Abstract
In many cases bilateral cortical activation in older adults has been associated with better task performance, suggesting that a greater reliance on interhemispheric interactions aids performance. Interhemispheric communication is primarily mediated via the corpus callosum (CC), however with advancing age the anterior half of the CC undergoes significant atrophy. Here we determine whether there are age differences in the relationship between cross-sectional area of the CC and performance on cognitive tests of psychomotor processing speed and working memory. We found that older adults had significantly smaller callosal area in the anterior and mid-body of the CC than young adults. Furthermore, older adults with larger size in these callosal areas performed better on assessments of working memory and processing speed. Our results indicate that older adults with larger size of the anterior half of the CC exhibit better cognitive function, although their performance was still poorer than young adults with similar CC size. Thus, while the capability for interhemispheric interactions, as inferred from callosal size, may provide performance benefits for older adults, this capacity alone does not assure protection from general performance decline.
Keywords: Corpus callosum, aging, working memory, processing speed
1. INTRODUCTION
There is clear evidence that advancing age is associated with poorer performance on a wide range of speeded tasks (cf. Salthouse, 2000). Specifically, age-related performance declines have been observed in working memory tasks, processing speed, attention, and perceptual speed (Hedden et al., 2005; Salthouse, 2000). As noted by Salthouse (2000), it seems unlikely that causal mechanisms underlying these declines can be distinguished through behavioral research alone. Rather, inclusion of variables quantifying neural structure and function may be required to resolve the fundamental causes of age-related performance declines.
Transcallosal fibers connect largely homologous regions of the right and left hemispheres; thus, the corpus callosum (CC) mediates the transfer and integration of lateralized cognitive, motor, and sensory information between the hemispheres. Structural magnetic resonance imaging (MRI) has demonstrated that there is substantial interindividual variability in CC size and morphology for both young and older adults (Raz et al., 2010; Sullivan et al., 2010). One standard approach to studying callosal function involves parsing the callosum into segments using a geometric scheme (Witelson, 1989). Numerous studies relying on this approach have shown that variations in both CC macro- and microstructure partially explain individual differences in cognitive and motor function (Fling et al., in press; Stancak et al., 2003; Westerhausen et al., 2004).
Emerging evidence indicates that both the structure and physiological function of the corpus callosum exhibit age differences (Figure 1). The relative contributions of white matter morphology to age differences in cortical recruitment appear to be substantially more robust than those that result from gray matter (Colcombe et al., 2005). Declines in white matter volume with age begin later and appear to continue at a more accelerated rate than declines in gray matter volume (Courchesne et al., 2000; Ge et al., 2002; Jernigan et al., 2001); however it should be noted that these were cross-sectional studies. Moreover, the speed of white matter shrinkage appears to depend on vascular risk factors (Raz et al., 2005; Kennedy and Raz, 2009).
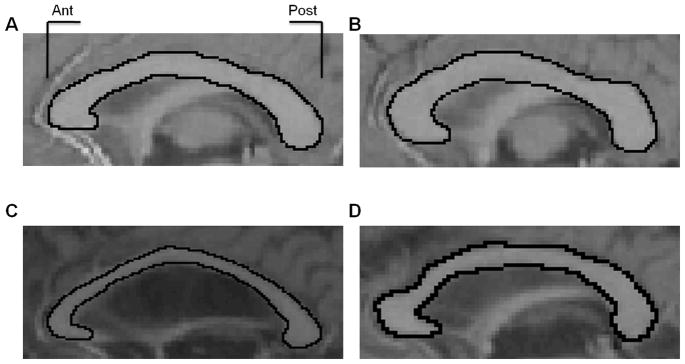
Figure 1.
Two representative examples of the corpus callosum viewed on a mid-sagittal slice from young adult participants (A, B), and two older adult participants (C, D). These images are displayed to demonstrate overall age differences in callosal size, as well as region-specific decreases within the anterior and mid-body of the corpus callosum. Ant = anterior; Post = posterior.
