Abstract
Epithelial Ovarian cancer has the highest mortality rate among gynaecological cancers. Altered glycosylation is associated with oncogenic transformation producing tumor-associated carbohydrate antigens. We investigated the potential of natural occurring anti-glycan antibodies in the diagnosis of ovarian cancer by using printed glycan array.
Anti-glycan antibodies bound to 203 chemically synthesized printed glycans were detected via biotin-streptavidin fluorescence system in serum of women with normal operative findings (healthy controls; n=24) and non-mucinous borderline or ovarian cancer of various FIGO stages (n=33). Data were validated measuring blood group associated di-, tri and tetra- saccharide antigens on known ABO blood groups.
Anti-glycan antibodies demonstrated high reproducibility (rc>0.9). Cluster analysis identified repetitive patterns of specific core carbohydrate structures: 11 N-linked glycans, 3 O-linked glycans, 2 glycosphingolipids. Biomarker detection revealed 24 glycans including P1 (Galα1-4Galβ1-4GlcNAcβ; p<0.001) significantly discriminating between (low-) malignant tumors and healthy controls. Comparable sensitivity and specificity with tumor marker CA125 was achieved by a panel of multivariate selected and linear combined anti-glycan antibody signals (79.2% and 84.8%, respectively).
Our findings demonstrate the potential of glycan arrays in the development of a new generation of biomarkers for ovarian cancer.
Keywords: Epithelial ovarian cancer, biomarker, glycan array, antibodies, serum
Introduction
Epithelial ovarian cancer is the fourth most common cause of cancer death in women in the Western world and the leading cause of death from gynecological malignancies1. This phenomenon is caused by the delay of initial diagnosis due to non-specific symptoms and non-existent screening methods, leading to 75% of patients being detected in late stage disease, with a five-year survival of 20%2. During the last ten years, a large amount of research has focused on using transcriptomics and proteomics to identify a more sensitive and specific tumor marker than the current clinical marker, CA1253, 4. Although this work has advanced the biological understanding of the heterogeneity of ovarian cancer, and has led to the discovery of various potential biomarkers, so far, all of these markers appear to share similar limitations as CA125.
Glycans are essential partners in many biological recognition processes, and the characterization of the “glycome” of cells, tissues and organisms has become one of the frontiers in the post-genomic era. Beside their potential oncogenic role, naturally occurring antibodies seem to play an important part in anti-tumor surveillance, probably by binding to the repetitive motif of carbohydrate epitopes5. During oncogenesis the immense biological potential of altered glycosylation is reflected by the occurrence of tumor-associated carbohydrate antigens6. Cancer associated carbohydrates are mostly located on the surface of cancer cells and are therefore potential targets for new diagnostic biomarkers7.
Carbohydrate arrays are powerful tools composed of a diverse repertoire of oligosaccharides immobilized on solid matrices and have therefore the potential to map out glycan-protein interactions in a high-throughput manner8. These arrays allow determination of the specificities of glycan binding proteins9, 10, examination of microbiologically relevant glycans11, study of carbohydrate-processing enzymes12, and anti-glycan immune responses13, 14. To date, there have been only a small number of reports describing the study of glycans and glycoconjugates within a specific biological system15, these reports include the screening of anti-glycan antibodies within human serum for diagnostic purposes13, 16, 17 or for the detection of the immune response to bacterial pathogens18. Anti-glycan antibodies in their malignancy-defining role identified by glycan array technology has been first described in breast cancer19, 20 and Hodgkin's lymphoma21.
Here, we used a recently described, standardized printed glycan array to characterize and evaluate the diagnostic potential of carbohydrate antibody recognition in human blood serum samples from healthy controls, ovarian borderline tumors and ovarian cancer patients. The library of printed glycans contains a large number of chemically synthesized carbohydrate structures of high purity: e.g., blood group antigens, pathogen related oligosaccharides, lactosamines, sulphatedcarbohydrates, sialylated-carbohydrates, fucosylated-carbohydrates and known tumor-associated carbohydrate antigens.
As the field of glycan arrays is just evolving, to our knowledge there are currently only three publications describing anti-glycan antibody binding to glycans in healthy patients13, 17, 22.
This is the first study on anti-glycan antibody profiling for the detection of ovarian cancer biomarkers and facilitates the largest glycan array incorporating more than 200 different carbohydrate structures.
Material and Methods
Clinical cohort
Serum samples were prospectively collected from 57 women at the Department of Gynecology University Hospital Zurich after written informed consent was granted (SPUK, Canton of Zurich, Switzerland). Optimal healthy controls should not have signs of endometriosis or pelvic inflammation, which is why we selected only patients who underwent surgery and had normal operative findings. Serum was collected immediately prior to surgery for healthy controls (n=24) and non-mucinous epithelial ovarian borderline tumors and ovarian cancers (EOC, n=33) (Table 1). Mucinous ovarian cancer patients were excluded from this study as they are increasingly proven to have a distinct genetic background compared to other epithelial ovarian cancers23, 24. Further care was taken to exclude patients with a past history of cancer or chronic infectious diseases of non-gynaecological and gynaecological origin. Histopathological diagnosis was independently confirmed by a pathologist specialising in gynecological oncology (R.C.), and patients with inconsistent diagnoses, mixed or mucinous pathologies excluded from the study. Venous blood samples (12 ml) were collected in EDTA blood tubes (BD Vacutainer®, 0.184 M EDTA, BD Diagnostics, Franklin Lakes, NJ, USA) and stored on ice for a maximum of 3 h until further processing. Blood samples were centrifuged at 3000×g at 4°C for 10 min, and aliquots of the supernatant plasma were frozen at −80°C. Comprehensive present and past medical history including routine imaging and CA125 serum tumor marker measurements (standardized ELISA, Fujirebio Diagnostics Inc., Goteborg, Sweden) were obtained and stored in an in-house database.
