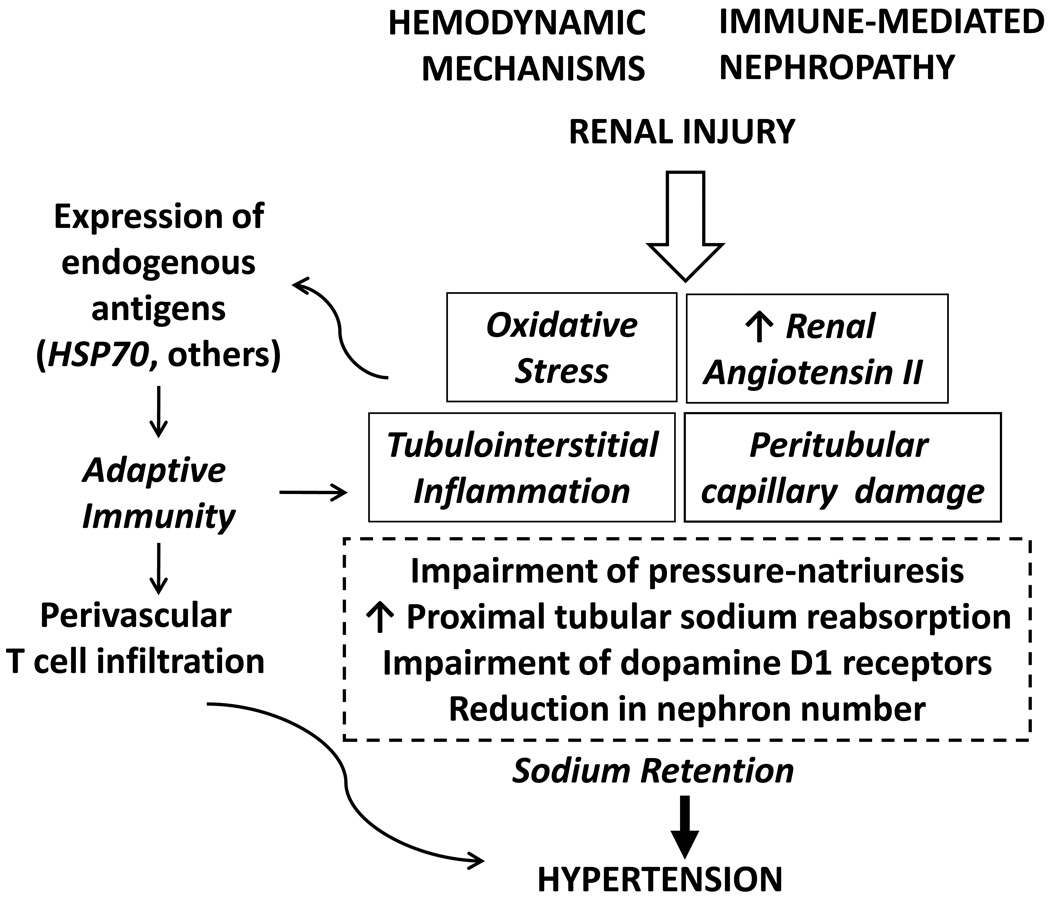
Figure 3 shows a pathogenic scheme of salt-sensitive hypertension that is emerging from the studies cited in this review. Tubulointerstitial inflammation, loss of peritubular capillaries, oxidative stress and augmented renal angiotensin II activity support one another and constitute key elements in the development of impaired pressure natriuresis, reduced dopamine D1 receptor function, tendency to increased proximal sodium reabsorption end, eventually, loss of nephron units. Activation of adaptive immune mechanisms by neoantigen expression sustains and aggravates the tendency to sodium retention that characterizes salt-driven hypertension. Investigations directed to define the role of autologous antigens driving the T cell reactivity may uncover potential new therapeutic targets in the treatment of hypertension
Figure 3.
Schematic representation of the pathogenesis of salt-sensitive hypertension. Mechanisms described in the text.