Numerous studies have reported that older adults have extensive reductions in both CC volume (Muller-Oehring et al., 2007; Langan, et al., 2010) and microstructural integrity (Fling et al., In press; Ota et al., 2006; Salat et al., 2005; Sullivan et al., 2010). These age-related differences follow an anterior to posterior gradient, such that white matter quality and quantity is poorest in anterior relative to posterior fiber bundles for older adults (Davis et al., 2009; O’Sullivan et al., 2001; Salat et al., 2004; Sullivan et al., 2010; Zahr et al., 2009). Furthermore, recent work has demonstrated that the relationship between callosal structure and interhemsipheric function appears to shift in the aging brain (cf. Fling et al., 2011). Colcombe and colleagues (2005) therefore suggest that impaired interhemispheric communication may play a more significant role than the structure of local gray matter in age deficits in cognition, due to a limited potential for compensatory bilateral processing.
Age differences in brain activation patterns are often posited to be the result of changes in neural structure. Increased reliance on interhemispheric communication with age may serve a compensatory role in cognitive and motor performance. For example, older adults tend to engage both brain hemispheres for tasks that are associated with unilateral processing in young adults (for reviews see Reuter-Lorenz & Lustig, 2005; Seidler et al., 2010). In many cases these bilateral activation patterns have been associated with better task performance among older adults (Cabeza et al., 2002; Reuter-Lorenz & Cappell, 2008), suggesting that a greater reliance on interhemispheric interactions aids performance. Reuter-Lorenz and colleagues (1999) have shown that older adults benefit from bihemispheric processing across all levels of task complexity on a divided visual field letter-matching task, whereas young adults only demonstrate similar benefits on the most complex tasks. Interestingly, less lateralized task processing during both cognitive (Muller-Oehring et al., 2007) and motor (Langan et al., 2010) tasks has been shown to be associated with reductions in CC cross-sectional area in older adults. Thus, structural differences of the CC appear to impact cortical activity both at rest and during task performance, with significant implications for behavior.
Such evidence motivates the present examination of age differences in the relationship between cross-sectional area of the CC and performance on a variety of cognitive and speeded psychomotor tasks. The assessments used in the current study primarily rely upon anterior and sensorimotor cortical areas and are of relatively low complexity; thus we hypothesized that larger area in the anterior half of the CC would be associated with performance benefits for older, but not young adults.
2. METHODS
2.1 Participants
All participants were community-dwelling individuals recruited from the greater Ann Arbor area. Fifty-seven young adults (31 male; mean age 21.8 ± 3.2 years; range 18–30 years) were recruited from the population at the University of Michigan; all young adults were either undergraduate or graduate students and had completed an average of 14.9 years of education. Fifty-five older adults (27 male; 72.1 ± 5.2 years; range 65–80 years) also participated in this study and had completed an average of 15.3 years of education (all older adults completed high school). Participants were strongly right-handed as determined by the Edinburgh Handedness Inventory (M: 0.88; Oldfield, 1971).
2.2 Neuropsychological assessments and health questionnaire
Participants completed a series of questionnaires to assess general cognitive function including the Mini-Mental State Exam (MMSE; Folstein et al., 1975) and the Mattis dementia rating scale (Mattis, 1988). These assessments were administered to potentially exclude participants exhibiting signs of dementia and to confirm that older adult participants were representative of their age group. As in previous studies from our lab, a minimum MMSE score of 27 and a Mattis score of 123 were required for participation (Anguera et al., 2011). The Dysexecutive Questionnaire (DEX; Wilson et al. 1996) was also administered to all participants to determine whether individuals had difficulty with abstract thinking, planning, or other tasks associated with executive functioning. Finally, a health history questionnaire was provided to allow us to exclude participants with a history of stroke, diabetes, alcoholism, arthritis, or neurological disease.
All participants performed a battery of neuropsychological tests to assess specific cognitive processes. Short-term verbal working memory was assessed with the reading span task (a measure of the storage and processing aspects of verbal working memory, Daneman & Carpenter, 1980), as well as the forward and backward digit span tasks from the Wechsler Adult Intelligence Scale – Revised (WAIS-R; Wechsler, 1997). The forward and backward digit span tests are similar in that they both rely on attention and working memory, but the backward digit span also assesses planning and transformation of information within working memory (Schofield & Ashman, 1986). Finally, psychomotor processing speed was assessed by the digit-symbol substitution test (from the WAIS-R; Joy et al., 2004). For all of these neuropsychological assessments, a higher score indicates better performance.