Table 1.
Clinicopathological characteristics of the patient cohort.
| No. of patients | % of patients | |
|---|---|---|
| Healthy control | 24 | 42.1 |
| Ovarian borderline tumor | 2 | 3.5 |
| Ovarian cancer | 31 | 54.4 |
|
| ||
| Age (y) (n = 57) | ||
| <50 | 11 | 19.3 |
| ≥50 | 46 | 80.7 |
|
| ||
| Tumor Stage (FIGO)(n = 32) | ||
| I | 4 | 12.5 |
| II | 3 | 9.4 |
| III | 17 | 53.1 |
| IV | 8 | 25.0 |
|
| ||
| Tumor Grade (n = 28) | ||
| G1 | 1 | 3.6 |
| G2 | 9 | 32.1 |
| G3 | 18 | 64.3 |
|
| ||
| Histological subtype (n = 33) | ||
| Serous | 19 | 57.6 |
| Endometrioid | 7 | 21.2 |
| Clear cell | 1 | 3.0 |
| Transitional cell | 4 | 12.1 |
| Undifferentiated | 2 | 6.1 |
|
| ||
| Residual disease (n = 23) | ||
| < 1cm | 12 | 52.2 |
| ≥ 1cm | 11 | 47.8 |
|
| ||
| Preoperative CA125 (n = 53) | ||
| < 35 U/ml | 18 | 34.0 |
| ≥ 35 U/ml | 35 | 66.0 |
|
| ||
| Blood group (n = 52) | ||
| A | 22 | 42.3 |
| B | 9 | 17.3 |
| AB | 2 | 3.8 |
| 0 | 19 | 36.6 |
Printed glycan array
Printed glycan array slide fabrication and high-throughput profiling were performed as previously described13. Glycans were diluted in 300 mM phosphate buffer pH 8.5 containing 0.005% Tween 20 and printed by robotic pin deposition on N-hydroxysuccinimide activated glass slides (Nexterion Slide H, Schott, Jena, Germany). The entire glycan library containing 203 structures of 95%–98% purity (Lectinity Holdings, Moscow, Russia) was printed at a 50 μM concentration in eight replicates. To create images documenting the deposition of each feature, printed glycan library slides were scanned for salt deposition using a ProScanArray HT Microarray Scanner (PerkinElmer, Waltham, USA) with the red reflect scan protocol (633 nm excitation, neutral density filter). Following the salt scan, free N-hydroxysuccinimide activated groups were blocked with 50 mM ethanolamine in 50 mM borate buffer at a final pH of 9.2. Slides were then rinsed with deionised water, dried and stored at room temperature in a desiccator.
Sample preparation
Each serum sample was diluted 1:15 with phosphate buffered saline (PBS) containing 0.1% v/v Tween20 and 3% w/v BSA, thoroughly vortexed for 15 s and incubated at 37°C for 15 min to dissolve potential lipid aggregates. Insoluble residual sample components were removed by centrifugation for 30 s in a table-top centrifuge at maximum speed. Samples were transferred to the array slides and gently rocked in a sealed humidified incubator for 2 h at 37°C. Unbound sample components were removed with a series of washes with 0.1% and 0.001% Tween 20 in PBS. ImmunoPure goat anti-human IgA + IgG + IgM conjugated to long chain biotin (“Combo”, Pierce, Rockford, IL, USA) diluted 1:100 in PBS containing 0.1% Tween20 and 3% BSA was added, and the slides were incubated at room temperature in a humidified chamber for 45 min. Following washing steps, as described above, bound antibodies were visualized by incubating the slides with fluorescent dye streptavidin Alexa Fluor555 (Molecular Probes, Invitrogen, Carlsbad, CA, USA), diluted 1:1000 in PBS containing 0.1% Tween 20 at room temperature for 30 min.
Data quantification
Fluorescence signals corresponding to glycan-bound antibodies were quantified using ImaGene analysis software version 6.1 and 7.5 (BioDiscovery, El Segundo, CA, USA). For each slide, the salt scan and fluorescence images were aligned to assure quantification accuracy. Signals were measured as the median total signal intensity (medTSI) for each glycan and were expressed as the median of eight within-array replicates.
Statistical analysis
Data analysis was performed using the open source statistical programming language R (http://CRAN.R-project.org/, version 2.8.1). P-values less than 0.05 were classed as statistically significant. Epitopic and structural preference of antibody binding to individual carbohydrate structures was analysed by using hierarchical clustering. We performed unsupervised agglomerative hierarchical clustering using Ward's method25 with 1-correlation distance of the array fluorescence signals. Cancer-specific glycans were identified by univariate linear modelling, and sets of glycans selected by support vector machine algorithm (R package GALGO)26 for multivariate analysis. Hereby, their stability was established within 700 independent statistical runs and the most stable glycans further combined in a linear discriminant model (R package `MASS')27. The binary classifier in each model was analyzed by receiver operating characteristics (ROC; R package ROCR28) to determine its sensitivity and specificity. These models were also used to compare identified classifiers to CA125. The best cut-off between observed false negative and false positive rates was determined using the well established “precision-recall break-even point”, where positive and negative predictions are made at the same rate as their prevalence in the data28.
