Abstract
For accurate processing of auditory information, neurons in auditory brainstem nuclei have to fire at high rates with high temporal accuracy. These two requirements can only be fulfilled when the intrinsic electrical properties of these neurons are matched to the pattern of incoming synaptic stimulation. This review article focuses on three families of potassium channels that are critical to shaping the firing pattern and accuracy of neurons. Changes in the auditory environment can trigger very rapid changes in the phosphorylation state of potassium channels in auditory brainstem nuclei. Longer lasting changes in the auditory environment produce changes in the rates of translation and transcription of genes encoding these channels. A key protein that plays a role in setting the overall sensitivity of the auditory system to sound stimuli is FMRP (Fragile X Mental Retardation Protein), which binds channels directly and also regulates the translation of mRNAs for the channels.
Keywords: MNTB, Kv3.1, Kv1, KNa, Slack, FMRP
Introduction
The central auditory system in mammals discriminates among numerous stimuli in the auditory environment. Appropriate processing of auditory inputs, including the decoding of timbre, pitch and spatial locations of sounds, allows one to identify specific features of a sound in a noisy environment, such as one particular voice, and to ignore other sounds located at a slightly different location. To carry out this task, many neurons in the auditory brainstem nuclei are capable of firing at high rates (up to ~800 Hz) with very high temporal precision (Banks et al., 1992; Taschenberger et al., 2000; Taschenberger et al., 2002; Trussell, 1999; Wu et al., 1993). In low-frequency hearing animals, phase-locking brainstem neurons accurately lock their action potentials to the phase of incoming auditory stimuli with frequencies up to 2 kHz. At higher frequencies, and in high-frequency hearing animals, such neurons lock their action potentials to the envelope of sounds that are amplitude modulated at these lower frequencies (Joris, 1996; Joris et al., 1995). The ability of such neurons to follow auditory stimuli relies in major part on their intrinsic electrical properties, which allow them to generate the appropriate firing rate with the needed precision of timing, and on their ability to change their electrical properties in response to changes in the auditory environment.
Auditory brainstem circuits for sound localization
The basic neural circuitry required for discrimination of the spatial location of sounds is located in the auditory brainstem, within the cochlear nuclei and superior olivary complex. Phase-locking bushy cells in the anteroventral cochlear nucleus (AVCN) relay information on the timing and intensity of sounds presented to the ipsilateral cochlea to the lateral superior olivary (LSO) nuclei and the medial superior olivary (MSO) nuclei. The nervous system makes a comparison of intensity and time of arrival of sounds at the two ears in the LSO and MSO. A key component of this circuit is the medial nucleus of the trapezoid body (MNTB). The principal neurons of this nucleus receive excitatory inputs from the AVCN globular bushy cells and relay this information via glycinergic inputs to both the LSO and MSO nuclei. (Banks et al., 1992; Brand et al., 2002; Brownell, 1975; Kopp-Scheinpflug et al., 2003a; Smith et al., 1998; Taschenberger et al., 2000; Wu et al., 1993). This inhibitory input is required for the processing of both intensity and time differences (Brand et al., 2002; Moore et al., 1983). Excitation of MNTB neurons by globular bushy cells occurs at the giant calyx of Held synapse where glutamatergic excitatory input from the AVCN surrounds the somata of the MNTB neurons (Morest, 1968), and this synaptic contact has proved to be a model system for answering a number of basic questions in neurobiology. For example, rigorous investigations of the mechanisms of neurotransmitter release are possible at this synaptic terminal because patch clamp electrodes can be used to gain direct chemical and electrical entry into the interior of the presynaptic terminal (Borst et al., 1996; Borst et al., 1995; Forsythe, 1994; Schneggenburger et al., 2006; Taschenberger et al., 2002; Trussell, 1999; Wang et al., 1998a). The majority of studies investigating the regulation of intrinsic excitability of auditory neurons have also been carried out in the MNTB, and these are the focus of this review.
Classes of potassium channels
The last few decades have seen an enormous growth in the molecular basis of ionic conductances, and it is now believed that the genes encoding most plasma membrane channels have been identified. Without question, channels selective for potassium form the most diverse class of ion channels. Over a hundred genes encode subunits of potassium channels compared to nine genes described for voltage-dependent sodium channels and ten genes for voltage-dependent calcium channels (Hille, 2001). Genetic and pharmacologic manipulation of the levels of various ion channel subunits has allowed investigators to test the role of specific subunits in the generation of different types of firing patterns. The existence of a wide variety of potassium channels, coupled with the fact that the properties of many of these channels can be rapidly modified by intracellular biochemical events such as activation of protein kinases and second messenger pathways, provides the possibility of fine-tuning the excitability of a neuron to an exquisite degree. Because their biological function is linked to their ability to fire at high rates with high temporal precision, auditory brainstem neurons have proved invaluable for probing the role of specific ion channels in control of synaptic transmission and neuronal firing patterns (Joris, 1996; Kaczmarek et al., 2005; Oertel, 1999; Schneggenburger et al., 2006; Trussell, 1999).
As will be described in this review, the intrinsic excitability of these neurons is dynamic and can rapidly change to adapt to different acoustic environments. Although most of the work examining regulation of ion channels in auditory brainstem has used rodents, the ion channels and signaling pathways that underlie rapid changes in neuronal excitability are highly conserved between rodent and humans. Thus it would not be surprising to find that such plasticity of intrinsic excitability also occurs in humans and may contribute to, for example, improvements in auditory discrimination tasks following training.
This review article will focus on three types of potassium channels that are known to shape the intrinsic excitability of MNTB neurons: “high-threshold” voltage-dependent Kv3 family potassium channels, “low-threshold” voltage-dependent Kv1 family channels, and the Slack/Slick sodium-activated channels. Neurons can rapidly respond to various patterns of stimulation using biochemical mechanisms to modulate the properties of potassium channels.
Rapid firing requires Kv3.1 channels
First defined in the squid giant axon, delayed rectifier channels provide current for the repolarization of action potentials (Hodgkin et al., 1952). Kv3.1 channels are voltage-dependent potassium channels belonging to the delayed rectifier channel family (Swanson et al., 1990) located on presynaptic terminals as well as immediately adjacent to subsynaptic membranes of MNTB neurons (Wang et al., 1998c). However, the properties of Kv3.1 diverge substantially from most channels in this classification. Kv3.1 currents recorded using patch clamp techniques on heterologous cell lines expressing the Kv3.1 gene, activate and deactivate very rapidly in response to voltage changes (Kanemasa et al., 1995) (Fig. 1A). Kv3.1 currents become activated when a cell depolarizes to potentials more positive than −10 mV. Because of its rapid deactivation, however, very few Kv3.1 channels remain open after the end of an action potential. For most other delayed rectifier channels, their slow rate of deactivation after an action potential results in the relative refractory period. In numerical simulations, neurons with Kv3.1 currents have narrow action potentials and can fire at high rates because of the lack of a relative refractory period (Kanemasa et al., 1995).
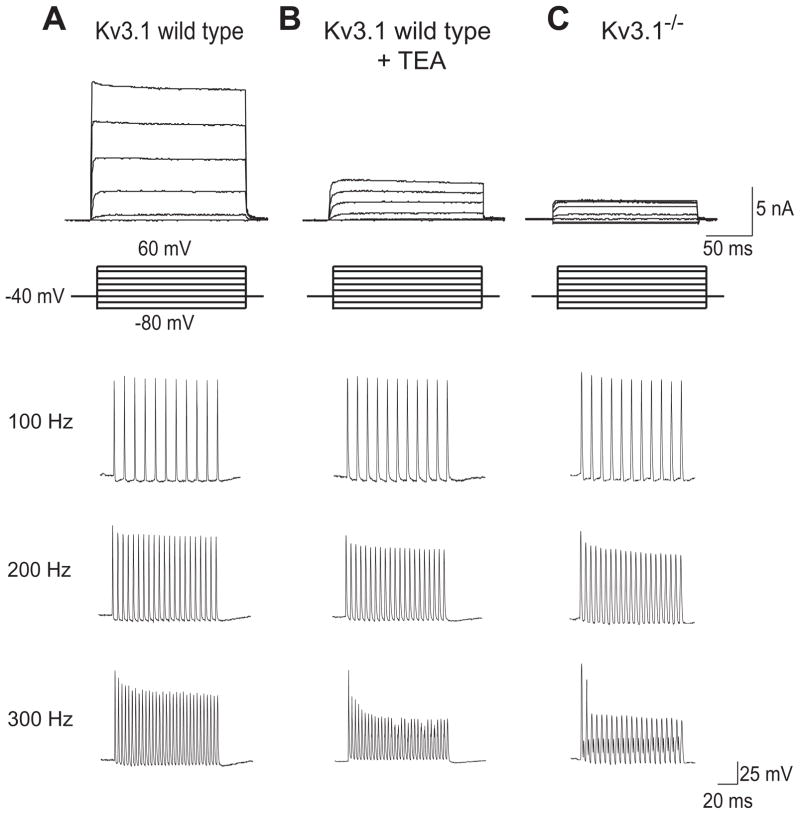
Fig. 1.