2.3 Corpus callosum structural measures
All neuroimaging data were collected in the magnetic resonance imaging (MRI) scanner at the University of Michigan’s functional MRI Laboratory (3.0T MRI scanner: General Electric, Waukesha, WI) with a standard GE head coil. Whole brain high-resolution structural MR images were collected using one of the following three T1-weighted gradient-echo pulse sequences acquired in the sagittal plane (field of view/repetition time/echo time/voxel size): sequence 1) 240 × 240mm, 10.2ms, 3.4ms, 0.94 × 0.94 × 1.4mm (N = 28); sequence 2) 240 × 240mm, 250ms, 3ms, 1.4 × 1.4 × 3.2mm (N = 34); or sequence 3) 220 × 220mm, 200ms, 3.7ms, 1 × 1 × 1.2mm (N = 50). Using a custom MATLAB program that that has previously been described (Fling et al., in press; Langan et al., 2010), we manually outline the corpus callosum (CC) from the mid-sagittal slice of a high-resolution T1 image, which is subsequently divided into seven regions as previously described by Witelson (1989). Based upon recent diffusion tensor tractography work from Hofer & Frahm (2006), these seven regions approximately correspond to distinct anatomical connections of the caudal/orbital prefrontal, inferior premotor cortices (region 1), prefrontal cortices (2), premotor cortices (3), pre-supplementary motor and supplementary motor areas (4), primary motor cortices (5), primary somatosensory cortices (6), superior temporal, posterior parietal, occipital and inferior temporal cortices (7) (Figure 2). Although Hofer and Frahm (2006) outline a scheme with five CC segments, we maintained all seven CC regions from the Witelson (1989) segmentation to increase the sensitivity for determining relationships between regions of the callosal midbody and task performance. Despite the inclusion of 7 regions, connectivity of callosal regions was interpreted relative to Hofer & Frahm (2006). The same custom MATLAB program was utilized to calculate intracranial area (ICA); an outline was drawn along the interior border of the skull with a straight line connecting the nasion and the inion. The same mid-sagittal slice was used to measure the CC and ICA for each participant. Four independent raters made all callosal measurements and inter-rater reliability was assessed with both Krippendorff’s alpha (Hayes and Krippendorff, 2007) and intraclass correlation coefficient (ICC). The mean callosal cross-sectional area across raters was used in subsequent analyses.
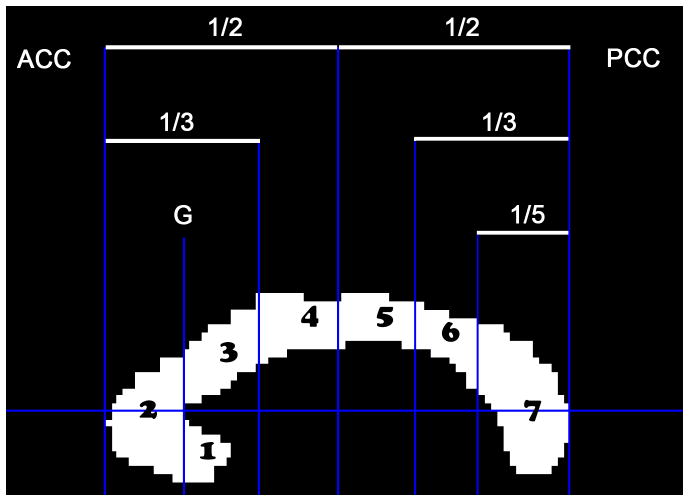
Figure 2.
Geometric segmentation of the corpus callosum based on Witelson (1989). The cross sectional area of the midsagittal CC was parsed into 7 regions: (1) rostrum, (2) genu, (3) rostral truncus, (4) anterior intermediate truncus, (5) posterior intermediate truncus, (6) isthmus, (7) splenium. ACC = anterior border of corpus callosum; PCC = posterior border of corpus callosum; G = genu.
