SUMMARY
In adult mammalian brains, neurogenesis persists in the subventricular zone of the lateral ventricles (SVZ) and the dentate gyrus (DG) of the hippocampus. Although evidence suggest that adult neurogenesis in these two regions is subjected to differential regulation, the underlying mechanism is unclear. Here we show that the RNA-binding protein FXR2 specifically regulates DG neurogenesis by reducing the stability of Noggin mRNA. FXR2 deficiency leads to increased Noggin expression and subsequently reduced BMP signaling, which results in increased proliferation and altered fate specification of neural stem/progenitor cells in DG. In contrast, Noggin is not regulated by FXR2 in the SVZ, because Noggin expression is restricted to the ependymal cells of the lateral ventricles, where FXR2 is not expressed. Differential regulation of SVZ and DG stem cells by FXR2 may be a key component of the mechanism that governs the different neurogenic processes in these two adult germinal zones.
INTRODUCTION
Adult mammalian brains have two neurogenic regions: the subgranular zone of the dentate gyrus (DG) of the hippocampus, which generates excitatory glutamatergic granule neurons in the DG, and the subventricular zone (SVZ) of the lateral ventricles, which produces inhibitory GABAergic and dopaminergic interneurons of the olfactory bulb (Lledo et al., 2006; Ming and Song, 2005; Mu et al., 2010). Since the discovery of adult neurogenesis, DG and SVZ neurogenesis have been known to respond differently to neurotrophic factors treatment and physiological and pathological conditions (Li and Zhao, 2008; Zhao et al., 2008). For example, environmental enrichment and physical activity boost neurogenesis in the DG, but not in the SVZ (Brown et al., 2003; Kempermann et al., 1997; Nilsson et al., 1999). In addition, cranial irradiation represses cell proliferation in both the SVZ and DG, but the DG suffers long-term effects, whereas the SVZ recovers with time (Hellstrom et al., 2009)
Although multipotent neural stem/progenitor cells (NPCs) exist widely in adult brains, neurogenesis is known to be restricted by the local stem cell niche (Goldman, 2004; Mu et al., 2010; Zhao et al., 2008). However, recent literature suggests that NPCs residing in different regions of the brain may be intrinsically programmed to differentiate into restricted types of neurons (Merkle et al., 2007). NPCs derived from the adult SVZ (SVZ-NPCs) are shown to have better self-renewal capability than NPCs derived from the adult DG (DG-NPCs) (Bull and Bartlett, 2005; Seaberg and van der Kooy, 2002), which could be due to their intrinsic differences in BMP signaling (Bonaguidi et al., 2008). Nonetheless, despite these observations, the precise molecular mechanism underlying the differential regulation of SVZ and DG neurogenesis is still largely a mystery.
Fragile X relative protein 2 (FXR2) belongs to a family of fragile X mental retardation proteins (FMRP, FXR1, and FXR2), which can bind to RNA and associate with polyribosomes (Darnell et al., 2009). These proteins share high sequence similarity in certain functional domains, but diverge in the C-termini and in the nucleolar localization signal sequence, suggesting that they may possess both overlapping and distinct functions (Coffee et al., 2010; Kirkpatrick et al., 2001). FMRP and FXR2 are highly enriched in mammalian brains (Agulhon et al., 1999; Bakker et al., 2000). Although only FMRP deficiency has been linked to human fragile X syndrome, both FMRP and FXR2 mutant (KO) mice exhibit fragile X-like behavioral deficits, including hippocampus-dependent learning impairment. Furthermore, FXR2 and FMRP double mutant mice display exaggerated learning deficits compared with single mutant mice (Bontekoe et al., 2002; Brennan et al., 2006; Hayashi et al., 2007; Spencer et al., 2006; Zhao et al., 2005). Since FMRP deficiency affects adult hippocampal neurogenesis (Guo et al., 2011; Luo et al., 2010), the behavioral phenotype of Fxr2 KO mice suggests that FXR2 may also regulate adult neurogenesis. Interestingly, Fxr2 KO mice display learning impairments and changes in synaptic plasticity that are somewhat distinct from those of FMRP-deficient mice (Spencer et al., 2006; Zhang et al., 2009), indicating that FXR2 may also regulate adult hippocampal neurogenesis via mechanisms distinct from FMRP (Luo et al., 2010). Until now, the role of FXR2 in the adult brain has not been well studied, and its role in adult neurogenesis remains unexplored.
Here we show that FXR2 deficiency leads to altered stem cell proliferation and differentiation specifically in the DG and not in the SVZ. We find that in DG-NPCs, FXR2 represses the expression of Noggin, an antagonist of BMP signaling. Either reducing the action of Noggin or enhancing BMP signaling rescues the stem cell phenotypes resulting from FXR2 deficiency. In the SVZ, however, Noggin expression is restricted to ependymal cells, where FXR2 is not expressed; therefore, Noggin expression is not regulated by FXR2. Our study reveals a novel regulatory mechanism of adult hippocampal neurogenesis by the brain-enriched RNA-binding protein FXR2. The differential regulation of SVZ and DG stem cells by FXR2 may be a key component of the mechanism governing the differential neurogenic processes in these two adult germinal zones.
RESULTS
FXR2 Deficiency Affects NPC Proliferation and Differentiation in the DG but Not in the SVZ
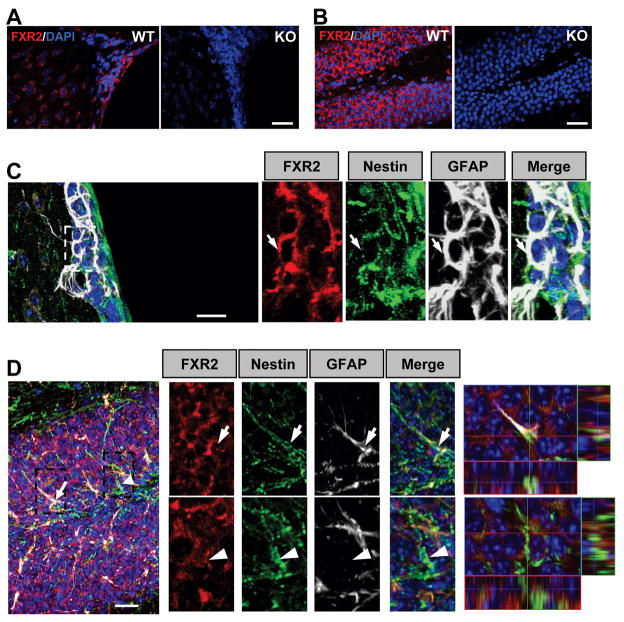
To investigate the role of FXR2 in adult neural stem cells, we first demonstrated that FXR2 is indeed expressed in the two adult germinal zones, SVZ (Figure 1A) and the DG (Figure 1B). We detected FXR2 expression in most of the granule neurons in the DG with no compensatory increased expression of FMRP in Fxr2 KO mice (Figure S1A), which is consistent with the literature (Bakker et al., 2000; Bontekoe et al., 2002). In both the SVZ and DG, FXR2 was localized in Nestin-positive (Nestin+) NPCs, Nestin and GFAP double-positive (Nestin+GFAP+) radial glia-like NPCs, and doublecortin-positive (DCX+) immature neurons (Figure 1C and 1D; Figure S1B), but not in either astrocytes or oligodendrocytes (Figure S1C–S1E).
Figure 1. FXR2 is Expressed in the NPCs of the Adult DG and SVZ.
(A–B) FXR2 is expressed in the SVZ (A) and the DG (B). Red, FXR2; Blue, DAPI. Scale bars = 50 μm.