Variability and reproducibility
Quality control intra-chip analysis using replicates and inter-chip reproducibility were previously determined as described13. To assess the experimental variability of the array, samples from 32 patients (13 controls and 19 tumors) were randomly chosen for replicate profiling in two independent experiments on different days. The correlation concordance coefficient (rc) was calculated to study technical reproducibility (R package epiR)29. In order to detect technology based systematic errors, mean, standard deviation (SD), and coefficient of variation (CV) were assessed.
Biological and technical controls
Alpha-rhamnose was used as a “positive biological control” due to the known high expression levels of anti-rhamnose antibodies in healthy control individuals14. Aminoglucitol, an opened reduced form of D-glucose that is not a structural component of glycosylation, was used as a “negative biological control” as it is negative for anti-glycan binding at similar values to the technical background binding control13.
Results
Printed glycan array reproducibility
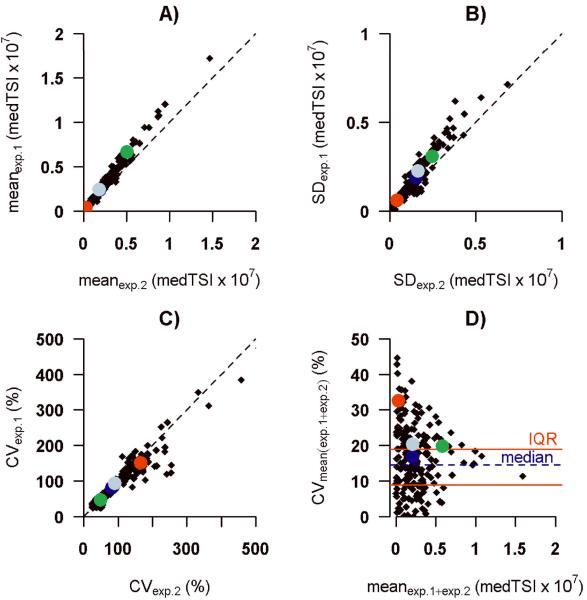
Experiments on two separate days showed a high concordance across patients for the mean medTSI values (rc = 0.96; Figure 1A), standard deviation (rc = 0.94; Figure 1B) and coefficient of variation (rc = 0.97; Figure 1C). The CV across mean signal intensities for repeats had a median of 14.2% and interquartile range of 8.9%–19.0% (Figure 1D). The “biological positive control” α-rhamnose showed a CVexp.1 equal to 49.7% and CVexp.2 equal to 46.8%, respectively. We examined intra-slide variability using the carbohydrate structure Fucα1-2(GalNAcβ1-3)Galβ, which was represented twice on the array in two different positions and had a CV of less than 10% in each experiment (CVexp.1 = 8.7%; CVexp.2 = 9.9%).
Figure 1. Overall inter-slide variability.
Comparison of mean (A), SD (B), CV (C) of fluorescence intensity detected in two independent glycan array experiments (n=32); relationship between CV and mean (D). Each point represents a different glycan on the array. Alpha-rhamnose (green); aminoglucitol (red); Fucα1-2(GalNAcβ1-3)Galβ (blue); experiment 1 (exp.1); experiment 2 (exp.2)
ABO blood group antigens as proof of principle
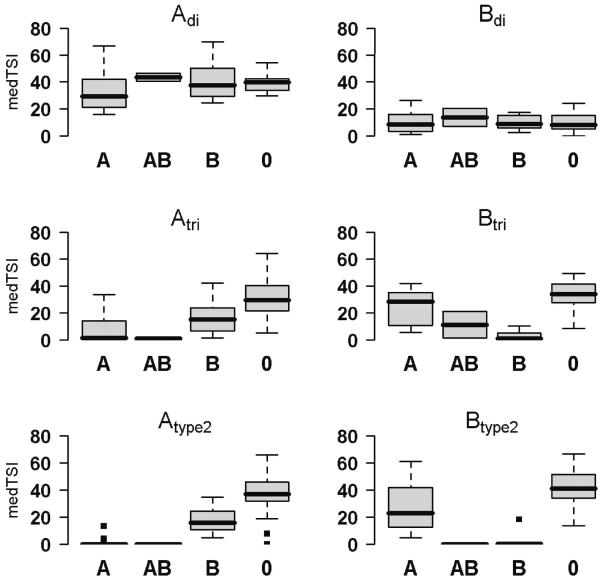
Anti-A and anti-B blood group antibody binding to corresponding ABO-blood group antigens printed onto the array was used as biological validation of the array. Blood group antibody patterns were defined by standardized clinical agglutination tests and compared to the array-based results. The lowest significant P values indicating high diversity of anti-glycan antibody levels were detected for A and B tri- and tetrasaccharides. Antibody levels against two printed trisaccharide structures (GalNAcα1-3(Fucα1-2)Galβ (A trisaccharide [Atri]) and Galα1-3(Fucα1-2)Galβ (B trisaccharide [Btri])), were significantly discriminant (P < 0.001) for individual blood groups (minimal blood group determinant). Low or no signals were detected for antibodies against Atri in blood groups A and AB, and against Btri in blood groups B and AB. In contrast, high antibody levels were found in both trisaccharides in blood group O, for Atri in blood group B and for Btri in blood group A (Figure 2). A significant difference in antibody levels (P < 0.001) was observed for tetrasaccharides GalNAcα1-3(Fucα1-2)Galβ1-4GlcNAcβ (Atype 2) and Galα1-3(Fucα1-2)Galβ1-4GlcNAcβ (Btype 2), which are known to be major ABO antigens. Antibody levels for Atype2 differed significantly between blood groups B and O (P < 0.001). A similar result was found for Btype 2 with low antibody levels in blood groups AB and B compared to A and O (P = 0.005), which significantly differed from lower antibody levels in blood groups A versus O. Differentiating antibody binding across blood groups was not possible using the disaccharides GalNAcα1-3Galβ (Adi; P = 0.084) and Galα1-3Galβ (Bdi; P = 0.92), which are lacking fucose residues (Figure 2).