MNTB neurons express the Kv3.1 voltage-dependent potassium channel that is required for high rates of firing (Figure is modified from (Macica et al., 2003)). (A) Top traces show the high threshold outward current evoked by depolarization from −40 mV to test potentials between −80 and 40mV in a mouse MNTB neuron. This current is missing in neurons treated with the Kv3.1 blocker 1 mM TEA (B) or from mice in which the Kv3.1 gene has been eliminated (Kv3.1−/−) (C). The lower current clamp traces show the firing properties of MNTB neurons briefly stimulated (for 0.3 msec with 2nA) at frequencies ranging from 100–300 Hz. In Kv3.1−/− animals or wild type neurons treated with TEA, MNTB neurons are incapable of following the stimulation at 300 Hz.
MNTB and AVCN neurons express high levels of Kv3.1 channels (Li et al., 2001a; Perney et al., 1997; Wang et al., 1998c) and are capable of generating action potentials at high rates (Martina et al., 1998; Massengill et al., 1997; McDonald et al., 2006; Perney et al., 1992; Weiser et al., 1995). A potassium current matching Kv3.1 in its kinetic and pharmacological characteristics has been demonstrated in patch clamp recordings from MNTB neurons (Wang et al., 1998b) and this component of potassium current is lacking in mice in which the Kv3.1 gene has been deleted (Kv3.1−/− ) (Fig. 1C) (Macica et al., 2003). The firing pattern of MNTB neurons from Kv3.1−/− mice does not differ from wild type cells when stimulated with intracellular current pulses at frequencies less than ~200 Hz. But at stimulus rates above 200 Hz, MNTB neurons from Kv3.1−/− mice become incapable of sustaining action potential firing beyond the first or second stimulus (Fig. 1C). These experiments on Kv3.1−/− mice, coupled with a variety of pharmacological approaches, have demonstrated that Kv3.1 is essential for MNTB neurons to fire at high rates (Kaczmarek et al., 2005). Moreover, increasing the level of Kv3.1 current either in real neurons or in numerical simulations of MNTB neurons (Fig. 2) further increases the rate at which a neuron can be stimulated and still maintain sustained firing of action potentials (Kaczmarek et al., 2005; Song et al., 2005).
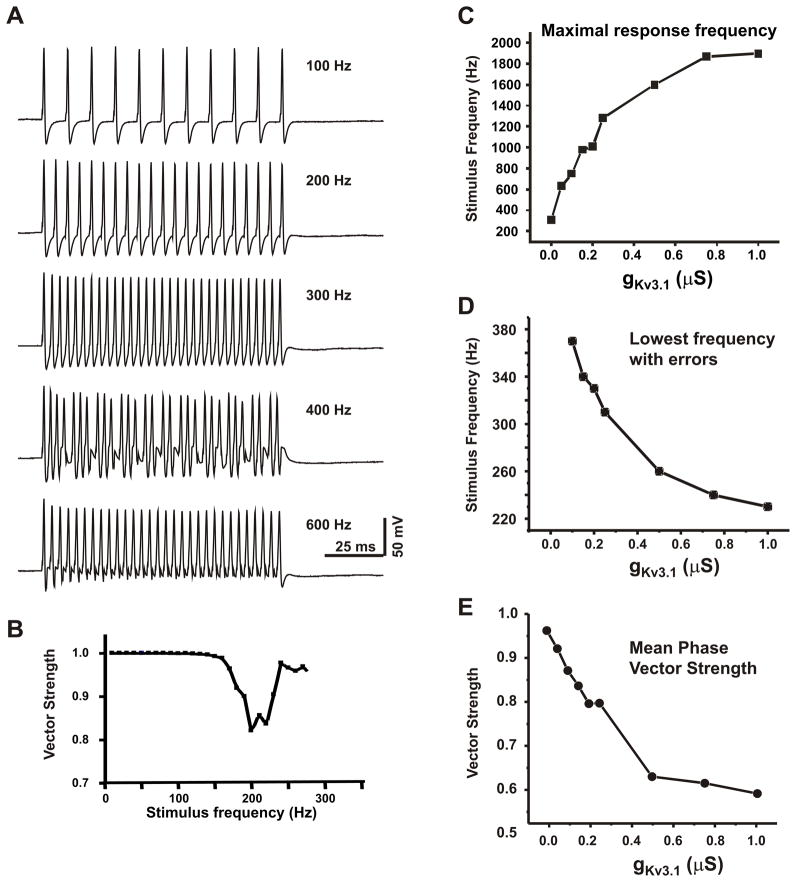
Fig. 2.
MNTB neuron firing patterns recorded in a mouse brainstem slice. (A) Phase locking to each stimulus pulse is lost at stimulation rates above 400 Hz, when the neuron is stimulated with intracellular current pulses. (Modified from (Wang et al., 1998a)). (B) A plot of the strength of the phase vector linking the timing of evoked action potentials to the onset of the stimulus pulse for a different MNTB neuron (Song et al., 2005; Yang et al., 2007). A dip in the plot reflects the onset of an irregular firing pattern. (C–E) Increases in Kv3.1 current promote responses to high rates of stimulation at the expense of temporal accuracy. Plots are derived from numerical simulations of rat MNTB neurons (Song et al., 2005) demonstrating the effects of increasing levels of Kv3.1 current on the maximal stimulus to which the neuron continues to respond with a sustained train of action potentials (C), the lowest frequency at which irregular firing patterns occur (D) and the strength of phase vector averaged over the stimulus rates from 200 Hz to the maximal response frequency (E).
MNTB neurons expressing high levels of Kv3.1 channels make mistakes in timing
In addition to allowing cells to fire at rapid rates, high levels of Kv3.1 can promote the occurrence of the irregular firing patterns that degrade timing information. Figure 2A illustrates the response of an MNTB neuron in a brain slice to current pulses applied at frequencies from 100 Hz to 600 Hz (Wang et al., 1998b). This neuron precisely locks an action potential in response to every stimulus at stimulation rates below 400Hz. At 400Hz, however, the response pattern becomes irregular. Different action potentials evoked during the train have different latencies to the onset of the stimulus pulses and some of the stimuli fail to trigger action potentials. Once the stimulation rate is raised to 600Hz, there is a restoration of the regular firing pattern, although now only every other stimulus pulse evokes an action potential. Accuracy of timing is restored at this higher stimulus rate because, in fact, the neurons fire at a lower rate (300 Hz) in response to the 600 Hz stimulus than in response to the 400 Hz stimulus. Clearly, however, information is lost when cells only respond to a subset of stimuli.
A firing pattern such as that observed for 400 Hz stimulation in Figure 2B poses a problem for the processing of auditory information. While higher levels of Kv3.1 current in a neuron are required for firing at high rates, they also promote such irregular firing responses to regular patterns of input. The loss of accuracy with high levels of Kv3.1 can be attributed to the fact that, at higher current amplitudes, even the rapidly-deactivating Kv3.1 channels begins to contribute to the relative refractory period, producing a progressive delay in the timing of successive action potentials evoked by a regular stimulus. This can readily be quantified in real neurons and in numerical simulations by calculating the strength of a phase vector that measures the temporal relation between a stimulus and its evoked action potential. The value of this phase vector falls in magnitude as errors in timing occur (Fig. 2B). When the amplitude of Kv3.1 current in a neuron is increased, the strength of the phase vector begins to drop at progressively lower stimulus frequencies (Fig. 2D), and the temporal accuracy of a neuron over its full range of firing rates is reduced (Fig. 2E) (Song et al., 2005; Yang et al., 2007).
