Abstract
Objective
To determine whether reduction in brain grey matter volume associated with hypertension persisted or was remediated among hypertensive patients newly treated over the course of a year.
Methods
Forty-one hypertensive patients were assessed over the course of a one-year successful anti-hypertensive treatment. Brain areas identified previously in cross-sectional studies as differing in volume between hypertensive and normotensive individuals were examined with a semi-automated measurement technique (ALP, automated labeling pathway). Volumes of grey matter regions were computed at baseline and after a year of treatment and compared to archival data from normotensive individuals.
Results
Reductions in regional grey matter volume over the follow-up period were observed despite successful treatment of blood pressure. The comparison group of older, but normotensive individuals showed no significant changes over a year in the regions tested in the treated hypertensive group.
Conclusions
These novel results suggest that essential hypertension is associated with regional grey matter shrinkage and successful reduction of blood pressure may not completely counter that trend.
Keywords: Grey matter volume, brain atrophy, magnetic resonance imaging, hypertension
Multiple in vivo and postmortem studies of brain morphology demonstrate that hypertensive individuals show greater loss of tissue than age–matched controls [1]. Hypertension, even at mild levels, acts as a negative modifier of aging as it enhances morphological alterations in brain indices typically associated with advanced age. Such effects include expansion of ventricular and sulcal spaces, decreased total and regional grey matter volume, increased burden of white matter abnormalities (e.g., white matter hyperintensities, WMH), and deterioration of the micro-structural organization of the white matter [2–7]. Although differences in imaging technology, nomenclature, analytic approach, and areas examined across studies impede generalizations, existing literature suggests in addition that the negative effects of hypertension on the brain involve structures that are relatively age-invariant. Across studies, several regions emerge as especially vulnerable to negative modification by essential hypertension. These include prefrontal cortex [6,8,9], hippocampus [6,10,11], the inferior temporal cortex [7,12], and inferior parietal lobule [13]. Hypertension also affects brain regions that are only moderately vulnerable to aging, e.g., supplementary motor areas [8,14], cuneus [12], thalamus [15], and entorhinal cortex [13]. Some studies show that regions that are usually resistant to aging such as the primary visual cortex shrink in hypertensive individuals [7]. The effects of hypertension may differ between the sexes. Some studies find the vulnerability only in men [8,12,14], whereas others report such effects only in women [12], with some (albeit not consistent) indication of lateralization.
The role of various anti-hypertensive medications in the modifying effect of hypertension is unclear, as participants have been typically studied while medicated, although some samples were comprised of never-medicated patients [8] or patients who discontinued medication prior to testing [15]. Notably, most extant studies of hypertension and brain morphology were cross-sectional, and the longitudinal studies [13] included too few hypertensives to afford a sufficient statistical power for discovering relatively subtle effects. In light of the discussed inconsistency of the literature and the confounding of diagnosed hypertension with medication, we designed this longitudinal study. In a one-year follow-up, we examined the impact of pre-existing untreated hypertension and the potentially curative influence of its alleviation on the regional brain shrinkage.
In addition to changes in brain structure, we examined the impact of hypertension and anti-hypertensive treatment on neuropsychological measures. Although both aging and hypertension are associated with declines in cognitive performance [16], the literature concerned with specific associations between structural change and cognitive function is inconsistent [1,8,15,17].