(C–D) FXR2 (red) is expressed in GFAP (white) and Nestin (green) double-positive NPCs in the SVZ (C) and the DG (D), as well as in a large number of granule neurons (B and D). Arrows in C and D point to FXR2+Nestin+GFAP+ cells. Arrowhead in C points to FXR2+Nestin+GFAP- cells. Scale bar = 20 μm. The white dotted boxes indicate the regions with high-magnification pictures shown below (arrow: GFAP and Nestin double-positive stem cells). (Also see Figure S1)
To explore the function of FXR2 in adult neurogenesis, we assessed the proliferation and differentiation of NPCs in Fxr2 KO mice and wild-type (WT) controls using a saturation BrdU pulse-labeling method that could label the entire pool of proliferating NPCs within a 12 hour period (Figure 2A) (Hayes and Nowakowski, 2002; Luo et al., 2010). Quantitative analysis at 12 hours following the last BrdU injection showed that, in the DG of the hippocampus, Fxr2 KO mice had ~20% more BrdU+ cells compared with WT littermates (Figure 2B and 2C, n=6, p<0.05). Nestin+ immature cells in the DG are known to contain at least two populations: Nestin+GFAP+ radial glia-like cells (also called type 1, Figure 2D) and Nestin+GFAP− nonradial glia-like cells (also called type 2a, Figure 2G). Both types can incorporate BrdU (Ables et al., 2010; Kempermann et al., 2004; Ming and Song, 2005). In the Fxr2 KO DG, both total Nestin+ cells (n = 6, p < 0.001) and Nestin+GFAP+ radial glia-like NPCs (Figure 2E and 2F, n = 6, p < 0.001) exhibited increased BrdU incorporation, whereas Nestin+GFAP− nonradial glia-like NPCs did not (Figure 2H, n = 6, p = 0.7313). The volume (size) of the DG did not differ between WT and Fxr2 KO mice (data not shown). These results indicate that FXR2 deficiency leads to increased proliferation of radial glia-like NPCs in the adult DG.
Figure 2. FXR2 Deficiency Affects Stem Cell Proliferation and Differentiation in the Adult DG but Not in the SVZ.
(A) Experimental scheme for assessing neural stem cell proliferation and early stage new cell survival and differentiation in adult mice.
(B) Examples of WT and Fxr2 KO brain sections stained with an antibody against BrdU (red) and DAPI (blue) for in vivo hippocampal cell proliferation and differentiation analyses. Scale bars = 50 μm.
(C) Quantification of the number of BrdU+ cells in the DG normalized to volume of the DG. Fxr2 KO mice showed increased BrdU incorporation indicating proliferation analyzed at 12 hours after the last BrdU injection compared with WT mice (p < 0.05, n = 6). There were still more BrdU+ cells in the DG of Fxr2 KO mice when analyzed at one week post-BrdU injection (p < 0.05, n = 5).
(D) Example of a Nestin+ (green), GFAP+ (blue), and BrdU+ (red) cell used for quantification in E and F. This cell is localized in a brain section analyzed at 12 hours post-BrdU injection.
(E–F) The DG of Fxr2 KO mice exhibited increased proliferation (E) and the total number (F) of radial glia-like NPCs (BrdU+Nestin+GFAP+) analyzed at 12 hours after the last BrdU injection (n = 6 WT; n = 6 KO).
(G) Example of a Nestin+ (green), GFAP− (blue), and BrdU+ (red) cell used for quantification in H at 12 hours post-BrdU injection.
(H) The DG of Fxr2 KO mice exhibited no change in nonradial glia-like NPCs BrdU+Nestin+GFAP−) analyzed at 12 hours after the last BrdU injection (n = 6 WT; n = 6 KO).
(I) Examples of brain sections stained with antibodies against immature neuronal marker DCX (green) and BrdU (red) at one week post-BrdU injection to assess early stage neuronal differentiation. Scale bars = 20 μm.
(J) A higher percentage of BrdU+ cells in the DG of Fxr2 KO mice were DCX+, suggesting increased neuronal differentiation compared with WT mice at one week post-BrdU injection (n = 6, p < 0.01).
(K) Examples of WT and Fxr2 KO brain sections stained with an antibody against BrdU (red) for in vivo SVZ cell proliferation and differentiation analyses at 12 hours post-BrdU injection. Scale bars = 20 μm.
(L) Brain regions containing the SVZ were labeled with antibodies against Nestin (green), GFAP (white), and BrdU (red) at 12 hours post-BrdU injection. Scale bars = 20 μm.
(M) Brain sections containing the rostral migratory stream (RMS) were stained with antibodies against DCX (green) and BrdU (red) at one week post-BrdU injection. Data are presented as mean ± SEM; *, p < 0.05, **, p < 0.01, Student’s t-test. Scale bars = 20 μm.
We next assessed the fate of new cells in the DG at one week post BrdU injection. We found that FXR2-deficient mice still had ~25% more BrdU+ cells (Figure 2B and 2C, n = 5, p < 0.05), and the survival rate of BrdU+ cells from 12 hours to one week post-BrdU injection was no different between WT and Fxr2 KO mice (n = 6, p = 0.99). On the other hand, BrdU+ cells in the Fxr2 KO DG differentiated into more DCX+ neurons compared with WT mice (Figure 2I and 2J, n = 6, p < 0.001). Therefore, FXR2 deficiency leads to enhanced proliferation and neuronal differentiation of NPCs in the DG, without affecting the short-term survival of new cells.
We then assessed neurogenesis in the SVZ of adult Fxr2 KO mice. To our surprise, Fxr2 KO mice showed no significant differences in BrdU incorporation (Figures 2K, n = 5, p = 0.525) and the proliferation of either Nestin+GFAP+ cells (Figures 2L, n = 6, p = 0.6472) or Nestin+GFAP− cells (n = 6, p = 0.8538) compared to WT mice. Furthermore, at one week after BrdU injection, the percentage of DCX+ neuroblasts among BrdU+ cells in the rostral migratory stream (RMS, Figure 2M) was essentially the same for WT and Fxr2 KO mice (n = 5, p = 0.8871). Taken together, these results suggest that the loss of FXR2 specifically alters neurogenesis in the adult DG, but not in the adult SVZ.
FXR2 Regulates the Proliferation and Differentiation of NPCs Derived from the Adult DG, but Not Those from the SVZ
To determine whether the different phenotypes in the DG and SVZ in the Fxr2 KO mice were due to intrinsic differences in NPCs, we isolated NPCs from both regions (Figures 3A and 3J). Early passage primary NPCs isolated from both DG and SVZ were positive for the progenitor markers Nestin and Sox2 (Figure 3B) and expressed FXR2 (Figure S2A and S2B). In fact, 96.7±0.84 % of total cultured NPCs and 98.8±0.82 % of Nestin+Sox2+ NPCs expressed FXR2 (Figure S2C).
Figure 3. Loss of FXR2 Leads to Increased Proliferation of DG-NPCs but Not SVZ-NPCs.
(A) Schematic drawing showing the isolation of DG-NPCs. Special care was taken to avoid any contamination from lateral ventricles.
(B) Both WT and KO DG-NPCs incorporated BrdU (white) during a 16-hour BrdU pulse labeling under proliferating conditions and expressed neural progenitor markers Nestin (Green) and Sox2 (Red). Scale bars = 20 μm.
(C, D) Quantitative analysis showing that Fxr2 KO DG-NPCs, particularly those Nestin and Sox2 double-positive cells (D), incorporated more BrdU compared with WT controls (N = 3).
(E) Sample images of DG-NPC neurospheres isolated from Fxr2 KO and WT mice. Scale bars = 100 μm.
(F, G) Fxr2 KO DG yielded more primary neurospheres (F) with larger sizes (G) than WT DG (n = 3).
(H, I) The self-renewal ability of neurospheres isolated from WT and KO adult DG was assessed by secondary and tertiary neurosphere formation. Fxr2 KO DG-NPCs had increased self-renewal ability, as demonstrated by the increased of number (H) and size (I) of neurospheres compared with WT (n = 3).
(J) Schematic drawing showing the isolation of adult SVZ-NPCs.
Data are presented as mean ± SEM; *, p < 0.05, **, p < 0.01, Student’s t-test. (Also see Figure S2 and Figure S3)
We found that Fxr2 KO DG-NPCs exhibited significantly higher BrdU incorporation compared with WT cells, particularly in the Sox2/Nestin double-positive populations (Figure 3C and 3D, n = 3, p < 0.05). In addition, DG-NPCs isolated from Fxr2 KO brains yielded ~25% more primary neurospheres that were ~40% larger (in diameter) than WT controls (Figure 3E–3G, n = 3, p < 0.001). To determine the self-renewal capability of these neurospheres, primary spheres were individually dissociated into single cells and plated at clonal density. Fxr2 KO DG-NPCs yielded ~40% more secondary and tertiary spheres with ~30% increased size compared to WT cells (Figure 3H and 3I, n = 3, p < 0.001). These results indicate that FXR2 deficiency leads to increased proliferation and self-renewal of DG-NPCs. However, SVZ-NPCs derived from WT and Fxr2 KO mice (Figure 3J) had the same BrdU incorporation rate (n = 3, p = 0.8268) and displayed the same primary neurosphere formation as well as similar self-renewal abilities (n = 3, p > 0.05; Figure S2d–S2F). Therefore, FXR2 deficiency does not affect the self-renewal of SVZ-NPCs.
