Abstract
Tinnitus is a phantom sensation of sound in the absence of external stimulation. However, external stimulation, particularly electric stimulation via a cochlear implant, has been shown to suppress tinnitus. Different from traditional methods of delivering speech sounds or high-rate (>2,000 Hz) stimulation, the present study found a unique unilaterally-deafened cochlear implant subject whose tinnitus was completely suppressed by a low-rate (<100 Hz) stimulus, delivered at a level softer than tinnitus to the apical part of the cochlea. Taking advantage of this novel finding, the present study compared both event-related and spontaneous cortical activities in the same subject between the tinnitus-present and tinnitus-suppressed states. Compared with the results obtained in the tinnitus-presentstate, the low-rate stimulus reduced cortical N100 potentials while increasing the spontaneous alpha power in the auditory cortex. These results are consistent with previous neurophysiological studies employing subjects with and without tinnitus and shed light on both tinnitus mechanism and treatment.
Keywords: Tinnitus, Electric stimulation, Cochlear Implant, Loudness, Cortical potentials, N100, Alpha rhythm
1. Introduction
Tinnitus is a phantom sensation of sound that affects 10–15% of the general population and may cause many audiological, cognitive and neurological issues ranging from hearing and attention deficits to depression and even suicide (e.g., Henry et al., 2005; Lockwood et al., 2002). Despite intensive research into therapeutic options, including surgery, pharmacotherapy, and stimulation with lasers and magnetic fields, there is no FDA-approved treatment for tinnitus at present.
Although significant progress has been made to the elucidation of tinnitus mechanisms over the past 15 years, much remains to be learned. Historically, tinnitus had been thought to reside solely within the ear itself. As an extreme example, van Gogh actually cut his ear off given the mistaken notion that this may rid him of his debilitating tinnitus. Recent animal and human imaging studies have linked tinnitus to abnormal, usually hyper-excitable brain activities that are likely caused by absent or disordered auditory signals, and can be modulated by the limbic system (e.g., Kaltenbach et al., 2005; Rauschecker et al., 2010; Roberts et al., 2010). Such diffuse central disorders have been very difficult to document objectively and non-invasively. Earlier work examined the association of tinnitus with an onset, auditory cortical negative component, N100 (e.g., Attias et al., 1993; Jacobson et al., 2003; Kadner et al., 2002), while recent work focused on quantitative changes of auditory state-state responses (ASSR) and spontaneous brain activities in the resting state (e.g., Diesch et al., 2010; Weisz et al., 2005).
Clinically, sound therapy has been used to either mask or desensitize tinnitus (Jastreboff, 2007), which, when combined with counseling, can be effective in tinnitus management (Jastreboff, 1990). As a unique form of sound therapy, electric stimulation with galvanic direct-current was used to treat tinnitus at least 120 years ago (Althaus, 1886). Modern electric stimulation via a cochlear implant can suppress tinnitus, presumably as a secondary effect of treating deafness (e.g., Miyamoto et al., 1997). In particular, high-rate (>2,000 Hz) electric stimulation has been shownto restore pseudo spontaneous neural activity and to suppress tinnitus (Rubinstein et al., 2003). Encouraged by these findings, cochlear implantation has now been used to specifically treat patients with unilateral tinnitus and deafness (Kleinjung et al., 2009; Van de Heyning et al., 2008). At present, only the standard program optimized for speech recognition is used in tinnitus management via cochlear implants. Given the opposite goal between speech recognition and tinnitus suppression, with the former restoring sound and the latter restoring silence, it is possible that using cochlear implants to recognize speech and suppress tinnitus requires different stimulation patterns and parameters.
We present our findings in a unique, unilaterally-deafened subject who received a cochlear implant solely for the purpose of tinnitus control. This individual experiences total subjective tinnitus suppression only in response to low-rate electrical stimulation. In addition, we recorded and compared within-subject cortical potentials recorded during low-rate and high-rate stimulation. The objective electrophysiological measures showed that low-rate stimulation abated the abnormal cortical activities in this subject, and were consistent with the subjective report of total tinnitus suppression.
2. Methods
2.1. Subject
A 46-year-old male audio engineer and musician presented with a 1.5-year history of debilitating tinnitus following an idiopathic profound sudden sensorineural hearing lossin the right ear. His symptoms were refractory to habituation therapy and maximal medical treatment, including high doses of benzodiazepines, antidepressants, and other hypnotics. Despite the fact that he had normal hearing in his left ear, he was offered cochlear implantationin his right ear with the hope of controlling his tinnitus with electrical stimulation. Implantation was considered for this unconventional indication given the profound impact of his symptoms and lack of other options. The patient underwent implantation with a Clarion HiRes 90K™ device from Advanced Bionics Corporation (Valencia, CA), and used his device regularly. He reported some auditory benefit, although his tinnitus persisted despite the implementation of a variety of conventional stimulation strategies.