The current longitudinal investigation focused on one-year changes in brain morphology among persons who received a diagnosis of hypertension and were treated with medication to lower their blood pressure (BP). If heightened BP proximally induces morphological changes in the brain, it is plausible that reducing BP may normalize brain morphology. Even if such intervention would not reverse the damage, it could at least attenuate further structural deterioration. Hence, we compared the effect of two medications that have been proven effective in reducing BP in uncomplicated hypertension: a beta-blocker and an angiotensin converting enzyme (ACE) inhibitor. Although both interventions were expected to reduce BP, the ACE inhibitor was expected to have a more favorable neuroprotective effect given its reported action of normalizing the vascular wall [18,19]. To assess brain morphology, we used techniques designed to extend methods employed in prior studies by using full brain coverage, an automated identification of specific brain regions, and a focused statistical approach. We used a previously validated and widely used Automated Labeling Procedure, ALP. In this approach, after segmentation into grey matter, white matter, and cerebrospinal fluid compartments, the algorithm identifies, labels, and quantifies the volume of 194 areas based on whole brain, structural magnetic resonance images [20,21]. We selected brain regions of interest (ROIs) according to the extant reports identifying them as discriminating between hypertensive and normotensive individuals, as discussed above. A comparison group consisted of healthy normotensive individuals with MRI scans acquired on two occasions separated by one year; those data were available from the public dataset of the Alzheimer's Disease Neuroimaging Initiative (ADNI) study.
Methods
Participants
Hypertensive participants were community volunteers recruited from a major metropolitan area in the USA. They were between 35 and 65 years of age, and had arterial BP in excess of the cut-off established by the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure [22]: an average diastolic 5th phase BP of 90 mm Hg, systolic BP of 140 mm Hg, or both. For safety reasons volunteers were referred for separate clinical treatment rather than accepted in the study if diastolic pressure was above 109 mmHg or systolic pressure above 179 mmHg. BP measures (ausculatory technique with a mercury manometer) were taken with a participant sitting after at least a five-minute rest. Baseline BP was calculated from the average of the last 2 of 3 readings performed on two occasions. Participants were required to have either no prior pharmacologic treatment for hypertension or no more than 6 months of BP medication within the past 5 years, with no BP medication taken in the 6 months preceding enrollment. For detailed inclusion/exclusion criteria for this study see an earlier publication [23]. Screening was designed to exclude secondary hypertension, use of drugs/substances interfering with accurate and safe treatment/assessment, and presence of other serious disease, notably coronary or cerebrovascular disease. Of 81 volunteer patients, 36 did not meet the inclusion criteria and of the remaining 45, 41 completed the entire study and 40 had a full set of structural MRI and neuropsychological measures. Among participants analyzed for either MRI or neuropsychological results, 27 entered the study with both systolic and diastolic pressure elevated, 9 with systolic elevated only and 6 with diastolic elevated only. Participants were not selected for handedness or race; 95% of completing individuals reported being right-handed, 85% were Caucasian. Participants were similar to non-completing individuals in age, education, and personality factors. However, continuing participants were significantly (χ2 p<.05) more likely to be male, Caucasian, and married. The University of Pittsburgh Institutional Review Board approved all procedures as consistent with ethical principles and subjects provided informed consent.
The imaging data for a comparison group of normotensive participants were obtained from a public database compiled by the ADNI (www.loni.ucla.edu/ADNI). The ADNI was launched in 2003 as a collaboratively funded public/private effort directed over a period of five years to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer's disease (AD). Appropriate structural MR samples separated by one year from normotensive individuals were selected. We identified such data using the control volunteers from the ADNI dataset. ADNI controls were defined by reported good health, below-cut-off blood pressure, and absence of antihypertensive medication. These images were acquired from several different sites using 1.5T Siemens and GE scanners, in accordance with the published ADNI protocol [24]. The ADNI protocol uses a similar volumetric spoiled gradient-recalled (SPGR) sequence to that used in our study. Age and gender were considered in selecting individuals for use, but exact matching was not possible. All of the members of the comparison group were of Caucasian race. This normotensive dataset was segmented and analyzed with ALP using the procedures described below. Procedures and testing described below only apply to hypertensive participants.
Design
Hypertensive participants were tested in 3 to 4 sessions which included BP assessment, medical screening and history, physical examination, administration of self-report questionnaires, neuropsychological examination, MRI, and functional positron emission tomography examinations [23]. Briefly, the functional scans included rest and checkerboard scans as well as a sequence of scans assessing working memory, see [23]. All examinations were repeated after 1 year, except for the screening and self-report instruments. Due to its representativeness of other blood pressure measures, its utility in epidemiological work, and our need to control number of analyses performed, systolic blood pressure was used in all analyses.