Consistent with our in vivo findings, Fxr2 KO DG-NPCs exhibited a ~30% increase in neuronal differentiation (Figure 4A and 4B, n = 3, p < 0.001) and a ~60% decrease in astrocyte differentiation (Figure 4D and 4E, n = 3, p < 0.001) compared with WT controls. The reduction in astrocyte differentiation was not a result of increased death of GFAP+ astrocytes (Figures S2G and S2H). To validate our immunocytochemical data, we assessed differentiation of NPCs by measuring the promoter activity of a pan-neuronal transcription factor, Neurogenic differentiation 1 (NeuroD1) and the promoter activity of astrocyte GFAP (Liu et al., 2010; Luo et al., 2010). In Fxr2 KO DG-NPCs, NeuroD1 promoter activity increased by ~30% (Figure 4C, n = 3, p < 0.05), while GFAP promoter activity decreased by ~70% (Figure 4F, n = 3, p < 0.001). On the other hand, SVZ-NPCs derived from Fxr2 KO mice showed no significant difference in either neuronal or astrocyte differentiation compared with WT cells (n = 3, p >0.5). Next, we found that expressing exogenous FXR2 in Fxr2 KO DG-NPCs rescued the proliferation (Figure 4G, n = 3, p < 0.05), neuronal differentiation (Figure 4H, n = 3, p < 0.05), and astrocyte differentiation (Figure 4I, n = 3, p < 0.05) deficits of Fxr2 KO DG-NPCs. Therefore, FXR2 regulation of DG-NPCs is likely intrinsic to the NPCs.
Figure 4. Loss of FXR2 Leads to Increased Neuronal but Decreased Astrocyte Differentiation of DG-NPCs but Not of SVZ-NPCs.
(A–C) Fxr2 KO DG-NPCs differentiated into more neurons as demonstrated by immunostaining cells using neuronal marker Tuj1+ (A red) followed by quantitative analysis of Tuj1+ cells (B), as well as transfected NeuroD1 promoter activity (C). A cotransfected Renilla luciferase (R-Luc) plasmid was used as a transfection control (n = 3). Scale bars = 20 μm.
(D–F) Fxr2 KO NPCs from the DG differentiated into fewer GFAP+ astrocytes as shown by a reduced percentage of GFAP+ cells (D, E) and reduced GFAP promoter activity (F) (n = 3). Scale bars = 20 μm.
(G–I) Introducing exogenous FXR2 into Fxr2 KO DG-NPCs corrected the proliferation (G), neuronal differentiation (H), and astrocyte differentiation (I) deficits of Fxr2 KO NPCs to the WT levels. (n = 3)
Data are presented as mean ± SEM; *, p < 0.05, **, p < 0.01, Student’s t-test. (Also see Figure S2).
Even though Fxr2 KO mice exhibit no obvious deficits during embryonic development (Bontekoe et al., 2002), FXR2 deficiency may nonetheless have a developmental impact on adult NPCs. We therefore acutely knocked down FXR2 in adult DG-NPCs using lentivirus expressing a small inhibitory RNA against FXR2 (shFXR2, Figure S3A), which resulted in increased proliferation, increased neuronal differentiation, and decreased astrocyte differentiation (Figure S3B–S3D, n = 3, p < 0.05). We then acutely deleted FXR2 in NPCs in the DG of the adult WT mice using retrovirus that only infected dividing cells (Liu et al., 2010; Smrt et al., 2010) (Figure S3E–S3H). Viral infection resulted in increased proliferation (Figure S3I–S3M) and increased neuronal differentiation (Figure S3N). Therefore, acute knockdown of FXR2 in adult NPCs results in phenotypes similar to those we observed in Fxr2 KO NPCs, both in vitro and in vivo. Taken together, our results provide further evidence that FXR2 plays a role in regulating the proliferation and differentiation of NPCs specifically in the adult DG.
FXR2 Represses Noggin Protein Expression in DG-NPCs
To determine how FXR2 regulates NPCs in the DG, we first used real-time PCR-based neural stem cell pathway arrays to identify genes that exhibited altered expression levels in Fxr2 KO DG-NPCs relative to WT cells (Figure S4A). Among the genes with >2-fold changes in Fxr2 KO DG-NPCs (Figure S4B), we selected Shh (sonic hedgehog), Notch2 (Notch gene homolog 2), Sox3 (SRY-box containing gene 3), and Noggin for further analyses, due to their well-known functions in NPCs (Lim et al., 2000; Ninkovic and Gotz, 2007; Palma et al., 2005; Solecki et al., 2001; Wang et al., 2006). The upregulation of Noggin in Fxr2 KO DG-NPCs was particularly interesting, because Noggin has been shown to promote the self-renewal of DG-NPCs, but not SVZ-NPCs (Bonaguidi et al., 2008).
FXR2 is known to bind mRNAs and regulate protein translation (Darnell et al., 2009; Kirkpatrick et al., 2001). Using immunoprecipitation of FXR2 and its bound RNAs (RNA-IP), we confirmed that FXR2 bound to Noggin mRNA (Figure 5A and 5B), but not to Shh, Notch2, or Sox3 mRNAs (Figure S4C). In addition, biotin-labeled synthetic Noggin mRNA indeed bound FXR2 protein in NSC protein lysate, whereas an antisense control RNA did not (Figure 5C). Furthermore, on separately isolated DG-NPCs, we confirmed that Noggin mRNA levels were elevated in the Fxr2 KO DG-NPCs (Figure 5D). The increased Noggin mRNA levels could be due to either increased gene transcription or increased mRNA stability. We treated WT and KO NPCs with actinomycin D to inhibit gene transcription and found that Noggin mRNA had a longer half-life in Fxr2 KO DG-NPCs than in WT cells (Figure 5E, n = 3), while the half-life of Notch2, Shh, and Sox3 mRNA showed no significant difference (Figure S4D–S4F). We then manipulated FXR2 levels in WT and Fxr2 KO DG-NPCs and found that acute knockdown of FXR2 in WT NPCs resulted in a longer half-life of Noggin mRNA, while exogenous FXR2 reduced the Noggin mRNA half-life in Fxr2 KO DG-NPCs (Figure 5E). Therefore FXR2 expression levels directly affect the stability of Noggin mRNA in DG-NPCs. Finally, we confirmed that primary DG-NPCs express both FXR2 and Noggin protein (Figure S5A), and that the level of Noggin protein was significantly higher in both the cell lysate and the conditioned medium of Fxr2 KO DG-NPCs compared with WT cells (Figure 5F and 5G). Therefore, FXR2 represses Noggin protein expression in DG-NPCs by decreasing the half-life of Noggin mRNA.
Figure 5. FXR2 Regulates Noggin Expression in DG-NPCs.
(A) Western blot analysis showing the presence of FXR2 in both the input and the antibody immunoprecipitated FXR2-containing mRNA complexes (RNA-IP) from both WT and Fxr2 KO DG-NPCs.
(B) RT-PCR analysis of input and FXR2-IP RNAs indicated that FXR2 could bind to Noggin mRNA in WT DG-NPCs. GAPDH mRNA was used as a control.
(C) Biotin-labeled synthetic Noggin mRNA (S), but not a control antisense RNA (AS) pulled down FXR2 protein from DG-NPC lysates. Western blot analysis showing the presence of FXR2 in both the input and Noggin sense mRNA pull down conditions.
(D) DG-NPCs derived from Fxr2 KO mice had elevated Noggin mRNA levels as assessed by real-time PCR analysis (p < 0.05; n = 3).