His pure tone thresholds in the left ear were normal (<20 dB HL) at all octave frequencies from 250 to 8,000 Hz except for a mild loss of 35 dB HL at 4,000 Hz. Post-surgically, his thresholds in the deafenedright ear were at 100 dB HL or higher. Otoacoustic emission and auditory brainstem responses were normal in the left ear but totally absent in the right ear. The subject showed no sign of hyperacusis, as his loudness growth in response to either acoustic stimulation in the left ear or electric stimulation in the right ear was typical and covered the normal dynamic range. Sentence recognition scores in quiet were 100% for the left ear, 0% for the right ear without the cochlear implant, and 70% with the cochlear implant.
The patient described his tinnitus as a constant high pitched screech, subjectively rated as 4 to 6 on a 0–10 scale, with 10 being maximum imaginable loudness. Superimposed on this, the patient experienced episodic “spikes” of intolerable noise rated as 10/10. He was able to match his tinnitus in his deaf right ear to a pure tone of 4,000–8,000 Hz and 70–80 dB SPL in his normal left ear.
2.2. Psychophysical method
The methods of electric stimulation and experimental procedures used have been described elsewhere (Tang et al., 2006). Briefly, electric stimuli were generated and controlled by a research interface, the BionicEar Data Collection System software (BEDCS v1.17.208, 2006, Advanced Bionics Corporation, Valencia, CA). The stimuli were charge-balanced, biphasic pulses and delivered to the subject at various loudness levels using monopolar mode (one electrode located in the cochlea and the other located extracochlear, deep to the temporalis muscle). Stimulus rate ranged from 10 to 5,000 Hz and stimulus duration was 360 seconds. Before the onset of the stimulus, the subject reported his tinnitus baseline level on a loudness scale from 0 to 10, with 0 being inaudible and 10 uncomfortably loud. At the onset of the stimulus and every 30 seconds afterwards, the subject reported the loudness rating of both his tinnitus and the stimulus. To examine after effects of electric stimulation, the subject continued to report the loudness rating of his tinnitus for another 60–360 seconds after the stimulus was turned off.
2.3. Neurophysiological method
Auditory cortical potentials were recorded in the same subject during either an experimental condition in which electric stimulation produced total tinnitus suppression (Electrodes 1 and 0 at a rate of 80 Hz) or a control condition in which electric stimulation produced no suppression (Electrodes 1 and 0 with a rate of 200 Hz). A 64-channel Neuroscan SynAmps recording system (Compumedics Neuroscan, Charlotte, NC) was used, in which only 62 channels yielded valid measurements because the implant was directly below electrodes CP7 and P8. All electrode impedances were kept below 10 kΩ. Lateral and vertical eye movements were monitored using two bipolar electrodes above and below the right eye and two bipolar electrodes on the left and right outer canthi for defining the electro-oculogram. Signals were digitized at 1000 Hz, amplified, and band-pass filtered between 0.05 and 200 Hz. Independent component analysis (ICA) was used to remove electric stimulation and eye movement artifacts. Electric stimulation artifacts were identified based on the frequency spectra of the independent components that were maximal at the stimulation rate, namely, 80 or 200 Hz. Typically, the top 5 independent components were contributed by the artifacts and needed to be removed to reconstruct the actual cortical potentials.
The present auditory stimulation and recording parameters were chosen to minimize neural events arising from subcortical (i.e., brainstem) sources while emphasizing neural events from cortical sources. For example, 200 stimulus repetitions were used because the signal to noise ratio of cortical responses is considerably greater than that of brainstem responses, with the latter typically requiring a few 1000 repetitions. In addition, the present low filter cutoff frequency and digitization rate should attenuate brainstem responses, which occur at much shorter latencies and higher frequencies compared to cortical responses.
Three types of cortical responses were measured and compared between the tinnitus-present and tinnitus-suppressed states. First, cortical responses (N100) to abrupt frequency changes from an ongoing 4,000 Hz tone to a 6,000 Hz tone (returning to the 4,000 Hz baseline after 100 ms) were examined while the subject passively watched a closed captioned movie (Dimitrijevic et al., 2008). The frequency change stimulus was repeated 200 times and presented at 80 dB SPL to the subject’s left (unimplanted) ear using Etymotic -2A insert earphones.
Second, stimulus-aligned epochs were extracted using a −1 to 1s window relative to onset of the frequency change stimulus. Time-frequency decompositions were performed using five-cycle Morlet wavelet (Maris et al., 2007). Percent change from the pre-stimulus baseline oscillatory power was calculated as a function of frequency and deemed to deviate from the baseline significantly if p<0.05 using a bootstrap procedure (Delorme et al., 2004).