Acknowledgements
Support for this work was provided by FONACYT grant FC-2005000283 (Dr Rodriguez-Iturbe), CONACYT grant 79661 (Martha Franco) and by NIH NHLBI HL-68607 (Dr. Richard J. Johnson)
List of abbreviations
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
Nitric oxide
- AII
Angiotensin II
- MMF
Mycophenolate Mofetil
- GFR
Glomerular filtration rate
- DOCA
Deoxycorticosterone
- IL
Interleukin
- FoxP3
forkhead box P3
- TGFβ
Transforming Growth Factor β
- TNFα
Tumor necrosis factor α
- NADPH
Nicotinamide adenine dinucleotide phosphate
- TonEBP
Tonicity-responsive enhancer binding protein
- VEGF-C
vascular endothelial growth factor-C
- HSP
Heat Shock Protein
Footnotes
Disclosure: Dr Bernardo Rodriguez-Iturbe and Dr. Richard Johnson have a patent application with the University of Colorado on preventing or treating hypertension by inducing tolerance to HSP70. The other authors have no conflicts of interest.
References
- 1.Weinberg M, Fineberg N. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiologic mechanisms of salt sensitive hypertension. Am J Kidney Dis. 2007;50:655–672. doi: 10.1053/j.ajkd.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RJ, Herrera J, Schreiner G, Rodríguez-Iturbe B. Acquired and subtle renal injury as a mechanism for salt-sensitive hypertension: Bridging the hypothesis of Goldblatt and Guyton. N Engl J Med. 2007;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt-sensitive hypertension. Hypertens Res. 2010;33:975–980. doi: 10.1038/hr.2010.148. [DOI] [PubMed] [Google Scholar]
- 5.Guyton AC. Blood pressure control: special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 6.Guyton AC, Coleman TG, Cowley AW, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation: overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Iturbe B. Renal infiltration of immunocompetent cells: cause and effect of sodium sensitive hypertension. Clin Exp Nephrol. 2010;14:105. doi: 10.1007/s10157-010-0268-1. [DOI] [PubMed] [Google Scholar]
- 8.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of Disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nature Clin Prac Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 9.Tian N, Moore RS, Braddy S, et al. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H3388–H3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RJ, Gordon KL, Suga S, Duijvestijn AM, Griffin K, Bidani A. Renal injury and salt-sensitive hypertension after exposure to catecholamines. Hypertension. 1999;34:151–159. doi: 10.1161/01.hyp.34.1.151. [DOI] [PubMed] [Google Scholar]
- 12.Raqy PE, Suga S, Lin X-H, Huang X, Johnson RJ. Chronic potassium depletion induces renal injury, salt-sensitivity and hypertension in young rats. Kidney Int. 2001;59:1850–1858. doi: 10.1046/j.1523-1755.2001.0590051850.x. [DOI] [PubMed] [Google Scholar]
- 13.Bohle A, Muller G, Whermann M, Mackensen-Haen S, Xiao J-C. Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies and chronic interstitial nephritides. Kidney Int. 1996;49 suppl 54:S2–S9. [PubMed] [Google Scholar]
- 14.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowley AW. Renal medullary oxidative stress, pressure-natriuresis and hypertension. Hypertension. 2008;52:777–786. doi: 10.1161/HYPERTENSIONAHA.107.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 17.Asghar M, Chug G, Lokhandwala MF. Infammation compromises renal dopamine D1 receptor function in rats. Am J Physiol Renal Physiol. 2009;297:F1543–F1549. doi: 10.1152/ajprenal.00366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurokawa K. Kidney, salt and hypertension: how and why. Kidney Int. 1996;49 Suppl 55:S46–S51. [PubMed] [Google Scholar]
- 19.Quiroz Y, Pons H, Gordon KL, et al. Mycophenolate mofetil prevents the salt-sensitive hypertension resulting from short-term nitric oxide synthesis inhibition. Am J Physiol Renal Physiol. 2001;281:F38–F47. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- 20.Wang C-T, Chin SY, Navar LG. Impairment of pressure diuresis and renal autoregulation in Ang II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D, Navar LG. Acute angiotensin infusions elicit pressure natriuresis in mice and reduce fractional sodium reabsorption. Hypertension. 2008;52:137–142. doi: 10.1161/HYPERTENSIONAHA.108.111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiffrin E. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens. 2010;18:181–186. doi: 10.1097/MNH.0b013e3283360a2e. [DOI] [PubMed] [Google Scholar]