Kv3.1 current in MNTB neurons is rapidly adjusted by phosphorylation
Neurons with little or no Kv3.1 current cannot generate errors of timing because they are not able to fire at sufficiently high rates (Fig. 2C). Thus neurons with low Kv3.1 current levels would be expected to fire with high accuracy during periods of low auditory input, when firing rates are lower, but fail to respond adequately when sound levels are increased. Studies have demonstrated that the level of Kv3.1 current in MNTB neurons can be rapidly adjusted in response to changes in the auditory environment by changing the phosphorylation state of the Kv3.1 protein.
Kv3.1 exists as two isoforms, termed the a and b isoforms. The expression of the two isoforms is developmentally regulated by alternative splicing (Perney et al., 1992). Kv3.1b is the major form in auditory neurons of the adult, while Kv3.1a, which has a shorter cytoplasmic C-terminal, is expressed at higher levels only early in development. The developmental increase in the adult Kv3.1b isoform occurs close to the onset of hearing between postnatal days 8 and 15 (Macica et al., 2003).
Both casein kinase 2 and protein kinase C (PKC) regulate Kv3.1b currents. As stated earlier, a defining feature of Kv3.1 channels is that they predominantly activate at positive membrane potentials. This activation is dependent on constitutive phosphorylation by casein kinase 2 (Macica et al., 2001). Treatment of MNTB neurons or Kv3.1 transfected cells with casein kinase 2 inhibitors shifts the voltage-dependence of Kv3.1 currents to negative potentials and causes them to inactivate more rapidly. Casein kinase 2 modulates both Kv3.1a and Kv3.1b currents. In contrast, although phosphorylation of Kv3.1 by PKC occurs at several serine residues on both isoforms, a change in current only occurs for the adult Kv3.1b isoform (Macica et al., 2003). This can be attributed to a residue, serine 503, that is located on the longer C-terminal of the Kv3.1b isoform. When this site is phosphorylated the amplitude of the Kv3.1b current is suppressed by 30–40% (Kanemasa et al., 1995; Macica et al., 2003).
Under resting conditions, Kv3.1b channels in the presynaptic calyxes of Held and in MNTB neurons are basally phosphorylated. This basal phosphorylation can be detected using a phospho-specific antibody to serine 503 (Fig. 3) (Song et al., 2005). Experiments in brain slices have shown that rapid stimulation (600 Hz) of the presynaptic input from the AVCN causes a dephosphorylation at serine 503 (Song et al., 2005). At the end of stimulation, the recovery of basal phosphorylation, which is mediated by several different PKC isoforms, occurs within a minute or less (Song et al., 2006). This synaptic stimulation-induced dephosphorylation of serine 503 is accompanied by an increase in the Kv3.1 component of current in MNTB neurons (Fig. 3B) and increases the ability of the neurons to respond to high rates of synaptic stimulation (Fig. 3D). As predicted, however, errors of timing can detected at lower stimulation rates where, prior to synaptic stimulation, no errors occurred (Fig. 3E).
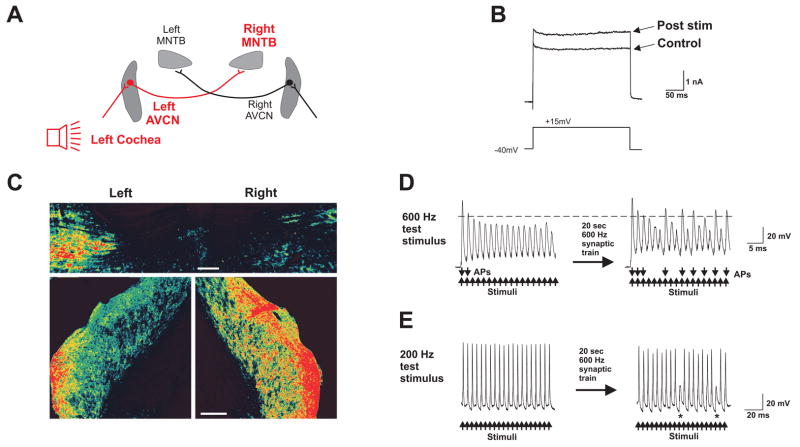
Fig. 3.
Acoustic stimulation decreases Kv3.1 phosphorylation in the rat auditory brainstem. (A) Diagram of the auditory pathways activating AVCN and MNTB nuclei. (B) High threshold outward currents evoked by a 250 ms step from −40 mV to 15 mV in an MNTB neuron before and after synaptic stimulation at 600 Hz for 20 s. (C) Rats with the right ear plugged were presented with a 600 Hz click train for 5 min. The right MNTB (upper panel) and left AVCN (lower panel) had decreased immunostaining for phospho-Kv3.1b. (D) Prior to synaptic stimulation, the amplitude of evoked responses declines so that only the first two current pulses produced overshooting action potentials. After synaptic stimulation, multiple overshooting action potentials were generated by the intracellular current pulses. Action potentials evoked by intracellular current injection (600 Hz, 0.2 ms, 2 nA pulses) before and after a 600 Hz conditioning stimulus train delivered for 20 s to the axons in the trapezoid body. Downward arrows indicate action potentials (APs) that reached 0 mV (indicated by the dotted line). (E) With a lower test frequency (200 Hz) failures to trigger action potentials (denoted by asterisks) were observed after, but not before, the 20 s synaptic stimulus train. (Figure is modified from (Song et al., 2005)).
The mechanism by which stimulation at high rates triggers dephosphorylation of Kv3.1b at serine 503 appears to be a direct consequence of the enhanced rate of synaptic transmission. Dephosphorylation can be prevented by antagonists of glutamate AMPA and NMDA receptors. Through an as yet uncharacterized mechanism, high rates of transmission trigger activation of a serine/threonine phosphatase and, in brain slices, the dephosphorylation can also be blocked completely by inhibitors of protein phosphatases 1 or 2A (Song et al., 2005).
Large changes in the phosphorylation state of Kv3.1b can also be detected in vivo in response to changes in the auditory environment (Song et al., 2005). When rats are maintained in a quiet environment, Kv3.1b in neurons of the AVCN and MNTB exists largely in its phosphorylated state. Exposure of such animals for five minutes to sound stimuli at physiological levels (600 Hz click trains, 70 dB) produces a ~80–90 % decrease in phosphorylation of this channel subunit. When animals are exposed binaurally to these sound stimuli, dephosphorylation occurs bilaterally in neurons of these brainstem nuclei. If, however, sound stimulation is applied to one ear only, as when the contralateral ear is plugged, dephosphorylation of channel subunits occurs only in the ispilateral AVCN and contralateral MNTB, providing an internal control (Fig. 3C).
These experiments demonstrate that the intrinsic excitability of neurons in the auditory brainstem is subject to rapid modification by the auditory environment. An increase in potassium current produced by dephosphorylation of serine 503 in Kv3.1b allows neurons to generate action potentials at high rates when an animal enters a loud auditory environment. In contrast, in a quiet environment, Kv3.1b currents are reduced through basal phosphorylation by PKC. This provides for maximal temporal accuracy when auditory stimuli evoke only low rates of action potential firing.
Sound-induced dephosphorylation of Kv3.1b occurs in the presynaptic calyces of Held, as well as in the postsynaptic somata (Song et al., 2005). The biological significance of the changes in phosphorylation in the presynaptic terminals has, however, not yet been explored. In contrast to the postsynaptic soma, where genetic deletion of Kv3.1 alone nearly fully eliminates the high threshold potassium current (Macica et al., 2003), both Kv3.1 and Kv3.3 subunits are expressed presynaptically and they may combine to form heteromeric channels. The regulation of Kv3.3 by PKC is opposite in direction from that of Kv3.1b (Desai et al., 2008) and the actions of this enzyme on Kv3.1b/Kv3.3 heteromeric channels or on the native presynaptic Kv3 current have not yet been established.
Auditory stimulation produces intermediate-term changes in levels of Kv3.1 channel subunits and alters tonotopic gradients
Phosphorylation of ion channels such as Kv3.1b, as well as the activation of other related second messenger pathways (Steinert et al., 2008), allows very rapid modulation of auditory neurons in response to rapid changes in the acoustic environment. More persistent changes in pattern of auditory stimulation, however, can lead to longer-lasting changes in ion channels. For example, recent evidence has demonstrated that the levels of Kv3.1 in MNTB neurons of rats and mice are significantly increased 30 minutes after acoustic stimulation by rapidly modulated sounds at physiological intensity levels (Fig. 4A,C) (Leao et al., 2010; Strumbos et al., 2010a; Strumbos et al., 2010b).