Third, spontaneous cortical activities were compared between tinnitus-present and tinnitus-suppressed conditions. The pre-stimulus baseline was used to measure spontaneous EEG activity and consistedof 200, one-second segments of EEG prior to the acoustic frequency change stimulus. Differences in the spontaneous oscillatory power between tinnitus-present and tinnitus-suppressed states were assessed across scalp topographies using a non-parametric t-test cluster analysis (Maris and Oostenveld, 2007). A minimum of two electrodes constituted one cluster and differences of p<0.025 were considered significant. The reference distribution was created by means of a Monte Carlo method with 500 random samples.
3. Results
3.1. Psychophysical results
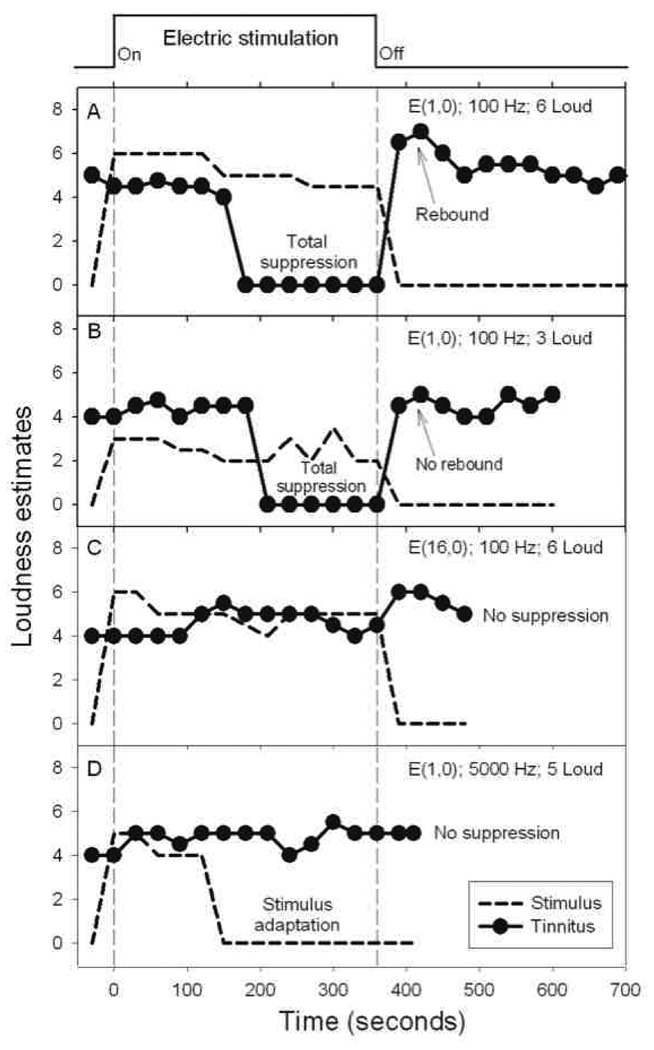
Initial search focused on high-rate (>2,000 Hz) stimuli with different electrode configurations and stimulation patterns, but yielded no success. Frequently the subject reported total adaption to the high-rate stimulation but no effect on his tinnitus (Figure 1D).
Figure 1.
Tinnitus response to electric stimulation. Perceived loudness (y-axis) of tinnitus (circles) and electric stimulus (dashed lines) as a function of time in four experimental conditions. The top trace shows the time course of the 360-second stimulus. (A) Tinnitus response to a 100-Hz, 100 µA, bi-phasic (107.8 µSecond/phase) pulse train, presented at a comfortable loudness level and delivered to the most apical electrode in the cochlea and a remote reference electrode, denoted as E(1,0). (B) Tinnitus response to the same stimulus as in (A), except for presenting at a softer loudness level. (C) Tinnitus response to the same stimulus as in (A), except for stimulating the most basal electrode E(16,0). (D) Tinnitus response to a 5,000-Hz, 150 µA, bi-phasic (32.3 µSecond/phase) pulse train delivered to the most apical electrode E(1,0).
After numerous unsuccessful sessions with high-rate stimulation, the subject reported a surprising result when a 100-Hz low-rate stimulus was presented at a comfortable level to the subject’s most apical intra-cochlear electrode (Figure 1A). During the first 150 seconds of this low-rate stimulus, nothing happened as the subject perceived both the stimulus and his tinnitus. Surprisingly at the 180-second mark, the subject, for the first time in two years, could not hear any of the high-pitched tinnitus. All he heard was a calming, pleasant tone produced by the low-rate stimulus. The subject described this brief period as a high-level of continued relief.
