Summary
Full functional recovery after traumatic peripheral nerve injury is rare. We postulate three reasons for the poor functional outcome measures observed. Axon regeneration is slow and not all axons participate. Significant misdirection of regenerating axons to reinnervate inappropriate targets occurs. Seemingly permanent changes in neural circuitry in the central nervous system are found to accompany axotomy of peripheral axons. Exercise in the form of modest daily treadmill training impacts all three of these areas. Compared to untrained controls, regenerating axons elongate considerably farther in treadmill trained animals and do so via an autocrine/paracrine neurotrophin signaling pathway. This enhancement of axon regeneration takes place without an increase in the amount of misdirection of regenerating axons found without training. The enhancement also occurs in a sex-dependent manner. Slow continuous training is effective only in males, while more intense interval training is effective only in females. In treadmill trained, but not untrained mice the extent of coverage of axotomized motoneurons is maintained, thus preserving important elements of the spinal circuitry.
Keywords: axon regeneration, exercise, neurotrophins, synaptic stripping, peripheral nerve
Introduction
Injured axons in peripheral nerves regenerate better than those in the central nervous system, but the functional outcomes observed clinically after peripheral nerves are injured are often so poor that some form of long term disability results (Brushart 1998; Frostick et al. 1998). The three reasons most often given for these poor outcomes are: 1) that the regeneration of axons in injured peripheral nerves is slow and not all axons participate, leading to a functionally inadequate reinnervation of muscles (Fu and Gordon 1995; Fu and Gordon 1997; Gordon 2009); 2) that regenerating motor axons are misdirected and reinnervate functionally inappropriate targets (Evans et al. 1991; de Ruiter et al. 2008); and 3) that plastic changes in the central nervous system (CNS) that accompany peripheral axotomy alter the relationship of circuitry in the CNS and the reinnervated muscles (Alvarez et al. 2010). Currently, there is no accepted medical treatment for traumatic peripheral nerve injuries that addresses these concerns. The standard of care is to provide a tension free repair of cut nerves and allow the process of regeneration to proceed.
Exercise as a means of improving brain health and function has received considerable recent attention, (Adlard and Cotman 2004; Adlard et al. 2004) at least in part because it has been shown to induce the synthesis of both brain derived neurotrophic factor (BDNF) and its receptor, trkB, in rats and to promote recovery after CNS injury (Gomez-Pinilla et al. 2001; Hutchinson et al. 2004; Molteni et al. 2004; Ploughman et al. 2005). Therapeutic exercise thus could form a useful means of stimulation to the growth of regenerating peripheral axons that would require very little clinical intervention. Because it activates motor and primary afferent neurons naturally, via their own neural circuits, one might expect that exercise could produce enhanced axon regeneration without increasing the misdirection of those axons to inappropriate targets. Additionally, this natural activation might have effects on the CNS consequences of peripheral nerve injury. Indeed, we have found that exercise in the form of treadmill training has beneficial effects on all three of the critical aspects of recovery from peripheral nerve injury listed above. In the review that follows, we will delineate these effects and discuss the need for and direction of future studies.
Treadmill training enhances axon regeneration in cut peripheral nerves
Physical activity during the recovery period has been shown to improve motor function after spinal cord injury, both clinically and in experimental animals.(Skinner et al. 1996; Edgerton et al. 1997; Hutchinson et al. 2004). Improvements in both sensory and motor functions have been described. Along with the successful effects of treadmill training in spinal cord injured cats by Rossignol and colleagues (Chau et al. 1998), Edgerton and colleagues have advocated treadmill exercise as a treatment for patients with spinal cord injury. They showed that both voluntary exercise (Gomez-Pinilla et al. 2002; Engesser-Cesar et al. 2005) and treadmill training (Ying et al. 2005; Heng and de Leon 2009) resulted in substantial increases in the expression of BDNF and neurotrophin-3 (NT-3) in the spinal cord, and that this was a likely source of support of the local neuronal circuitry caudal to the injury site (Courtine et al. 2009). Both the Edgerton group (Edgerton et al. 2004) and Basso and colleagues (Hutchinson et al. 2004) provided strong evidence for improvement in functional recovery in animals with spinal cord injuries. Although limitations to this approach have been recognized (Barbeau et al. 2006), a number of studies have been published establishing the potential value of treadmill training in spinal cord injury (reviewed by (Wessels et al. 2010)). Treadmill training for spinal cord injured patients is now a part of many rehabilitation therapy clinics worldwide.
Until recently, the effects of applied exercise during the recovery period following peripheral nerve injury had been less extensively studied. Application of exercise prior to peripheral nerve injury is thought to have a protective effect and is said to “prime” adult dorsal root ganglion neurons for increased axon regeneration (Molteni et al. 2004). (Marqueste et al. 2004) showed that treadmill exercise following transection of the common fibular nerve induced better functional recovery of muscle sensory axons. The effects of exercise on motor function after peripheral nerve injury were equivocal. Some had concluded that exercise has beneficial effects (van Meeteren et al. 1997; van Meeteren et al. 1998) but others have argued that it does not (Soucy et al. 1996).
Because treadmill exercise was known to increase the expression of neurotrophins and their receptors in spinal motoneurons (Funakoshi et al. 1995; Gomez-Pinilla et al. 2002; Hutchinson et al. 2004; Molteni et al. 2004), we evaluated the effects of modest daily treadmill training on axon regeneration following peripheral nerve injury. We have used mice in which a subset of the sensory and motor axons in peripheral nerves are marked completely by yellow fluorescent protein (YFP) (Feng et al. 2000) to study the elongation of fluorescent regenerating axon profiles into segments of the same nerves harvested from strain-matched mice without fluorescence. Repairing the nerves with these grafts enabled easy visualization of YFP+ axons without interference of fluorescent products of degenerating axons. It also enabled us to use mouse genetics to manipulate the regenerating axons and the environment through which they elongate separately. Using a simple treadmill training program similar to one of those used successfully by Basso and her colleagues (Hutchinson et al. 2004), we showed that one hour of daily continuous slow walking begun on the third day following transection and surgical repair of different peripheral nerves in wild type mice resulted in a striking increase in the length of regenerating axons through grafts from wild type littermates (Fig. 1). These enhancing effects of regular training were observed as early as one week after nerve transection and persisted for at least two weeks following the cessation of daily training. Similar enhancement was found in mice where cut nerves were repaired using simple end-to-end anastomosis of the cut stumps by using retrograde labeling of motoneurons as an assay of regeneration (English et al. 2009) and in rats with similar transection and repair, restoration of evoked compound muscle action potentials (M responses) occurred at earlier post-transection times than in untrained controls (Boeltz et al. 2010). Treadmill training thus results in enhancement of axon regeneration in cut peripheral nerves.