2.4 Data analysis
We used SPSS 18.0 to run statistical analyses on metrics of cognitive and motor performance. Independent sample t-tests were used to compare group performance on all neuropsychological tests and handedness; all t-tests were two-tailed, unless otherwise noted. Mixed model repeated measures ANOVA was used to analyze CC cross-sectional area: CC region was included as the within-subjects variable and age group and gender as the between-subjects variables. Intracranial area and structural MR acquisition parameters (TR/TE/and voxel size) were included as covariates in this statistical analysis, since three different image acquisitions were used for different subsets of the participants (all with the same MRI scanner). Significance was set at α = 0.05. The Huynh-Feldt epsilon was computed to test for sphericity; we evaluated tests using the adjusted degrees of freedom in cases of sphericity violation. Significant main effects were subjected to post-hoc independent (age group, gender) and paired t-tests (CC region). We also performed linear regression to assess relationships between cognitive task performance. This resulted in our computing a composite cognitive score using the Z scores for performance on the digit symbol substitution, reading span, and forward and backward digit span tasks. To assess relationships between size of callosal regions and task performance we used a general linear model with cognitive task performance as the dependent variable and age group and size of callosal region as predictors while controlling for ICA. All data are presented as mean (± standard error) unless otherwise noted.
3. Results
3.1 Neuropsychological assessments
Young adults performed significantly better than older adults on all assessments of working memory and processing speed (P < 0.05 for all tasks; Table 1). In addition, performance on all cognitive variables was significantly correlated (P < 0.01; Table 2). Therefore, we computed one composite variable for all cognitive measures, which we use in all subsequent regression analyses.
Table 1.
Group values for all assessments. YA – young adults; OA – older adults; S.E.M. –standard error of the mean; MMSE – mini-mental state exam; Mattis – Mattis dementia rating scale; DEX – dysexecutive questionnaire; FDS – forward digit span; BDS – backward digit span.
| YA (N = 57) | OA (N = 55) | Significance | ||
|---|---|---|---|---|
| Mean | Mean | t | p | |
| Edinburgh | 0.86 (0.02) | 0.89 (0.2) | −1.88 | 0.08 |
| Education | 14.9 (1.3) | 15.3 (2.6) | −0.9 | 0.16 |
| MMSE | 29.7 (0.09) | 29.1 (0.2) | 3.0 | 0.004 |
| Mattis | 141.8 (0.2) | 140.2 (0.5) | 3.0 | 0.003 |
| DEX | 18.9 (1.1) | 15.6 (1.1) | 2.1 | 0.037 |
| Digit Symbol | 90.0 (1.5) | 64.4 (1.7) | 11.4 | 0.001 |
| Reading Span | 32.8 (0.6) | 26.0 (0.8) | 6.6 | 0.001 |
| FDS Trial | 12.4 (0.3) | 10.4 (0.3) | 4.4 | 0.001 |
| BDS Trial | 8.3 (0.3) | 7.0 (0.3) | 3.2 | 0.002 |
Table 2.
Correlation matrix for all callosal regions and cognitive assessments, while controlling for intracranial area. All data in the table are Pearson r values. Because performance on all cognitive variables were significantly correlated, a cognitive composite score of performance on all four tasks is also included. FDS – forward digit span; BDS –backward digit span; Cog Comp = cognitive composite score.
| CC3 | CC4 | CC5 | CC6 | CC7 | Age | Digit Sym. | Read. Span | FDS | BDS | Cog Comp | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC2 | 0.25** | 0.56*** | 0.5*** | 0.3*** | 0.25** | −0.32*** | 0.30*** | 0.3*** | 0.1 | 0.12 | 0.25** |
| CC3 | 0.40*** | 0.35*** | 0.35*** | 0.54*** | −0.11 | 0.05 | 0.002 | 0.15 | −0.08 | 0.04 | |
| CC4 | 0.7*** | 0.40*** | 0.33*** | −0.42*** | 0.38*** | 0.33*** | 0.16 | 0.1 | 0.31*** | ||
| CC5 | 0.66*** | 0.34*** | −0.24** | 0.23* | 0.18* | 0.01 | 0.06 | 0.15 | |||
| CC6 | 0.29*** | −0.11 | 0.01 | 0.15 | 0.03 | 0.1 | 0.1 | ||||
| CC7 | −0.11 | −0.03 | −0.04 | −0.05 | −0.14 | −0.08 | |||||
| Age | −0.75*** | −0.52*** | −0.40*** | −0.28** | −0.62*** | ||||||
| Digit Sym. | 0.61*** | 0.42*** | 0.37*** | 0.77*** | |||||||
| Reading Span | 0.48*** | 0.48*** | 0.82*** | ||||||||
| FDS | 0.54*** | 0.78*** | |||||||||
| BDS | 0.76*** |
P < 0.05;
P <0.01;
P < 0.001
3.2 Callosal size
Inter-rater reliability of the CC cross-sectional area measurements within region 1 (rostrum) was poor (Krippendorff’s alpha < 0.3; ICC < 0.4). However, measurements within the remaining 6 CC regions (2–7) were highly reliable (Krippendorff’s alpha > 0.92; ICC > 0.88). Due to the large variability among measures of CC region 1 both within and across raters, this region was excluded from further analyses.
