Abstract
Objective
A prevailing concept in neuroscience has been that the adult mammalian central nervous system is incapable of restorative axon regeneration. Recent evidence, however, has suggested that reactivation of intrinsic cellular programs regulated by Akt/mTor signaling may restore this ability.
Methods
To assess this possibility in the brain, we have examined the ability of adeno-associated virus mediated transduction of dopaminergic neurons of the substantia nigra with constitutively active forms of the kinase Akt and the GTPase Rheb to induce re-growth of axons after they have been destroyed by neurotoxin lesion.
Results
Both constitutively active myristoylated Akt and hRheb(S16H) induce regrowth of axons from dopaminergic neurons to their target, the striatum. Histological analysis demonstrates that these new axons achieve morphologically accurate reinnervation. In addition, functional re-integration into target circuitry is achieved, as indicated by partial behavioral recovery.
Interpretation
We conclude that regrowth of axons within the adult nigro-striatal projection, a system that is prominently affected in Parkinson’s disease, can be achieved by activation of Akt/mTor signaling in surviving endogenous mesencephalic dopaminergic neurons by viral vector transduction.
INTRODUCTION
A long-standing belief in neuroscience has been that the adult mammalian central nervous system is incapable of an axonal regenerative response, unlike the peripheral nervous system, where axons are able to regrow, reach their targets, and restore function1–3. This absence of a regenerative response has been largely attributed to an unfavorable local environment following injury that is due to glial scar and inhibitory local proteins derived from damaged myelin3. More recently it has been emphasized that this regenerative failure is also due to downregulation in adult brain of cell autonomous neuronal molecular signals that mediate axon growth during development2,4,5. This concept, that failure of an adult regenerative response may be due in part to downregulation of essential molecular signals, raises the possibility that it may be feasible to restore the regenerative response by reactivation of these signals. Such a possibility was given support by the findings of Park and colleagues, who observed that activation of mTor signaling in adult retinal ganglion neurons prior to optic nerve injury promoted regeneration of their axons6.
However, whether such a regenerative response might also be possible within the parenchyma of the brain has remained highly uncertain due to the formidable challenges to axon growth that are present. The optic nerve, as a fasciculated and anatomically delimited nerve trunk lying in the periphery, provides a physical conduit for axon growth and guidance. In contrast, the large majority of axonal projections in brain are not assembled into discrete, myelinated bundles; they follow complex trajectories, and pass through diverse cellular environments. In spite of these anticipated challenges to axon pathfinding in the mature brain, it has recently been shown, remarkably, that fetal dopaminergic neuroblasts, when implanted into the lesioned substantia nigra (SN) of the adult brain, are capable of extending axons along the damaged nigro-striatal pathway to reach their normal target, the striatum, and achieve a functional integration into host circuitry7.
In view of this evidence that the injured adult nigro-striatal projection may provide a permissive environment for axon regrowth, we sought to determine whether activation of Akt kinase or Rheb GTPase, upstream activators of mTor, within residual surviving adult dopamine neurons of the SN may be sufficient to stimulate an axon regeneration response that restores function. In addition, we sought to evaluate whether such a response could be achieved after injury, rather than before; as such a sequence would bear greater relevance to any potential for clinical therapeutics.
For these investigations we utilized a highly destructive and well-characterized neurotoxin model that is induced by intra-striatal injection of 6-hydroxydopamine (6OHDA)8. This neurotoxin induces retrograde degeneration of dopaminergic axons that is maximal during the first week post-lesion, and complete by three weeks9,10 ( and see Supplementary Figure 1).
METHODS
Mice and animal care procedures
Adult (8 week) male C57Bl/6 mice weighing ~25 g were obtained from Charles River Laboratories (Wilmington, MA). TH-GFP transgenic mice, which express green fluorescent protein driven by the tyrosine hydroxylase (TH) promoter11 were generously made available by Drs. K Kobayashi and H Okano and maintained on a C57Bl/6 background. All injection procedures, described below, were approved by the Columbia University Animal Care and Use Committee.
Production of Adeno-Associated Virus (AAV) vectors
All vectors used for these studies were AAV1 serotype. To achieve activation of mTor, we utilized mutant constitutively active forms of either the kinase Akt or the GTPase Rheb, both of which are upstream mediators12. For Akt, we utilized a myristoylated form (Myr-Akt)13,14. To simplify subsequent analysis of relevant effector pathways, as discussed further below, we used a mutant in which the phosphoacceptor serine at position 473 had been changed to phenylalanine. For Rheb we utilized a mutant that is resistant to GTPase activation by the tuberous sclerosis complex (hRheb(S16H))15,16. Myr-Akt(S473F) was produced as previously described17 and as detailed in Additional Supplementary Material. We have previously shown that AAV Myr-Akt successfully transduces SN dopamine neurons17. AAV hRheb(S16H) likewise was able to successfully transduce dopamine neurons, and to activate mTor, demonstrated by immunostaining for phosphorylated 4E-BP1, an mTor substrate (Supplementary Figure 2).
Intra-striatal 6OHDA injection
The intra-striatal 6OHDA model was induced essentially as originally described for rats8, with modifications for adult mice18.
Intra-nigral AAV injection
Mice were anesthetized with ketamine/xylazine solution and placed in a stereotaxic frame (Kopf Instruments) with a mouse adapter. The tip of 5.0 μL syringe needle (26S) was inserted to stereotaxic coordinates AP: −0.35cm; ML: +0.11cm; DV: −0.37cm, relative to bregma. Viral vector suspension in a volume of 2.0 μL was injected at 0.1 μL/min over 20 minutes.