Figure 1.
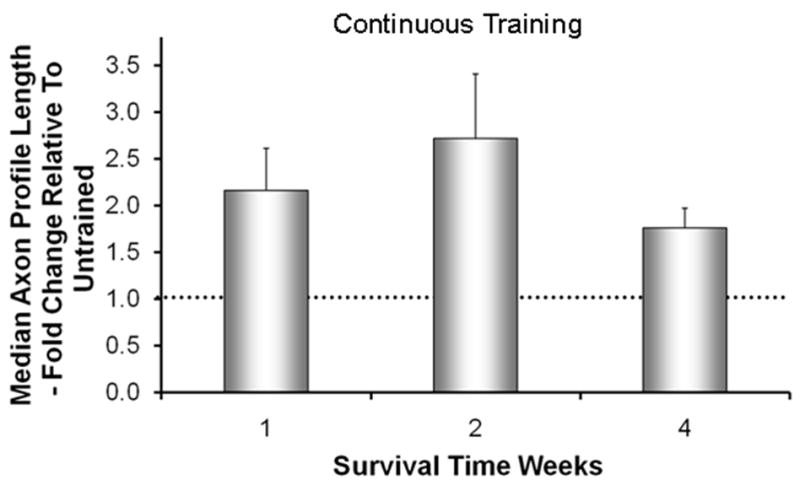
The effects of Continuous treadmill training on axon regeneration in peripheral nerves. Cut common fibular nerves in thy-1-YFP-H mice were repaired with a short length of nerve harvested from a non-fluorescent littermate. Animals were trained continuously for one hour per day at a slow treadmill speed (10 m/min), five days per week for no more than two weeks. Using images of optical sections made through these grafts, the lengths of YFP+ regenerating axon profiles were measured at different survival times. Average median axon profile lengths, expressed as a percentage of untrained controls (±SEM, N=4 for each), are shown. The horizontal dashed line at 1.0 indicates the length of regenerating axons in untrained controls. Data are from Sabatier et al (2008).
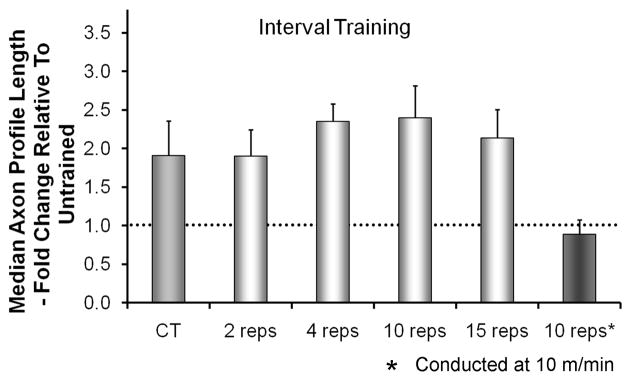
We chose to impose treadmill exercise on our animals rather than allow them to exercise voluntarily, because we wanted to be able to control the intensity, duration, and pattern of training. However, when compared to the patterns of training used by mice voluntarily (De Bono et al. 2006), our slow continuous training paradigm was quite different. During voluntary exercise, mice run at much greater speeds, up to 80% of their maximum running speed, and they do so for relatively short durations. The durations of these intervals is approximately two minutes and bouts of running are separated by rest periods of five minutes (De Bono et al. 2006). Mice repeat this interval training many times each night, and females run approximately twice as many intervals as males (De Bono et al. 2006). Based on these observations, we treated mice with cut and graft-repaired nerves using an interval training paradigm. Animals covered the same distance (600 m), but at a faster speed (20 m/min), and in two minute intervals separated by five minutes of rest. This interval training for two weeks resulted in regenerating axons that were approximately twice as long as untrained controls, the same enhancement of axon regeneration found with slow continuous walking (Sabatier et al. 2008). Reducing the number of training intervals to as few as two had no significant effect on the amount of enhancement of axon regeneration (Fig. 2). Running for a single two-minute interval or more extensive interval training at slow treadmill speeds were both ineffective in enhancing axon regeneration. Thus, both slow continuous walking and faster running at intervals are paradigms that enhance axon regeneration in cut peripheral nerves.
Figure 2.
The effects of Interval treadmill training on axon regeneration in peripheral nerves. The paradigm used was similar to that described for Figure 1 except for the pattern of treadmill training. Animals were trained at a faster treadmill speed (20 m/min), for two minutes and then rested for five minutes. This interval was repeated different numbers of times. Training was conducted five days/week for two weeks. Average median axon profile lengths, expressed as a percentage of untrained controls (±SEM, N=4 for each), are shown. The horizontal dashed line at 1.0 indicates the length of regenerating axons in untrained controls. Data are from Sabatier et al (2008).
Using different training paradigms, injury models and/or outcome measures, others have also found beneficial effects of treadmill training on axon regeneration after peripheral nerve injury in rodents. (Ilha et al. 2008) used a modest speed of treadmill locomotion of only 9 m/min for 60 minutes and found improvements in Sciatic Function Index (SFI) scores and morphology of regenerating nerve fibers in rats after sciatic nerve crush. Seo and colleagues also used the sciatic nerve crush model, but employed a higher training intensity of 18 m/min for 60 minutes (two bouts of 30 minutes of treadmill locomotion, undisclosed rest interval) and found better axonal regeneration as a result (Seo et al. 2006). However, recovery from nerve crush is generally much better than recovery from nerve transection and repair since in the former injury the endoneurial tubes remain largely intact (Sunderland 1990).
To our knowledge there is only one investigation, aside from those conducted in our lab and referenced above, that test the effect of treadmill training on recovery after peripheral nerve transection. In that study it was found that treadmill training after sciatic nerve transection increases the number of regenerated myelinated axons and prevents the development of hyper-reflexia (Asensio-Pinilla et al. 2009). Rats were walked on a treadmill at only 5 m/min for a total of 60 min/day (30 min’s exercise, 10 min’s rest, 30 min’s exercise). Therefore, it can be concluded from the studies to date that treadmill training after peripheral nerve injury results in better axon regeneration both 1) irrespective of whether nerve crush or transection is used and 2) irrespective of the parameters of the training program.