However, immediately after terminating the low-rate electric stimulus, the subject heard an unpleasant “rebound” in tinnitus that was louder than the baseline and took 100 seconds to recover (Figure 1A). Two stimulus manipulations were devised to avoid this undesirable rebound. The first manipulation was to present the low-rate stimulus at a softer level, which also produced total tinnitus suppression albeit requiring an additional 30 seconds to do so (Figure 1B). Tinnitus was continuously suppressed during low-rate stimulation and returned to the pre-stimulation baseline but without any rebound when the stimulus was turned off. The second effective manipulation was to introduce a gradual offset ramp in the low-rate electric stimulus waveform (60–180 seconds, data not shown).
In contrast to total suppression using the most apical electrode, the same low-rate stimulation applied to the most basal electrode produced neither stimulus adaptation nor tinnitus suppression (Figure 1C). Subsequent parametric studies revealed that tinnitus suppression depended on a relatively narrow range of stimulation rate between 20 and 100 Hz, stimulation place for the 4 most apical electrodes, and stimulation level between 1 and 6 loud. Subthreshold electric stimulation was attempted but did not produce any tinnitus suppression.
3.2. Neurophysiological results
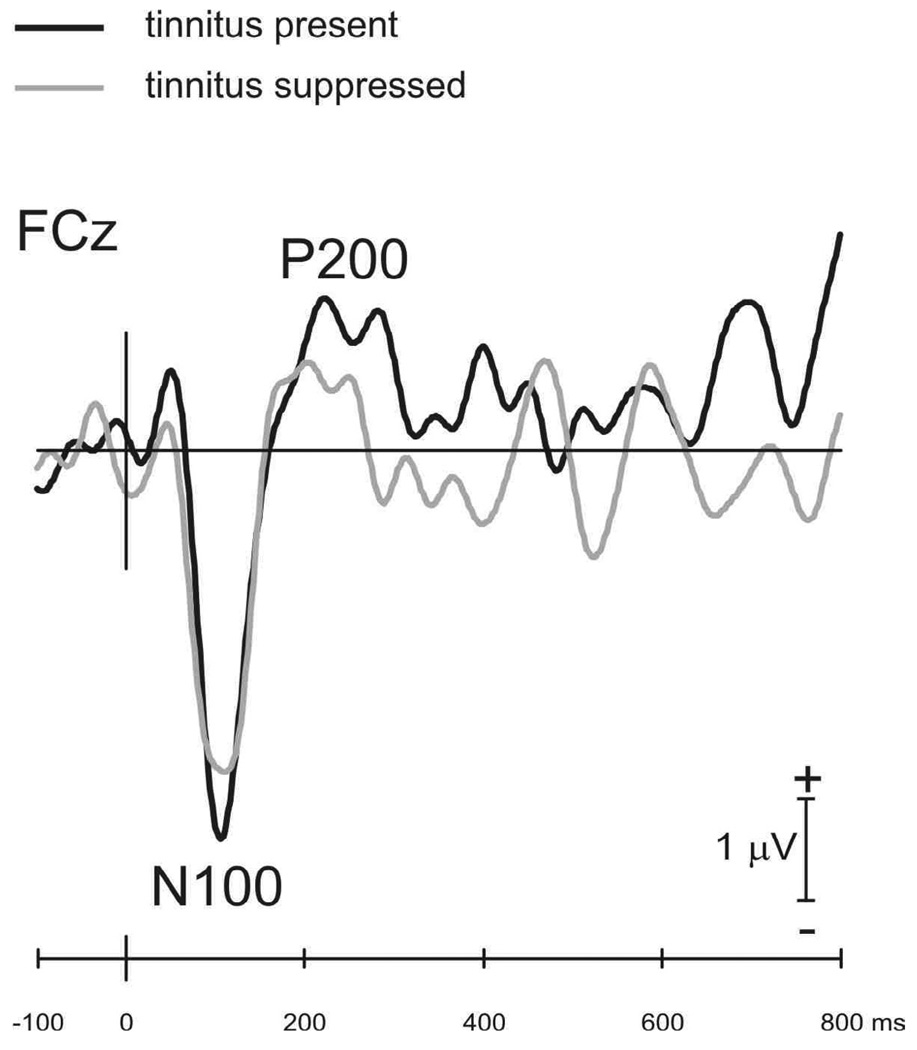
Taking advantage of the above psychophysical results, cortical responses to a frequency change in a continuous tone were recorded between a 200-Hz electric stimulus that produced no tinnitus suppression (solid trace in Figure 2) and an 80-Hz stimulus that produced total suppression (grey trace). Compared with N100 under the condition when tinnitus was present (−3.9 mV in magnitude and 106 ms in latency), N100 was reduced and delayed (−3.2 mV and 110 ms) when tinnitus was suppressed.
Figure 2.
Cortical potentials recorded at electrode FCz in response to a 50% increase in frequency in a 4000 Hz continuous tone. Cortical potentials were compared between an experimental condition when an 80-Hz electric stimulus totally suppressed tinnitus (grey trace) and a control condition when a 200-Hz electric stimulus produced no effect on tinnitus (solid trace).