Figure 2. ABO blood group anti-glycan antibody detection.
Boxplots (75th and 25thpercentile) of medTSI (×105) values within each blood group. Values in different groups (A, AB, B, O) compared using Kruskal-Wallis test: Adi P = 0.064; Bdi P = 0.90; Atri P < 0.001; Btri P < 0.001; Atype 2 P < 0.001; Btype 2 P < 0.001.
Anti-glycan antibody distribution across printed glycans and serum samples
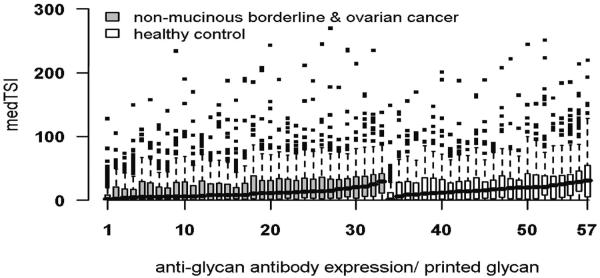
A large spectrum of anti-glycan antibody signals was detected with the mean medTSI for all carbohydrate-bound antibody levels per patient between 8.59×105 and 38.21×105 medTSI (Figure 3). The interquartile range varied from 8.08×105 to 49.7×105 medTSI and the CV from 79.7% to 188.4%. Anti-glycan antibody levels in cancer patients were not as a whole altered as compared to healthy control patients and the observed biological variability was not associated with any clinical or experimental covariates, including age of patients (Figure 3). of the highest anti-glycan antibody distribution independent of diagnosis the highest expression (mean ± SD in medTSI) was observed for glycan 3'-O-Su-Galβ1-3GlcNAcβ (146×105 ± 68×105). As proposed, α-rhamnose (50×105±24×105) showed high signals whilst aminoglucitol (2.5×105 ± 4.2×105) had a low mean result. The glycan which was examined twice on the array to analyze any intra-slide variability (Fucα1-2(GalNAcβ1-3)Galβ) showed close levels in both analyses (Supplementary Figure 1).
Figure 3. Anti-glycan antibody profiles for individual serum samples.
Antibody signals (medTSI × 105) bound to 203 individual carbohydrate structures, grouped by disease status and sorted by median per group.
Anti-glycan antibodies bind to specific epitope core structures
Cluster analysis was performed and revealed 53 structurally similar clusters within the detected anti-glycan antibodies (Supplementary Table 1). Glycans that were structurally identical but printed using different chemical spacers clustered together, as did the eight biotin controls and the blood group A and B antigens. Other carbohydrate structures which showed similarities were found for 21 clusters (Supplementary Table 2): N-linked glycans (11 clusters), O-linked glycans (3 clusters), glycosphingolipids (2 clusters) and not clearly sub-specifiable structures (5 clusters). N-linked carbohydrates included 1) linear lactosamine structures [Galβ1-4GlcNAcβ]1–3; 2) linear glucosamine structures [GlcNAcβ1-4]3–6; 3) sulphated lactosamine structures 4' or 6'-O-Su Galβ1-4GlcNAcβ; 4) single core lactosamine structures Galβ1-4GlcNAcβ with terminally coupled “monsters”13, representing artificial carbohydrates which are not present in biological objects, namely β2-6Neu5Gc, β2-6Neu5Ac or Galα1-4Galβ1-4GlcNAcβ; 5) single core lactosamine structures Galβ1-4GlcNAcβ modified by Galα or O-sulfation; 6) Lewis c (Galβ1-3GlcNAcβ) structures; 7) single core lactosamine structures modified by neuraminic acid (NeuAcα2-3Galβ1-4GlcNAcβ; 8) single core lactosamine structures with additional N-acetylglucosamine (GlcNAcβ1-6Galβ1-4GlcNAcβ; and 9) single core N-actelylglucosamine structures (GlcNAcβ). Interestingly, within this cluster four out of eight carbohydrate structures (Fucβ1-3GlcNAcβ, Galβ1-3GlcNAcβ, 3'-O-Su-Galβ1-3GlcNAcβ and NeuAcα2-6Galβ1-3GlcNAcβ) were highly correlated in their signal intensities, with correlation coefficients ranging from 0.76 to 0.90. O-linked carbohydrates included 1) Thomsen-Friedenreich structures Galβ1-3GalNAcα; 2) sialylated Tn antigen structures Neu5Acα2-6GalNAcα; and 3) lactosamine on Tn antigen Galβ1-4GlcNAcβ1-3GalNAcα whilst glycosphingolipids included Galβ1-3GlcNAcβ1-3Galβ1-3Glcβ.
Non-mucinous ovarian cancer biomarker detection
Linear modelling revealed 24 carbohydrate structures for which the amount of anti-glycan antibodies was significantly lower in the non-mucinous ovarian borderline and cancer cohort as compared to the healthy patient cohort (Table 2). The glycan structure with the most significant discriminatory ability was P1 (Galα1-4Galβ1-4GlcNAcβ; P < 0.001). Compared to the presently used tumor marker CA125, which had a sensitivity of 76% and specificity of 73.9% in our cohort, the identified anti-glycan antibodies revealed comparable values, particularly the top candidate P1 (sensitivity 70.8% and specificity 78.8%. A combination of both P1 and CA125 did improve neither sensitivity nor specificity (76.0% and 73.9%, respectively).
Table 2.