(E) DG-NPCs were treated with actinomycin D to inhibit gene transcription, and the percentage of Noggin mRNA in NPCs was quantified using real-time PCR at the indicated time point (n = 3). FXR2 deficiency (Fxr2 KO or WT+shFXR2) resulted in increased Noggin mRNA stability, whereas elevated FXR2 expression (WT+FXR2 or KO +FXR2) led to decreased Noggin mRNA stability.
(F) Western blot analysis and quantification showing that the Noggin protein level was increased in Fxr2 KO DG-NPCs compared with WT cells (n = 3, p < 0.01).
(G) Conditioned medium from Fxr2 KO DG-NPCs displayed significantly elevated Noggin levels determined using an ELSA assay (n = 3. P < 0.05).
(H) Schematic drawing of Noggin and BMP signaling pathway in DG-NPCs.
(I) The levels of p-Smad1/5 (ratio of p-Smad1/5 over total Smad1/5), an indicator of BMP signaling, were decreased in Fxr2 KO DG-NPCs compared with WT cells (n = 3, p < 0.01). (Also see Figure S4)
(J, K) Exogenous FXR2 rescued the secreted Noggin protein levels (J) as well as Smad1/5 phosphorylation (K).
(L,M) Acute knockdown of FXR2 in WT DG-NPCs led to increased secreted Noggin protein levels (L) and decreased Smad1/5 phosphorylation (M).
Noggin inhibits BMP signaling by preventing BMP from interacting with their receptors (Figure 5H) (Klingensmith et al., 2010; Rosen, 2006). Accordingly, we assessed the activity of the BMP signaling in Fxr2 KO DG-NPCs by analyzing the phosphorylation of Smad1/5 (p-Smad1/5), an indicator of BMP pathway activation (Miyazono et al., 2005). We found that KO DG-NPCs had a reduced ratio of p-Smad1/5 compared with total Smad1/5 (Figure 5I). Introducing exogenous FXR2 into Fxr2 KO DG-NPCs resulted in rescue of both secreted Noggin protein levels (Figure 5J) and p-Smad1/5 levels (Figure 5K and Figure S5B). On the other hand, acute knockdown of FXR2 in WT DG-NPCs resulted in increased secreted Noggin protein (Figure 5L) as well as reduced p-Smad1/5 (Figure 5M and Figure S5C). Therefore, FXR2 regulates the BMP signaling in DG-NPCs by controlling Noggin levels.
Since FXR2 is highly expressed in DG neurons, we also assessed BMP signaling in hippocampal tissue (Figure S5D–S5F). Indeed, Noggin protein levels were significantly higher (Figure S5G), while p-Smad1/5 levels were significantly lower (Figure S5H) in the hippocampal tissue of Fxr2 KO mice compared with WT mice. Thus, by inhibiting Noggin protein expression, FXR2 promotes BMP signaling in both DG-NPCs and in the hippocampus.
Manipulating BMP Signaling Rescues Deficits in FXR2-Deficeient DG-NPCs
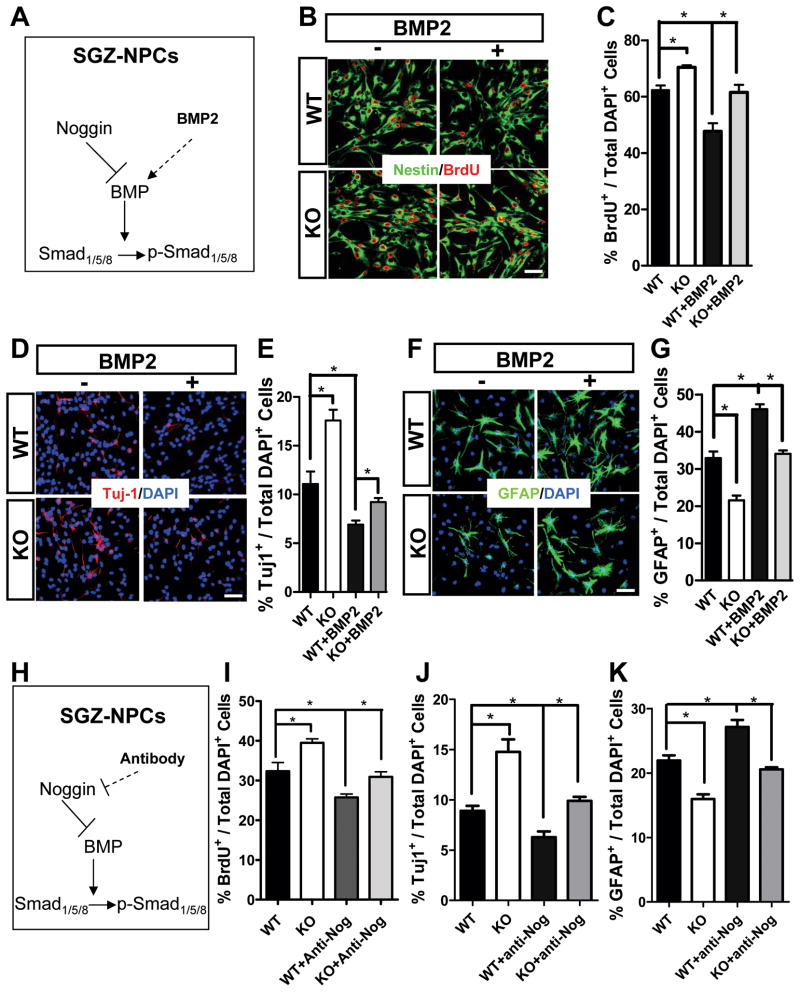
We reasoned that either adding exogenous BMP2 or blocking endogenous Noggin should rescue the phenotypes of Fxr2 KO DG-NPCs (Figure 6A). Indeed, BMP2 treatment reduced the high proliferation rate of Fxr2 KO DG-NPCs (Figure 6B and 6C, n = 3) and rescued both the neuronal (Figures 6D and 6E, n = 3) and astrocyte (Figure 6F and 6G, n = 3) differentiation phenotypes of Fxr2 KO DG-NPCs to the WT control levels. In addition, an anti-Noggin blocking antibody rescued the proliferation and differentiation deficits of Fxr2 KO DG-NPCs (Figure 6H–6K, n = 3). Next, to confirm that enhanced Noggin expression by Fxr2 KO DG-NPCs indeed had a biological effect on NPC functions, we treated WT DG-NPCs with conditioned medium collected from Fxr2 KO DG-NPCs. The conditioned medium from KO cells promoted the proliferation of WT cells, which could be blocked by an anti-Noggin blocking antibody (Figure S5I and S5J). Therefore Noggin and BMP signaling are likely downstream effectors of FXR2 in the regulation of DG neurogenesis.
Figure 6. Manipulation of BMP Signaling Rescues Phenotypes of FXR2-Deficient DG-NPCs.
(A) Schematic drawing showing that both adding exogenous BMP2 and blocking endogenous Noggin should affect intracellular BMP signaling.
(B) Both WT and KO NPCs incorporated BrdU (red) under proliferating conditions with or without exogenous BMP2. Scale bar = 20 μm.
(C) Quantitative analysis showing that exogenous BMP2 reduced the high proliferation rate of Fxr2 KO DG-NPCs to the WT control levels (n = 3, p < 0.05).
(D, F) Differentiating DG-NPCs with or without BMP2 treatment were analyzed using antibodies against Tuj1+ (D, red) for neurons and GFAP+ (G, green) for astrocytes. Scale bar = 20 μm.
(E, G) Quantification analyses of differentiated DG-NPCs demonstrate that exogenous BMP2 rescued the neuronal (E) and astrocyte (G) differentiation phenotypes of Fxr2 KO DG-NPCs. (n = 3, p < 0.05).
(H) Schematic drawing showing that a Noggin-blocking antibody should block Noggin activity and enhance BMP signaling.
(I–K) Quantification analysis showing that Noggin blocking antibody rescued the proliferation (I), neuronal differentiation (J), and astrocyte differentiation (K) phenotypes of Fxr2 KO DG-NPCs (n = 3, p < 0.05). Data are presented as mean ± SEM; *, p < 0.05, **, p < 0.01, Student’s t-test.