Immunohistochemistry
Immunostaining for TH was performed as described previously19 and as detailed in Additional Supplementary Material.
Quantitative determination of SN dopamine neuron numbers and striatal TH immunoperoxidase staining density
SN dopamine neuron numbers were determi n e d b y stereologic analysis under blinded conditions using StereoInvestigator software (MicroBrightField, Williston, VT). The optical density of striatal TH immunostaining was determined with an Imaging Research (St. Catherines, Ontario) Analytical Imaging Station.
Quantification of GFP-positive and Tomato-tau-positive axons in the medial forebrain bundle (MFB)
Quantification of axons was performed on TH-GFP transgenic mice, which express green fluorescent protein driven by the TH promoter11. Mice were perfused intracardially with 0.9% NaCl followed by 4.0% paraformaldehyde in 0.1 M phosphate buffer (pH 7.1), and then posfixation for 48 hours. The brains were sectioned horizontally on a Vibratome at 50 μm. A section containing the posterior third ventricular recess and the A13 dopamine cell group was selected for analysis as described20. Confocal microscopy (Leica TCS SP5 AOBS MP System) was used to acquire images through the entire medial-to-lateral extent of the MFB. Proceeding from a point midway between the anterior A13 cells and the posterior third ventricle recess, images were acquired with a 20X objective with a zoom factor of 8 applied. Seven contiguous fields (97 μm × 97 μm) were scanned. Each field was scanned in the Z-axis with twenty 0.1 μm thickness optical planes from dorsal to ventral, for a total vertical distance of 2.0 μm in the center of the section. These twenty optical planes were then merged to obtain a single maximal projection of the sampled volume. In order to count the number of axons passing in the rostro-caudal dimension through each sample volume, two horizontal sampling lines were drawn on the image at a separation distance of 10 μm in the center of the maximal projection. Every intact axon crossing both lines was counted as positive. An identical approach was used to count tomato-positive axons. In addition, tortuous tomato-positive axons in the MFB were identified by epifluorescence.
Rotational behavior following amphetamine injection
Rotational behavior tests in AAV hRheb(S16H)- and AAV Myr-Akt-injected mice were performed at 12 and 7 weeks after virus injection (15 and 10 weeks after the 6OHDA lesion, respectively). Mice were injected with amphetamine (2.5 mg/kg i.p.; Sigma) and placed in a plastic hemispherical bowl. Contralateral and ipsilateral turns were counted by a computerized rotometer system (San-Diego Instruments, San Diego, CA) for 60 min, and results were expressed as net turns per 60 min.
RESULTS
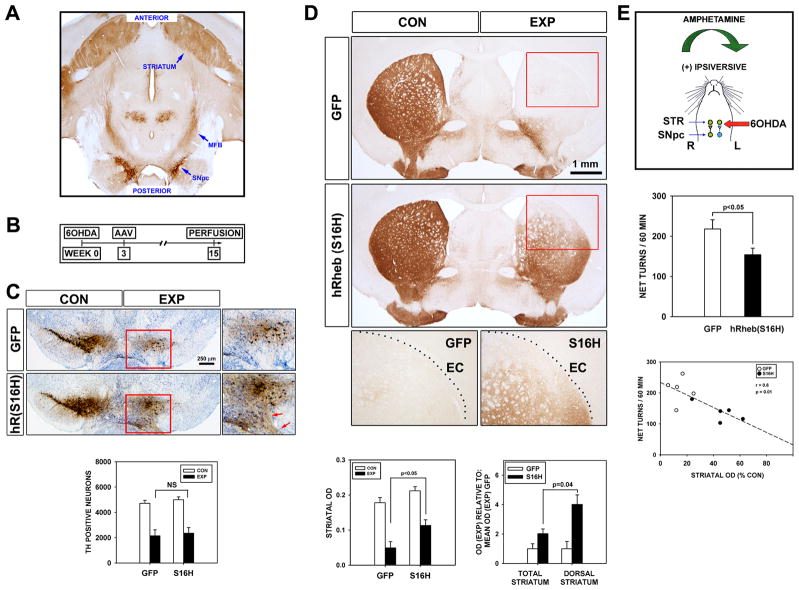
To better simulate the human disease context, we first lesioned the nigro-striatal system by intra-striatal injection of 6OHDA, waited three weeks for axon degeneration to take place9,10 (and see Supplementary Figure 1), and then injected the SN with AAV vectors (Fig 1B). Following transduction of SN neurons with AAV hRheb(S16H), an assessment at 15 weeks post-lesion revealed that although there was no effect on the number of remaining dopamine neurons (Fig. 1C), there was a striking reinnervation of the lesioned striatum by dopaminergic axons (Fig. 1D). To ascertain whether this reinnervation of the striatal target successfully re-integrated with intact circuitry, and achieved functionality, we examined effects on a behavioral response. Following unilateral striatal dopaminergic denervation, the administration of amphetamine induces a preponderance of dopamine release on the intact side, resulting in an ipsiversive rotational behavior. Mice treated with AAV hRheb(S16H) showed improvement as a diminished rotational response (Fig. 1E). The degree of behavioral improvement correlated with the extent of striatal reinnervation (Fig. 1E).
FIGURE 1.