- 23.Harrison DG, Guzik TJ, Goronzy J, Weyand C. Is hypertension an immunologic disease? Curr Cardiol Rep. 2008;10:464–469. doi: 10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 24.Bataillard P, Freiche J-C, Vincent M, Touraine J-L, Sassard JU. Effects of neonatal thymectomy on blood pressure and immunological characteristics of the genetically hypertensive rats of the Lyon strain. J Hypertens. 1986;4 suppl 3:5455–5467. [Google Scholar]
- 25.Svendsen UG. Spontaneous hypertension and hypertensive vascular disease in the NZB strain of mice. Acta Pathol Microbiol Scand A. 1977;85:548–554. doi: 10.1111/j.1699-0463.1977.tb03887.x. [DOI] [PubMed] [Google Scholar]
- 26.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus-dependent phase of DOCA and salt hypertension in mice. Acta Path Microbiol Scand A. 1976;84:523–528. doi: 10.1111/j.1699-0463.1976.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 27.Svendsen UG. The importance of thymus in the pathogenesis of the chronic phase of hypertension in mice following partial infarction of the kidney. Acta Pathol Microbiol Scand A. 1977;85:539–547. doi: 10.1111/j.1699-0463.1977.tb03886.x. [DOI] [PubMed] [Google Scholar]
- 28.Bendich A, Belisle EH, Strausser HR. Immune system modulation and its effects on blood pressure of the spontaneously hypertensive male and female rat. Biochem Biophys Res Commun. 1981;99:600–607. doi: 10.1016/0006-291x(81)91787-3. [DOI] [PubMed] [Google Scholar]
- 29.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DL, Sturgis LC, Labazi H, et al. Angiotensin II hypertension in attenuated in interleukin-g knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of Nuclear Factor kappa B prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005;315:51–57. doi: 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]
- 32.Müller DN, Dechend R, Mervaal EMA, et al. NF-kB inhibition ameliorates Angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35(part 2):193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Iturbe B, Quiroz Y, Nava M, et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 34.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in Dahl salt-hypertensive rats. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 35.Tian N, Gu JW, Braddy SJ, Rose RA, Hughson MD, Manning RD., Jr Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1018–H1025. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Iturbe B, Pons H, Quiroz Y, et al. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 37.Bravo Y, Quiroz Y, Pons H, et al. Vimentin and heat shock protein expression are induced in the kidney by angiotensin and by nitric oxide inhibition. Kidney Int. 2003;64 suppl 86:S46–S51. doi: 10.1046/j.1523-1755.64.s86.9.x. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez V, Quiroz Y, Nava M, Pons H, Rodríguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol. 2002;283:F1132–F1141. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 39.Vanegas V, Ferrebuz A, Quiroz Y, Rodríguez-Iturbe B. Hypertension in Page (cellophane wrapped) kidney is due to interstitial nephritis. Kidney Int. 2005;68:1161–1170. doi: 10.1111/j.1523-1755.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 40.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68:2180–2188. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 41.Mervaala E, Müller DN, Park J-K, et al. Cyclosporin A protects against angiotensin II-induced end-organ damage in double transgenic rats harboring human renin and angiotensinogen genes. Hypertension. 2000;35(1 Pt 2):360–366. doi: 10.1161/01.hyp.35.1.360. [DOI] [PubMed] [Google Scholar]
- 42.Khraibi AA, Norman RA, Jr, Dzielak DJ. Chronic immunosuppression attenuates hypertension in Okamoto spontaneously hypertensive rats. Am J Physiol. 1984;247:H722–H726. doi: 10.1152/ajpheart.1984.247.5.H722. (Heart Circ Physiol 16) [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Iturbe B, Zhan Chang- DE, Quiroz Y, Sindhu RK, Vaziri ND. Antioxidant-rich diet improves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension. 2003;41:341–346. doi: 10.1161/01.hyp.0000052833.20759.64. [DOI] [PubMed] [Google Scholar]
- 44.Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- 45.Nava M, Quiroz Y, Vaziri ND, Rodríguez-Iturbe B. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284:F447–F454. doi: 10.1152/ajprenal.00264.2002. [DOI] [PubMed] [Google Scholar]
- 46.Crosswhite P, Sun Z. Ribonucleic Acid Interference Knockdown of Interleukin 6 Attenuates Cold-Induced Hypertension. Hypertension. 2010;55:1484–1491. doi: 10.1161/HYPERTENSIONAHA.109.146902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao J, Nangaku M, Miyata T, et al. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension. 2003;42:31–38. doi: 10.1161/01.HYP.0000075082.06183.4E. [DOI] [PubMed] [Google Scholar]