Future studies may, nevertheless, do well to give careful consideration to the selection of an appropriate treadmill training paradigm. Treadmill training has traditionally been used as a strategy in research to induce physiological adaptations in rodents that facilitate aerobic power (American Physiological Society 2006). To that end, it is well-known that if exercise intensity is high, less volume is required (Pollock and Wenger 1998). Based on the available data we would conclude with some degree of caution that this principle also applies to the use of treadmill training for enhancing axon regeneration after peripheral nerve injury. For example, as few as four minutes of high intensity treadmill locomotion (i.e., two two-minute intervals at 20 m/min in mice (Sabatier et al. 2008)) improves axon regeneration to the same extent as sixty minutes of low intensity treadmill locomotion (i.e., 5 or 10 m/min). Thus only a minimum period of activation of the spinal circuits may be necessary to stimulate axon regeneration provided that intensity is high. It is also important to consider the potential for increasingly negative effects on recovery as treadmill training volume and intensity increase (Kim and Lee 2010). Therefore, future studies are warranted to determine the most effective minimal balance of exercise time and intensity to maximize the effect of treadmill training on the functional outcomes of axon regeneration.
The mechanism of action of treadmill training in enhancing axon regeneration involves neuronal neurotrophins
Using different knockout mice and our grafting protocol, we were able to begin to dissect apart the cellular basis for the effectiveness of treadmill training in enhancing axon regeneration. If axons of wild type host mice were constrained to regenerate into grafts from mice in which the gene for BDNF was knocked out conditionally in all cells, or even only in Schwann cells, very little elongation of regenerating axons was noted (Wilhelm et al. 2009). However, if these mice were treated with two weeks of daily treadmill training, using either a continuous or an interval training paradigm, then regenerating axons were twice as long as found in untrained wild type mice whose nerves were repaired with grafts from wild type mice (controls). A similar result was found in mice in which cut nerves were repaired with grafts from wild type mice that were made acellular by repeated freezing and thawing. Thus the effects of treadmill training are found irrespective of the environment (pathway) through which axons regenerate.
If instead of using wild type (YFP) mice in these experiments, we used mice in which the gene for BDNF was knocked out conditionally in neurons (Young et al. 2008), then a different outcome was obtained. Very sparse axon elongation was found in these conditional BDNF knockout mice when the regenerating axons were constrained to grow through nerve grafts from BDNF knockout mice. Such sparse regeneration was found regardless of whether the animals had been treated with treadmill training (Fig. 3). The effect of treadmill training on axon regeneration thus requires BDNF produced by the regenerating axons themselves. We believe that treadmill training evokes an increased expression of neuronal BDNF and that this stimulates elongation of regenerating axons by autocrine or/and paracrine action through trkB receptors on the growth cones of those axons.
Figure 3.
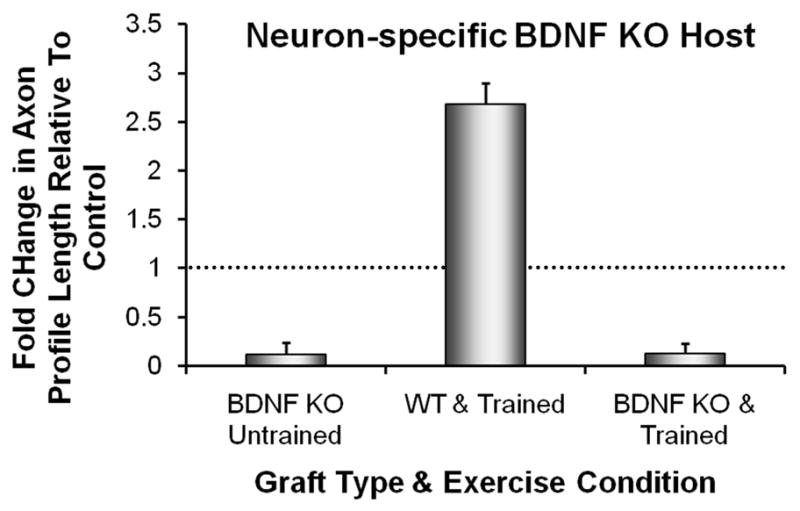
Axon regeneration is not enhanced by treadmill training in neuron-specific BDNF knockout mice. Cut common fibular and tibial nerves in female SLICK::BDNFf/f host mice were repaired with a short length of nerve harvested from non-fluorescent, strain-matched graft donor mice. Some graft donor mice were wild type (WT) and others were systemic BDNF knockout mice (BDNF KO). In the host mice in these experiments, the gene for BDNF was knocked out in motoneurons expressing YFP. The cut YFP+ axons in these mice were constrained to regenerate into an environment lacking (BDNF KO) or containing (WT) BDNF. Animals were trained using an interval training paradigm and the lengths of profiles of YFP+ regenerating axons measured in grafts after two weeks were compared to those found in untrained WT mice whose axons regenerated into WT grafts (Controls). Average median axon profile lengths, expressed as a percentage of untrained Controls (+SEM, N=4 for each), are shown from measurements made in grafts from BDNF KO mice (Untrained on the left, treadmill Trained on the right) and in WT mice (center). The horizontal dashed line at 1.0 indicates the length of regenerating axons in untrained Controls. Data are from Wilhelm et al (2009).
These effects of treadmill training are unlikely due to mobilization of activity-dependent molecules from the target muscles. The enhanced regeneration was found two weeks post-transection, a time well before any muscle reinnervation. In all of the experiments described above, the cut nerves were repaired with grafts from donor mice but they were not re-attached to the target muscles. Thus any muscle-derived growth promoting molecules could influence axon regeneration only if they acted systemically. Since the enhancement of axon regeneration produced by treadmill training was absent in neuron-specific BDNF knockout mice, where muscle BDNF expression is presumed to be normal, we contend that any systemic effect of treadmill training arising from the target muscles was not sufficient to enhance axon regeneration. The observed effects of treadmill training must be due to changes in the neurons themselves.
It is also possible that treadmill training increases the expression of the trkB receptor in the growth cones of regenerating axons. This increase would be expected to result in an increased sensitivity to BDNF in some neurons and novel sensitivity to BDNF in others. Indeed, after different forms of treadmill training, the number of primary afferent neurons that express trkB protein is increased markedly (Wilhelm & English, unpublished), and the trkB content in motoneurons is increased (Macias et al. 2007). Consistent with these observations, if axons from neuron-specific BDNF knockout mice are constrained to regenerate into a nerve graft from a strain-matched wild type mouse, and these mice are treadmill trained, then regenerating axons are much longer than found in untrained control mice (Fig. 3) (Wilhelm et al. 2009). Whether increased expression of trkB is essential for the enhancing effects of treadmill training awaits study of conditional trkB knockout mice.