Medication Procedures
Patients were treated for 1 year within a randomized, double-blind design. Two medications were administered: lisinopril (the ACE inhibitor) or atenolol (the beta-blocker). The choice of drugs was based on the initial hypothesis that lisinopril would alter brain function more than atenolol [25]. The dose was adjusted in a 6-week titration phase of the study to achieve a BP of < 140/90. The dose of hydrochlorothiazide was added to achieve BP control in 11 of the patients who had an insufficient BP response to their assigned treatment. Details of dosing and other procedures including functional positron emission tomography (PET) results are available elsewhere [25].
Structural MRI Measures
All subjects underwent a structural MRI on a GE Signa 1.5T scanner (Milwaukee, WI) to provide a detailed image for current morphometry as well as mapping PET results. A brief scout T1-weighted image was followed by an axial series oriented to the plane along anterior and posterior commissures: fast spin echo T2 weighted, proton density weighted, and fast fluid-attenuated inversion recovery [26]. A field of view of 24 cm and image matrix of 256 by 192 pixels were used for all axial MR series. A volumetric spoiled gradient-recalled (SPGR) sequence with parameters optimized for maximal contrast among grey matter, white matter, and spinal fluid was acquired in the coronal plane (TE/TR = 5/25 milliseconds, flip angle 40°, 1 excitation, slice thickness 1.5 mm, no gap) for purposes of the current analyses. This report focuses on brain morphology measures from the SPGR images. Severity of white matter hyper-intensities, ventricle size, and sulcal size were judged from the axial scans and the relationship of these to hypertension treatment has been previously reported [27]. Participants showing evidence of significant lacunar or other infarcts identified from the MRI films by a board certified neuroradiologist (n=2) were excluded prior to treatment for hypertension. Significant infarcts were those judged to potentially influence functional images and/or influence cognitive processing.
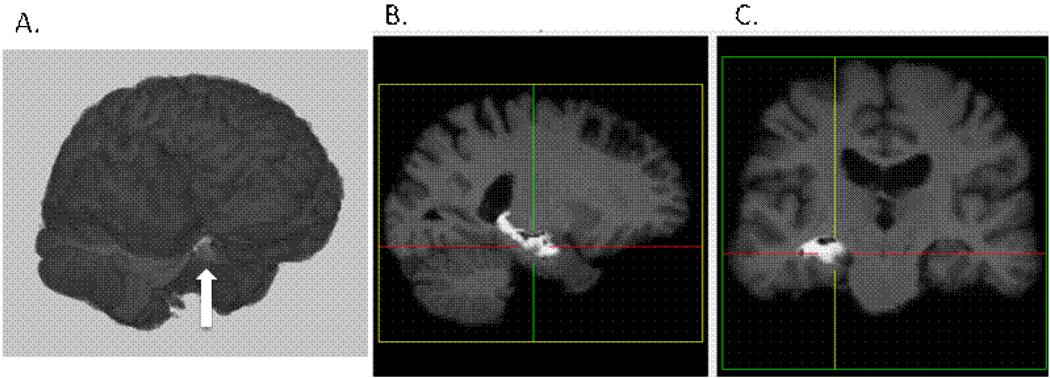
To determine volumes of grey and white matter and cerebrospinal fluid (CSF), we employed a procedure referred to as the Automated Labeling Pathway (ALP). We have applied this in previous structural MRI studies to automatically label specific anatomic regions, e.g., [28–35]. ALP is used to register the colin27 template brain [36] to a 3D T1-weighted high-resolution image of each subject. Prior to the registration, the skull and scalp are stripped from both colin27 and the subject’s 3D T1-weighted image using the Brain Extraction Tool (BET, [30]), and followed by semi-automated erosion and dilation to remove additional skull and scalp [35]. We use a fully deformable registration model similar to that described by Chen [37] and implemented the procedure using the registration library in ITK [38]. This method starts with a grid-based piecewise linear registration and then uses the demons registration algorithm [32] as a fine-tuning procedure for a voxel-level spatial deformation. The fully deformable registration allows for a high degree of spatial deformation, which seems to give it a particular advantage over other standard registration packages, such as AIR and SPM [34]. After registration of the template to the individual subject space, the ROIs from the template are applied to label regions on the subject’s MRI, i.e., volumes are estimated in the participant’s ‘native space’. The number of grey, white, and CSF voxels in each of these regions are then counted to produce a table of ROI volumes for each region and each subject. The anatomic regions of interest are from a previously published atlas (90 manually traced regions) [33], the Brodmann atlas (82 regions, included with the MRIcro software package), [29] and from locally generated regions defined from manual tracing [34]. Figure 1 illustrates the delineation by ALP of the grey matter volume of a particular area, the hippocampus, in one participant.