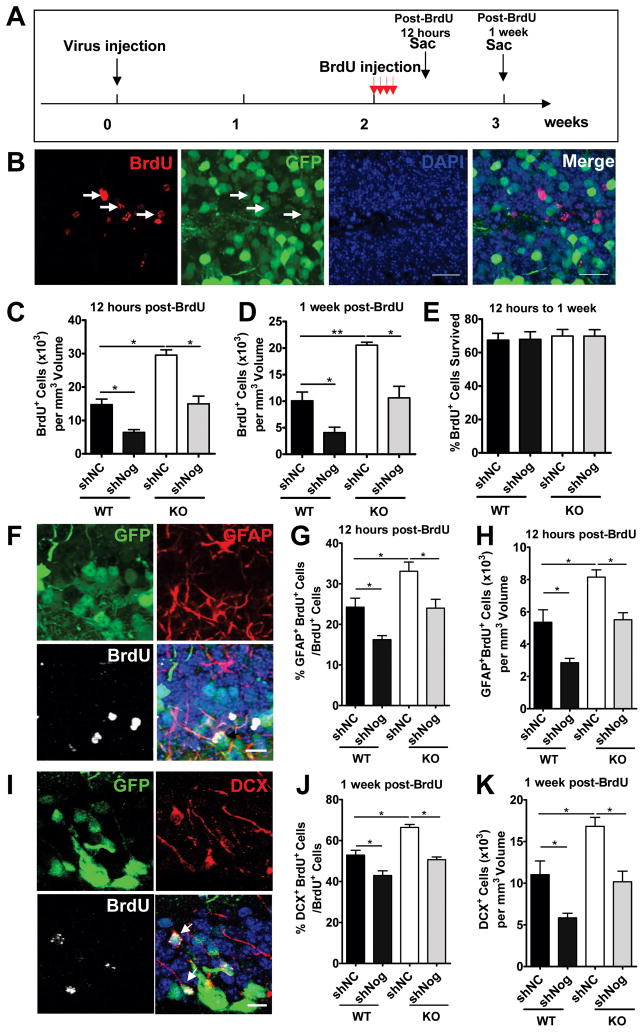
Noggin has been shown to promote the self-renewal of type 1 cells in the DG (Bonaguidi et al., 2008). We therefore hypothesized that elevated Noggin levels might be responsible for the increased cell proliferation we observed in Fxr2 KO mice. Accordingly, we investigated whether reducing endogenous Noggin in the DG could rescue the neurogenic phenotype of Fxr2 KO mice (Figures S6A–S6C). The lentivirus expressing either a shRNA against Noggin (shNog) or a control shRNA (shNC), as well as GFP, were injected into the DG of Fxr2 KO and WT littermates (Figure 7A) based on a published protocol (Clelland et al., 2009). We waited for two weeks after lentiviral grafting to allow for sufficient depletion of endogenous Noggin before giving mice BrdU. We saw that in animals with successful viral grafting, and therefore used for data analysis, a large number of DG cells were infected by the recombinant lentivirus in both WT and KO mice (GFP+ cells in Figure S6D; Figures 7B, 7F, and 7I). Lenti-shNog-infected cells had reduced levels of Noggin protein (Figure S6E). As expected, reduction of Noggin in the DG had a significant effect on the proliferation of DG-NPCs as assessed at both 12 hours (Figure 7C) and one week (Figure 7D) post-BrdU injection, with no effect on the survival of BrdU+ cells (Figure 7E). The effect of shNog on cell proliferation was at least in part due to its effect on the GFAP+ radial glia-like population (Figure 7F–7H; Figure S6F), which is similar to the function of FXR2. In addition, acute knockdown of Noggin resulted in enhanced neuronal differentiation of adult NPCs, hence enhanced number of new neurons analyzed at one week post-BrdU injection (Figure 7I–7K). Furthermore, knocking down endogenous Noggin in adult Fxr2 KO mice (KO+ shNog) led to significantly decreased NPC proliferation (Figure 7C–7H) and neuronal differentiation (Figure 7J and 7K) to levels similar to WT controls (WT+shNC). These in vivo data further support the model that FXR2 regulates DG neurogenesis by repressing Noggin protein levels.
Figure 7. Knocking Down Endogenous Noggin in the DG Rescues the Proliferation and Differentiation Deficits of DG-NPCs.
(A) Experimental scheme for assessing the effect of knocking down endogenous Noggin expression using lentivirus-shRNA on neural stem cell proliferation and differentiation in the adult DG.
(B) Examples of an adult hippocampal brain section stained with an antibody against GFP (green), BrdU (red), and DAPI (blue) for in vivo proliferation analyses.
(C–D) Knocking down Noggin could rescue the proliferation deficits of Fxr2 KO mice to similar levels as in WT mice as determined both at 12 hours (C, n = 3, p < 0.05) and at one week post-BrdU (D, n = 3, p < 0.05 and p < 0.01) injection.
(E) There is no difference in the survival of BrdU+ cells from 12 hours to one week in all conditions.
(F) Examples of an adult hippocampal brain section stained with an antibody against GFP (green), GFAP+ (red), BrdU (white), and DAPI (blue). Arrow points to a cell that is positive for all markers.
(G, H) Knocking down Noggin rescued the proliferation deficits of radial glia-like NPCs (G, percentage of GFAP+BrdU+/BrdU+ cells) and the total number radial glia-like NPCs (H, GFAP+ BrdU+ cells) of Fxr2 KO mice to levels similar to those of WT mice (n = 3, p < 0.05). (I) Examples of an adult hippocampal brain section stained with an antibody against GFP (green), DCX+ (red), BrdU (white), and DAPI (blue). Arrows point to cells that is positive for all markers
(J, K) Knocking down Noggin could rescue the neuronal differentiation deficits (J, percentage of DCX+BrdU+/BrdU+ cells) and the total number of new young neurons (K, DCX+ cells) of Fxr2 KO mice to levels similar to those of WT mice (n = 3, p < 0.05).
Data are presented as mean ± SEM; *, p < 0.05, **, p < 0.01, Student’s t-test. Scale bars in B = 20 μm. Scale bars in F and I = 10 μm. (Also see Figure S5.)
FXR2 and Noggin Colocalize in DG but Not in SVZ Cells
Next, we investigated why FXR2 deficiency had no effect on SVZ-NPCs. Although FXR2 was expressed at comparable levels in both SVZ-NPCs and DG-NPCs, we were only able to detect extremely low levels of Noggin protein in SVZ-NPCs, and the expression levels of Noggin were no different between cultured WT and KO SVZ-NPCs (Figure S7A). In addition, KO SVZ-NPCs did not show altered p-Smad1/5 levels compared with WT cells (Figure S7B), suggesting that FXR2 deficiency does not alter either Noggin expression or BMP signaling in SVZ-NPCs. The lack of effect from FXR2 deficiency on Noggin and BMP signaling in SVZ-NPCs could have two possible explanations: (1) Noggin and the BMP pathway do not regulate the functions of SVZ cells as they do in the case of DG cells; or (2) FXR2 does not regulate Noggin expression, and therefore BMP signaling in SVZ cells as it does in DG cells.
To distinguish between these two hypotheses, we first assessed the effects of exogenous Noggin and BMP2 on SVZ-NPCs compared with DG-NPCs. We found that exogenous Noggin promoted the proliferation of DG-NPCs (Figure S7F), but not SVZ-NPCs (Figure S7C), consistent with literature (Bonaguidi et al., 2008; Lim et al., 2000). On the other hand, Noggin promoted neuronal differentiation and decreased astrocyte differentiation in both SVZ-NPCs (Figure S7D and S7E) and DG-NPCs (Figures S7G and S7H) to similar extents, consistent with its reported role in inhibiting glia cell fate (Chmielnicki et al., 2004; Kohyama et al., 2010; Lim et al., 2000). We then found that exogenous BMP2 inhibited proliferation, repressed neuronal differentiation, and promoted astrocyte fate to similar extents in both WT and KO SVZ-NPCs (Figure S7I–S7K). Therefore, BMP2 had similar effects on both DG-NPCs (Figure 6B–6G) and SVZ-NPCs. We therefore predicted that FXR2 must not regulate Noggin expression in SVZ-NPCs as it does in DG-NPCs.