The role of neural activity in treadmill training
Because of the involvement of BDNF in enhancing axon regeneration found following treadmill training, one might assume that it is the activation of the neurons whose axons are regenerating that initiates the stimulation of axon elongation. Activity in neurons is known to drive the expression of BDNF (Vaynman and Gomez-Pinilla 2005). Thus it is assumed that activation of motoneurons via the spinal pattern generators for locomotion during treadmill training is a critical component of its mechanism of action. The results of our studies of slope training are consistent with this assumption. When animals walk up a 20° slope, the activity in ankle extensor muscles, such as soleus, is increased slightly, but activity in flexor muscles, such as tibialis anterior, is nearly twice the intensity as found during level walking (Sabatier et al. 2010b). Based on this observation, we predicted that treadmill training conducted with the treadmill inclined upward would have a more pronounced enhancing effect on axon regeneration in motoneurons innervating flexor than extensor muscles. Mice in which the sciatic nerve had been cut and repaired by end-to-end anastomosis were trained on an upslope-inclined treadmill for two weeks and then different retrograde fluorescent tracers were applied to the common fibular (flexor) and tibial (extensor) nerves 4 mm distal to the original transection. Numbers of retrogradely labeled motoneurons whose axons had regenerated at least 4 mm were counted in the spinal cord four days later. Counts were compared to those obtained from mice that had been trained on a level treadmill. Significance of differences in mean numbers of motoneurons labeled in the different groups was evaluated using a one-way analysis of variance (ANOVA) and post-hoc paired testing (Fisher’s least significant differences). Probabilities of <0.05 were considered significant. As predicted, the number of labeled motoneurons was increased significantly for both nerves. The increased number of motoneurons labeled from the common fibular nerve in upslope trained mice was especially notable because no significant increase had been found for continuous level treadmill training (Fig. 4) (English et al. 2009). A significant enhancement of axon regeneration was produced by upslope treadmill training in flexor motoneurons whose activity is also increased during upslope locomotion (Sabatier et al. 2010a). If such findings can be corroborated using different approaches that provide more than correlational evidence, we would contend that the paradigm used for training when different nerves are injured could be prescribed based on the optimal activation of the motoneurons whose regeneration would be desired.
Figure 4.
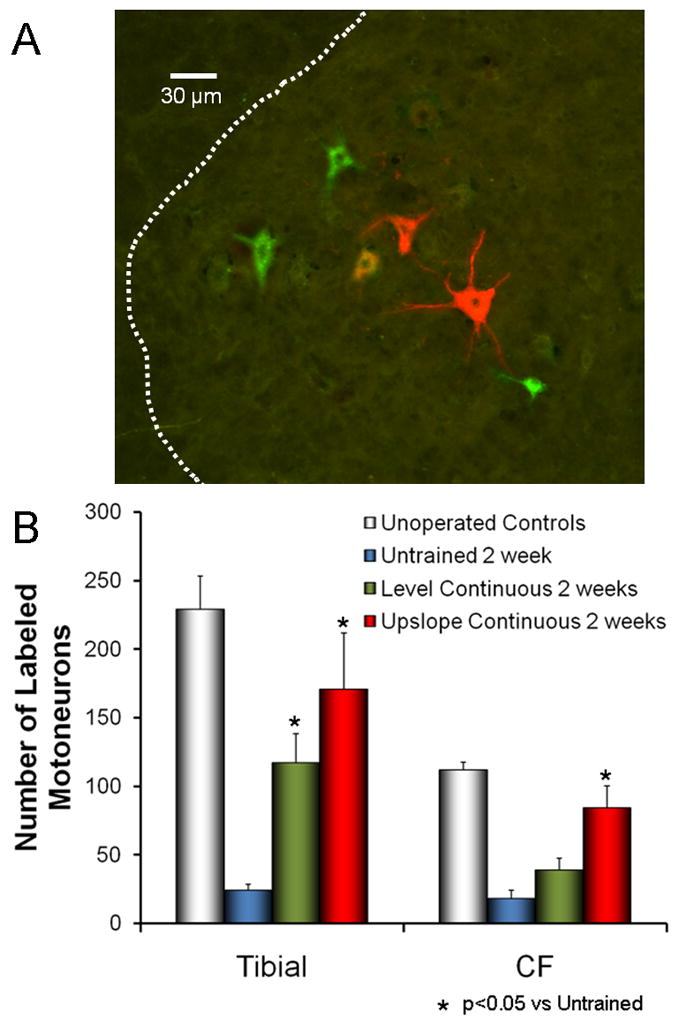
The effects of upslope training on axon regeneration in cut peripheral nerves. The sciatic nerves of mice were cut and repaired by end-to-end anastomosis. Some mice were trained using a continuous training paradigm with the treadmill level and others with the treadmill inclined upwards at 20 degrees. Two weeks after transection, the tibial and common fibular branches were cut 4 mm distal to the original transection and exposed to different retrograde fluorescent tracers. A. Examples of motoneurons retrogradely labeled from red and green fluorescent tracers applied to the tibial and common fibular nerves, respectively. B. Labeled motoneurons with axons regenerating that distance into these two branches were counted in histological sections of the lumbar spinal cord. Comparisons were made between the two groups of trained mice and to intact and untrained (both lesioned and unlesioned) mice. Each bar represents the mean number of labeled motoneurons (±SEM, N=4 for each).
Does treadmill training stimulate axonal protein synthesis?
Since the discovery of axonal transport, it has been assumed that the synthesis of axonal proteins occurred in the somata of neurons and that these proteins were transported down the axon by microtubules at a relatively slow average daily rate (Weiss and Hiscoe 1948). It is now known that this form of anterograde transport is mediated by the same molecular motor, kinesin, used in the much faster transport of organelles, but because the progression of protein transport is not constant, but interrupted, the average rate is slow (Brown 2003). If applied to the elongation of neurites from the proximal segments of cut nerves, then axon regeneration in cut peripheral nerves would be expected to proceed at this same rate, on the order of 1–3 mm/day (Lasek and Hoffman 1976). A compelling case has been made that cytoskeletal proteins required for axon regeneration, such as actin and tubulin, which are constitutively transported in axons, may be sufficient to account for the extent of elongation of axons past injury sites in cut peripheral nerves (Hoffman 2010). However, if mice are treadmill trained during that first post-injury week, more than twice as many axons will have elongated nearly twice as far as in untrained mice. Although one might imagine that treadmill training might increase the synthesis of cytoskeletal proteins, because such stimulation would be found only after the injury, the rapid onset of this effect of treadmill training would be difficult to account for entirely by the traditional view of somatal synthesis and slow transport of axonal proteins.