Significantly discriminative ovarian cancer carbohydrate structures between non-mucinous ovarian borderline tumors and cancers versus healthy controls, measured by linear modelling.
| Carbohydrate Structure | Common abbreviation | p value | |
|---|---|---|---|
| 1. | Galα1-4Galβ1-4GlcNAcβ | P1 | 0,0008 |
|
| |||
| 2. | GlcNAcβ1-4GlcNAcβ-Asn | Chitobiose-Asn | 0,0019 |
|
| |||
| 3. | Galβ1-4GlcNAcβ1-6Galβ1-4GlcNAcβ | n/a | 0,0025 |
|
| |||
| 4. | GlcNAcβ1-6Galβ1-4GlcNAcβ | n/a | 0,0041 |
|
| |||
| 5. | Galα1-4Galβ1-4Glcβ | Pk | 0,0077 |
|
| |||
| 6. | Galβ1-4(6-O-Su)GlcNAcβ | n/a | 0,0081 |
|
| |||
| 7. | GlcNAcβ1-3Galβ1-4GlcNAcβ | n/a | 0,0089 |
|
| |||
| 8. | Galα1-4GlcNAcβ1-3Galβ1-4GlcNAcβ | n/a | 0,0095 |
|
| |||
| 9. | GlcNAcβ1-3Manβ | n/a | 0,0106 |
|
| |||
| 10. | GlcNAcβ1-3Galβ1-4GlcNAcβ | n/a | 0,0120 |
We next aimed to identify a panel of anti-glycan antibodies that could generate a higher sensitivity and specificity for the differentiation between borderline and cancer patients from healthy control patients (Table 3). Interestingly, the top candidate P1 was again the most stable glycan using this selection algorithm. In comparison to the univariate approach, the first three multivariate selected and linear combined glycans (svmLDA03; Galα1-4Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-4-(6-Su)GlcNAcβ, Neu5Acβ2-6GalNAcα) did improve the specificity by 8% (Table 3). Combination of a panel of six glycans (svmLDA06; Galα1-4Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-4-(6-Su)GlcNAcβ, Neu5Acβ2-6GalNAcα, Neu5Acβ2-6Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-3-(6-Su)GalNAcα, Galβ1-4GlcNAcβ1-6Galβ1-4GlcNAcβ) generated a higher discriminative sensitivity (79.2%) and specificity (84.8%) (Table 3). These results were not improved by adding the next four significant glycans (svmLDA10; Galα1-4Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-4-(6-Su)GlcNAcβ, Neu5Acβ2-6GalNAcα, Neu5Acβ2-6Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-3-(6-Su)GalNAcα, Galβ1-4GlcNAcβ1-6Galβ1-4GlcNAcβ, Galβ1-4(6-OSu)GlcNAcβ, Galα1-3Galβ1-4(Fucα1-3)GlcNAcβ, Galα1-3(Galα1-4)Galβ1-4GlcNAcβ, GlcNAcβ1-3Galβ1-4GlcNAcβ). Similar carbohydrate motifs are found within svmLDA10, namely core single lactosamine (Galβ1-4GlcNAcβ) and Tn antigen (GalNAcα) (Supplementary Table 3).
Table 3.
Discriminative power of univariate/multivariate selected candidates. Sensitivity and specificity calculated for tumor marker CA125, individual discriminative anti-glycan antibodies and anti-glycan antibody panels (svmLDA03; Galα1-4Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-4-(6-Su)GlcNAcβ, Neu5Acβ2-6GalNAcα, svmLDA06; Galα1-4Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-4-(6-Su)GlcNAcβ, Neu5Acβ2-6GalNAcα, Neu5Acβ2-6Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-3-(6-Su)GalNAcα, Galβ 1-4GlcNAcβ1-6Galβ1-4GlcNAcβ, svmLDA10: Galα1-4Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-4-(6-Su)GlcNAcβ, Neu5Acβ2-6GalNAcα, Neu5Acβ2-6Galβ1-4GlcNAcβ, Neu5Acα2-3Galβ1-3-(6-Su)GalNAcα, Galβ1-4GlcNAcβ1-6Galβ1-4GlcNAcβ, Galβ1-4(6-O-Su)GlcNAcβ, Galα1-3Galβ1-4(Fucα1-3)GlcNAcβ, Galα1-3(Galα1-4)Galβ1-4GlcNAcβ, GlcNAcβ1-3Galβ1-4GlcNAcβ); LN = Galβ1-4GlcNAc.
| Precision- recall break even point | ||
|---|---|---|
| Sensitivity (%) | Specificity (%) | |
|
|
||
| CA125 | 76.0 | 73.9 |
| P1 | 70.8 | 78.8 |
|
|
||
| Chitobiose-Asn | 66.7 | 75.0 |
|
|
||
| GNb6'LN | 66.7 | 75.7 |
|
|
||
| GlcNAcβ1-6'LN | 66.7 | 54.2 |
|
|
||
| Pk | 62.5 | 72.7 |
|
|
||
| svmLDA03 | 75.0 | 81.8 |
|
|
||
| svmLDA06 | 79.2 | 84.8 |
|
|
||
| svmLDA10 | 79.2 | 84.8 |
|
|
||
| LDA/P1-CA125 | 76.0 | 73.9 |
|
|
||
Discussion
Using printed glycan array technology, we identified patterns of core carbohydrate structures in ovarian cancer patients which are specifically recognized by anti-glycan antibodies. We assume that the core of identified carbohydrate structures could play an important role in antibody recognition. The binding affinity or specificity of anti-glycan antibodies seems to be higher in the case of specific core structures13, especially containing GalNAcα-, Galβ1-4GlcNAc- or Galβ1-4Glcβ-. Potentially, benign human cell core structures could be occupied by interactions of different types of membrane-associated proteins as well as other carbohydrate structures. Therefore, binding of anti-glycan antibodies seems to be limited to these potential cell surface carbohydrate epitopes. During oncogenic transformation of cells and rearrangements of extra-cellular matrix, these encrypted binding sites could become available for circulating anti-glycan antibodies. This means that glycans and their molecular environment are presenting epitopes and signal the status of a cell to the immune system leading to a corresponding response.