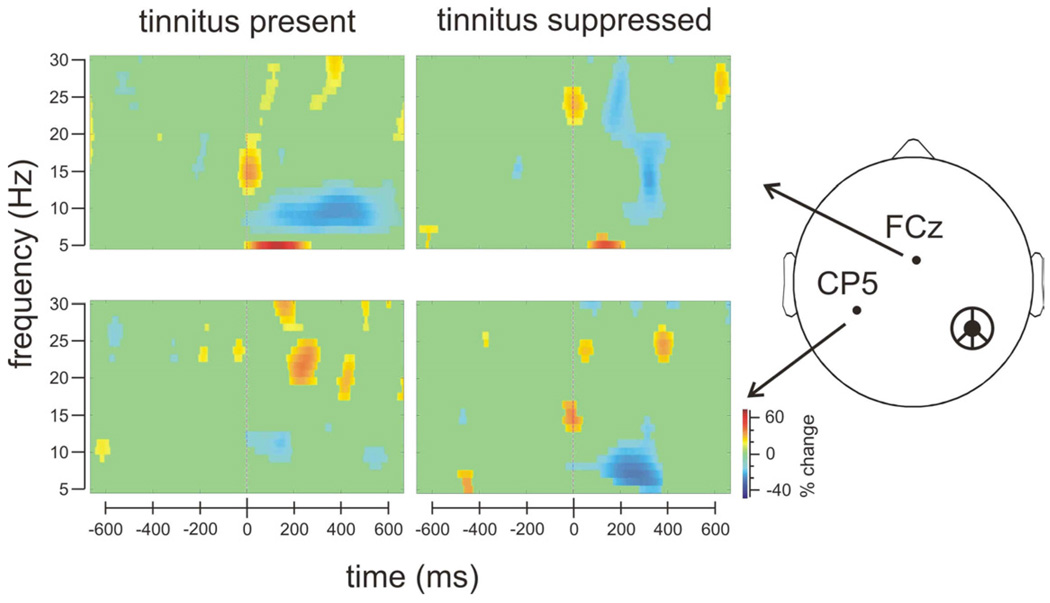
Time-frequency representations of the cortical responses to the frequency change stimulus were contrasted between the tinnitus-present state (left panelsin Figure 3) and tinnitus-suppressed state (right panels). At the frontal-center electrode FCz (top panels), the frequency change stimulus elicited significant alpha desynchronization (7–9 Hz over 100–500 ms post-stimulus period, top-left panel) when tinnitus was present, but no such desynchronization when tinnitus was suppressed (top-right panel). At the contralateral temporal electrode CP5 (bottom panels), there was no significant alpha desynchronization when tinnitus was present (bottom-left panel) but significant alpha desynchronization when tinnitus was suppressed (bottom-right panel). The increased N100 and P200 potentials with tinnitus perception (Figure 2) are likely related to reduced post-stimulus alpha power in the frontal-center region.
Figure 3.
Time-frequency representations of cortical responses to a frequency change stimulus that were recorded a frontal-center electrode, FCz (top panels) and a temporal electrode, CP5 (contralateral to the cochlear implant, bottom panels). Scale is relative to the pre-stimulus baseline as percent increases (red) and percent decreases (blue).
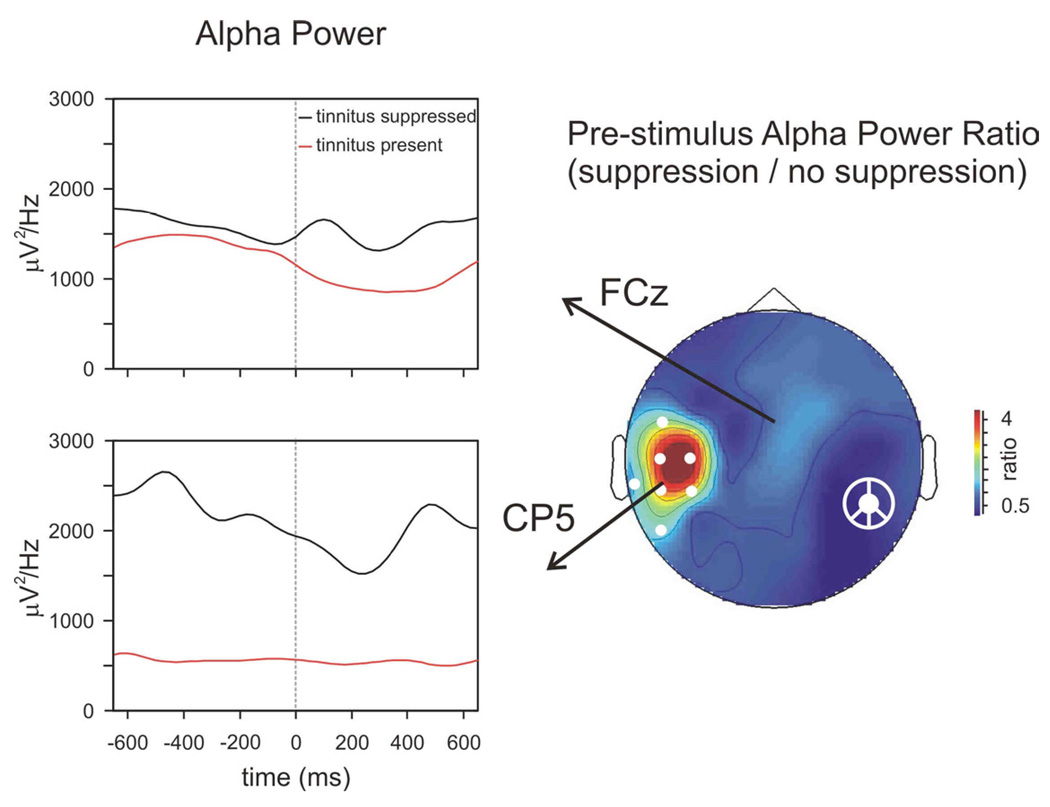
Figure 4 directly compares alpha power (7–9 Hz) with and without tinnitussuppression. The time course of raw alpha power changes is shown for electrodes FCz and CP5. The topography of the pre-stimulus alpha power ratio between tinnitus suppressed and tinnitus present states shows that tinnitus suppression is associated with increased activity at temporal sites contralateral to the cochlear implant. This pre-stimulus baseline activity can be considered a measure of differences in spontaneous cortical activity between the tinnitus-present and tinnitus-suppressed states.
Figure 4.
Comparison of alpha power (7–9 Hz) between tinnitus-present and tinnitus-suppressed states. The pre-stimulus baseline alpha activity was significantly reduced during tinnitus (significant clusters shown as white circles). The ratio of the pre-stimulus alpha power between the tinnitus-suppressed and tinnitus-present states is plotted topographically.
4. Discussion
Taking advantage of the novel finding that low-rate electric stimulation abolished tinnitus, the present study was able to directly compare cortical activities in the same subject between his tinnitus-present and tinnitus-suppressed conditions. This within-subject design departed from a between-subjects design involving individuals with and without tinnitus in most previous neurophysiological studies (e.g., Attias et al., 1993; Kadner et al., 2002; Osaki et al., 2005; Weisz et al., 2005), but produced similar findings. The present results shed light on both tinnitus mechanism and management.