It is now widely accepted that axonal proteins can be synthesized in axons (Tobias and Koenig 1975). The mRNAs for several axonal proteins are found in axons (Bassell et al. 1998; Krichevsky and Kosik 2001). Further, these mRNAs are known to be transcribed in the neuronal somata and transported in the axons much more rapidly than proteins, after binding to RNA binding proteins known as zip code proteins (Bassell et al. 1998). Based on the rates of transport of these mRNAs, one might anticipate that the relatively rapid regeneration of some axons in cut or crushed peripheral nerves might utilize proteins that are translated and synthesized at or close to the site of injury. Indeed, in mice in which mRNA transport into axons is compromised, axon regeneration after crush injury is diminished (Willis et al, 2011). The response of growth cones to BDNF, which we believe is required for the enhancing effects of treadmill training (see above), is dependent on local protein synthesis (Yao et al. 2006). We would speculate that the rapid enhancement of axon regeneration produced by treadmill training is the result of a stimulation of axonal protein synthesis. Whether the proteins synthesized locally in response to treadmill training are limited to structural proteins required to construct new axons or whether they also involve signaling proteins such as BDNF and its receptor, trkB, is not known at this time.
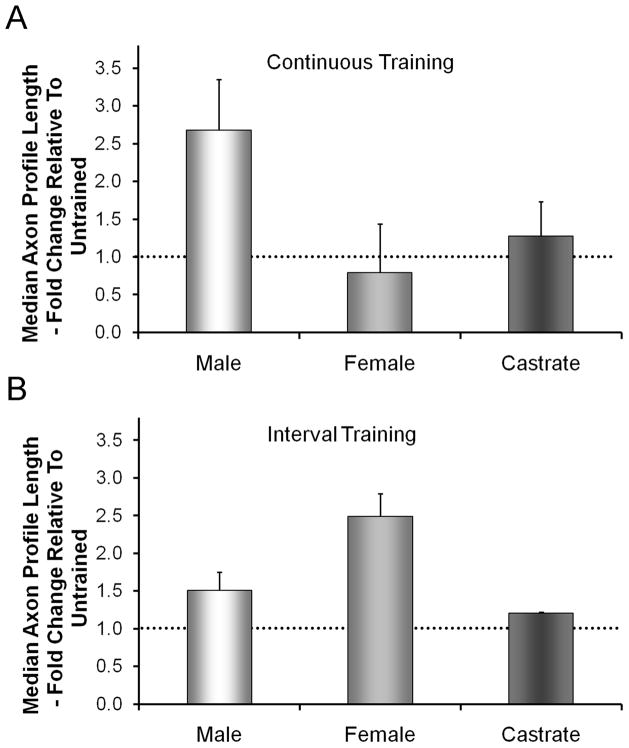
Sex differences in the enhancing effects of treadmill training
When mice are allowed to exercise voluntarily on an exercise wheel, the females run nearly twice as far each night as the males (De Bono et al. 2006). In addition, testosterone is known to be a potent regulator of neuronal expression of BDNF and its receptor, trkB (Osborne et al. 2007; Sharma et al. 2010; Verhovshek et al. 2010). Thus we wondered whether the effects of treadmill training on axon regeneration in cut nerves would be the same in male and female mice. When we examined the sex of the animals used in our original studies, we found that males and females were not represented equally. When we conducted additional experiments to correct for this inequality and analyzed the data separately for males and females, we found a striking sex difference in the effects of our different training paradigms on axon regeneration. No sex difference was found in untrained mice, but after continuous training for one hour a day for two weeks, the lengths of regenerating axons were significantly greater than untrained controls only in males. No significant increase in axon profile lengths were found in females or castrated males (Fig. 5A). In contrast, following daily interval training, significant enhancement of axon regeneration was found only in females and not in either intact or castrated males (Fig. 5B). We believe that these results mean that the mechanism of enhancement of axon regeneration produced by the two different training paradigms must be different. The involvement of training-related androgens in continuously trained males seems clear, but the cellular mechanism by which this effect is exerted awaits further study. The basis for the success of interval training in females is less clear, but may involve localized, non-gonadal production of testosterone by neurons or Schwann cells (Garcia-Ovejero et al. 2005). More work on this interesting topic is needed.
Figure 5.
Sex differences in the effects of treadmill training on axon regeneration in cut peripheral nerves. In each graph, average median axon profile lengths, expressed as a percentage of untrained controls (±SEM, N=4 for each), are shown. The horizontal dashed line at 1.0 indicates the length of regenerating axons in untrained controls. A. If mice are trained using a continuous training paradigm, enhancement is found only in males and not females or castrated males. B. If mice are exercised at intervals, enhanced regeneration is found only in females, not in males or castrated males.
Effects of treadmill training on misdirection of regenerating axons
In the mammalian CNS, motoneurons innervating functionally different groups of muscles are topographically localized (McHanwell and Biscoe 1981; Nicolopoulos-Stournaras and Iles 1983; Swett et al. 1986; Yakovenko et al. 2002). That is, the motor nuclei of different muscle groups lie in spatially distinct locations in the brainstem and spinal cord. Following peripheral nerve injury, individual muscles or even groups of muscles are likely to be reinnervated by a somewhat different cadre of motoneurons than before transection of the nerve. For example, (Ito and Kudo 1994) found that motoneurons reinnervating the guinea pig posterior digastric muscle four weeks after crush of the facial nerve were found in parts of the motor nucleus which do not normally contribute to this innervation. A number of labs have reported that the locations of motoneurons reinnervating different limb muscles were much less spatially restricted than found in intact animals (Brushart and Mesulam 1980; Brushart et al. 1983; Gramsbergen et al. 2000; English 2005). Since the designation of this innervation as different from the pre-denervation condition was made on the basis of the spatial location of the motoneurons in the CNS, we have termed such reinnervation as topographically inappropriate. This inappropriate reinnervation of peripheral targets is the result of misdirection of regenerating axons in the periphery. Such misdirection is said to be a major contribution to the poor functional outcomes noted clinically after peripheral nerve injury (Sperry 1941; Sunderland 1978; Fawcett and Keynes 1990; Fu and Gordon 1997; de Ruiter et al. 2008).
We exploited the topographic organization of the sciatic motor nucleus to study the effects of treadmill training on the extent of misdirection of regenerating axons following sciatic nerve transection and end-to-end repair. Using retrograde fluorescent tracers applied to the cut ends of peripheral nerves, we confirmed that motoneurons whose axons are found in the common fibular nerve are restricted to the rostral-most 60% of the sciatic motor nucleus in intact mice. Thus, any motoneurons whose cell bodies are found in the caudal-most 40% of this nucleus after nerve injury are defined as topographically inappropriate and reflect misdirection of regenerating tibial axons (English 2005). In untrained mice, we found a modest amount of such misdirection; approximately 20% of regenerating motor axons in the common fibular nerve in reinnervated animals had innervated targets of the tibial nerve prior to sciatic nerve transection (English 2005). In mice treated with treadmill training, significantly more motoneurons were labeled with retrograde tracers, indicating that the training had enhanced axon regeneration, but only about 10% of motoneurons with regenerating axons in the common fibular nerve were from motoneurons in topographically inappropriate locations (English et al. 2009). Thus at least for the motoneurons studied, treadmill training results not only in an enhancement of axon regeneration but, at least at the level of reinnervation of functionally antagonistic muscles about the ankle joint, significantly less misdirection of those axons.