Figure 1.
Left Hippocampal segmentation with ALP from one subject in the sample. A. 3D surface rendering with left hippocampal segmentation identified by arrow, B. Sagittal view, C. Coronal view
Regions selected for hypothesis testing were areas showing statistically significant differences between hypertensive and control individuals in the studies reviewed in the prior section. We examined the nine regions of interest (ROIs), defined bilaterally, using Brodmann area (BA) nomenclature for cortical areas. These choices were made in order to reduce the probability of spurious results, i.e. control experiment-wise error and to provide a specific terminology for the areas examined. Note that prior studies did not employ common nomenclatures or similar degrees of specificity in their labeling of regions; thus, it was impossible to derive ROIs with specific spatial coordinates from the descriptions provided in these studies. Table 1 lists the ROIs examined. We further correlated grey matter regions and systolic BP between the current control sample and the pre-treatment hypertensive sample. In exploratory analyses, areas that showed a significant correlation were also tested for change across the year of treatment for the hypertensives and the comparable year for the controls.
Table 1.
Brain regions of interest examined based on prior cross-sectional reports.
| Region of Interest | Brodmann Area | Lobe |
|---|---|---|
| Supplementary Motor Area | BA6 | Frontal |
| Medial Frontal | BA8 | Frontal |
| Superior Prefrontal | BA9 | Frontal |
| Cuneus | BA18/19 | Occipital |
| Mid Temporal | BA21 | Temporal |
| Entorhinal Cortex | BA28 | Temporal |
| Inferior Parietal | BA39 | Parietal |
| Hippocampus | ---- | Sub-cortical |
| Thalamus | ---- | Sub-cortical |
Neuropsychological Assessment
Cognitive functioning was assessed with a two hour battery of well-known neuropsychological tests used routinely in clinical practice. The battery emphasized tests of memory and executive functions previously shown to be influenced by hypertension [16]. Tests include an estimate of intelligence (National Adult Reading Test (NART) revised for US samples, [39], verbal learning and memory (Rey Auditory Verbal Learning Test and Newspaper Story Test, incidental memory after the Digit Symbol Substitution test [40,41], nonverbal learning and memory (Rey Complex Figure, Wechsler Faces), semantic memory (Verbal fluency, [42]; Boston Naming Test, [43], working memory (Four Word Working Memory, [44], Wechsler Memory Scale 3 Letter Number Sequencing and Spatial Span), psychomotor efficiency (Grooved Pegboard, [45], attention/mental flexibility (Trail Making, [46], WAIS Digit Symbol Substitution, and Stroop Color/Word Interference, [47]).