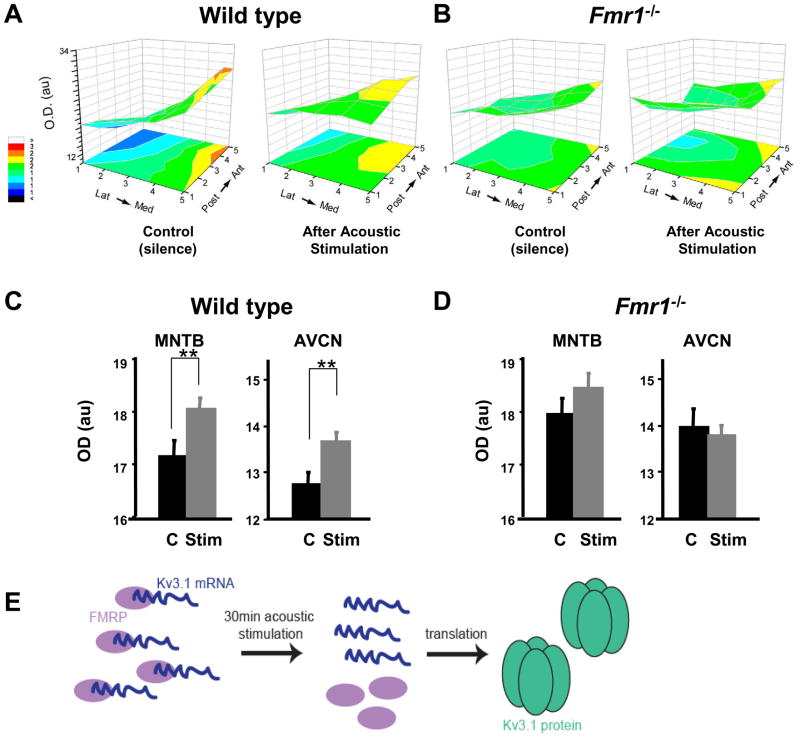
Fig. 4.
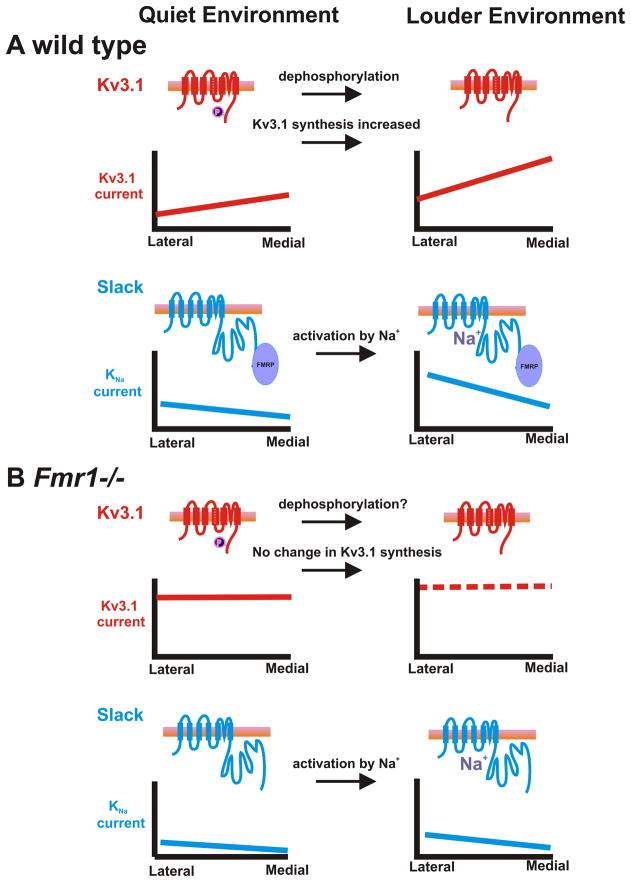
Acoustic environment regulates Kv3.1b levels and tonotopicity in wild type mice but not Fmr1−/− mice. (A) 3-D representation of levels of Kv3.1 in the MNTB of wild type animals maintained in silence for 30 min or exposed to a 32 kHz pure tone modulated at 380-420 Hz for the same time. The stimulus produced a net increase in Kv3.1 immunoreactivity in both the MNTB and the AVCN (C), as well as a flattening of the tonotopic gradient (A). (B) In mice lacking FMRP, there is little or no Kv3.1b gradient either in silence or after acoustic stimulation, which fails to produce any change in levels of Kv3.1 immunoreactivity (D) in either the MNTB or the AVCN. (Panels A-D are modified from (Strumbos et al., 2010b).) (E) Schematic overview of the hypothesis that FMRP binds Kv3.1b mRNA and represses its translation.
Other evidence also indicates that overall levels of Kv3.1 channels are not static in auditory neurons and are dependent upon ongoing neural activity. In fact, Kv3.1 is expressed along a tonotopic gradient, with low levels of the channel in the lateral, low-frequency end of the MNTB and high levels in the medial, high frequency aspect of the nucleus (Brew et al., 2005; Li et al., 2001a). Gradients of expression in the MNTB may be determined by gradients of ephrins or other regulatory factors (Cramer, 2005). The maintenance of the Kv3.1 gradient, however, appears to require ongoing synaptic activity in auditory circuits. Mouse strains that undergo age-related hearing loss lose the gradient of Kv3.1 expression in their MNTB whereas mice that maintain normal hearing with age maintain a Kv3.1 gradient (Leao et al., 2006; von Hehn et al., 2004). Moreover, in addition to increasing the overall levels of Kv3.1 in the MNTB, acoustic stimulation for 30 minutes alters the distribution of Kv3.1 along the tonotopic axis (Strumbos et al., 2010a).
The FMRP pathway is required for activity-dependent changes in Kv3.1 levels
The ability of auditory stimulation to change levels of Kv3.1 in MNTB neurons and to influence its tonotopic distribution appears to depend on an mRNA-binding protein termed FMRP (Fragile X Mental Retardation Protein) (Fig. 4). One of the leading hypotheses for the role of FMRP is that it acts to repress the translation of its cargo mRNAs, but that such mRNAs can be released for translation in response to neuronal stimulation (Bassell et al., 2008; Huber et al., 2002; Laggerbauer et al., 2001; Li et al., 2001b; Pfeiffer et al., 2009; Zukin et al., 2009).
Kv3.1 mRNA was one of the first identified mRNAs that bind to FMRP (Darnell et al., 2001). A schematic overview of the role of FMRP regulating Kv3.1 mRNA is shown in Fig. 4E. Consistent with the notion that FMRP acts to repress translation of Kv3.1 mRNA, both immunoreactivity for the Kv3.1 protein and Kv3.1 potassium currents in MNTB neurons are elevated in Fmr1−/− mice compared to wild type mice (Strumbos et al., 2010b). Additionally, Kv3.1 tonotopic gradients are disrupted in Fmr1−/− mice (Fig. 4A, B). In contrast to wild type animals, there is no significant difference in Kv3.1 levels across the tonotopic axis in MNTB or AVCN neurons from Fmr1−/− mice (Fig. 4C,D, see also Fig. 6).
Fig. 6.
An overview of how FMRP is likely to regulate both Kv3.1 and Slack currents in the MNTB in quiet vs loud environments. (A) In wild type mice moving from a quiet to loud environment, Kv3.1 becomes dephosphorylated and there is an increase in the synthesis of Kv3.1 channels. Both of these events lead to an overall increase in Kv3.1 currents across the tonotopic gradient. The increased synthesis of Kv3.1 believed to occur through FMRP loss of repression Kv3.1 mRNA. Additionally, FMRP binds to Slack channels and activates Slack currents when there is rise in intracellular sodium. (B) In contrast, in Fmr1−/− mice, Kv3.1 levels are high and do not differ along the tonotopic gradient and there is a decrease in Slack currents in the MNTB. Auditory stimulation fails to increase synthesis of Kv3.1 channels. Although the phosphorylation of Kv3.1 in Fmr1−/− animals has not been studied, there is no a priori reason to believe that it differs in such animals. Thus neurons from Fmr1−/− animal are expected to be capable of firing at higher rates than those from wild type animals and with reduced temporal accuracy.