To assess this possibility, we first confirmed that FXR2 indeed does not bind Noggin mRNA in SVZ-NPCs (Figure S7L). Because FXR2 and Noggin are expressed in both the DG and SVZ, we reasoned that a lack of FXR2 regulation of Noggin in the SVZ might be due to cell type-restricted expression of these two proteins. To precisely identify the cells expressing Noggin, we used both Noggin antibody staining and a transgenic “knock-in” mouse strain expressing β-gal under the Noggin promoter (NogginlacZ) (McMahon et al., 1998). Expression of β-gal in this strain is an accurate and precise reporter of Noggin expression (Stottmann et al., 2001). Indeed, we found that FXR2 and Noggin are not colocalized in the same cells in the SVZ (Figure 8A, Figure S8A–S8C). Noggin expression is restricted to s100β+ ependymal cells that also express Nestin (Figure 8B and 8C, Figure S8B), consistent with a previous report (Lim et al., 2000). By contrast, FXR2 is expressed only in s100β-negative NPCs (Figure1, Figure 8A and 8C, and Figure S8C), and not in s100β+Nestin+ ependymal cells (Figure 8A and 8B, Figure S8A).
Figure 8. Noggin and FXR2 are Colocalized in the DG but Not in the SVZ.
(A) Noggin (as shown by β-gal staining in NogginlacZ mice) and FXR2 were not expressed in the same cells of the SVZ.
(B) Noggin (red) is expressed in S100β+ ependymal cells (green) in the SVZ.
(C) Since ependymal cells also expressed Nestin (green), Noggin (red) partially overlaps with Nestin. However, Noggin is not expressed in GFAP (white) or Nestin double-positive stem cells in the SVZ. Blue, DAPI.
(D) Noggin (red) and FXR2 (green) were coexpressed in the same cells of the DG. Noggin (red) is expressed in GFAP+ (green) radial glia-like NPCs in the DG.
(E) Noggin (red) is expressed in GFAP+ and Nestin (green) double-positive radial glia-like NPCs in the DG.
(F, G) Models of FXR2 and Noggin function in adult SVZ- (F) and DG- (G) NPCs. In the SVZ (F), Noggin is expressed by ependymal cells, while FXR2 is expressed by stem cells; therefore, FXR2 does not regulate Noggin expression. However, in the DG (G), FXR2 and Noggin are coexpressed in the stem cells (and also granule neurons); therefore, the expression levels of Noggin are under FXR2 control. FXR2 thus specifically regulates the fate of DG-NPCs by repressing Noggin expression, thereby enhancing BMP signaling. All scale bars = 20 μm. (Also see Figure S6 and Figure S7.)
In the DG, however, we found that Noggin is expressed in Nestin+GFAP+ radial glia-like NPCs (Figure 8E; Figure S8E and S8G), consistent with an earlier study (Bonaguidi et al., 2008). Importantly, these cells also express FXR2 (Figure 1), and FXR2 expression colocalizes with Noggin in both NPCs and neurons of the DG (Figure 8D, Figure S8D and S8F). These spatiotemporal expression data further support the regulatory role of FXR2 in DG-NPCs, but not in SVZ-NPCs. Taken together, our data argue for a model in which FXR2 specifically regulates DG-NPCs by directly repressing Noggin expression in DG-NPCs. Because Noggin expression in the SVZ is not regulated by FXR2, FXR2 deficiency therefore has minimal impact on SVZ-NPCs (Figure 8F and 8G).
DISCUSSION
The molecular mechanism behind the differential regulation of SVZ and DG neurogenesis has gone largely unexplored. By unveiling a novel regulatory mechanism governing adult hippocampal neurogenesis, our data show that a brain-enriched RNA-binding protein could play important roles in the differential regulation of NPCs residing in different brain regions.
Both the Extrinsic Stem Cell Niche and Intrinsic NPC Properties May Contribute to the Differences between Adult DG and SVZ Neurogenesis
A limited number of publications have analyzed both DG and SVZ neurogenesis and reported different phenotypes between these two regions (Belvindrah et al., 2002; Liu et al., 2007). However, the molecular mechanism underlying the differences between DG and SVZ neurogenesis is largely a mystery.
The cytoarchitecture of the two adult neurogenic regions are quite different. There are four key cell types in the SVZ: ciliated ependymal cells that face the ventricle lumen, providing a barrier and filtration system for cerebrospinal fluid; slowly proliferating stem cells; actively proliferating progenitor cells, and proliferating neuroblasts (Doetsch et al., 1999; Seri et al., 2004). Ependymal cells were proposed to be SVZ stem cells (Johansson et al., 1999), but mounting evidence indicates that ependymal cells are not proliferative and do not have the properties of NPCs (Capela and Temple, 2002; Doetsch et al., 1999). Since FXR2 expression is restricted to NPCs and Noggin expression is restricted to the ependymal cells, this differential expression prevents the direct regulation of Noggin expression by FXR2. We detected very low levels of Noggin protein in the early passage SVZ-NPCs, which could be due to contamination of residual ependymal cells during SVZ dissection.
The DG lies deep within the hippocampal parenchyma. Type 1 radial glia-like (GFAP+Nestin+) cells are found to have stem cell properties which can generate type 2a (GFAP−Nestin+) transient amplifying NPCs that differentiate into type 3 (DCX+) neuroblasts in the DG (Kriegstein and Alvarez-Buylla, 2009; Ming and Song, 2005; Seri et al., 2004; Zhao et al., 2008). We found that Noggin and FXR2 are colocalized in the DG type 1 cells and FXR2 deficiency leads to increased proliferation of these cells. An ependymal-equivalent cell type has not been found in the DG. However, the neurons in the DG are in much closer proximity to stem cells compared with those in the SVZ; therefore, granule neurons may create a plausible stem cell niche in the DG, and increased neuronal Noggin expression in the DG neurons of Fxr2 KO mice may be partially responsible for the phenotypes of DG–NPCs in Fxr2 KO mice. In summary, our data support the notion that the differences both in the intrinsic properties of NPCs and in the stem cell niche may contribute to the differences in neurogenesis seen between the DG and the SVZ.
FXR2, Noggin, and BMP Signaling May be Key Components of the Mechanism Underlying Differential Regulation of Adult DG and SVZ NPCs
Noggin plays important roles in many types of stem cells and helps maintain pluripotency in cultured stem cells (Chambers et al., 2009; Chaturvedi et al., 2009). With regard to adult neurogenesis, Noggin inhibits BMP signaling to promote NPC proliferation and neuronal differentiation, while inhibiting glial differentiation (Chmielnicki et al., 2004; Lim et al., 2000). Our data, together with previous study (Bonaguidi et al., 2008), suggest that Noggin and BMP may be key components of the mechanism underlying the differential regulation of DG and SVZ neurogenesis. Bonaguidi et al. demonstrated that DG-NPCs have a high level of intrinsic BMP signaling, and that Noggin promotes the proliferation of isolated DG-NPCs; they concluded that Noggin is responsible for maintaining the self-renewal of type 1 DG stem cells in vivo (Bonaguidi et al., 2008). This is consistent with our observation that Fxr2 KO mice exhibited increased Noggin expression and self-renewal of Type 1 cells in the DG. Both the findings of Bonaguidi et al. and our study show that Noggin has no effect on the proliferation of SVZ-NPCs; however, our interpretation of this result is different. Bonaguidi et al. propose that this lack of an effect from Noggin might be due to the very low level of intrinsic BMP signaling in SVZ-NPCs, but they did not analyze the effect of Noggin and BMP on the differentiation of SVZ-NPCs. We found that although Noggin has no effect on the proliferation of SVZ-NPCs, it has similar effects on the differentiation of both DG-NPCs and SVZ-NPCs, consistent with the literature (Chmielnicki et al., 2004; Lim et al., 2000). We also found that exogenous BMP2 had similar effects on both the proliferation and differentiation of both DG-NPCs and SVZ-NPCs. Therefore, our data suggest that the BMP signal transduction pathway is intact in both DG-NPCs and SVZ-NPCs. The lack of any effect from Noggin manipulation on SVZ-NPC proliferation could stem from other causes, such as the presence of another yet-to-be-identified inhibitor of BMP signaling in SVZ-NPCs. Based on our data, we suggest that the main reason FXR2 deficiency has no effect on SVZ-NPCs is simply because FXR2 does not regulate Noggin expression in the SVZ.