At present no technology is available to study anti-glycan antibody reactions as sensitive and reproducible as within high-throughput glycan array technology. Other detection methods as ELISA and suspension array are under evaluation, but the main advantage of the glycan array remains the unlimited number of glycans which can be screened in a high-throughput manner. Due to the limitations of other technologies for validation purposes, we decided to perform a biological validation using the well established ABO blood group antigens. ABO-specific antibodies (isohaemagglutinins) belong to a group of naturally occurring antibodies which can be determined by hemagglutination, ELISA30 and FACS31. In our experimental setting anti-glycan antibodies could indeed detect ABO-specific antibodies using a pool of immunoglobulin subtypes, concordant with classical hemagglutination data. However, only tri- and tetra-saccharides, and no disaccharides, were able to discriminate within blood groups, whilst anti-disaccharide antibodies seemed to be less specific and bound to corresponding blood group antigens independent of ABO blood groups13, 17. Highly specific binding of corresponding antibodies was found for A/B tri- and tetrasaccharides showed which was concordant with the well known distribution of anti- A/B antibodies. Affinity of ABO blood group antibodies increased due to the number of sugar units in A/B antigens, being lowest in A/B disaccharides and highest in A/B tetrasaccharides, with fewer outliers observable in longer carbohydrate structures. We also observed a degree of auto-reactivity, probably due to the heterogeneity of the A and B blood groups31. These findings, although used as biological validation, are the first published experiments on the ABO blood group system using a glycan array approach.
Since there are no carbohydrates that are yet established as truly positive and negative antibody binding reference structures, α-rhamnose was used in this study as positive and aminoglucitol as negative biological control. The overall variability of signals for anti-glycan antibodies bound to carbohydrate antigens measured in this study are similar to the literature as is the coefficient of variation for the proposed positive biological control α-rhamnose17, 32, hereby demonstrating their validity as controls.
The printed glycan array was previously established in a different patient cohort to ours studying the immunoprofiles of serum anti-glycan antibodies in 106 healthy female blood donors13, detecting a broad variety of antibody profiles, independent of clinical parameters. Although similar individual anti-glycan antibody patterns have been shown in another group of patients22, other studies have observed discrepancies due to differing age groups33 or varying antibody titres against the carbohydrate mannan of Candida albicans34, 35. As interpretations on a cohort without proven negative intra-operative findings could harvest mistakes, we have taken special care to ensure that healthy patients did not have underlying inflammatory conditions which could influence their detected immune status. We used the printed glycan array as a method to identify anti-glycan antibodies which were able to discriminate between the clinically most important setting, namely patients with suspicious findings on ultrasound and an intermediate or high CA125 level. P1, a member of the P blood group system demonstrated the highest significance and similar sensitivity/specificity levels as CA125 in detecting nonmucinous ovarian borderline tumors and cancers from healthy patients. The P blood group system has two common phenotypes, P1 and P2 (also called Pk or CD77; Galα1-4Galβ1-4Glc), which share a common structure (Galα1-4Galβ1-4Glc(NAc)). The physiological function of P1 and the P blood group system in general is still unknown. Pk antigen has been associated with acute leukaemia36, whilst P1 is present in hematopoietic37 and mesothelioma cell lines38. The presence of P1 in epithelial cells has been correlated to urinary tract infections due to the terminal part of P1, Galα1-4Gal, being a bacterial adhesion molecule in a pyelonephrotic strain of Escherichia coli39. The detection of P1 within this experimental setting as a potential tumor-associated carbohydrate antigen in ovarian cancers could implement that similarly to mesothelioma cells also ovarian cancer cells contain a P1 epitope. In this case the lowered levels of anti-P1 antibodies in the serum of cancer patients could be explained due to the higher amount of P1 cell-bound antibodies.
Except for P1 no other anti-glycan antibody reached a similar performance as CA125, which could be due to the currently limited panel of synthetic carbohydrates within the printed glycan array. A panel of anti-glycan antibodies improved sensitivity and specificity compared to all individual candidates, including P1. The benefit of using a panel has been shown in previous proteogenomic studies40, 41 and is probably also due to the heterogeneity of ovarian cancers. Improved differentiation was achieved using six glycans with no improvement by combining an even higher number of glycans. Panels of anti-glycan antibodies did not reach a better sensitivity and specificity than 84%. Possible explanations why sensitivities and specificities above 84% could not be reached are 1) the cohort heterogeneity; 2) unknown underlying immunological conditions; 3) background binding effects; 4) low-titer reactivities of potential tumor-associated signals; 5) cross-reactivity, and 6) limited number of synthesized glycans on the array. We also need to be aware that using anti-glycan antibody detection via array technology cannot offer information about inducing stimuli, neither are we able to define the affinity or specificity of a collective of antibodies binding to individual glycan structures. A positive binding signal of human immunoglobulins to glycans demonstrates therefore only a variety of polyclonal antibodies binding to a saccharide that possibly represents different forms of biological epitopes.