Deletion of the gene encoding FMRP in mice also blocks the ability of acoustic stimulation to increase levels of Kv3.1 channels following exposure to 30 minutes of amplitude-modulated acoustic stimuli (Fig. 4B,D). The results of these experiments suggest that FMRP is necessary for fine-tuning tonotopic gradients of Kv3.1 expression in the MNTB. In numerical simulations, a Kv3.1 tonotopic gradient within the MNTB may permit accurate encoding of acoustic stimulus features such as carrier frequency and modulation rate such that disruption of the Kv3.1 tonotopic gradient, as seen in Fmr1−/− animals, hinders the processing of auditory information related to sound localization (Strumbos et al., 2010b).
Long-term transcriptional regulation of Kv3.1 channels
Activity also regulates the promoter for the Kv3.1 gene (Gan et al., 1998; Gan et al., 1996; Gan et al., 1999; Liu et al., 1998a). Upstream of the transcription start site for Kv3.1 there is a cyclic AMP/calcium response element (CRE) that has been demonstrated to bind the transcription factor CREB (CRE-binding protein). Following a rise in calcium or cAMP, as occurs during repetitive neuronal firing, CREB is phosphorylated and binds the CRE in the Kv3.1 promoter. Removal of the CRE produces a functional Kv3.1 promoter that is insensitive to second messenger signaling (Gan et al., 1996). In developing inferior colliculus neurons, elevations of intracellular calcium ions produce an increase in the transcription of the Kv3.1 gene and enhance Kv3.1 currents (Liu et al., 1998b). Although the detailed time course for regulation of Kv3.1 by transcriptional mechanisms is not yet established, the work with inferior colliculus neurons suggests that relatively large changes in Kv3.1 current levels resulting from altered transcription of the Kv3.1 gene can occur over a few hours (Liu et al., 1998b). Kv3.1 transcription would also be expected to be enhanced by elevation of cytoplasmic calcium levels during high frequency synaptic stimulation of the AVCN and MNTB neurons.
Changes in the expression of potassium channels in culture have also been studied under conditions of chronic depolarization (high K+ medium) or lack of neuronal stimulation (low K+ medium) in MNTB neurons maintained in organotypic brainstem slices (Tong et al., 2010). Specifically, the levels of Kv3.3 mRNA were increased with chronic depolarization, and this change was reversed with addition of a CREB antagonist. Surprisingly, despite the presence of the CRE element in the promoter of the Kv3.1 gene, Kv3.1 mRNA was unchanged with chronic high K+ treatment (Tong et al., 2010). This suggests that chronic and persistent stimulation of MNTB neurons induces multiple pathways of potassium channel regulation (Kaczmarek, 2010).
Kv1 family channels are required for accuracy of timing
In auditory brainstem neurons, an important set of voltage-gated potassium channels controlling the membrane properties close to the threshold of firing are the Kv1 Shaker family of channels (reviewed by Johnston et al., 2010). Members of this channel subfamily such as Kv1.1, Kv1.2, Kv1.6 (Dodson et al., 2002; Grigg et al., 2000) are expressed in neurons of the AVCN and MNTB and provide a low threshold current that activates between –67 mV and –40 mV (Brew et al., 1995; Brew et al., 2003; Dodson et al., 2003; Liu et al., 1998b; Manis et al., 1991; Rathouz et al., 1998; Wang et al., 1998b). Activation of these currents at the resting potential endows neurons with a rapid time constant for depolarizing stimuli, allowing the action potentials to lock faithfully to synaptic inputs (Kopp-Scheinpflug et al., 2003b; Manis et al., 1991).
The regulation of Kv1 channels by intracellular signaling in auditory neurons is not well understood at this time. Kv1 family channels are selectively targeted to the axons of neurons of the AVCN and MNTB (Dodson et al., 2002), where they may not be subject to rapid modulation by second messengers generated at subsynaptic sites. In congenitally deaf mice, however, the loss of sensory input leads to a loss of Kv1 family channels in MNTB neurons (Taschenberger et al., 2002). Additionally, the full complement of potassium channels setting the threshold of MNTB neurons has not been established. ERG channels (Kv11, Ether-á-go-go-related channels) located at the somata (Hardman et al., 2009) and Kv2.2 channels, located at the initial segment (Johnston et al., 2008) are known to also influence their firing patterns, and the modulation of these channels in the MNTB has yet to be fully explored.
A recent study has found that the Kv1.3 Shaker family channel is expressed in the calyx of Held presynaptic terminal that engulfs the MNTB principal neurons (Gazula et al., 2010). This contrasts sharply with the location of the other Kv1 family channels, which are found selectively in the axons. Like the Kv3.1 channel, Kv1.3 is expressed in a tonotopic gradient in the MNTB, but the direction of this gradient is opposite to that for Kv3.1, with highest levels of Kv1.3 found in the lateral low-frequency region of the MNTB. In the central olfactory system, lack of Kv1.3 has been found to produce a profound change in the sensitivity to sensory inputs (Fadool et al., 2004). It is not yet known, however, whether regulation of Kv1.3 in the auditory system alters sensitivity to stimuli.
Regulation of timing of action potentials by sodium-activated potassium channels
Another class of potassium channels, sodium-activated potassium channels (KNa channels) may also contribute to the timing of action potentials in auditory brainstem neurons. Like Kv1 family channels, KNa channels open near the resting potential of MNTB neurons (Bhattacharjee et al., 2003; Joiner et al., 1998). Elevations in intracellular sodium or chloride levels increase the activity of these channels (Bhattacharjee et al., 2003; Yuan et al., 2003). Mammalian KNa channels are composed of subunits encoded by two different mammalian genes called Slack (Sequence like a calcium-activated K+ channel, also known as Slo2.2) and Slick (Sequence like intermediate conductance K+ channel, also known as Slo2.1) (Fig. 5). Slack and Slick are found at high levels in MNTB neurons and are widely expressed throughout the brain (Bhattacharjee et al., 2002; Bhattacharjee et al., 2005).
Fig. 5.
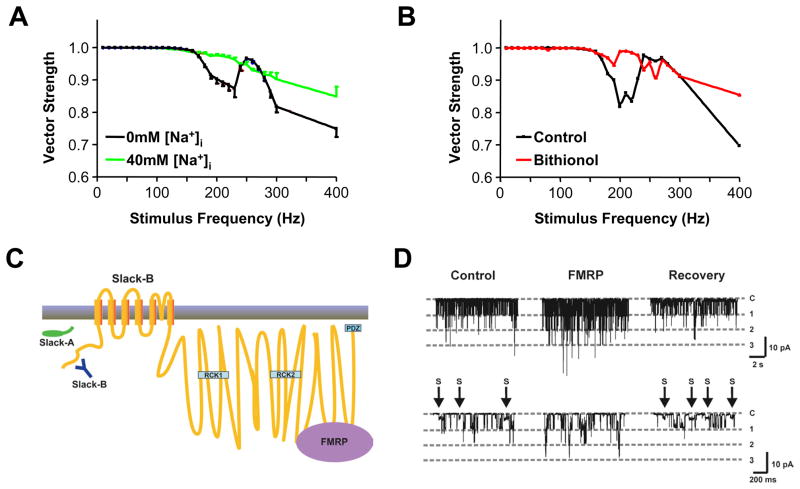
KNa channels influence timing accuracy of MNTB neurons. (A,B) Plots of the strength of the phase vector linking the timing of action potentials evoked in mice MNTB neurons to the onset of stimulus pulse applied at different rates. At stimulus rates that produce irregular patterns of firing (corresponding to dips in the plots) improved timing accuracy is observed when intracellular sodium is elevated (40mM) (A) or neurons are exposed to the Slack pharmacological activator, bithionol (B) (modified from (Yang et al., 2007)). (C) Schematic diagram of the transmembrane topology of Slack subunits. Slack-A and Slack-B, two different splice variant of Slack, differ at their N-termini. Both subunits have two RCK domains (Regulators of Conductance of K channels) and bind to FMRP in the C-terminus. (D) Single channel recording from Xenopus oocytes expressing rat Slack-B channels before, during and after (recovery) from application of recombinant FMRP(1-298aa) to the cytoplasmic face of the patch. Addition of FMRP eliminates subconductance states (marked as S) and potently stimulates channel opening (modified from (Brown et al., 2010)).