For callosal cross-sectional area a main effect of CC region (F5,535 = 10.3, P < 0.001) and an age group × CC region interaction were found (F5,535 = 6.6, P < 0.001), as well as a main effect of age group (F1,107 = 6.4, P < 0.05). Further, the use of three different MRI acquisition parameter sets had no significant effect on measures of callosal size (P > 0.4), nor were there any significant interactions between MR sequence and CC region, or age group (P > 0.35). Post-hoc independent sample t-tests indicated that older adults had significantly smaller cross-sectional area in callosal regions 2 (t = 3.4, P < 0.001) and 4 (t = 4.4, P < 0.001; Figure 3) than young adults. These regions contain transcallosal fibers that connect pre-frontal cortices (CC2) and supplementary motor areas (CC4). Although not significant when corrected for multiple comparisons (α = 0.05/6) we also note a similar trend within CC region 5 (t = 2.1, P < 0.04), a region containing fibers that connect the primary motor cortices. Furthermore, the results revealed no main effect of gender (F1,107 = 0.3, P < 0.8), no gender × CC region interaction (F5,535 = .47, P < 0.7), no gender × age group interaction (F1,107 = .46, P < 0.5), and no CC region × gender × age interaction (F5,535 = 2.0, P > 0.1).
Figure 3.
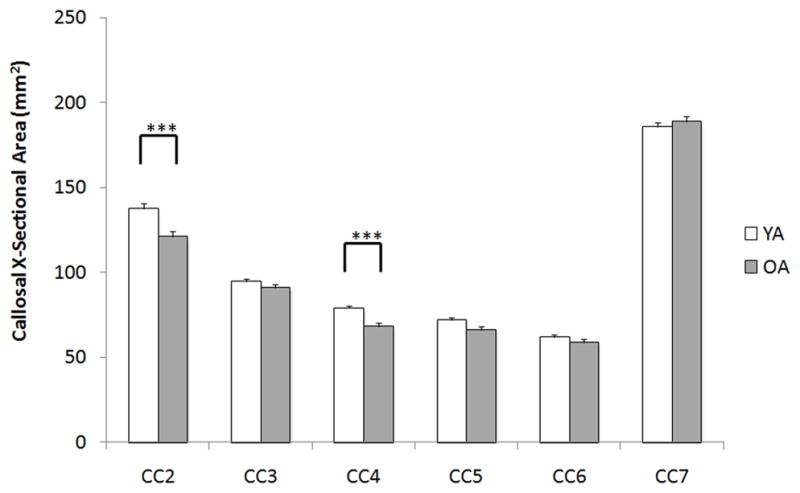
Raw corpus callosum cross-sectional area of regions 2–7 (region 1 is excluded due to lack of measurement reliability). Older adults (OA) had significantly smaller callosal area than young adults (YA) in CC region 2 (**P < 0.01) and region 4 (***P < 0.001) while controlling for intracranial area. Data are mean + 1 S.E.M. X-sectional = cross-sectional.