Treadmill training and CNS plasticity
Following nerve transection in the periphery, a series of changes occurs in the circuitry of the spinal cord or brainstem. Nearly half of the synaptic inputs onto the somata and proximal dendrites of motoneurons are withdrawn following peripheral axotomy, a process known as synaptic stripping (Blinzinger and Kreutzberg 1968; Hamberger et al. 1970; Lindå et al. 1992; Oliveira et al. 2008). Although the withdrawal of injured primary afferent terminals following peripheral nerve injury can contribute to synaptic stripping (Mendell et al. 2001), there is considerable evidence that a change in motoneuron properties, rather than signals from damaged sensory inputs, initiates synaptic stripping. For example, synaptic stripping is found on brainstem motoneurons following transection of the facial nerve, which does not contain sensory axons (Liebermann 1971; Titmus and Faber 1990). Unlike axotomy-induced changes in the periphery, which can be reversed, albeit imperfectly and imprecisely, once the withdrawal of afferent terminals from motoneurons has occurred, it appears permanent. Reduced numbers of synaptic terminals from primary afferent neurons are found on motoneurons many months after nerve transection, whether or not the nerve was repaired and axon regeneration was successful (Hughes et al. 2004; Alvarez et al. 2010; Chen et al. 2010). This permanent loss is highly correlated with the permanent loss of the stretch reflex in self-reinnervated muscles (Haftel et al. 2005; Alvarez et al. 2010). In addition, over a much longer time course, there is a nearly 50% increase in contact of these motoneurons by synaptic boutons containing GAD-67, the rate limiting enzyme in the synthesis of the inhibitory neurotransmitter, gamma amino butyric acid (Rose & English, unpublished). These changes in neuronal circuitry thus could have an important impact on functional recovery following nerve regeneration.
We have begun to study the effects of treadmill training on axotomy-induced synaptic stripping. The rationale for these studies comes from a series of recent papers that showed that stripping could be reversed by persistent application of recombinant BDNF and/or NT-3 to the proximal stumps of cut abducens nerves (Davis-Lopez de Carrizosa et al. 2009a; Davis-Lopez de Carrizosa et al. 2009b). Some of these treatments were initiated after a two week delay, during which time synaptic inputs were stripped from the axotomized motoneurons. After these delayed treatments, coverage of the motoneuron somata by identified synaptic terminals was found to the same extent and in a composition similar to that found in intact motoneurons, suggesting that the neurotrophin treatments resulted in a restoration of stripped inputs. Because treadmill training results in an increased expression of these neurotrophins in spinal motoneurons (Gomez-Pinilla et al. 2001), we reasoned that it might affect axotomy-induced synaptic stripping. Indeed, after two weeks of modest daily treadmill training, no loss of synaptic inputs from the axotomized motoneurons was found (Fig. 6) (Krakowiak et al. 2010). Using mice in which the gene for BDNF was knocked out conditionally, we found that this effect of treadmill training was at least partially dependent on the availability of BDNF (Krakowiak et al. 2010). Although it is possible that the effect of treadmill training on axotomy-induced synaptic stripping might be viewed as preventing synaptic withdrawal, by analogy to the results of treatments with BDNF treatments cited above and based on preliminary results in our lab (Wilson and English, unpublished), we believe that the training induces some sort of restoration of stripped inputs to the motoneurons.
Figure 6.
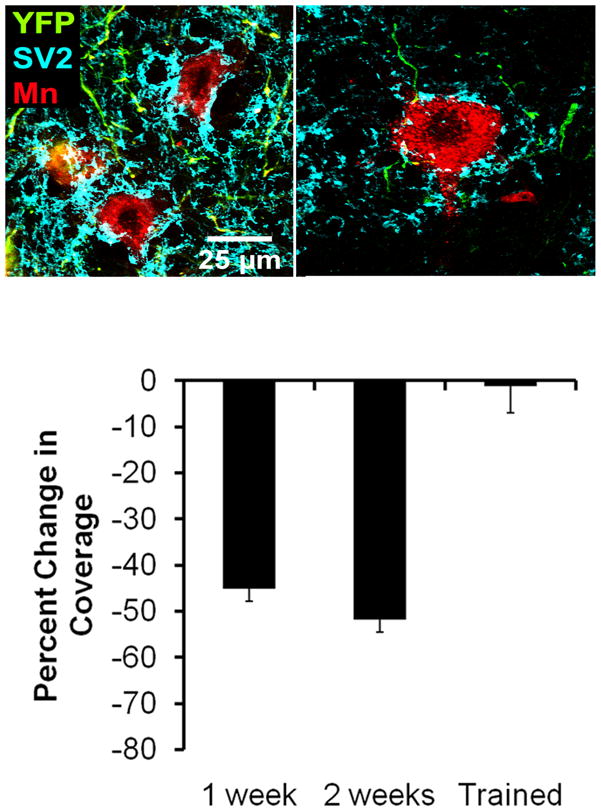
Effects of treadmill training on synaptic stripping following peripheral nerve transection. Images of labeled motoneurons in untrained intact mice and one week following sciatic nerve transection are shown in the top two panels. In these mice, a subset of neurons express yellow fluorescent protein (YFP, shown in green in these panels) and motoneurons innervating the gastrocnemius muscle were marked by injection of a red fluorescent retrograde tracer (cholera toxin B-Alexafluor 555) four days prior to sciatic nerve transection (without repair). Expression of the ubiquitous synaptic vesicle protein, SV2, was demonstrated using immuno-histofluorescence, using a secondary antibody conjugated to Alexafluor 647 (shown as cyan in these panels). Note the robust SV2 immunoreactivity in the intact mouse and the relative paucity of synaptic coverage one week after nerve transection. In a series of trained and untrained mice, the proportion of the soma and proximal dendrites of retrogradely labeled motoneurons contacted by SV2 immune-positive structures was determined. The average change in this synaptic coverage was determined by comparison to coverage in intact and untrained mice and is shown in the bottom panel. Each bar represents the average percent change in synaptic coverage (±SEM, N=4 for each) in untrained mice after 1 or 2 weeks and mice treadmill trained for two weeks. Note that the reduction in coverage of nearly 50% observed in untrained mice is completely absent in trained mice.