4.1. What is the neural basis of tinnitus?
Differences in ongoing brain oscillations have been quantified across tinnitus and control groups, showing that tinnitus is associated with increases in delta (1–4 Hz) and gamma (>50 Hz) activities, but decreases in alpha (8–12 Hz) activity (Weisz et al., 2007). Alpha oscillation is not simply an idling rhythm; its reduction may reflect thalamocortical reorganization in response to sensory injury and can reset the cortical activity phase to influence the event-related potentials (e.g., Hanslmayr et al., 2007; Llinas et al., 1999). The present results are consistent with previous findings of spontaneous alpha reduction in tinnitus subjects and support the notion that decreased alpha power represents a hyperexcitable cortical state during tinnitus (Weisz et al., 2007). When the stimulus frequency was inside the “tinnitus zone” as in this case (4000 Hz), greater than normal N100 was observed (Kadner et al., 2002). The present study clearly demonstrated in the same subject that, when the subjective tinnitus was abolished by the low-rate electric stimulation, normal cortical state was restored as reflected by reduced N100 and increased alpha power.
4.2. How can low-rate electric stimulation abolish tinnitus?
Tinnitus most likely reflects cortical changes in response to sensory deprivation. Although several cortical changes from tonotopic reorganization to increased spontaneous activity have been related to tinnitus, the neural basis has not been unequivocally confirmed (e.g., Eggermont et al., 2004; Roberts et al., 2006). In patients with cochlear implants, electric stimulation of otherwise silent nerve terminals (Kiang et al., 1972) may restore partially normal central activation patterns (Gilley et al., 2008). The patterned electric stimulation is sufficient for speech perception but may not be strong enough to interfere complicated pathological changes associated with tinnitus. High-rate stimulation can restore spontaneous activity at the peripheral level (Rubinstein et al., 1999), but is not effective in inducing robust central activity (Middlebrooks, 2008). In contrast, low rate stimulation below 100 Hz can induce robust, strong, and highly-synchronized thalamocortical activities (Lu et al., 2001; Snyder et al., 2000). Perhaps this strong stimulus-induced central activity is needed to interfere or suppress the tinnitus-induced abnormal central activity. The finding that this low-rate stimulus suppresses tinnitus through an apical but not a basal intra-cochlear electrode suggests an additional cochlear-place-sensitive mechanism. This place mechanism might be related to restoration of lateral inhibition from the stimulus-induced low-frequency region to the tinnitus-located high-frequency region at either the brainstem level or the cortical level or both (e.g., Don et al., 1978; Gerken, 1996; Kadner et al., 2002).