3.3 Relationships between callosal size and performance
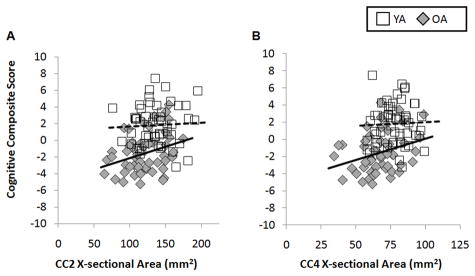
For the regions where CC cross-sectional area significantly differed between groups (CC2 and CC4), we assessed whether size of CC region predicted performance on the composite cognitive variable in a differential fashion between age groups. We report a significant main effect for group (χ21,104 = 552.6; P < 0.001), size of CC2 (χ298,104 = 3561.6; P < 0.001), and size of CC4 (χ286,95 = 279.8; P < 0.001). Further, we observed a significant interaction for age group × CC2 size (χ25,104 = 200.9; P < 0.001) and for age group × CC4 size (χ28,95 = 31.7; P < 0.01). When investigating linear regression between the cognitive composite variable and size of CC2 (while controlling for ICA) when separated by age group, a positive relationship was observed in older adults (r = 0.32) whereas no relationship was found in young adults (r = 0.05; see Figure 4A). Similarly the relationship between the cognitive composite variable and the size of CC4 revealed a positive relationship in older adults (r = 0.29), while little to no relationship was observed in young adults (r = 0.04; Figure 4B). Thus, size of CC2 and CC4 are positively correlated with cognitive performance in older adults and unrelated to cognitive performance in young adults.
Figure 4.
A) Relationships between size of CC2 and the cognitive composite variable in each age group. While older adults demonstrate a positive relationship (r = 0.32), no relationship is observed in young adults (r = 0.05). B) Relationships between size of CC4 and the cognitive composite variable in each age group. Again older adults demonstrate a positive relationship (r = 0.29), whereas there is no association observed in young adults (r = 0.04). Dashed lines represent linear regression fit to young adult data. Solid lines indicate linear regression fit to older adult data. X-sectional = cross-sectional.
Finally, to demonstrate the callosal region specificity of these relationships we also correlated the cognitive composite variable with size of CC7, a region that was actually larger in the older adult group (OA: 188.7mm2; YA: 185.7mm2). We chose this as a control region because CC7 is an area containing fibers that connect cortical regions serving a minimal role during the current tasks (e.g. occipital cortices). We report no relationship between size of CC7 and cognitive performance in either the young adult (r = 0.03) or older adult (r = −0.09) group, supporting the specificity of the effects reported for CC2 and CC4.
4. Discussion
We report that adults over the age of 65 have reduced size within the genu and anterior truncus of the corpus callosum when controlling for intracranial area (ICA). For all participants, performance on cognitive tasks assessing psychomotor processing speed and working memory were all highly correlated with one another, and as a result we computed a composite score of cognitive function. Consistent with our hypothesis, we observed a positive relationship between the size of the genu and anterior truncus of the corpus callosum with our cognitive composite measure in older, but not young adults. The observed result of age-group and callosal region specific relationships between callosal size (controlled for intracranial cranial area) and task performance implies that our findings are not driven by general callosal atrophy, but rather reflect age effects specific to certain callosal regions.
Age differences in the relationship between neural activity and cognitive function have been studied extensively in recent years (for reviews see Reuter-Lorenz and Lustig, 2005; Reuter-Lorenz and Park, 2010). Additionally, research has shown that changes in neural structure play a significant role in age-related performance declines. Solely investigating the genu and the splenium of the CC, Kennedy and Raz (2009) found that poorer white matter microstructure of the genu (which is approximately equivalent to CC2 in the current study) was associated with decreased processing speed and working memory performance in older adults. In addition to fibers within this anterior CC region, our results indicate that fibers passing through CC4 also play an important role during tasks assessing psychomotor speeded response and working memory. This particular callosal region contains transcallosal fibers projecting into the medial motor areas including cingulate motor and supplementary motor areas (Hofer and Frahm, 2006). These medial motor areas have previously been implicated in tasks assessing psychomotor processing speed (Venkatraman et al., 2010) and working memory (Jansma et al., 2001; Mu et al., 2005). The findings of the current study, coupled with those of Kennedy and Raz (2009), suggest that older adults with larger macrostructure and better microstructure of the genu and anterior truncus of the CC experience relatively spared cognitive function, potentially through the preserved capability of interhemispheric interactions. For example, Sullivan and colleagues (2001) noted that age-related declines in the corpus callosum were associated with reduced interhemispheric communication efficiency.