This is the first study publishing the identification of patient-specific anti-glycan antibody patterns in ovarian cancers. We found a discriminating anti-glycan antibody panel including the top candidate P1 which can diagnose ovarian borderline tumors and cancers with higher sensitivity and specificity than CA125. Except for P1, no other individual anti-glycan antibody in this experimental approach has reached a similar performance as CA125. This could be due to the current limited panel of synthetic carbohydrates within the printed glycan array. It certainly also reflects the heterogeneity even in a non-mucinous ovarian cancer cohort, and further clear-cut cohort selections will be necessary to improve diagnostic performance. At present, glycan array technology has the potential to detect individual and panels of anti-glycan antibodies and is therefore a promising tool for future ovarian cancer biomarker discovery.
Statement.
This is the first publication using a large printed glycan array (> 200 synthesized carbohydrate structures) for diagnostic purposes in cancer patients.
We are also the first group world-wide who describes the possibility to detect anti-glycan antibodies as a useful method for diagnosis in patients with epithelial ovarian cancer. This leads the field of biomarker discovery in an exiting new direction which involves the individual immunological state of a patient.
Supplementary Material
Acknowledgements
This work was supported by the Cancer League of the Canton of Zurich (V.H.S.); Oncosuisse (02115-08-2007 to V.H.S.); Swiss National Foundation (320000-12543 to V.H.S.), European Science Foundation (V.H.S.); Cellexicon, Inc. La Jolla, CA, USA; National Cancer Institute, USA, grant 1U01CA128526 (to M.E.H).
Abbreviations
- (EOC)
Epithelial ovarian cancer
- (HC)
healthy control
- (medTSI)
median total signal intensity
- (rc)
concordance correlation coefficient
- (CV)
coefficient of variation
- (ROC)
receiver operating characteristics
References
- 1.Ozols RF. Challenges for chemotherapy in ovarian cancer. Ann Oncol. 2006;17(Suppl 5):v181–7. doi: 10.1093/annonc/mdj978. [DOI] [PubMed] [Google Scholar]
- 2.Fishman DA, Bozorgi K. The scientific basis of early detection of epithelial ovarian cancer: the National Ovarian Cancer Early Detection Program (NOCEDP) Cancer Treat Res. 2002;107:3–28. doi: 10.1007/978-1-4757-3587-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Sasaroli D, Coukos G, Scholler N. Beyond CA125: the coming of age of ovarian cancer biomarkers. Are we there yet? Biomark Med. 2009;3:275–88. doi: 10.2217/bmm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacob F, Goldstein DR, Fink D, Heinzelmann-Schwarz V. Proteogenomic studies in epithelial ovarian cancer: established knowledge and future needs. Biomarkers Med. 2009;3:743–56. doi: 10.2217/bmm.09.48. [DOI] [PubMed] [Google Scholar]
- 5.Lutz HU, Binder CJ, Kaveri S. Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol. 2009;30:43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. PNAS. 2002;99:10231–3. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–42. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 8.Shin I, Park S, Lee MR. Carbohydrate microarrays: an advanced technology for functional studies of glycans. Chemistry. 2005;11:2894–901. doi: 10.1002/chem.200401030. [DOI] [PubMed] [Google Scholar]
- 9.Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-throughput carbohydrate microarray analysis of 24 lectins. Angew Chem Int Ed Engl. 2006;45:3607–10. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 10.Moller I, Marcus SE, Haeger A, Verhertbruggen Y, Verhoef R, Schols H, Ulvskov P, Mikkelsen JD, Knox JP, Willats W. High-throughput screening of monoclonal antibodies against plant cell wall glycans by hierarchical clustering of their carbohydrate microarray binding profiles. Glycoconj J. 2008;25:37–48. doi: 10.1007/s10719-007-9059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Disney MD, Seeberger PH. The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem Biol. 2004;11:1701–7. doi: 10.1016/j.chembiol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Shin I. Carbohydrate microarrays for assaying galactosyltransferase activity. Org Lett. 2007;9:1675–8. doi: 10.1021/ol070250l. [DOI] [PubMed] [Google Scholar]
- 13.Huflejt ME, Vuskovic M, Vasiliu D, Xu H, Obukhova P, Shilova N, Tuzikov A, Galanina O, Arun B, Lu K, Bovin N. Anti-carbohydrate antibodies of normal sera: findings, surprises and challenges. Mol Immunol. 2009;46:3037–49. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Dotan N, Altstock RT, Schwarz M, Dukler A. Anti-glycan antibodies as biomarkers for diagnosis and prognosis. Lupus. 2006;15:442–50. doi: 10.1191/0961203306lu2331oa. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamoorthy L, Mahal LK. Glycomic analysis: an array of technologies. ACS Chem Biol. 2009;4:715–32. doi: 10.1021/cb900103n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bovin NV, Huflejt ME. Unlimited glycochip. Trends Glycosci. Glycotechnol. 2008;20:245–58. [Google Scholar]