To limit the number of comparisons, we reduced pre-treatment neuropsychological data to four factors via a principal component analysis with varimax rotation. The factors were labeled: Verbal Memory (Logical Memory, 1st recall; Auditory Immediate Memory, Auditory Delayed Memory; 4-Word Short Term Memory, and Verbal Paired Associates), Visuospatial Memory (Family Pictures immediate and delayed, Visual Immediate Memory and delayed, and General Memory Index,), Psychomotor Speed (Grooved Pegboard preferred and non-preferred hands), Spatial Construction (Object Assembly and Block Design). Working memory performance on the n-back tasks tested both in the scanner and in the neuropsychological testing was also examined. Working memory was assessed using a non-parametric A’ sensitivity index derived from the proportion of hit and false alarms observed in the responses of the subjects [48,49].
Analysis
Volumes of the target ROI’s served as dependent variables in a general linear model that also contained pre-post one year as a repeated measure factors, gender and hypertensive versus control as grouping factors and age and intra-cranial volume (average of pre and post estimates) as continuous independent variables. Age has known effects and the intra cranial volume adjusted for individual differences in head/brain size. The ROI’s served as a multivariate dependent variable with significant effects then assessed with univariate follow-up tests. Multivariate analysis with a Type III decomposition was applied to estimate well interaction effects of primary interest and account for dependency in the assessment occasions [50]. Analyses were also performed separately within the hypertensive and normotensive groups to confirm results from the multivariate analyses as it was possible that covariance in the presence of group differences might violate assumption of covariance analysis [51]. The sample size did not permit adding all possible covariates simultaneously, but a number of potentially important covariates were added individually to ensure that they were not major confounders of the results. These were body mass index, initial BP, educational level, and for hypertensive analyses, type of medication and BP response to mediation. Areas previously implicated in longitudinal studies of brain aging were also examined to see if comparable change hypertensive and control individuals occurred in these areas over the one year period [17]. Following these analyses, correlations were computed between pre-treatment systolic BP in the patients and initial systolic BP in the controls and bilateral regional grey matter estimates. Areas showing significant correlations were then tested in an exploratory linear model identical to the one performed for the pre-planned ROI analysis. We used a different approach with the white matter and cerebrospinal fluid (CSF) volumes as we lacked any prior results from these subjects to guide the analyses. We tested the difference over a year in total brain white matter and CSF. Any trend could then be followed up by regional testing.
Results
Table 2 shows the characteristics of the hypertensive and normotensive samples. The normotensive sample was small due to the limited number of individuals with one-year test/retest in the ADNI database that were normotensive and demographically and physically comparable to the hypertensive samples. As shown in the table, neither age nor body weight were strictly comparable between the samples. The table presents characteristics of our sample taken as a whole because atenolol and lisinopril had comparable effects on BP in the two treated hypertensive groups—a decrease of approximately 22 mmHg systolic and 18 mmHg diastolic. Table 3 illustrates further the overall success of the pharmacological treatment. Although some variability in response to medication is evident, titration was largely successful by week 6 in reaching normotensive levels in the majority of the patients, and further blood pressure reduction is evident through week 38. Further description of the hypertensive sample and treatment is available [23, 27]. Although uniformly tested in our statistical models, the two medication groups yielded essentially identical results. None of the effects involving medication as a grouping factor approached significance, and therefore, medication will not be discussed further.
Table 2.
Demographic characteristics of the hypertensive and normotensive samples.