Constitutively active hRheb(S16H) induces extensive reinnervation of the striatum by dopaminergic axons. (A) A horizontal section of the mouse brain immunostained for TH demonstrates the anatomy of the mesencephalic dopaminergic projection to the striatum. The neurons giving rise to this projection reside in the substantia nigra pars compacta (SNpc), and their axons pass anterior within the medial forebrain bundle (MFB) to their target, the striatum. (B) An extensive lesion of this system is induced by the intra-striatal injection of 6OHDA. At 3 weeks after lesion, when axon degeneration is complete, neurons of the SNpc were transduced by intra-nigral injection of AAV hRheb(S16H). (C) At 15 weeks post-lesion, coronal sections of the SNpc immunostained for TH reveal no protection of dopamine neurons by hRheb(S16H) in comparison to AAV GFP-injected controls, as shown by stereologic counts (n = 5 both groups). The brown immunoperoxidase staining in the medial SNpc observed at low power in the hRheb(S16H) condition is due to an extensive plexus of neurites within the remaining SNpc and SN pars reticulata (red arrows, inset). This plexus is not observed in normal mice, so its presence suggests the occurrence of aberrant sprouting induced by hRheb(S16H). (D) In spite of the lack of protection of neurons, hRheb(S16H) induces an extensive reinnervation of the striatum by dopaminergic fibers. The reinnervation extends fully to the dorso-lateral border of the striatum, defined by the external capsule (EC). This effect is revealed quantitatively as a more than 2-fold increase in the optical density of TH peroxidase staining in the hRheb(S16H)-treated mice (n = 5 both groups; p < 0.001, ANOVA; p < 0.05, Tukey post-hoc). The reinnervation induced by hRheb(S16H) is especially striking in the dorso-lateral region of the striatum, where there is a 4-fold increase in optical density. (E) To ascertain whether this anatomical restoration is accompanied by motor recovery, we examined the rotational response to amphetamine. Mice treated with hRheb(S16H) showed improvement as a diminished rotational behavior (n = 7 both groups; p < 0.05, t-test). In keeping with this improvement being due to striatal dopaminergic reinnervation, there was a significant inverse correlation between increased striatal TH staining and diminished ipsiversive rotations (r = 0.8, p = 0.01).
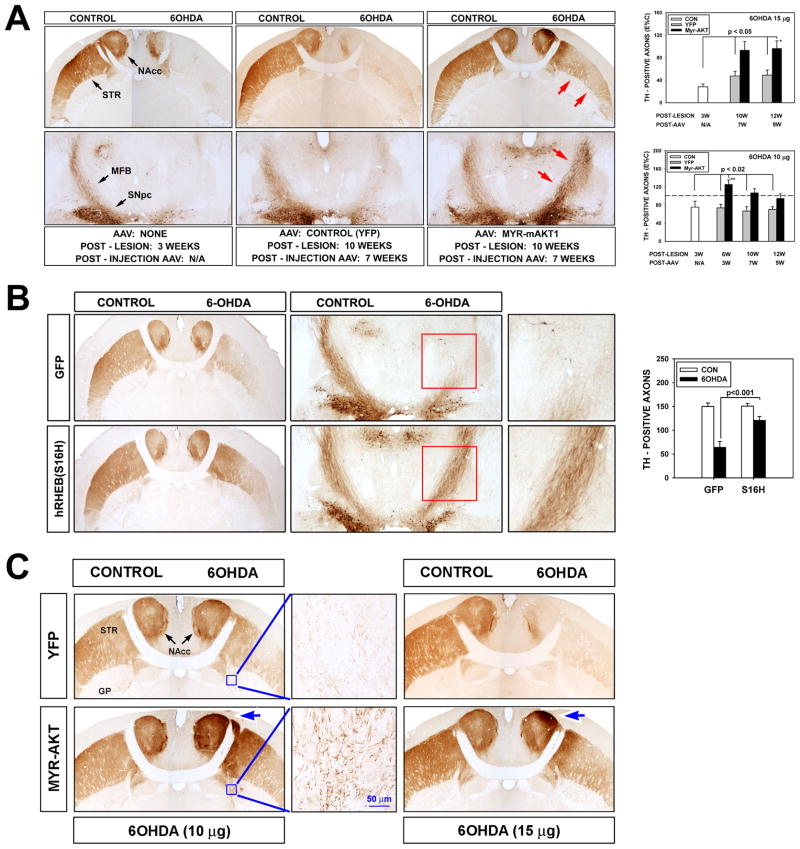
Striatal reinnervation was accompanied by substantial restoration of the number of TH-positive axons within the MFB by both Myr-Akt and hRheb(S16H). For Myr-Akt, at 10 and 12 weeks post-lesion, axon counts were 93 ± 15 and 96 ± 14 percent of contralateral, non-lesioned control values respectively, whereas axon counts in AAV YFP control injections were 47± 8 and 49 ± 9, respectively (p = 0.001, ANOVA) (Fig. 2A). For hRheb(S16H) axons counts were restored to 72 ± 8 percent of contralateral values, in comparison to 42 ± 5 for control injections (p= 0.001, ANOVA) (Fig. 2B).
FIGURE 2.