KNa channels are expressed at the soma and readily detected in isolated patches of excised membrane from the soma of an MNTB neuron (Yang et al., 2007). In addition, KNa channels are also detected on axons as well as in the axon initial segment. Elevations in intracellular sodium significantly increase KNa currents in MNTB neurons (Yang et al., 2007). Thus KNa channels are localized appropriately for modulation by second messenger pathways triggered by synaptic stimulation. Moreover these channels localized near zones where changes in sodium influx via AMPA receptors in the postsynaptic membranes or sodium channels in the axon initial segment could activate KNa channels.
During high rates of synaptic stimulation, increases in intracellular sodium are probably not the sole factor increasing KNa current. PKC regulates both Slack and Slick subunits (Yuan et al., 2003). Native KNa channels in MNTB neurons are likely to represent a heteromer of Slack and Slick subunits (Chen et al., 2009; Yang et al., 2007) although this needs to be tested directly. If KNa channels are indeed heteromers of Slack and Slick in the MNTB, then PKC activity will strongly suppress KNa currents. Conversely KNa current is predicted to increase with activation of phosphatases as occurs during exposure of animals to louder sound environments or high frequency synaptic stimulation (Song et al., 2005).
When KNa channels become activated, the temporal accuracy of firing is improved, as has been found both in current-clamp recordings from MNTB neurons as well as in numerical simulations (Yang et al., 2007). As described earlier for Kv3.1 channels, one can measure the strength of the phase vector for different stimulation rates, for conditions where the amplitude of KNa currents is altered (Fig. 5A). Improved fidelity of locking action potentials to the stimulus is observed when the intracellular solution contains 40mM Na+ over traditional Na+-free internal solutions. This is most evident for those frequencies at which a “dip” in phase vector strength occurs due to timing errors of the sort shown in Figure 2B.
An increase in temporal fidelity can also be achieved by treatment of MNTB neurons with bithionol, a pharmacological activator of Slack channels (Fig. 5B). Bithionol has been shown to produce an increase in current in cells transfected with the Slack gene, even in the absence of any change in internal sodium (Yang et al., 2006). Treatment of MNTB neurons with this agent increases the native KNa current, and also increases the fidelity with which action potentials lock to depolarizing stimuli (Yang et al., 2007). As was described earlier, activity-induced increases in Kv3.1b currents render the cells more capable of firing at high rates, but decrease temporal accuracy. An increase in KNa currents at these high rates, however, serves to improve temporal fidelity.
FMRP regulates Slack potassium channels
One of the unusual characteristics of KNa subunits is the large size of their cytoplasmic C-terminal domains. The transmembrane spanning domains of the Slack channel, which is the largest known potassium channel subunit, comprise only 18% of the entire protein. This raises the possibility that the extended cytoplasmic domains have functions beyond the basic sensing of cytoplasmic sodium concentrations and the control of channel gating. The cytoplasmic domains of many channels interact with cytoplasmic signaling pathways and channel gating can influence cellular signaling as well as vice versa. These types of interactions with signaling pathways have been termed “non-conducting” functions of ion channels (Kaczmarek, 2006). It has been found that the cytoplasmic C-terminal domain of Slack interacts directly with the mRNA-binding protein FMRP (Brown et al., 2010), which, as described above, regulates the translation of Kv3.1 channels, as well as many other neuronal mRNAs. The association of Slack channels with FMRP, which occurs at the distal C-terminus of Slack, results in a potent stimulation of Slack channel activity (Fig. 5C,D).
The level of KNa current in MNTB neurons from Fmr1−/− animals, measured using patch clamp techniques, is significantly reduced compared to that in wild type animals (Brown et al., 2010). Because the level of Slack protein is unchanged in Fmr1−/− animals, this finding is consistent with the loss of the channel-activating protein FMRP. These findings also raise the hypothesis that activation of the Slack channel may serve as a sensor for increased neuronal activity and that its association with FMRP transduces increased neuronal activity into changes in the translation of mRNAs, such as the Kv3.1mRNA. An overview of how loss of FMRP affects the levels and tonotopic distribution of Kv3.1 and Slack channels, and how these are expected to change in different auditory environments is presented in Fig. 6.
Implications of the mechanisms for modulation of the excitability of brainstem neurons
Acoustic training improves the ability of humans and animals to discriminate acoustic information (Mossbridge et al., 2006; Polley et al., 2006) and can lead to the ability to detect minute differences in spatial location of sounds (Munte et al., 2001; Nager et al., 2003). Such abilities require a high degree of temporal accuracy in underlying neuronal circuits. Long-lasting changes in the organization of regions of the cerebral cortex have been observed in animals after training to improve acoustic detection of pitch or timing (Polley et al., 2006). Major computations that lead to these temporal discriminations, however, occur in the auditory brainstem. The work presented in this review has shown that the activity of channels is regulated at several levels to optimize the accuracy with which information is encoded in these circuits. Reversible modulation of channels via phosphorylation occurs within seconds of a change in the auditory environment. Local regulation of protein translation may alter levels of channel proteins within tens of minutes. Changes in synthesis of channel subunits that are brought about by changes in transcription probably occur over a period of hours to days. Each of these mechanisms is likely to respond to both changes in incoming sensory information and to inputs from higher levels, allowing individuals to learn to discriminate novel aspects of the auditory environment.
We have also described work demonstrating that both Kv3 channels and Slack channels are regulated by the FMRP signaling pathway. Loss of FMRP in humans results in the most common genetic form of intellectual disability called Fragile X syndrome. A major feature of Fragile X syndrome in humans and in mice lacking FMRP (Fmr1−/−) is a hypersensitivity to auditory stimuli (Miller et al., 1999). Fragile X syndrome includes a constellation of emotional and intellectual disabilities including autism (Hagerman et al., 2009). Children with autism and Fragile X syndrome have difficulty in temporal processing of complex auditory information and speech disturbances (Hall et al., 2009; Hanson et al., 1986; Russo et al., 2009b; Russo et al., 2008), even though they have normal hearing and normal range of auditory brainstem reflexes (Tharpe et al., 2006). A subset of children with autism spectrum disorders have reduced phase locking and increased frequency errors in measurements of brainstem activity compared to age-matched children with neurotypical development (Russo et al., 2008). Additionally children with autism have trouble processing sounds with background noises (Alcantara et al., 2004; Russo et al., 2009a). It would not be surprising if errors in the regulation of potassium channels were found to contribute to such deficits in decoding complex auditory information.