The specificity and composition of the input to motoneurons in treadmill trained animals is not yet known but we think that acquiring these data will be important. If treadmill training restores sensory input to motoneurons, although a misdirection of regenerating axons in the periphery (see above) exists, then the net effect on functional recovery might not be desirable.
We believe that establishing the therapeutic window for application of treadmill training as a therapy for patients with peripheral nerve injury will be important. The optimal window would begin early enough to influence both axon regeneration in the periphery and to have an effect on synaptic stripping but late enough that the nature of the training might be used to restore or even shape the nature of the synaptic inputs onto stripped motoneurons in some relationship to the specificity of the targets reinnervated by sensory and motor axons while at the same time permanently restoring those inputs.
Conclusions
Limitations in three different aspects of the biology of responses to traumatic injury to peripheral nerves have been postulated to contribute to the poor functional outcomes observed in human patients. Treadmill training applied following traumatic peripheral nerve injury results in demonstrable improvements in each of these three areas. Regenerating axons grow considerably farther in treadmill trained animals than they do in untrained controls. This enhancement of axon regeneration is achieved without an increase in the misdirection of the regenerating axons in the periphery and without the expected axotomy-induced decrease in synaptic contacts onto motoneurons in the CNS. At each of these levels of inquiry, important questions remain. Clearly a lot more work needs to be done. Most forms of therapeutic exercise are aimed at increasing muscle strength, range of motion of joints, or cardiovascular effects. Since all of the effects of treadmill training described above occur before any muscle reinnervation is found, the effects of treadmill training must be the result of exercising the nervous system. This form of therapeutic exercise thus represents a promising approach to a patient-driven treatment for peripheral nerve injuries.
Acknowledgments
This work was completed with support from NIH grants NS057190 (AWE) and K12GM000680 (JCW and MJS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Bullinger KL, Titus HE, Nardelli P, Cope TC. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injury. Ann NY Acad Sci. 2010;1198:231–241. doi: 10.1111/j.1749-6632.2010.05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Physiological Society. Resource Book for the Design of Animal Exercise Protocols. American Physiological Society; 2006. [Google Scholar]
- Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Basso M, Behrman A, Harkema S. Treadmill training after spinal cord injury: good but not better. Neurology. 2006;67:1900–1901. doi: 10.1212/01.wnl.0000249080.15391.6d. author reply 1901–1902. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Zhang H, Byrd AL, Femino AM, Singer RH, Taneja KL, Lifshitz LM, Herman IM, Kosik KS. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci. 1998;18:251–265. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Boeltz T, Ireland M, Mathis K, Nicolini J, Poplavski K, Rose SJ, Wilson E, Sabatier MJ, English AW. Treadmill training and functional recovery after peripheral nerve injury. Abstr Soc Neurosci. 2010 doi: 10.1152/jn.00946.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003;160:817–821. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Nerve repair and grafting. In: Green D, Hotchkiss R, Pederson W, editors. Green’s operative hand surgery. New York: Churchill Livingstone; 1998. pp. 1381–1403. [Google Scholar]
- Brushart TM, Mesulam MM. Alteration in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science. 1980;208:603–605. doi: 10.1126/science.7367884. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Tarlov EC, Mesulam MM. Specificity of muscle reinnervation after epineurial and individual fascicular suture of the rat sciatic nerve. J Hand Surg [Am] 1983;8:248–253. doi: 10.1016/s0363-5023(83)80152-x. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Early locomotor training with clonidine in spinal cats. J Neurophysiol. 1998;79:392–409. doi: 10.1152/jn.1998.79.1.392. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Chen L, Sun C, English AW, Wolpaw JR, Chen XY. H-Reflex up conditioning encourages recovery of EMG activity and H reflexes after sciatic nerve transection and repair in rats. J Neurosci. 2010;30:16128–16136. doi: 10.1523/JNEUROSCI.4578-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Lopez de Carrizosa M, Morado-Diaz C, Tena J, Benitez-Temino B, Pecero M, Morcuende SR, de la Cruz R, AP Complimentary actions of BDNF and Neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci. 2009a;29:575–587. doi: 10.1523/JNEUROSCI.5312-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Lopez de Carrizosa MA, Morado-Diaz CJ, Morcuende S, de la Cruz RR, Pastor AM. Nerve growth factor regulates the firing patterns and synaptic composition of motoneurons. J Neurosci. 2009b;30:8308–8319. doi: 10.1523/JNEUROSCI.0719-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- de Ruiter GC, Malessy MJ, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, Kaufman KR, Dyck PJ, Windebank AJ. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, de Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Use-dependent plasticity in spinal stepping and standing. Adv Neurol. 1997;72:233–247. [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- English AW, Cucoranu D, Mulligan A, Sabatier M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J Comp Neurol. 2009;517:245–255. doi: 10.1002/cne.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PJ, Bain JR, Mackinnon SE, Makino AP, Hunter DA. Selective reinnervation: a comparison of recovery following microsuture and conduit nerve repair. Brain Res. 1991;559:315–321. doi: 10.1016/0006-8993(91)90018-q. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Ann Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48:273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26:E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A, Ijkema-Paassen J, Meek MF. Sciatic nerve transection in the adult rat: abnormal EMG patterns during locomotion by aberrant innervation of hindleg muscles. Exp Neurol. 2000;161:183–193. doi: 10.1006/exnr.1999.7233. [DOI] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Wang QB, Prather JF, Pinter MJ, Cope TC. Central suppression of regenerated proprioceptive afferents. J Neurosci. 2005;25:4733–4742. doi: 10.1523/JNEUROSCI.4895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger A, Hansson HA, Sjöstrand J. Surface structure of isolated neurons. Detachment of nerve terminals during axon regeneration. J Cell Biol. 1970;47:319–331. doi: 10.1083/jcb.47.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng C, de Leon RD. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp Neurol. 2009;216:139–147. doi: 10.1016/j.expneurol.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PN. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp Neurol. 2010;223:11–18. doi: 10.1016/j.expneurol.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Polgar E, Shehab SA, Todd AJ. Peripheral axotomy induces depletion of the vesicular glutamate transporter VGLUT1 in central terminals of myelinated afferent fibres in the rat spinal cord. Brain Res. 2004;1017:69–76. doi: 10.1016/j.brainres.2004.05.054. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Ilha J, Araujo RT, Malysz T, Hermel EE, Rigon P, Xavier LL, Achaval M. Endurance and resistance exercise training programs elicit specific effects on sciatic nerve regeneration after experimental traumatic lesion in rats. Neurorehabil Neural Repair. 2008;22:355–366. doi: 10.1177/1545968307313502. [DOI] [PubMed] [Google Scholar]