- 48.Gratze P, Dechend R, Stocker C, et al. Novel role for inhibitor of differentiation 2 in the genesis of angiotensin II-induced hypertension. Circulation. 2008;117:2645–2656. doi: 10.1161/CIRCULATIONAHA.107.760116. [DOI] [PubMed] [Google Scholar]
- 49.Viel EC, Lamarié CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol. 2009;298:H938–H944. doi: 10.1152/ajpheart.00707.2009. [DOI] [PubMed] [Google Scholar]
- 50.Kvakan H, Kleinewietfeld M, Qadri F, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 51.Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17:568–573. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- 52.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17 Suppl 3:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 53.Hughson MD, Gobe GC, Hoy WE, Manning RD, Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. 2008;52:18–28. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 54.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol. 1958;34:685–715. [PMC free article] [PubMed] [Google Scholar]
- 55.Jurewicz M, McDermott DH, Sechler JL, et al. Human T and natural killer cells posses a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 56.Hotch NE, Guzik TJ, Chen W, et al. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franco M, Martínez F, Quiroz Y, et al. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Reg Integr Comp Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 58.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1136–R1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lob HE, Marvar PJ, Guzik TJ, et al. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Titze J, Machnik A. Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens. 2010;19:385–392. doi: 10.1097/MNH.0b013e32833aeb3b. [DOI] [PubMed] [Google Scholar]
- 61.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 62.Machnik A, Dhalmann A, Kopp C, et al. Mononuclear phagocyte system depletion blocks interstitial Tonicity-Responsive Enhyancer Binding Protein/Vascular Endothelial Growth Factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55:755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 63.Norman RA, Jr, Galloway PG, Dzielak DJ, Huang M. Mechanisms of partial renal infarct hypertension. J Hypertens. 1988;6:397–403. [PubMed] [Google Scholar]
- 64.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol. 1982;128:1211–1216. [PubMed] [Google Scholar]
- 65.Tuttle RS, Boppana DP. Antihypertensive effect of interleukin 2. Hypertension. 1990;15:89–94. doi: 10.1161/01.hyp.15.1.89. [DOI] [PubMed] [Google Scholar]
- 66.Dzielak DJ. AIDS, lupus, rheumatoid arthritis-hypertension? Hypertension. 1990;15:95–96. doi: 10.1161/01.hyp.15.1.95. [DOI] [PubMed] [Google Scholar]
- 67.Kristensen BO, Andersen PL. Autoantibodies in untreated and treated essential hypertension. Acta Med Scan. 1978;203:55–59. doi: 10.1111/j.0954-6820.1978.tb14831.x. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells and salt-sensitive hypertension: All for one and one for all. Am J Physiol Renal Physiol. 2004;286:F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 69.Kaufmann SHE. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 70.Young RA. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- 71.Ishizaka N, Aizawa T, Ohno M, et al. Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension. 2002;39:122–128. doi: 10.1161/hy1201.096818. [DOI] [PubMed] [Google Scholar]
- 72.Bravo J, Quiroz Y, Pons H, et al. Vimentin and heat shock protein expression are induced in the kidney by angiotensin and by nitric oxide inhibition. Kidney Int. 2003;64 suppl 86:S46–S51. doi: 10.1046/j.1523-1755.64.s86.9.x. [DOI] [PubMed] [Google Scholar]
- 73.Parra G, Quiroz Y, Salazar J, et al. Experimental induction of salt-sensitive hypertension is associated with lymphocyte proliferative response to HSP70. Kidney Int Suppl. 2008;111:S55–S59. doi: 10.1038/ki.2008.513. [DOI] [PubMed] [Google Scholar]
- 74.Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegård J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens. 2002;20:1815–1820. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 75.Miossec P, Kom T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 76.Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes Angiotensin II-Iinduce hypertension and vascular dysfunction. Hypertension. 2010;55(part 2):500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]