Post-transcriptional Regulation of Adult Neural Stem Cells by Fragile X Family RNA-binding Proteins
Both FMRP and FXR2 are enriched in the brain; however, mutation of FXR2 has not been associated with human mental retardation disorders. It is possible that FXR2 mutations in humans contribute to mild learning deficits without the distinct features seen in FMRP deficiency. Although FXR2 and FMRP do not compensate for each other at the protein expression level; nonetheless, functional compensation between FMRP and FXR2 has been established clearly in double mutant mice by their exaggerated behavioral deficits (Bontekoe et al., 2002; Spencer et al., 2006), circadian rhythm changes (Zhang et al., 2008), and synaptic transmission alterations (Zhang et al., 2009). On the other hand, evidence also points to different functions for FXR2 and FMRP. For example, loss of FMRP expression leads to alterations in long-term synaptic plasticity, including enhanced mGluR-dependent long-term depression (LTD) in hippocampal CA1 cells, as well as loss of protein synthesis-dependence for its maintenance (Hou et al., 2006; Huber et al., 2002; Nosyreva and Huber, 2006). Surprisingly, Fxr2 KO mice have decreased mGluR-LTD that remains protein synthesis-dependent, whereas FMRP and FXR2 double mutant mice have a dramatically exaggerated LTD (Zhang et al., 2009). It is likely that FXR2 and FMRP are functioning in different pathways that could synergistically affect certain biological processes, despite their opposite individual effects. In a similar situation, we previously showed that FMRP deficiency in mice leads to impaired hippocampal neurogenesis and hippocampal dependent learning and that FMRP regulates DG-NPCs via the Wnt signaling pathway (Guo et al., 2011; Luo et al., 2010). However, in the current study, we discovered that DG-NPCs in Fxr2 KO mice have increased neuronal differentiation with no change in Wnt signaling in Fxr2 KO DG-NPCs. In addition, FMRP inhibits Gsk3β protein expression by repressing translation without affecting Gsk3β mRNA stability, whereas FXR2 represses Noggin protein expression by reducing the stability of Noggin mRNA. Furthermore, FMRP deficiency results in increased proliferation of both stem cells and transient amplifying cells in the adult DG (Luo et al., 2010), and loss of FXR2 only affects stem cell proliferation in the DG. Therefore, both FMRP and FXR2 can regulate adult hippocampal NPCs by binding to the mRNAs of NPC regulators, but their mechanisms, as well as their functional outputs, are different. Post-transcriptional regulation of critical regulatory mRNAs by RNA-binding proteins is likely to be a common mechanism during critical cellular processes (Bhattacharyya et al., 2008; Callan et al., 2010; Tervonen et al., 2009; Yang et al., 2009), but evidence for this in adult mammalian neurogenesis is rather limited. Our data are among the first to demonstrate that RNA-binding proteins can play important roles in the differential regulation of NPCs residing in different adult brain regions. Future studies examining the role of FXR2 in generating the inhibitory interneurons of the olfactory bulb and excitatory neurons of the DG, particularly in comparison with FMRP, will further contribute to our knowledge of these important RNA-binding proteins in adult neurogenesis and plasticity.
EXPERIMENTAL PROCEDURES
(Please refer to Supplemental Experimental Procedures for details)
Mice
All animal procedures were performed according to protocols approved by the University of New Mexico Animal Care and Use Committee. The Fxr2 KO mouse strain on the C57B/L6 genetic background published previously (Bontekoe et al., 2002) was obtained from the Emory University fragile X consortium. The NogginLacZ transgenic mice were maintained and genotyped as described previously (Stottmann et al., 2001).
In vivo Cell Proliferation, Differentiation Analysis and Immunohistology
In vivo neurogenesis analyses were performed essentially as we have previously described (Guo et al., 2011; Luo et al., 2010; Smrt et al., 2007; Zhao et al., 2003). Mice were given 4 injections of BrdU (50 mg/kg) within 12 hours to label all dividing cells in adult germinal zones within this time period based on a published paradigm (Hayes and Nowakowski, 2002). Mice were then euthanized either at either 12 hours or one week following the final BrdU injection. Antibody information is provided in Supplemental Methods. Quantification of BrdU+ cells in the DG and SVZ and phenotypic analysis of BrdU+ cells were performed as described previously.
Isolation, Culture, and in vitro Analyses of Adult NPCs
NPCs used in this study were isolated from the DG and SVZ of 8- to 10-week-old male Fxr2 KO mice and WT littermate controls based on published methods (Babu et al., 2007; Bull and Bartlett, 2005; Luo et al., 2010; Seaberg and van der Kooy, 2002). NPC maintenance, proliferation and differentiation analyses, and neurosphere assays were performed as described in our publications (Barkho et al., 2008; Liu et al., 2010; Luo et al., 2010; Szulwach et al., 2010).
Cell Transfection and Luciferase Assay
Transfection of NPCs was carried out using a Stemfect kit (Stemgent, San Diego, CA) and luciferase activity was detected using the Dual-Luciferase Reporter 1000 System (Promega, # E1980), based on the manufacturer’s protocols and our publications (Liu et al., 2010; Smrt et al., 2010).
In vivo and in vitro Acute Knockdown of FXR2 in NPCs using FXR2-siRNA
For in vivo acute knockdown of FXR2, retroviral grating was performed as described (Liu et al., 2010; Smrt et al., 2007; Smrt et al., 2010; Szulwach et al., 2010)
RNA Immunoprecipitation (IP)
RNA-IP was carried out as described (Brown et al., 2003; Luo et al., 2010). WT and Fxr2 KO NPCs (2 × 106) and a monoclonal antibody against FXR2 (F1554, Sigma-Aldrich) were used.
RT-PCR, Real-Time PCR, and Neural Stem Cell Pathway Arrays
RT-PCR and real-time PCR were performed using standard methods as described (Liu et al., 2010; Luo et al., 2010). Differential gene expression in Fxr2 KO DG-NPCs was determined using mouse Neural Stem Cell Pathway Arraya (Qiagen) according to the manufacturer’s instructions (Qiagen).
Actinomycin D Treatment (Noggin mRNA Stability Assay)
DG-NPCs were treated with 10 μg/ml of actinomycin D (Sigma-Aldrich) to inhibit gene transcription, based on a published method (Ghosh et al., 2009), and cells were collected at various time intervals. Noggin mRNA levels normalized to GAPDH were assessed by real-time PCR.
In vitro Transcription of Biotin-labeled Noggin mRNA and Binding Assay
Performance of this procedure was based on a previously published method with minor modifications (Deschenes-Furry et al., 2007).
ELISA Analysis of Secreted Noggin
To determine the amount of secreted Noggin protein in the cell medium, medium was collected after 24 hours of culture. Determination of secreted Noggin was performed using a mouse Noggin ELISA kit (ABIN425343, antibodies-online.com, Atlanta, USA), according to the manufacturer’s instructions.
Treating NPCs with Exogenous Noggin and BMP2, anti-Noggin Blocking Antibody, and Conditioned Medium
For growth factor and antibody treatment, the concentration of Noggin was 250 ng/ml (R&D Systems), BMP2 was 25 ng/ml (R&D Systems), and Noggin antibody was 2.5 ng/ml (R&D Systems). Conditioned medium was collected from WT and KO DG-NPCs after 24-hours in culture.
Recombinant Lentivirus and In vivo Lentiviral Grafting
Lentivirus expressing control shRNA (shCon) was published previously (Barkho et al., 2008; Liu et al., 2010). Noggin-shRNA plasmids were purchased (Qiagen) and their efficiency at knocking down endogenous Noggin was tested by transfecting P19 cells followed by Western blotting analyses. The shRNA exhibiting highest efficacy was then cloned into the lentiviral vector and in vivo lentiviral grafting was performed as described (Liu et al., 2010; Smrt et al., 2007; Smrt et al., 2010; Szulwach et al., 2010). Based on the published criteria (Clelland et al., 2009), only animals with lentivirus-infected (GFP+) cells in > 50% of the DG area of the adult hippocampus were included in the data analysis shown in Figure 7.