Constitutively active Akt (Myr-Akt) and hRheb(S16H) induce repopulation of the MFB by dopaminergic axons. (A) Horizontal sections of mouse brain, stained for TH, demonstrate restoration of dopaminergic axons within the MFB by Myr-Akt. At 3 weeks post-lesion, there are a few remaining dopaminergic neurons in the SNpc, but in this low magnification micrograph there is no remaining axonal immunostaining in the MFB or striatum (STR). Minimal residual staining is observed in the medial aspect of the nucleus accumbens (NAcc). Following intra-nigral administration of AAVs, very little TH immunostaining is observed in both MFB and striatum at 10 weeks post-lesion of mice given AAV YFP as control. However, robust axonal immunostaining is observed in the MFB (red arrows, bottom right panel) and in the striatum (red arrows, top right panel) of mice given AAV Myr-Akt. This striatal reinnervation induced by Myr-Akt successfully re-integrated with host circuitry, demonstrated as an improvement in amphetamine-induced rotations (Supplementary Figure 3). Restoration of axons is shown in the upper graph as number of TH-positive axons in the MFB following a high dose (15 μg) 6OHDA lesion. Axon numbers were reduced to 28% of the contralateral non-lesioned MFB at 3 weeks post-lesion. There was no significant change in mice given control YFP injections. In the mice given Myr-Akt, there was an approximately two-fold increase by 10 and 12 weeks post-lesion (n = 4 all groups; p = 0.001 ANOVA; p< 0.05 Tukey post-hoc, as shown). In a similar experiment performed with a lower does of 6OHDA (10 μg), Myr-Akt induced a greater number of axons on the lesioned side at 6 weeks post-lesion (n = 4 all groups; p = 0.002 ANOVA; p< 0.02 Tukey post-hoc, as shown) (the horizontal dotted line indicates 100%, or an equal number of axons on the lesioned and non-lesioned sides of the brain). This observation strongly suggests that Myr-Akt induces dopaminergic axon sprouting. (B) hRheb(S16H) also induces a restoration of dopaminergic axons within the MFB and striatum. Representative horizontal sections stained for TH are shown at 15 weeks post-lesion. The red rectangles encompass the MFB on the lesioned side, and are shown at higher magnification in the panels at the right. The effect of hRheb(S16H) is shown quantitatively as TH-positive axon counts (n = 5 AAV GFP; n = 7 AAV hRheb(S16H); p<0.001, ANOVA). (C) Abnormal patterns of dopaminergic innervation are observed in the globus pallidus (GP) and striatum (STR) following treatment with Myr-Akt. After low dose 6OHDA (10 μg), Myr-Akt induces a plexus of TH-positive axons in the medial globus pallidus (inset). Very few TH-positive axons are normally observed in the location. In addition, Myr-Akt induces abnormal foci of increased TH immunoperoxidase staining (greater than the contralateral control) in the medial striatum and nucleus accumbens (blue arrow). Similar abnormal foci are observed among mice treated with high dose 6OHDA (blue arrow).
Although the reinervation induced by Myr-Akt and hRheb(S16H) accurately recapitulated normal patterns of innervation in most respects, there were a number of abnormalities attributable to aberrant patterns of regrowth. In the SN an abnormal population of TH-positive neurites was identified in the mesencephalon ventral to the SNpc and medial to the SNpr (Fig. 1C, inset). In the globus pallidus, an abnormal population of TH-positive axons was identified medially, just posterior to the anterior limb of the anterior commissure (Fig. 2C). In the striatum itself, in both the low and high dose 6OHDA conditions, abnormal foci of increased density of peroxidase staining, greater than the contralateral control, non-lesioned side, were observed in the medial striatum and nucleus accumbens (Fig. 2C).
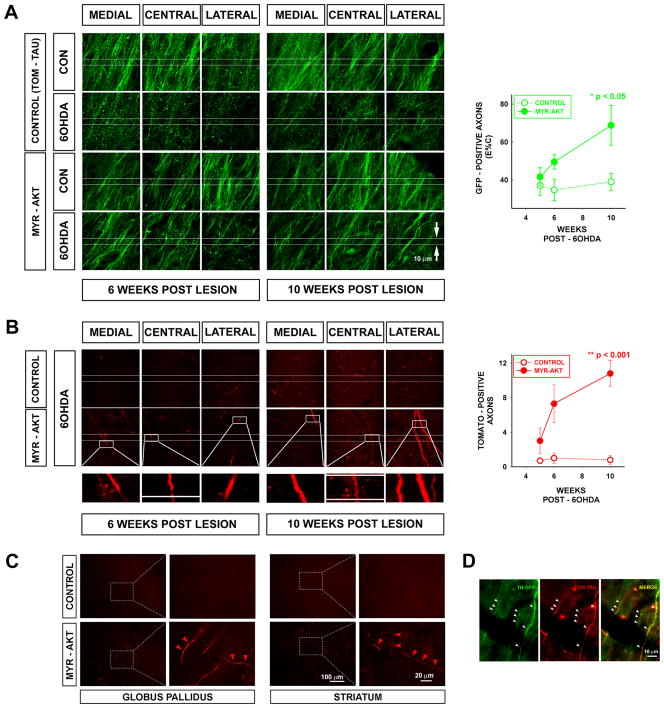
While immunostaining for TH, or other protein markers of dopamine axons, provides a useful static picture of the extent of striatal and MFB axon restoration, it is not useful for monitoring dynamic aspects of dopaminergic axon growth, because TH is highly regulated, and in some contexts, there is no expression even in structurally intact axons20,21. Therefore, in order to monitor the growth of new axons from the SN through the MFB towards the striatum, we utilized two techniques. First, we monitored the number of GFP-positive axons in TH-GFP mice at a fixed distance anterior to the SN at three time points. Second, we labeled SN neurons and their axons with the anterograde tracer tomato-tau (Tom-Tau) by AAV Tom-Tau co-injection with AAV Myr-Akt (or alone, as control), followed by counts of labeled axons in the MFB at the three time points post-injection. Both of these techniques demonstrated an increased number of axons in the MFB anterior to the SN following transduction of the 6OHDA-lesioned dopamine neurons with Myr-Akt (Fig. 3). In the analysis of the TH-GFP mice, there is a trend for an increased number of axons in the MFB at 6 weeks post-lesion (Fig. 3A). However, by 10 weeks, there is a significant increase in the number of these axons in comparison to control mice. In control mice, not treated with Myr-Akt, very few tomato-tau-positive axons entered the MFB at either 6 weeks (1.0 ± 0.6) or 10 weeks (0.8 ± 0.5) post lesion (Fig. 3B). However, mice treated with Myr-Akt showed many tomato-tau – positive axons in the MFB at 6 weeks (7.3 ± 2.2) and more so at 10 weeks (10.8 ± 1.5) (Fig. 3B). Mice treated with Myr-Akt also demonstrated tomato-tau-positive fibers in the globus pallidus and the striatum, whereas control mice (treated with AAV Tom-Tau) did not (Fig. 3C). We confirmed that some of the tomato-tau-positive fibers in the MFB in Myr-Akt-treated mice were dopaminergic, because they were co- labeled with GFP under the TH promoter (Fig. 3D).