- 17.Oyelaran O, McShane LM, Dodd L, Gildersleeve JC. Profiling Human Serum Antibodies with a Carbohydrate Antigen Microarray. J Proteome Res. 2009;8:4301–10. doi: 10.1021/pr900515y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blixt O, Hoffmann J, Svenson S, Norberg T. Pathogen specific carbohydrate antigen microarrays: a chip for detection of Salmonella O-antigen specific antibodies. Glycoconj J. 2008;25:27–36. doi: 10.1007/s10719-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 19.Huang CY, Thayer DA, Chang AY, Best MD, Hoffmann J, Head S, Wong CH. Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc Natl Acad Sci U S A. 2006;103:15–20. doi: 10.1073/pnas.0509693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huflejt ME, Blixt O, Vuskovic M, Xu H, Shaw LE, Reuben JM, Kuerer HM, Cristofanilli M. Glycan array identifies specific signatures of anti-glycan autoantibodies in sera of breast cancer patients: diagnostic, prognostic and therapeutic opportunities. 28th Annual San Antonia Breast Cancer Symposium.2005. p. 85. [Google Scholar]
- 21.Lawrie CH, Marafioti T, Hatton CS, Dirnhofer S, Roncador G, Went P, Tzankov A, Pileri SA, Pulford K, Banham AH. Cancer-associated carbohydrate identification in Hodgkin's lymphoma by carbohydrate array profiling. Int J Cancer. 2006;118:3161–6. doi: 10.1002/ijc.21762. [DOI] [PubMed] [Google Scholar]
- 22.Oyelaran O, Li Q, Farnsworth D, Gildersleeve JC. Microarrays with varying carbohydrate density reveal distinct subpopulations of serum antibodies. J Proteome Res. 2009;8:3529–38. doi: 10.1021/pr9002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry JP, Scolyer RA, Smith AN, Bali A, Vanden Bergh P, Baron-Hay S, Scott C, Fink D, Hacker NF, et al. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer. 2006;94:904–13. doi: 10.1038/sj.bjc.6603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzelmann-Schwarz VA, Scolyer RA, Scurry JP, Smith AN, Gardiner-Garden M, Biankin AV, Baron-Hay S, Scott C, Ward R, Fink D, Hacker NF, Sutherland RL, et al. Low meprin alpha expression differentiates primary ovarian mucinous carcinoma from gastrointestinal cancers that commonly metastasise to the ovaries. J Clin Pathol. 2007 doi: 10.1136/jcp.2005.034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward JH. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association. 1963;58:236–44. [Google Scholar]
- 26.Trevino V, Falciani F. GALGO: an R package for multivariate variable selection using genetic algorithms. Bioinformatics. 2006;22:1154–6. doi: 10.1093/bioinformatics/btl074. [DOI] [PubMed] [Google Scholar]
- 27.Venables WN, Ripley BD. Modern Applied statistics with Sed. Springer, New York; New York: 2002. [Google Scholar]
- 28.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21:3940–1. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 29.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 30.Buchs JP, Nydegger UE. Development of an ABO-ELISA for the quantitation of human blood group anti-A and anti-B IgM and IgG antibodies. J Immunol Methods. 1989;118:37–46. doi: 10.1016/0022-1759(89)90050-1. [DOI] [PubMed] [Google Scholar]
- 31.Stussi G, Huggel K, Lutz HU, Schanz U, Rieben R, Seebach JD. Isotype-specific detection of ABO blood group antibodies using a novel flow cytometric method. Br J Haematol. 2005;130:954–63. doi: 10.1111/j.1365-2141.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz M, Spector L, Gortler M, Weisshaus O, Glass-Marmor L, Karni A, Dotan N, Miller A. Serum anti-Glc(alpha1,4)Glc(alpha) antibodies as a biomarker for relapsing-remitting multiple sclerosis. J Neurol Sci. 2006;244:59–68. doi: 10.1016/j.jns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Faux JA, Agbarakwe AE, Misbah SA, Chapel HM. A comparison of specific IgG antibody levels to the cell wall mannan of Candida albicans in normal individuals and in patients with primary antibody deficiency. J Immunol Methods. 1992;153:167–72. doi: 10.1016/0022-1759(92)90319-o. [DOI] [PubMed] [Google Scholar]
- 34.Lehmann PF, Reiss E. Comparison by ELISA of serum anti-Candida albicans mannan IgG levels of a normal population and in diseased patients. Mycopathologia. 1980;70:89–93. doi: 10.1007/BF00443073. [DOI] [PubMed] [Google Scholar]
- 35.Greenfield RA, Stephens JL, Bussey MJ, Jones JM. Quantitation of antibody to Candida mannan by enzyme-linked immunosorbent assay. J Lab Clin Med. 1983;101:758–71. [PubMed] [Google Scholar]
- 36.Brodin NT, Dahmen J, Nilsson B, Messeter L, Martensson S, Heldrup J, Sjogren HO, Lundblad A. Monoclonal antibodies produced by immunization with neoglycoproteins containing Gal alpha 1-4Gal beta 1-4Glc beta-O and Gal alpha 1-4Gal beta 1-4GlcNAc beta-O residues: useful immunochemical and cytochemical reagents for blood group P antigens and a differentiation marker in Burkitt lymphoma and other B-cell malignancies. Int J Cancer. 1988;42:185–94. doi: 10.1002/ijc.2910420208. [DOI] [PubMed] [Google Scholar]
- 37.Dunstan RA, Simpson MB, Rosse WF. Presence of P blood group antigens on human platelets. Am J Clin Pathol. 1985;83:731–5. doi: 10.1093/ajcp/83.6.731. [DOI] [PubMed] [Google Scholar]
- 38.Spitalnik SL, Spitalnik PF, Dubois C, Mulshine J, Magnani JL, Cuttitta F, Civin CI, Minna JD, Ginsburg V. Glycolipid antigen expression in human lung cancer. Cancer Res. 1986;46:4751–5. [PubMed] [Google Scholar]
- 39.Lomberg H, Jodal U, Eden CS, Leffler H, Samuelsson B. P1 blood group and urinary tract infection. Lancet. 1981;1:551–2. doi: 10.1016/s0140-6736(81)92878-6. [DOI] [PubMed] [Google Scholar]
- 40.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, Bast RC., Jr. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–8. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, Yue L, Bray-Ward P, Ward DC. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:7677–82. doi: 10.1073/pnas.0502178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.