| Variable | Hypertensive (n=41) |
Normotensive (n=16) |
t | P |
|---|---|---|---|---|
| Age (years) | 52.4 (7.1) | 65.9 (3.9) | −7.2 | <.001 |
| Gender (% female) | 24 | 38 | χ2=.9 | Ns |
| Height (cm) | 173.5 (7.4) | 169.9 (8.4) | 1.6 | Ns |
| Weight (kg) | 88.9 (15.4) | 77.1 (17.7) | 2.6 | <.02 |
| BMI | 29.6 (4.8) | 26.5 (4.9) | 2.2 | <.04 |
| Systolic BP(mmHg) | 148 (11) | 126 (8) | 7.2 | <.001 |
| Diastolic BP(mmHg) | 95 (8) | 75 (7) | 8.4 | <.001 |
| Education (yrs) | 15 (2) | 16 (3) | −1.1 | Ns |
| Whole Brain Grey (cc)—PRE | 625.8 (75.7) | 635.0 (68.9) | −.4 | Ns |
| Whole Brain Grey (cc)—POST | 612.8 (79.9) | 627.5 (66.1) | −.6 | Ns |
Figures in parentheses in body of table are standard deviations. Abbreviations are cm, centimeter; kg, kilogram; BMI, BMI; BP, BP; cc, cubic centimeter. T values compare hypertensive and normotensive groups; chi square is used to compare gender composition of the groups. Pre-Post changes in whole brain grey are not significant overall or within each group. Note that due to sample size differences and possible method variance, the standard error the mean was larger in the ADNI control sample (SE=17) than in the hypertensive sample (SE=12).
Table 3.
Percentage of patients failing to meet normotensive levels of systolic and diastolic pressure by week of clinical visit.
| Week | 4 | 6 | 23 | 38 |
|---|---|---|---|---|
| % systolic >140 mmHg | 9.3 | 6.9 | 3.7 | 3.1 |
| % diastolic>90 mmHg | 5.6 | 5.6 | 3.1 | 2.5 |
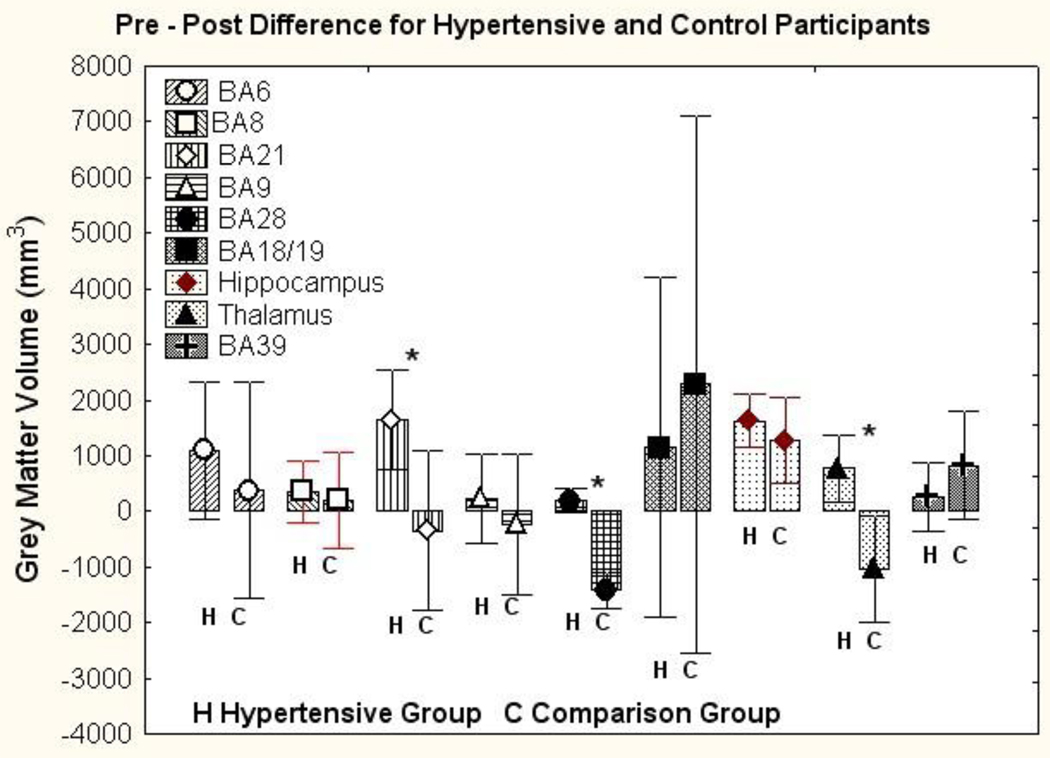
The primary focus of this study was on change in the regional volumes of brain volumes of the hypertensive patients over the course of treatment. These results were compared to the sample of normotensive individuals drawn from the ADNI database. The findings for the regional grey matter volumes in hypertensive individuals and normotensive comparisons are presented in Figure 2. The multivariate analysis of the pre to post-treatment change in the hypertensive and control group, with age, gender and intracranial volume as covariates, showed an interaction of diagnostic group (hypertensive versus comparison group) by occasion (readings separated by a year, i.e., pre vs post treatment in the hypertensive group) (F (9,43) = 8.09, Wilks