Abbreviations
- FMRP
Fragile-X Mental Retardation Protein
- AVCN
anteroventral cochlear nucleus
- LSO
lateral superior olive
- MSO
medial superior olive
- MNTB
medial nuclei of the trapezoid body
- Kv
voltage-gated potassium channel family
- PKC
protein kinase C
- CRE
cyclic AMP/calcium response element
- CREB
CRE-binding protein
- KNa
sodium-activated potassium channels
- Slack
KNa channel subunit
- Slick
KNa channel subunit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcantara JI, Weisblatt EJ, Moore BC, Bolton PF. Speech-in-noise perception in high-functioning individuals with autism or Asperger's syndrome. Journal of child psychology and psychiatry, and allied disciplines. 2004;45:1107–14. doi: 10.1111/j.1469-7610.2004.t01-1-00303.x. [DOI] [PubMed] [Google Scholar]
- Banks MI, Smith PH. Intracellular recordings from neurobiotin-labeled cells in brain slices of the rat medial nucleus of the trapezoid body. Journal of Neuroscience. 1992;12:2819–37. doi: 10.1523/JNEUROSCI.12-07-02819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–14. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the Slack potassium channel in the rat central nervous system. Journal of Comparative Neurology. 2002;454:241–54. doi: 10.1002/cne.10439. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, von Hehn CA, Mei X, Kaczmarek LK. Localization of the Na+-activated K+ channel Slick in the rat central nervous system. Journal of Comparative Neurology. 2005;484:80–92. doi: 10.1002/cne.20462. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. Journal of Neuroscience. 2003;23:11681–91. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–4. doi: 10.1038/383431a0. [see comment] [DOI] [PubMed] [Google Scholar]
- Borst JG, Helmchen F, Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. Journal of Physiology. 1995;489:825–40. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–7. doi: 10.1038/417543a. [see comment] [DOI] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. Journal of Neuroscience. 1995;15:8011–22. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Systematic variation of potassium current amplitudes across the tonotopic axis of the rat medial nucleus of the trapezoid body. Hearing Research. 2005;206:116–32. doi: 10.1016/j.heares.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. Journal of Physiology. 2003;548:1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nature neuroscience. 2010;13:819–21. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE. Organization of the cat trapezoid body and the discharge characteristics of its fibers. Brain Research. 1975;94:413–33. doi: 10.1016/0006-8993(75)90226-7. [DOI] [PubMed] [Google Scholar]
- Chen H, Kronengold J, Yan Y, Gazula VR, Brown MR, Ma L, Ferreira G, Yang Y, Bhattacharjee A, Sigworth FJ, Salkoff L, Kaczmarek LK. The N-terminal domain of Slack determines the formation and trafficking of Slick/Slack heteromeric sodium-activated potassium channels. J Neurosci. 2009;29:5654–65. doi: 10.1523/JNEUROSCI.5978-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer KS. Eph proteins and the assembly of auditory circuits. Hearing Research. 2005;206:42–51. doi: 10.1016/j.heares.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–99. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Desai R, Kronengold J, Mei J, Forman SA, Kaczmarek LK. Protein kinase C modulates inactivation of Kv3.3 channels. The Journal of biological chemistry. 2008;283:22283–94. doi: 10.1074/jbc.M801663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. Journal of Neuroscience. 2002;22:6953–61. doi: 10.1523/JNEUROSCI.22-16-06953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Billups B, Rusznak Z, Szucs G, Barker MC, Forsythe ID. Presynaptic rat Kv1.2 channels suppress synaptic terminal hyperexcitability following action potential invasion. Journal of Physiology. 2003;550:27–33. doi: 10.1113/jphysiol.2003.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK. Kv1.3 channel gene-targeted deletion produces "Super-Smeller Mice" with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron. 2004;41:389–404. doi: 10.1016/s0896-6273(03)00844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. Journal of Physiology. 1994;479:381–7. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Kaczmarek LK. When, where, and how much? Expression of the Kv3.1 potassium channel in high-frequency firing neurons. Journal of Neurobiology. 1998;37:69–79. doi: 10.1002/(sici)1097-4695(199810)37:1<69::aid-neu6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gan L, Perney TM, Kaczmarek LK. Cloning and characterization of the promoter for a potassium channel expressed in high frequency firing neurons. Journal of Biological Chemistry. 1996;271:5859–65. doi: 10.1074/jbc.271.10.5859. [DOI] [PubMed] [Google Scholar]
- Gan L, Hahn SJ, Kaczmarek LK. Cell type-specific expression of the Kv3.1 gene is mediated by a negative element in the 5' untranslated region of the Kv3.1 promoter. Journal of Neurochemistry. 1999;73:1350–62. doi: 10.1046/j.1471-4159.1999.0731350.x. [DOI] [PubMed] [Google Scholar]
- Gazula VR, Strumbos JG, Mei X, Chen H, Rahner C, Kaczmarek LK. Localization of Kv1.3 channels in presynaptic terminals of brainstem auditory neurons. The Journal of comparative neurology. 2010;518:3205–20. doi: 10.1002/cne.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg JJ, Brew HM, Tempel BL. Differential expression of voltage-gated potassium channel genes in auditory nuclei of the mouse brainstem. Hearing Research. 2000;140:77–90. doi: 10.1016/s0378-5955(99)00187-2. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–90. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Walter E, Sherman E, Hoeft F, Reiss AL. The neural basis of auditory temporal discrimination in girls with fragile X syndrome. Journal of neurodevelopmental disorders. 2009;1:91–99. doi: 10.1007/s11689-009-9007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson DM, Jackson AW, 3rd, Hagerman RJ. Speech disturbances (cluttering) in mildly impaired males with the Martin-Bell/fragile X syndrome. American journal of medical genetics. 1986;23:195–206. doi: 10.1002/ajmg.1320230114. [DOI] [PubMed] [Google Scholar]
- Hardman RM, Forsythe ID. Ether-a-go-go-related gene K+ channels contribute to threshold excitability of mouse auditory brainstem neurons. The Journal of physiology. 2009;587:2487–97. doi: 10.1113/jphysiol.2009.170548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3. Sinauer; Sunderland, Mass: 2001. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology. 1952;117:500–44. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J, Griffin SJ, Baker C, Skrzypiec A, Chernova T, Forsythe ID. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons. The Journal of physiology. 2008;586:3493–509. doi: 10.1113/jphysiol.2008.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Tang MD, Wang LY, Dworetzky SI, Boissard CG, Gan L, Gribkoff VK, Kaczmarek LK. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nature neuroscience. 1998;1:462–9. doi: 10.1038/2176. [DOI] [PubMed] [Google Scholar]
- Joris PX. Envelope coding in the lateral superior olive. II. Characteristic delays and comparison with responses in the medial superior olive. Journal of Neurophysiology. 1996;76:2137–56. doi: 10.1152/jn.1996.76.4.2137. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences. Journal of Neurophysiology. 1995;73:1043–62. doi: 10.1152/jn.1995.73.3.1043. [erratum appears in J Neurophysiol 1995 Jun;73(6):followi] [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK. Non-conducting functions of voltage-gated ion channels. Nature reviews. 2006;7:761–71. doi: 10.1038/nrn1988. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK. Controlling auditory excitability: the benefits of a cultured environment. The Journal of physiology. 2010;588:1387–8. doi: 10.1113/jphysiol.2010.189712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK, Bhattacharjee A, Desai R, Gan L, Song P, von Hehn CA, Whim MD, Yang B. Regulation of the timing of MNTB neurons by short-term and long-term modulation of potassium channels. Hearing Research. 2005;206:133–45. doi: 10.1016/j.heares.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Kanemasa T, Gan L, Perney TM, Wang LY, Kaczmarek LK. Electrophysiological and pharmacological characterization of a mammalian Shaw channel expressed in NIH 3T3 fibroblasts. Journal of Neurophysiology. 1995;74:207–17. doi: 10.1152/jn.1995.74.1.207. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Lippe WR, Dorrscheidt GJ, Rubsamen R. The medial nucleus of the trapezoid body in the gerbil is more than a relay: comparison of pre- and postsynaptic activity. Jaro. 2003a;4:1–23. doi: 10.1007/s10162-002-2010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rubsamen R. Decreased temporal precision of auditory signaling in Kcna1-null mice: an electrophysiological study in vivo. Journal of Neuroscience. 2003b;23:9199–207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Human molecular genetics. 2001;10:329–38. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Leao KE, Leao RN, Deardorff AS, Garrett A, Fyffe R, Walmsley B. Sound stimulation modulates high-threshold K(+) currents in mouse auditory brainstem neurons. Eur J Neurosci. 2010;32:1658–67. doi: 10.1111/j.1460-9568.2010.07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Sun H, Svahn K, Berntson A, Youssoufian M, Paolini AG, Fyffe RE, Walmsley B. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. The Journal of physiology. 2006;571:563–78. doi: 10.1113/jphysiol.2005.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kaczmarek LK, Perney TM. Localization of two high-threshold potassium channel subunits in the rat central auditory system. Journal of Comparative Neurology. 