FIGURE 3.
Reconstruction of the dopaminergic projection within the MFB by axons from the SNpc. Adult TH-GFP mice were injected with 6OHDA (15 μg) and 3 weeks later they were injected with AAV tomato-tau (TOM-TAU) mixed with AAV Myr-Akt (or tomato-tau alone as a control) into the SNpc. The number of GFP-positive axons passing through the MFB at a fixed point anterior to the SNpc at 5, 6 and 10 weeks after AAV injection was determined. In addition, the number of anterogradely-labeled tomato-tau-positive axons at the same location and times was determined. (A) Each panel represents a single confocal maximal projection of a 20 × 0.1 μm Z stack acquired from the MFB of a TH-GFP mouse. For each representative set of panels acquired from a single mouse, three images are shown from the central and adjacent medial and lateral MFB on both the non-lesioned control (CON) or the 6OHDA-lesioned side. In the mouse receiving tomato-tau alone as control, there is a loss of GFP-positive axons on the 6OHDA-lesioned side at 5 and 6 weeks. Quantitative analysis, shown in the graph to the right, reveals about 65% loss (n = 3, both time points). In the mouse receiving Myr-Akt, some axons have appeared at 6 weeks, but the quantitative difference in comparison to the tomato-tau alone controls does not achieve significance (n = 3, p = 0.1, t-test). However, by 10 weeks post-lesion the number of new axons in mice treated with Myr-Akt has increased by 45% (from 32.7 ± 3.2 at 6 weeks to 47 ± 5.0), and there is now a significant difference in comparison to control (n = 4 both groups, p < 0.05, t-test). (B) Representative confocal Z-stacks from central and adjacent medial and lateral MFB on the lesioned side are shown at 6 and 10 weeks post-lesion. While few tomato-labeled axons appear in the MFB of the control mice given tomato-tau alone, many are identified in the mice given tomato-tau co-administered with Myr-Akt. Analysis of tomato-positive axons reveals a significant effect at both 6 weeks (n = 3, both groups, p = 0.05, t-test) and 10 weeks (n = 4, p<0.001, t-test). (C) Myr- Akt induced the growth of tomato-tau positive axons (red arrowheads) not only in the MFB, but also in the globus pallidus and the striatum. (D) Injection of AAV TOM-TAU in the TH-GFP mice provided an opportunity to identify many of the tomato-tau positive fibers as dopaminergic. Three representative examples (white arrowheads) are shown, as indicated by double-labeling for GFP in dopaminergic axons and tomato-tau.
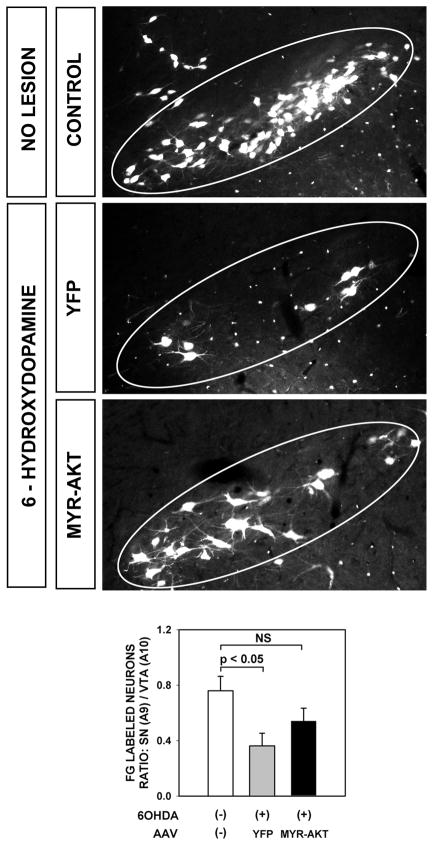
In order to confirm the presence of new axons arising from the SNpc and reaching the striatal target, we performed retrograde labeling by intra-striatal injection of Fluorogold at 10 weeks following 6OHDA lesion (Fig. 4). This analysis revealed a greater abundance of retrograde labeling in the SNpc of Myr-Akt-treated mice in comparison to AAV YFP-injected controls (Fig. 4).