Cabeza (2002) originally proposed the Hemispheric Asymmetry Reduction in Older Adults (HAROLD) model, describing the finding that older adults demonstrate decreased prefrontal cortex lateralization across different memory and cognitive tasks. Older adults exhibit similar over-recruitment when performing motor tasks (cf. Seidler et al., 2010 for a comprehensive review). Evidence suggests that over-recruitment patterns in older adults may reflect either i) compensatory activation where additional brain areas are positively associated with task performance (Cabeza, 2002; Heuninickx et al., 2008; Mattay et al., 2002; Naccarato et al., 2006; Reuter-Lorenz and Lustig, 2005) or ii) de-differentiation suggesting that brain structure-function relationships become less precise with age (Li & Lindenberger, 1999; Logan et al., 2002; Park et al., 2004; Riecker et al., 2006). In support of the de-differentiation hypothesis, increased bilateral activation in older adults has been suggested to be the result of decreased interhemispheric inhibition via the corpus callosum.
Fujiyama and colleagues (2009) found that older adults have a reduced ability to modulate inhibitory function in a task-dependent manner in comparison to young adults. Further, recent work by Muller-Oehring and colleagues (2007) investigating visual attentional processes indicates that callosal degeneration plays a pivotal role in the reduced functional cerebral lateralization seen in healthy aging. Specifically, they propose that selective degradation of the genu, as observed in the current study, leads to less robust interhemispheric inhibition, thus reducing lateralization and allowing interhemispheric crosstalk to influence cortical processing. Supporting this, we have recently shown that older adults with smaller CC size and poorer microstructure demonstrate task-specific performance declines on bimanual motor tasks requiring increased interhemispheric inhibition (Fling et al., In press). The current results fit in well with this literature demonstrating that older adults who maintain callosal size in specific regions that are prone to degradation demonstrate preserved performance on both cognitive and motor tasks. Thus, declines in callosal size and integrity, coupled with decreases in the inter-connectedness of the two hemispheres of the brain suggest that age-related cognitive and motor impairments may be due, at least in part, to reductions in interhemispheric inhibition. However, one notable limitation of the current study is a lack of measurement of vascular risk. As previously described, vascular risk is a mediator of age-related changes and differences in white matter integrity and volume (Raz et al., 2005; Kennedy & Raz, 2009).
Furthermore, the older adults exhibited lower scores on the MMSE, DEX, and Mattis dementia rating scale than the young adults. While the differences were slight and certainly are not atypical for studies comparing these two age groups, it may be that these differences in general cognitive status influenced the results. However, given the specificity of age differences in CC2 and CC4, and the fact that size of CC7—which did not exhibit significant age differences in size nor significant relationships with cognitive performance—we think it unlikely that such generalized factors are at play.
Our finding of age-specific relationships between callosal size and performance indicate older adults with larger macrostructure of the anterior half of the CC experience relatively spared performance on tasks related to psychomotor processing speed and working memory. Nevertheless, while the capability for interhemispheric interactions, as inferred from callosal size, may provide performance benefits for older adults, this capacity alone does not assure protection from general performance decline. That is to say, older adults with similar callosal size to young adults still exhibited reductions in performance. Further research is required to dissociate which task parameters are associated positively or negatively with CC size and microstructure, bihemispheric processing and task performance in young and older adults.
Age-related callosal decline has the capability to influence cognitive performance.
Older adults have selective loss of size in anterior callosal regions.
We correlate size of callosal sub-regions with cognition in a large sample size.
Increased anterior callosal size is favorable for older, but not young adults.