Statistical Analysis
Statistical analysis was performed using ANOVA and Student’s t-test, unless specified with the aid of SPSS v.17. All percentages were arcsine-transformed before statistical analysis. The Bonferroni correction was used to control type I error (Rice, 1989). We first normalized the treatment group by the control group for RT-PCR, and then one-sample t-test against mean of 1 was used on the normalized values. All data were shown as mean with standard error of mean (mean ± SEM). Probabilities of P < 0.05 were considered as significant.
Supplementary Material
Acknowledgments
We would like to thank Ms. Cheryl. T. Strauss for editing the manuscript, Nora Perrone-Bizzozero for teaching us the biotinylated-RNA pull down assay, Eric G. Schaller and Adeline C. Murthy for critical reading of the manuscript, and Sergei J. von Hoyningen-Huene and Adeline Murthy for technical assistance. This work was supported by grants from the International Rett Syndrome Foundation and the NIH (MH080434 and MH078972) to XZ and NIH (NS068932) to JK. DMC was supported by an NIH/NIMH Career Opportunity for Research (COR) training grant (MH19101). OLG was supported by NIH ARACDA/ASERT program.
Footnotes
Supplemental data include 8 figures and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Blanchet P, Kobetz A, Marchant D, Faucon N, Sarda P, Moraine C, Sittler A, Biancalana V, Malafosse A, et al. Expression of FMR1, FXR1, and FXR2 genes in human prenatal tissues. J Neuropathol Exp Neurol. 1999;58:867–880. doi: 10.1097/00005072-199908000-00009. [DOI] [PubMed] [Google Scholar]
- Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS One. 2007;2:e388. doi: 10.1371/journal.pone.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker CE, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen AT, Oostra BA, Willemsen R. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Exp Cell Res. 2000;258:162–170. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–3149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R, Rougon G, Chazal G. Increased neurogenesis in adult mCD24-deficient mice. J Neurosci. 2002;22:3594–3607. doi: 10.1523/JNEUROSCI.22-09-03594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, McMillan E, Wallace K, Tubon TC, Jr, Capowski EE, Svendsen CN. Normal Neurogenesis but Abnormal Gene Expression in Human Fragile X Cortical Progenitor Cells. Stem Cells Dev. 2008;17:107–117. doi: 10.1089/scd.2007.0073. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28:9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontekoe CJ, McIlwain KL, Nieuwenhuizen IM, Yuva-Paylor LA, Nellis A, Willemsen R, Fang Z, Kirkpatrick L, Bakker CE, McAninch R, et al. Knockout mouse model for Fxr2: a model for mental retardation. Hum Mol Genet. 2002;11:487–498. doi: 10.1093/hmg/11.5.487. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Albeck DS, Paylor R. Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 2006;5:467–471. doi: 10.1111/j.1601-183X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan MA, Cabernard C, Heck J, Luois S, Doe CQ, Zarnescu DC. Fragile X protein controls neural stem cell proliferation in the Drosophila brain. Hum Mol Genet. 2010 doi: 10.1093/hmg/ddq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi G, Simone PD, Ain R, Soares MJ, Wolfe MW. Noggin maintains pluripotency of human embryonic stem cells grown on Matrigel. Cell Prolif. 2009;42:425–433. doi: 10.1111/j.1365-2184.2009.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee RL, Jr, Tessier CR, Woodruff EA, 3rd, Broadie K. Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P. Dis Model Mech. 2010 doi: 10.1242/dmm.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, Darnell RB. Discrimination of common and unique RNA-binding activities among Fragile X mental retardation protein paralogs. Hum Mol Genet. 2009;18:3164–3177. doi: 10.1093/hmg/ddp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes-Furry J, Mousavi K, Bolognani F, Neve RL, Parks RJ, Perrone-Bizzozero NI, Jasmin BJ. The RNA-binding protein HuD binds acetylcholinesterase mRNA in neurons and regulates its expression after axotomy. J Neurosci. 2007;27:665–675. doi: 10.1523/JNEUROSCI.4626-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Aguila HL, Michaud J, Ai Y, Wu MT, Hemmes A, Ristimaki A, Guo C, Furneaux H, Hla T. Essential role of the RNA-binding protein HuR in progenitor cell survival in mice. J Clin Invest. 2009;119:3530–3543. doi: 10.1172/JCI38263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA. Directed mobilization of endogenous neural progenitor cells: the intersection of stem cell biology and gene therapy. Curr Opin Mol Ther. 2004;6:466–472. [PubMed] [Google Scholar]
- Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, Murthy AC, Goggin SL, Eisch AJ, Oostra BA, et al. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat Med. 2011 doi: 10.1038/nm.2336. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Hellstrom NA, Bjork-Eriksson T, Blomgren K, Kuhn HG. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27:634–641. doi: 10.1634/stemcells.2008-0732. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LL, McIlwain KA, Nelson DL. Comparative genomic sequence analysis of the FXR gene family: FMR1, FXR1, and FXR2. Genomics. 2001;78:169–177. doi: 10.1006/geno.2001.6667. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Matsui M, Yang YP, Anderson RM. Roles of bone morphogenetic protein signaling and its antagonism in holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:43–51. doi: 10.1002/ajmg.c.30256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama J, Sanosaka T, Tokunaga A, Takatsuka E, Tsujimura K, Okano H, Nakashima K. BMP-induced REST regulates the establishment and maintenance of astrocytic identity. J Cell Biol. 2010;189:159–170. doi: 10.1083/jcb.200908048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhao X. Epigenetic regulation of mammalian stem cells. Stem Cells Dev. 2008;17:1043–1052. doi: 10.1089/scd.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RR, Brown CE, Murphy TH. Differential regulation of cell proliferation in neurogenic zones in mice lacking cystine transport by xCT. Biochem Biophys Res Commun. 2007;364:528–533. doi: 10.1016/j.bbrc.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shan G, Guo W, Smrt RD, Johnson EB, Li X, Pfeiffer RL, Szulwach KE, Duan R, Barkho BZ, et al. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Gotz M. Signaling in adult neurogenesis: from stem cell niche to neuronal networks. Curr Opin Neurobiol. 2007;17:338–344. doi: 10.1016/j.conb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR. Analyzing Tables of Statistical Tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci. 2006;1068:19–25. doi: 10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Smrt RD, Eaves-Egenes J, Barkho BZ, Santistevan NJ, Zhao C, Aimone JB, Gage FH, Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, Teng ZQ, Luo Y, Peng J, Bordey A, et al. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28:1060–1070. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between Fragile X-related proteins. Hum Mol Genet. 2006;15:1984–1994. doi: 10.1093/hmg/ddl121. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Anderson RM, Klingensmith J. The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Dev Biol. 2001;240:457–473. doi: 10.1006/dbio.2001.0479. [DOI] [PubMed] [Google Scholar]
- Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, Santistevan NJ, Li W, Zhao X, Jin P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervonen TA, Louhivuori V, Sun X, Hokkanen ME, Kratochwil CF, Zebryk P, Castren E, Castren ML. Aberrant differentiation of glutamatergic cells in neocortex of mouse model for fragile X syndrome. Neurobiol Dis. 2009;33:250–259. doi: 10.1016/j.nbd.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Wang TW, Stromberg GP, Whitney JT, Brower NW, Klymkowsky MW, Parent JM. Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. J Comp Neurol. 2006;497:88–100. doi: 10.1002/cne.20984. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu S, Xia L, Wang J, Wen S, Jin P, Chen D. The bantam microRNA is associated with drosophila fragile X mental retardation protein and regulates the fate of germline stem cells. PLoS Genet. 2009;5:e1000444. doi: 10.1371/journal.pgen.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fang Z, Jud C, Vansteensel MJ, Kaasik K, Lee CC, Albrecht U, Tamanini F, Meijer JH, Oostra BA, et al. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am J Hum Genet. 2008;83:43–52. doi: 10.1016/j.ajhg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hou L, Klann E, Nelson DL. Altered hippocampal synaptic plasticity in the FMR1 gene family knockout mouse models. J Neurophysiol. 2009;101:2572–2580. doi: 10.1152/jn.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, Lein ES, Eadie BD, Willhoite AR, Muotri AR, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci U S A. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.