FIGURE 4.
To determine whether axons in Myr-Akt-treated mice successfully reached the striatal target, and if they originated, at least in part, from neurons of the SNpc, we performed retrograde labeling by intra-striatal injection of Fluorogold (FG) at 10 weeks following 6OHDA lesion. This analysis revealed that many SNpc neurons in the Myr-Akt condition were retrogradely labeled, indicating that they had arisen from the SNpc and reached the striatal target. Following intra-striatal 6OHDA injection, there is a relative loss of dopamine neurons in SNpc (A9) in comparison to VTA (A10). This loss is reflected as a decrease in the ratio of SN FG-positive neuron counts to VTA FG-positive counts (p = 0.04, ANOVA; p < 0.05 Tukey post-hoc; No 6OHDA lesion (n = 5) vs 6OHDA lesion/YFP (n = 6)). The ratio is partially restored following Myr-Akt (n = 7; p = 0.3, NS, Tukey).
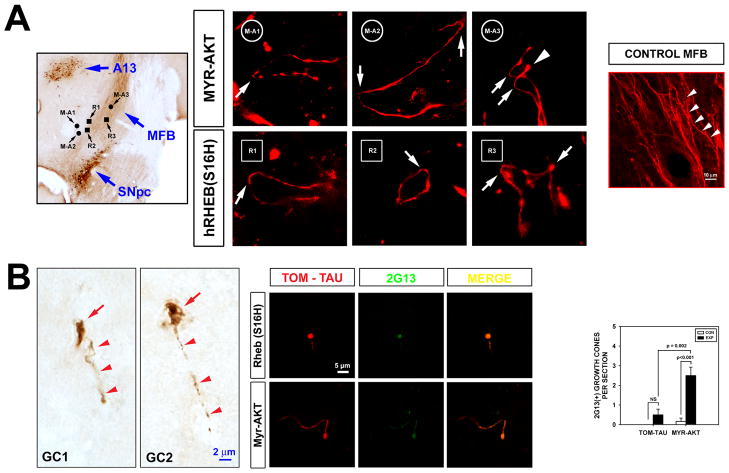
The presence of increased numbers of axons by anterograde tomato-tau-labeling at 6 weeks post-lesion (3 weeks after AAV transduction) would predict that active axonal growth should be identifiable within the MFB at 5 weeks post-lesion (2 weeks after AAV). We therefore sought the presence of axon growth in the MFB by use of anterograde tracing with tomato-tau to identify tortuous axons and the characteristic morphology of enlarged, bulbous growth cones at the tip of axons. We supplemented these observations by immunostaining with a monoclonal antibody (2G13), to detect growth cones22,23. These methods demonstrated tortuous axons and growth cones exclusively in the MFB following transduction with either Myr-Akt or hRheb(S16H) (Fig. 5).
FIGURE 5.
Axon regeneration in the MFB following transduction of SN neurons with either Myr-Akt or hRheb(S16H). (A) The upper left panel shows a sample horizontal section stained for TH, to illustrate the anatomical relationships among the substantia nigra pars compacta (SNpc), the MFB and the A13 dopaminergic nucleus. The locations where tortuous axons were identified in comparable sections following treatment with Myr-Akt or hRheb(S16H) are shown. Three circles identify sites where three tortuous axons were identified following transduction with Myr-Akt (M-A1, 2, 3: Myr-Akt axons 1, 2, and 3); three squares identify sites where axons were found following transduction with hRheb(S16H) (R1, 2, 3). Both AAV Myr-Akt and AAV hRheb(S16H) were mixed with AAV tomato-tau to permit visualization of axons. AAV injections were performed at 3 weeks following 6OHDA lesion (15 μg), and mice were sacrificed at 2 weeks post-AAV transduction. Following treatment with Myr-Akt and hRheb(S16H), tortuous axons with numerous hairpin turns (white arrows) are identified. Such abnormal, recursive growth patterns suggest new axon formation24. In one panel (M-A3) a pair of axons with hairpin turns are observed; one has a bulbous tip, suggestive of a growth cone (white arrowhead). In the panel on the right, a low power micrograph of tomato-tau labeling in a normal, unlesioned MFB reveals that normal axons are parallel and only slightly meandering in their course (while arrowheads). (B) Immunoperoxidase staining with 2G13 reveals growth cones within the MFB at 2 weeks after intra-nigral injection of AAV Myr-Akt (5 weeks after 6OHDA lesion). Representative profiles are shown from two mice. Each profile demonstrates the characteristic appearance of an axon (red arrowheads) terminating in a bulbous growth cone (GC) (red arrow). In the center panels growth cones are identified in the MFB following transduction of SN neurons with either Myr-Akt or hRheb(S16H) by double-labeling achieved by immunofluorescent (green) staining for 2G13 in combination with anterograde axon labeling with tomato-tau (TOM-TAU) (red). The characteristic appearance of axons terminating in a bulbous growth cone, labeled by 2G13, is observed. The graph shows the results of a quantitative analysis of the number of growth cones in the MFB at 2 weeks after intra-nigral injection of AAV Myr-Akt (5 weeks after 6OHDA lesion). Growth cones are significantly more abundant following treatment with Myr-Akt (n = 6) than tomato-tau alone (n = 4)(p < 0.001, ANOVA; Tukey posthoc analysis).