Acknowledgments
We thank N. Chapman and K. Olson for their help in data analysis. This work was supported by National Institutes of Health [T32-AG00114-21], and [R01-NS052514 RCW]; the UM Office of the Vice President for Research [RS]; and the UM National Institutes of Health Claude D. Pepper Older Americans Independence Center [AG08808 pilot grant and human subjects cores].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J of Cog Nuerosci. 2011;23(1):11–25. doi: 10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalp The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20(3):363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216 (3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. J of Verbal Learn and Verbal Behav. 1980;19:450–466. [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46 (2):530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Walsh CM, Bangert A, Reuter-Lorenz PA, Welsh RC, Seidler RD. Differential cllosal contributions to bimanual control in Yyung and older adults. J of Cog Neurosci. doi: 10.1162/jocn.2010.21600. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Peltier SJ, Bo J, Welsh RC, Seidler RD. Age differences in interhemispheric interactions: callosal structure, physiological function, and behavior. Front in Neurosci. 2011;5:38. doi: 10.3389/fnins.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Garry MI, Levin O, Swinnen SP, Summers JJ. Age-related differences in inhibitory processes during interlimb coordination. Brain Res. 2009;1262:38–47. doi: 10.1016/j.brainres.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I Volumetric MR imaging analysis. Am J Neuroradiol. 2002;23 (8):1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Krippendorff K. Answering the Call for a Standard Measure of Coding Data. Comm Methods and Measures. 2007;1:77–89. [Google Scholar]
- Hedden T, Lautenschlager G, Park DC. Contributions of processing ability and knowledge to verbal memory tasks across the adult life-span. Q J Exp Psychol A. 2005;58(1):169–190. doi: 10.1080/02724980443000179. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28 (1):91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS. Functional anatomical correlates of controlled and automatic processing. J of Cog Neurosci. 2001;13(6):730–743. doi: 10.1162/08989290152541403. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22 (4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Joy S, Kaplan E, Fein D. Speed and memory in the WAIS-III Digit Symbol –Coding subtest across the adult lifespan. Arch of Clin Neuropsych. 2004;19:759–767. doi: 10.1016/j.acn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J, Peltier S, Bo J, Fling BW, Welsh RC, Seidler RD. Functional implications of age differences in motor system connectivity. Front Syst Neurosci. 2010;4:17. doi: 10.3389/fnsys.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Lindenberger U. Cross-level uni cation: a computational exploration of the link between deterioration of neurotransmitter systems and the dedifferentiation of cognitive abilities in old age. In: Nilsson LG, Markowitsch H, editors. Cognitive Neuroscience of Memory. Hogrefe & Huber; Toronto: 1999. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33 (5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58 (4):630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia rating scale: Professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Mu Q, Nahas Z, Johnson KA, Yamanaka K, Mishory A, Koola J, Hill S, Horner MD, Bohning DE, George MS. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28(1):55–67. doi: 10.1093/sleep/28.1.55. [DOI] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Raassi C, Pfefferbaum A, Sullivan EV. Local-global interference is modulated by age, sex and anterior corpus callosum size. Brain Res. 2007;1142:189–205. doi: 10.1016/j.brainres.2007.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC. Does healthy aging affect the hemispheric activation balance during paced index-to- thumb opposition task? An fMRI study. Neuroimage. 2006;32 (3):1250–1256. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, et al. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31(4):1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA. 2004;101 (35):13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51(2):501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell K. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;18 (3):177–182. [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15(2):245–51. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci. 2010;65(4):405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller AC. Neural Recruitment and Cognitive Aging: Two Hemispheres Are Better Than One, Especially as You Age. Psychological Science. 1999;10(6):494–500. [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A. Functional significance of age-related differences in motor activation patterns. Neuroimage. 2006;32 (3):1345–1354. doi: 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14 (7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26 (8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54(1–3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Schofield NJ, Ashman AF. The Relationship between Digit Span and Cognitive Processing across Ability Groups. Intelligence. 1986;10:59–73. [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):13. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stančáak A, Cohen ER, Seidler RD, Duong TQ, Seong-Gi K. The size of corpus callosum correlates with functional activation of medial motor cortical areas in bimanual and unimanual movements. Cereb Cortex. 2003;13:475–485. doi: 10.1093/cercor/13.5.475. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging. 2010;31(3):464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman VK, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, Studenski S, Launer L, Pahor M, Williamson J, Rosano C. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010;49:3436–3442. doi: 10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, et al. Effect of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Cog Brain Research. 2004;21:418–426. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Evans JJ, Emslie H. The development of an ecologically valid test for assessing patients with dysexecutive syndrome. Neuropsych Rehab. 1996;8(3):213–228. [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112 (Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan ES. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage. 2009;44 (3):1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]