Note also that the present low-rate suppression differs from the masking traditionally used in treating tinnitus (Hazell et al., 1981; Vernon, 2000). First, the suppressor can be softer than the tinnitus whereas an effective masker has to be louder to cover the tinnitus. Second, the pitch of the suppressor is much lower than the tinnitus whereas the pitch of the masker needs to be close to the tinnitus. Third, the low-rate suppression does not produce any residual inhibition on tinnitus, which is sometimes observed in masking (e.g., Roberts et al., 2006). Finally, the suppression takes time (up to several minutes, see Figure 1) to develop whereas the masking, if effective, is usually instantaneous.
4.3. How should tinnitus be treated with electric stimulation?
At present, tinnitus treatment with cochlear implants is poorly understood and underdeveloped. Traditionally, improvement in tinnitus is considered to be more or less a side effect of treating deafness (e.g., Ito, 1997; Pan et al., 2009). There is now a trend, including the present study, towards using cochlear implants to specifically treat tinnitus in unilaterally deafened subjects who still have normal or significant residual contralateral hearing (Kleinjung et al., 2009; Van de Heyning et al., 2008). In the majority of these cases, electrical stimulation is still delivered via standard signal processing whose main function is to restore speech intelligibility, rather than silence tinnitus. The present pro-speech signal processing approach has certainly worked for some of the tinnitus sufferers, but clearly does not work for all. To provide relief to the presenttinnitus subject, a customresearch interface was made to produce the low-rate stimulation since the manufacturer’s speech processor can only generate high-rate stimulation. To achieve maximal efficacy and convenience, it would be ideal to have the same signal processing that is optimized for both speech recognition and tinnitus suppression. In reality, this goal would be difficult to achieve because of the seemingly conflicting goal of these two interventions. As a minimum, acustomized means to deliver both low- and high-rate individualized stimulation, would benefit tinnitus management in the present cochlear implant recipients.
5. Conclusions
The present study reported a novel method to treat tinnitus in a unilaterally-deafened subject: A low-rate (20–100 Hz) and a low-level (softer than the tinnitus) electric stimulus delivered by an apical intra-cochlear electrode totally abolished his tinnitus. By directly comparing evoked and spontaneous cortical activities between the tinnitus-present and tinnitus-suppressed states in the same subject, the present study demonstrates that the effective tinnitus suppressor restored normal brain activities in terms of decreasing the event-related N100 cortical potential in the frontal-center region and increasing the spontaneous alpha rhythm power in the temporal region. The present findings suggest that these neurophysiological responses be used as an objective measure of tinnitus and the efficacy of customized stimulation in tinnitus treatment.
Acknowledgement
We thank the subject for his spirited and cooperative participation in the present study. This work was first reported at the 2007 Midwinter Meeting of The Association for Research in Otolaryngology in Denver, CO and supported in part by a grant from the American Tinnitus Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Althaus J. On tinnitus aurium and its treatment by electricity. Lancet. 1886;128:205–206. [Google Scholar]
- Attias J, Urbach D, Gold S, Shemesh Z. Auditory event related potentials in chronic tinnitus patients with noise induced hearing loss. Hear Res. 1993;71:106–113. doi: 10.1016/0378-5955(93)90026-w. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diesch E, Andermann M, Flor H, Rupp A. Interaction among the components of multiple auditory steady-state responses: enhancement in tinnitus patients, inhibition in controls. Neuroscience. 2010;167:540–553. doi: 10.1016/j.neuroscience.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, Michalewski HJ, Zeng FG, Pratt H, Starr A. Frequency changes in a continuous tone: auditory cortical potentials. Clin. Neurophysiol. 2008;119:2111–2124. doi: 10.1016/j.clinph.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don M, Eggermont JJ. Analysis of the click-evoked brainstem potentials in man unsing high-pass noise masking. J. Acoust. Soc. Am. 1978;63:1084–1092. doi: 10.1121/1.381816. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Gerken GM. Central tinnitus and lateral inhibition: an auditory brainstem model. Hear Res. 1996;97:75–83. [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman MF. Cortical reorganization in children with cochlear implants. Brain Res. 2008;1239:56–65. doi: 10.1016/j.brainres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Klimesch W, Sauseng P, Gruber W, Doppelmayr M, Freunberger R, Pecherstorfer T, Birbaumer N. Alpha phase reset contributes to the generation of ERPs. Cereb Cortex. 2007;17:1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- Hazell JW, Wood S. Tinnitus masking-a significant contribution to tinnitus management. Br. J. Audiol. 1981;15:223–230. doi: 10.3109/03005368109081442. [DOI] [PubMed] [Google Scholar]
- Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J. Speech. Lang. Hear. Res. 2005;48:1204–1235. doi: 10.1044/1092-4388(2005/084). [DOI] [PubMed] [Google Scholar]
- Ito J. Tinnitus suppression in cochlear implant patients. Otolaryngol Head Neck Surg. 1997;117:701–703. doi: 10.1016/S0194-59989770056-1. [DOI] [PubMed] [Google Scholar]
- Jacobson GP, McCaslin DL. A reexamination of the long latency N1 response in patients with tinnitus. J Am Acad Audiol. 2003;14:393–400. [PubMed] [Google Scholar]
- Jastreboff MM. Sound therapies for tinnitus management. Prog. Brain Res. 2007;166:435–440. doi: 10.1016/S0079-6123(07)66042-7. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Kadner A, Viirre E, Wester DC, Walsh SF, Hestenes J, Vankov A, Pineda JA. Lateral inhibition in the auditory cortex: an EEG index of tinnitus? Neuroreport. 2002;13:443–446. doi: 10.1097/00001756-200203250-00016. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206:200–226. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Moxon EC. Physiological considerations in artificial stimulation of the inner ear. Ann. Otol. Rhinol. Laryngol. 1972;81:714–730. doi: 10.1177/000348947208100513. [DOI] [PubMed] [Google Scholar]
- Kleinjung T, Steffens T, Strutz J, Langguth B. Curing tinnitus with a Cochlear Implant in a patient with unilateral sudden deafness: a case report. Cases J. 2009;2:7462. doi: 10.1186/1757-1626-2-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N. Engl. J. Med. 2002;347:904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat. Neurosci. 2001;4:1131–1138. doi: 10.1038/nn737. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Cochlear-implant high pulse rate and narrow electrode configuration impair transmission of temporal information to the auditory cortex. J. Neurophysiol. 2008;100:92–107. doi: 10.1152/jn.01114.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto RT, Wynne MK, McKnight C, Bichey B. Electrical Suppression of Tinnitus via Cochlear Implants. Int Tinnitus J. 1997;3:35–38. [PubMed] [Google Scholar]
- Osaki Y, Nishimura H, Takasawa M, Imaizumi M, Kawashima T, Iwaki T, Oku N, Hashikawa K, Doi K, Nishimura T, Hatazawa J, Kubo T. Neural mechanism of residual inhibition of tinnitus in cochlear implant users. Neuroreport. 2005;16:1625–1628. doi: 10.1097/01.wnr.0000183899.85277.08. [DOI] [PubMed] [Google Scholar]
- Pan T, Tyler RS, Ji H, Coelho C, Gehringer AK, Gogel SA. Changes in the tinnitus handicap questionnaire after cochlear implantation. Am J Audiol. 2009;18:144–151. doi: 10.1044/1059-0889(2009/07-0042). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Moffat G, Bosnyak DJ. Residual inhibition functions in relation to tinnitus spectra and auditory threshold shift. Acta Otolaryngol Suppl. 2006:27–33. doi: 10.1080/03655230600895358. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing Ears: The Neuroscience of Tinnitus. J. Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein JT, Wilson BS, Finley CC, Abbas PJ. Pseudospontaneous activity: stochastic independence of auditory nerve fibers with electrical stimulation. Hear. Res. 1999;127:108–118. doi: 10.1016/s0378-5955(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Tyler RS, Johnson A, Brown CJ. Electrical suppression of tinnitus with high-rate pulse trains. Otol Neurotol. 2003;24:478–485. doi: 10.1097/00129492-200305000-00021. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Vollmer M, Moore CM, Rebscher SJ, Leake PA, Beitel RE. Responses of inferior colliculus neurons to amplitude-modulated intracochlear electrical pulses in deaf cats. J. Neurophysiol. 2000;84:166–183. doi: 10.1152/jn.2000.84.1.166. [DOI] [PubMed] [Google Scholar]
- Tang Q, Liu S, Zeng FG. Loudness adaptation in acoustic and electric hearing. J Assoc Res Otolaryngol. 2006;7:59–70. doi: 10.1007/s10162-005-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol. 2008;117:645–652. doi: 10.1177/000348940811700903. [DOI] [PubMed] [Google Scholar]
- Vernon JA. Masking of tinnitus through a cochlear implant. J Am Acad Audiol. 2000;11:293–294. [PubMed] [Google Scholar]
- Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2:e153. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T. The neural code of auditory phantom perception. J Neurosci. 2007;27:1479–1484. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]