DISCUSSION
In the identification of axon regeneration; it is important to exclude alternative axonal responses that may cause a false apparent appearance of “new” axons24. It is conceivable, for example, for an experimental treatment to forestall degeneration of axons on the first hand, and as they recover, they regain phenotypic markers and reappear, seeming to “re-grow”. Alternatively, the original injury may not have even induced degeneration in the complete population of axons, but only caused them to lose phenotype. If the treatment effectively restores phenotype, they will re-appear, without having actually re-grown24. With regard to the first possibility, we have in fact shown that both Myr-Akt and hRheb(S16H) are capable of forestalling retrograde degeneration of dopaminergic axons due to a variety of injuries21. However, this axon protection phenotype of Myr-Akt and hRheb(S16H) is observed exclusively in the period of acute axon injury. The present experiments were undertaken at 3 weeks post-lesion, by which time the degenerative process has run its course and ceased.
Careful consideration must be given to the second alternative explanation for the false appearance of “new” axons, that their phenotype has simply been restored. Several of our observations provide evidence against this possibility. First, we show for axons identified both by GFP under the TH promoter and by tomato-tau under the chicken beta actin promoter that between 6 and 10 weeks post-lesion there is an increase number of axons in the MFB. This observation is precisely what would be anticipated should regeneration of axons occur. The alternate hypothesis, that there has instead been a restoration of phenotype, would account for these observations only if there occurred an anterograde “wave” of restoration, occurring gradually over weeks, for both promoter-reporter systems, an unlikely event. Second, the postulated condition of “lost phenotype” for axons under this hypothesis would need to be characterized not only by the lack of expression of these protein markers and their anterograde transport, but also by the inability to take up and retrogradely transport Fluorogold, an unlikely state for living axons. Third, we have observed by use of anterograde axon tracing with tomato-tau following transduction with Myr-Akt or hRheb(S16H) that many axons demonstrate a tortuous appearance that is not normally observed among adult dopaminergic axons within the MFB (Fig. 5A). Such a morphology suggests that new axon growth has occurred24. Fourth, we have shown that while axon regrowth initiated by both Myr-Akt and hRheb(S16H) faithfully restores normal patterns of innervation by the dopaminergic projection in most respects, it is not perfect; abnormal populations of TH-positive neurites are observed in the mesencephalon and the pallidum, and foci of abnormal, intense peroxidase staining are observed in the striatum and nucleus accumbens. These observations cannot be attributed to a restoration of dopaminergic phenotype because they are not observed in the normal brain. Fifth, and most importantly, we have demonstrated the presence of axon growth cones by morphologic and immunohistochemical criteria at the anticipated time in the MFB following both Myr-Akt and hRheb(S16H) (Fig 4B). In conclusion, our observations, when considered together, suggest that both Myr-Akt and hRheb(S16H) have induced axon regeneration in this system. The regeneration is robust and lasting, it achieves target contact, and achieves functional re-integration within the adult brain.
Given the abundant evidence for the very limited ability of the adult brain to give rise to an axon regeneration response1–3, the ability of both Myr-Akt and hRheb(S16H) to induce a robust, accurate and functionally integrated axon regrowth in nigral dopamine neurons is unexpected and striking. While this robust response may be entirely attributable to a previously unknown ability of these constitutively active mutants to induce new axon growth, other differences between our approach and those of prior studies may also account for our observations. The large majority of prior studies of axon regrowth in adult brain have utilized acute injury models such as axotomy or stroke that result in disruption of brain or spinal cord parenchyma, formation of glial scar, and disruption of myelin, both of which are inhibitory to axon regrowth3,25,26. In the 6OHDA intra-striatal model, however, axonal degeneration is sub-acute and progressive, proceeding retrograde without disruption of brain parenchyma. In addition, very few axons of the MFB are myelinated. Thus, our particular lesion model may produce a more favorable brain environment for axon regrowth. It is also possible that nigral dopamine neurons may have a unique propensity for axon regeneration.
Park and colleagues have previously proposed that activation of mTor kinase signaling by conditional deletion of either PTEN, a negative regulator of PI3K/AKT, or tuberous sclerosis complex 1, a negative regulator of Rheb, promotes axon regeneration of retinal ganglion neurons6. This hypothesis is in keeping with many other experimental observations that have demonstrated an ability of Akt/Rheb/mTor signaling to enhance many features of axon growth, including not only axon length, but also number per neuron27, branching, caliber, and growth cone dynamics28 (reviewed in29,30). Our observations support the hypothesis proposed by Park and colleagues. mTor is a principal target of Akt signaling31 and we have shown that Myr-Akt activates mTor in transduced neurons of the SN21. The GTPase Rheb is a principal activator of mTor32,33. We herein demonstrate that transduction of SN neurons with hRheb(S16H) activates mTor, indicated by increased p4E-BP1. Thus, our observations and those of Park and colleagues suggest that activation of mTor signaling mediates the restored ability of adult neurons in the central nervous system to regenerate axons. mTor exists as two complexes, mTORC1, associated with raptor, and mTORC2, associated with rictor, and their downstream effector pathways are diverse12. Our observations, made with Myr-Akt(S473F), indicate that phosphorylation of Akt at S473 by mTORC2/rictor does not play an essential role.