Λ =.37, p<.001, partial η2=.63). Main effects were also observed for diagnostic group (F(9,43) = 2.60, Wilks Λ=.65, p<.02, partial η2=.35), measurement occasion (F(9,43) = 2.48, Wilks Λ=.66, partial η2=.34), and intra cranial volume (F(9,43) = 8.64, Wilks Λ=.36, p<.001, partial η2=.64). Other interactions involving the diagnostic grouping were not significant. The follow-up univariate tests showed significant group × occasion interactions for BA 21 (F(1,51) = 4.44, p<.05, partial η2=.08), BA28 (F(1,51) = 55.54, p<.001, partial η2=.52), and the thalamus (F(1,51) = 8.10, p<.01, partial η2=.14).
Figure 2.
Pre minus post year differences in grey matter volume in cubic millimeters among treated hypertensive individuals (H) and normotensive comparison individuals (N). Differences are presented separately for brain areas as classified within ALP (see text) that showed changes over the one-year period. Error bars reflect the 95% confidence intervals. Positive differences show shrinkage over the course of a year. For each area, left bar is change among hypertensive (H) individuals and right bar normotensive(N). Figure illustrates significant statistical interaction between pre vs post and hypertension vs control. Comparisons indicated with * are significant in comparison of individual areas.
Despite the loss of statistical power, separate multivariate analyses of the groups yielded useful information. Analysis of the hypertensive group showed a significant interaction between gender and treatment: F(9,29) = 4.10, Wilks Λ=.44, p<.002, partial η2=.56. Follow-up univariate analyses revealed that primary sources of the interaction were women showing greater grey matter loss in BA21 and the hippocampus relative to males. The means (standard error) relevant to this interaction were as follows. For BA21: males baseline volume =26040 mm3 (520) and follow-up volume = 25947 (527) mm3; for the females: baseline volume = 27228 mm3 (970) and follow-up volume = 24885 mm3 (982). For the hippocampus, the volumes were: males baseline volume=12261 mm3 (266) and follow-up volume = 11530 (182) mm3; for the females: pre = 14221 mm3 (340) and post = 11502 mm3 (497). No significant effects were observed in the analysis of the comparison group.