- Ito M, Kudo M. Reinnervation by axon collaterals from single facial motoneurons to multiple muscle targets following axotomy in the adult guinea pig. Acta Anat. 1994;151:124–130. doi: 10.1159/000147653. [DOI] [PubMed] [Google Scholar]
- Kim WS, Lee SU. Harmful effect of land-based endurance exercise in rats with diabetic nerve. Med Sci Sports Exerc. 2010;42:1625–1631. doi: 10.1249/MSS.0b013e3181d58e09. [DOI] [PubMed] [Google Scholar]
- Krakowiak JR, Wilhelm JC, English AW. Effect of treadmill training on synaptic stripping of axotomized mouse motoneurons. Abstr Soc Neurosci 2010 [Google Scholar]
- Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Lasek RJ, Hoffman PN. The neuronal cytoskeleton, axonal transport and axonal growth. Cold Spring Harb Symp Conf Cell Proliferation. 1976;3:1021–1049. [Google Scholar]
- Liebermann A. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Lindå H, Cullheim S, Risling M. A light and electron microscopic study of intracellularly HRP-labelled lumbar motoneurons after intramedullary axotomy in the adult cat. J Comp Neurol. 1992;318:188–208. doi: 10.1002/cne.903180205. [DOI] [PubMed] [Google Scholar]
- Macias M, Dwornik A, Ziemlinska E, Fehr S, Schachner M, Czarkowska-Bauch J, Skup M. Locomotor exercise alters expression of pro-brain-derived neurotrophic factor, brain-derived neurotrophic factor and its receptor TrkB in the spinal cord of adult rats. Eur J Neurosci. 2007;25:2425–2444. doi: 10.1111/j.1460-9568.2007.05498.x. [DOI] [PubMed] [Google Scholar]
- Marqueste T, Alliez JR, Alluin O, Jammes Y, Decherchi P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J Appl Physiol. 2004;96:1988–1995. doi: 10.1152/japplphysiol.00775.2003. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Munson JB, Arvanian VL. Neurotrophins and synaptic plasticity in the mammalian spinal cord. Journal of Physiology. 2001;533:91–97. doi: 10.1111/j.1469-7793.2001.0091b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983;217:75–85. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- Oliveira A, Thams S, Lidman O, Piehl F, Hökfelt T, Kärre K, Lindå H, Cullheim S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. PNAS. 2008;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007;85:303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-1 and insulin-like growth factor 1 after focal ischemia. Neuroscience. 2005;136:991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Wenger NK. Physical Activity and Exercise Training in the Elderly: A Position Paper from the Society of Geriatric Cardiology. Am J Geriatr Cardiol. 1998;7:45–46. [PubMed] [Google Scholar]
- Sabatier M, Redmon N, Schwartz G, English A. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, Kaufman M, English AW. Effects of upslope treadmill exercise on axon regeneration in peripheral nerves. Abstr Amer Cong Sports Medicine 2010a [Google Scholar]
- Sabatier MJ, To BN, Nicolini J, English AW. Effect of slope and sciatic nerve injury on ankle muscle recruitment and hindlimb kinematics during walking in the rat. J Exp Biol. 2010b doi: 10.1242/jeb.051508. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo TB, Han IS, Yoon JH, Hong KE, Yoon SJ, Namgung U. Involvement of Cdc2 in axonal regeneration enhanced by exercise training in rats. Med Sci Sports Exerc. 2006;38:1267–1276. doi: 10.1249/01.mss.0000227311.00976.68. [DOI] [PubMed] [Google Scholar]
- Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol. 2010;223:183–191. doi: 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–131. [PubMed] [Google Scholar]
- Soucy M, Seburn K, Gardiner P. Is increased voluntary motor activity beneficial or detrimental during the period of motor nerve regeneration/reinnervation? Can J Appl Physiol. 1996;21:218–224. doi: 10.1139/h96-018. [DOI] [PubMed] [Google Scholar]
- Sperry RW. The effect of crossing nerves to antagonistic muscles in the hind limb of the rat. J Comp Neurol. 1941;75:1–19. [Google Scholar]
- Sunderland S. Nerve and Nerve Injuries. Edinburgh: Livingstone; 1978. [Google Scholar]
- Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990;13:771–784. doi: 10.1002/mus.880130903. [DOI] [PubMed] [Google Scholar]
- Swett JE, PWR, Blanks RH, Swett AL, Conley LC. Motoneurons of the rat sciatic nerve. Exp Neurol. 1986;93:227–252. doi: 10.1016/0014-4886(86)90161-5. [DOI] [PubMed] [Google Scholar]
- Titmus M, Faber D. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol. 1990;35:1–51. doi: 10.1016/0301-0082(90)90039-j. [DOI] [PubMed] [Google Scholar]
- Tobias GS, Koenig E. Axonal protein synthesizing activity in motoneurons during the early outgrowth period following neurotomy. Exp Neurol. 1975;49:221–234. doi: 10.1016/0014-4886(75)90206-x. [DOI] [PubMed] [Google Scholar]
- van Meeteren NL, Brakkee JH, Hamers FP, Helders PJ, Gispen WH. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch Phys Med Rehabil. 1997;78:70–77. doi: 10.1016/s0003-9993(97)90013-7. [DOI] [PubMed] [Google Scholar]
- van Meeteren NL, Brakkee JH, Helders PJ, Gispen WH. The effect of exercise training on functional recovery after sciatic nerve crush in the rat. J Peripher Nerv Syst. 1998;3:277–282. [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, Osborne MC, Sengelaub DR. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology. 2010;151:253–261. doi: 10.1210/en.2009-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P, Hiscoe HB. Experiments on the mechanism of nerve growth. J Exp Zool. 1948;107:315–393. doi: 10.1002/jez.1401070302. [DOI] [PubMed] [Google Scholar]
- Wessels M, Lucas C, Eriks I, de Groot S. Body weight-supported gait training for restoration of walking in people with an incomplete spinal cord injury: a systematic review. J Rehabil Med. 2010;42:513–519. doi: 10.2340/16501977-0525. [DOI] [PubMed] [Google Scholar]
- Wilhelm JC, Cucoranu D, Gu J, Mulligan A, English AW. Limited peripheral axon regeneration in conditional BDNF knockout mice. Soc Neurosci Abstr. 2009:510.518. [Google Scholar]
- Yakovenko S, Mushahwar V, VanderHorst V, Holstege G, Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol. 2002;87:1542–1553. doi: 10.1152/jn.00479.2001. [DOI] [PubMed] [Google Scholar]
- Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci. 2006;9:1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Young P, Qiu L, Wang D, Zhao S, Gross J, Feng G. Single-neuron labeling with inducible Cre-mediated knockout in transgenic mice. Nature Neuroscience. 2008;11:721–728. doi: 10.1038/nn.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]