Our findings have implications for regenerative therapies of diseases of the central nervous system, because they support the concept that programs that mediate axon growth can be reactivated in the injured adult brain, with successful target contact and restoration of function. It remains unknown, however, to what extent these findings may generalize to other complex circuits of the brain. However, even if these findings are confined to the dopaminergic nigro-striatal projection, they nevertheless have implications for the treatment of Parkinson’s disease, which has been proposed to initially involve axons, and in which even partial reinnervation may be sufficient to restore normal motor function34.
Both Akt and Rheb are potent oncogenes35, so consideration of using any therapeutic approach, whether pharmacologic or gene therapy, to activate them raises obvious concerns. However, our observations here represent only an early proof-of-principal step, and concerns about oncogenesis may not be insurmountable. It may be possible to identify downstream mediators that retain the axon regeneration phenotype, and yet are devoid of oncogenic potential. Alternatively, specific intra-neuronal cellular targeting may circumvent oncogenic signaling36. Thus, the risk of oncogenesis may yield to future developments in molecular specificity and intra-cellular trafficking.
In conclusion, the prevailing concept that the adult brain is incapable of axon regeneration needs to be challenged, particularly in the context of neurodegenerative disease. The observations presented herein would suggest that induction of an axon regeneration response may be possible, even in the adult brain, and provide a basis for regenerative therapeutics that restore complex circuitry and function.
Supplementary Material
Acknowledgments
We are grateful to Dr. L. Greene for his thoughtful reading of the manuscript and comments and Dr. G. Di Paolo for insightful discussion. This work was supported by NIH NS26836 and NS38370, the Parkinson’s Disease Foundation, the Parkinson’s Alliance, and the RJG Foundation (REB).
References
- 1.Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benowitz LI, Yin Y. Combinatorial treatments for promoting axon regeneration in the CNS: strategies for overcoming inhibitory signals and activating neurons’ intrinsic growth state. Dev Neurobiol. 2007;67:1148–1165. doi: 10.1002/dneu.20515. [DOI] [PubMed] [Google Scholar]
- 3.Nash M, Pribiag H, Fournier AE, et al. Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol. 2009;40:224–235. doi: 10.1007/s12035-009-8083-y. [DOI] [PubMed] [Google Scholar]
- 4.Sun F, He Z. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Kim AH, Bonni A. The dynamic ubiquitin ligase duo: Cdh1-APC and Cdc20-APC regulate neuronal morphogenesis and connectivity. Curr Opin Neurobiol. 2010;20:92–99. doi: 10.1016/j.conb.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson LH, Grealish S, Kirik D, et al. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci. 2009;30:625–638. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- 8.Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6 hydroxydopamine a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 9.Ries V, Silva RM, Oo TF, et al. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. J Neurochem. 2008;107:1578–1588. doi: 10.1111/j.1471-4159.2008.05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedreen JC, Chalmers JP. Neuronal degeneration in rat brain induced by 6-hydroxydopamine; a histological and biochemical study. Brain Res. 1972;47:1–36. doi: 10.1016/0006-8993(72)90248-x. [DOI] [PubMed] [Google Scholar]
- 11.Sawamoto K, Nakao N, Kobayashi K, et al. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Pellman D, Garber EA, Cross FR, et al. An N-terminal peptide from p60src can direct myristylation and plasma membrane localization when fused to heterologous proteins. Nature. 1985;314:374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed NN, Grimes HL, Bellacosa A, et al. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci U S A. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan L, Findlay GM, Jones R, et al. Hyperactivation of mammalian target of rapamycin (mTOR) signaling by a gain-of-function mutant of the Rheb GTPase. J Biol Chem. 2006;281:19793–19797. doi: 10.1074/jbc.C600028200. [DOI] [PubMed] [Google Scholar]
- 16.Sato T, Umetsu A, Tamanoi F. Characterization of the Rheb-mTOR Signaling Pathway in Mammalian Cells: Constitutive Active Mutants of Rheb and mTOR. Methods Enzymol. 2008;438:307–320. doi: 10.1016/S0076-6879(07)38021-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ries V, Henchcliffe C, Kareva T, et al. Oncoprotein Akt/PKB: Trophic effects in murine models of Parkinson’s Disease. Proc Natl Acad Sci U S A. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva RM, Ries V, Oo TF, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kholodilov N, Yarygina O, Oo TF, et al. Regulation of the development of mesencephalic dopaminergic systems by the selective expression of glial cell line-derived neurotrophic factor in their targets. J Neurosci. 2004;24:3136–3146. doi: 10.1523/JNEUROSCI.4506-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng HC, Burke RE. The Wld(S) mutation delays anterograde, but not retrograde, axonal degeneration of the dopaminergic nigro-striatal pathway in vivo. J Neurochem. 2010;113:683–691. doi: 10.1111/j.1471-4159.2010.06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng HC, Kim SR, Oo TF, et al. AKT suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.5519-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stettler O, Bush MS, Kasper M, et al. Monoclonal antibody 2G13, a new axonal growth cone marker. J Neurocytol. 1999;28:1035–1044. doi: 10.1023/a:1007044207002. [DOI] [PubMed] [Google Scholar]
- 23.Maier IC, Baumann K, Thallmair M, et al. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steward O, Zheng B, Tessier-Lavigne M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- 25.Liu BP, Cafferty WB, Budel SO, et al. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YJ, Di Nardo A, Kramvis I, et al. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 29.Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci. 2009;66:2975–2984. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park KK, Liu K, Hu Y, et al. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67:715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 36.Dowling RJ, Topisirovic I, Alain T, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.