2001a;437:196–218. doi: 10.1002/cne.1279. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic acids research. 2001b;29:2276–83. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Kaczmarek LK. The expression of two splice variants of the Kv3.1 potassium channel gene is regulated by different signaling pathways. Journal of Neuroscience. 1998a;18:2881–90. doi: 10.1523/JNEUROSCI.18-08-02881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Kaczmarek LK. Depolarization selectively increases the expression of the Kv3.1 potassium channel in developing inferior colliculus neurons. Journal of Neuroscience. 1998b;18:8758–69. doi: 10.1523/JNEUROSCI.18-21-08758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macica CM, Kaczmarek LK. Casein kinase 2 determines the voltage dependence of the Kv3.1 channel in auditory neurons and transfected cells. Journal of Neuroscience. 2001;21:1160–8. doi: 10.1523/JNEUROSCI.21-04-01160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macica CM, von Hehn CA, Wang LY, Ho CS, Yokoyama S, Joho RH, Kaczmarek LK. Modulation of the kv3.1b potassium channel isoform adjusts the fidelity of the firing pattern of auditory neurons. Journal of Neuroscience. 2003;23:1133–41. doi: 10.1523/JNEUROSCI.23-04-01133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB, Marx SO. Outward currents in isolated ventral cochlear nucleus neurons. Journal of Neuroscience. 1991;11:2865–80. doi: 10.1523/JNEUROSCI.11-09-02865.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. Journal of Neuroscience. 1998;18:8111–25. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massengill JL, Smith MA, Son DI, O'Dowd DK. Differential expression of K4-AP currents and Kv3.1 potassium channel transcripts in cortical neurons that develop distinct firing phenotypes. Journal of Neuroscience. 1997;17:3136–47. doi: 10.1523/JNEUROSCI.17-09-03136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Differential expression of Kv3.1b and Kv3.2 potassium channel subunits in interneurons of the basolateral amygdala. Neuroscience. 2006;138:537–47. doi: 10.1016/j.neuroscience.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. American journal of medical genetics. 1999;83:268–79. [PubMed] [Google Scholar]
- Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J Neurosci. 1983;3:237–42. doi: 10.1523/JNEUROSCI.03-01-00237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morest DK. The collateral system of the medial nucleus of the trapezoid body of the cat, its neuronal architecture and relation to the olivo-cochlear bundle. Brain Research. 1968;9:288–311. doi: 10.1016/0006-8993(68)90235-7. [DOI] [PubMed] [Google Scholar]
- Mossbridge JA, Fitzgerald MB, O'Connor ES, Wright BA. Perceptual-learning evidence for separate processing of asynchrony and order tasks. Journal of Neuroscience. 2006;26:12708–16. doi: 10.1523/JNEUROSCI.2254-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munte TF, Kohlmetz C, Nager W, Altenmuller E. Neuroperception. Superior auditory spatial tuning in conductors. Nature. 2001;409:580. doi: 10.1038/35054668. [DOI] [PubMed] [Google Scholar]
- Nager W, Kohlmetz C, Altenmuller E, Rodriguez-Fornells A, Munte TF. The fate of sounds in conductors' brains: an ERP study. Cognitive Brain Research. 2003;17:83–93. doi: 10.1016/s0926-6410(03)00083-1. [DOI] [PubMed] [Google Scholar]
- Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annual Review of Physiology. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- Perney TM, Kaczmarek LK. Localization of a high threshold potassium channel in the rat cochlear nucleus. Journal of Comparative Neurology. 1997;386:178–202. [PubMed] [Google Scholar]
- Perney TM, Marshall J, Martin KA, Hockfield S, Kaczmarek LK. Expression of the mRNAs for the Kv3.1 potassium channel gene in the adult and developing rat brain. Journal of Neurophysiology. 1992;68:756–66. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–67. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. Journal of Neuroscience. 2006;26:4970–82. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathouz M, Trussell L. Characterization of outward currents in neurons of the avian nucleus magnocellularis. Journal of Neurophysiology. 1998;80:2824–35. doi: 10.1152/jn.1998.80.6.2824. [DOI] [PubMed] [Google Scholar]
- Russo N, Zecker S, Trommer B, Chen J, Kraus N. Effects of background noise on cortical encoding of speech in autism spectrum disorders. Journal of autism and developmental disorders. 2009a;39:1185–96. doi: 10.1007/s10803-009-0737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Nicol T, Trommer B, Zecker S, Kraus N. Brainstem transcription of speech is disrupted in children with autism spectrum disorders. Developmental science. 2009b;12:557–67. doi: 10.1111/j.1467-7687.2008.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, Kraus N. Deficient brainstem encoding of pitch in children with Autism Spectrum Disorders. Clin Neurophysiol. 2008;119:1720–31. doi: 10.1016/j.clinph.2008.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell and tissue research. 2006;326:311–37. doi: 10.1007/s00441-006-0272-7. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. Journal of Neurophysiology. 1998;79:3127–42. doi: 10.1152/jn.1998.79.6.3127. [DOI] [PubMed] [Google Scholar]
- Song P, Kaczmarek LK. Modulation of Kv3.1b potassium channel phosphorylation in auditory neurons by conventional and novel protein kinase C isozymes. Journal of Biological Chemistry. 2006;281:15582–91. doi: 10.1074/jbc.M512866200. [DOI] [PubMed] [Google Scholar]
- Song P, Yang Y, Barnes-Davies M, Bhattacharjee A, Hamann M, Forsythe ID, Oliver DL, Kaczmarek LK. Acoustic environment determines phosphorylation state of the Kv3.1 potassium channel in auditory neurons. Nature neuroscience. 2005;8:1335–42. doi: 10.1038/nn1533. [DOI] [PubMed] [Google Scholar]
- Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RA, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID. Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron. 2008;60:642–56. doi: 10.1016/j.neuron.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Strumbos JG, Polley DB, Kaczmarek LK. Specific and rapid effects of acoustic stimulation on the tonotopic distribution of Kv3.1b potassium channels in the adult rat. Neuroscience. 2010a;167:567–72. doi: 10.1016/j.neuroscience.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumbos JG, Brown MR, Kronengold J, Polley DB, Kaczmarek LK. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J Neurosci. 2010b;30:10263–71. doi: 10.1523/JNEUROSCI.1125-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, Luneau CJ, Antanavage J, Oliva C, Buhrow SA, et al. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990;4:929–39. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. Journal of Neuroscience. 2000;20:9162–73. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–43. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Tharpe AM, Bess FH, Sladen DP, Schissel H, Couch S, Schery T. Auditory characteristics of children with autism. Ear and hearing. 2006;27:430–41. doi: 10.1097/01.aud.0000224981.60575.d8. [DOI] [PubMed] [Google Scholar]
- Tong H, Steinert JR, Robinson SW, Chernova T, Read DJ, Oliver DL, Forsythe ID. Regulation of Kv channel expression and neuronal excitability in rat medial nucleus of the trapezoid body maintained in organotypic culture. The Journal of physiology. 2010;588:1451–68. doi: 10.1113/jphysiol.2009.186676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annual Review of Physiology. 1999;61:477–96. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- von Hehn CA, Bhattacharjee A, Kaczmarek LK. Loss of Kv3.1 tonotopicity and alterations in cAMP response element-binding protein signaling in central auditory neurons of hearing impaired mice. Journal of Neuroscience. 2004;24:1936–40. doi: 10.1523/JNEUROSCI.4554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998a;394:384–8. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. Journal of Physiology. 1998b;509:183–94. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Gan L, Perney TM, Schwartz I, Kaczmarek LK. Activation of Kv3.1 channels in neuronal spine-like structures may induce local potassium ion depletion. Proceedings of the National Academy of Sciences of the United States of America. 1998c;95:1882–7. doi: 10.1073/pnas.95.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Bueno E, Sekirnjak C, Martone ME, Baker H, Hillman D, Chen S, Thornhill W, Ellisman M, Rudy B. The potassium channel subunit KV3.1b is localized to somatic and axonal membranes of specific populations of CNS neurons. Journal of Neuroscience. 1995;15:4298–314. doi: 10.1523/JNEUROSCI.15-06-04298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Response of neurons in the lateral superior olive and medial nucleus of the trapezoid body to repetitive stimulation: intracellular and extracellular recordings from mouse brain slice. Hearing Research. 1993;68:189–201. doi: 10.1016/0378-5955(93)90123-i. [DOI] [PubMed] [Google Scholar]
- Yang B, Desai R, Kaczmarek LK. Slack and Slick K(Na) channels regulate the accuracy of timing of auditory neurons. J Neurosci. 2007;27:2617–27. doi: 10.1523/JNEUROSCI.5308-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Gribkoff VK, Pan J, Damagnez V, Dworetzky SI, Boissard CG, Bhattacharjee A, Yan Y, Sigworth FJ, Kaczmarek LK. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology. 2006;51:896–906. doi: 10.1016/j.neuropharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron. 2003;37:765–73. doi: 10.1016/s0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Richter JD, Bagni C. Signals, synapses, and synthesis: how new proteins control plasticity. Frontiers in neural circuits. 2009;3:14. doi: 10.3389/neuro.04.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]