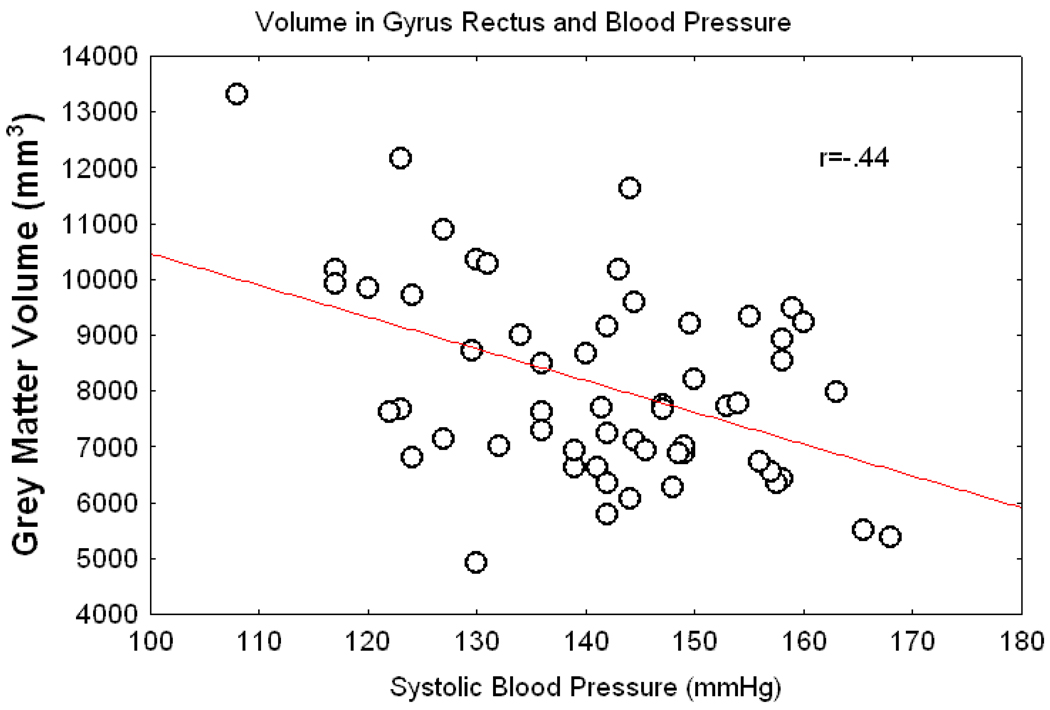
Following the analyses of areas derived from the literature, we conducted an exploratory correlational analysis of the baseline systolic BP and bilateral grey matter volumes. The analysis yielded significant correlations (p <.02 or better) for superior orbital frontal cortex, lateral orbital cortex, gyrus rectus, cerebellum, and BA 11. Figure 3 illustrates these findings by showing the scatter plot for the relation of the gyrus rectus grey matter volume and untreated systolic BP. Grey matter shrinkage over a year was then assessed for areas that showed correlation with systolic BP but had not been previously tested in a one-year longitudinal analyses. The only regional difference that emerged from this analysis was a pre-post difference in the gyrus rectus grey matter (F(1,51) = 6.22, p<.03). The means (standard error) constituting this interaction were for the hypertensive participants: pre=12358 mm3 (361) and post = 7718 (303) mm3; for the normotensive sample: pre = 12789 mm3 (570) and post = 9637 mm3 (479).
Figure 3.
Correlation between grey matter volume in the gyrus rectus and systolic BP for the entire sample using the pre-treatment BP of the patients and the BP at initial scan for the controls.
Correlations with cognitive performance scores were minimal. Correlations were computed for the areas showing change over the treatment period. Change in these areas was compared to change in the neuropsychological factor scores as well as the 2-back memory performance accuracy during the scanning. Correlations with the neuropsychological factors were not significant; none of the neuropsychological factors were even minimally correlated with the change in grey matter volumes in the tested areas.
Another set of analyses focused on aging effects in order to examine if areas known to change with aging were showing similar changes in hypertensive and control participants. We examined the regions that were identified as vulnerable to aging in a recent review of the literature [17]. These were temporal, and frontal cortices, hippocampus, entorhinal, cortex, caudate nucleus, and cerebellum. These analyses typically showed statistically marginal decreases in area volume over the year with essentially equivalent changes between normotensive and hypertensive individuals. The exception was the temporal lobe that showed a significant decrease in cortical volume across the year (F(1,55) = 5.3, p<.03, 11088 (sd=647) to 10333 (sd=687) mm3. The entire temporal lobe within each group evidenced significant shrinkage within the hypertensive group (F(1,54) = 5.8, p<.03, see mid temporal and entorhinal results above as well) but only marginal change in the controls:(F(1,13) = 3.6, p=.08.
We found no change in CSF and white matter volume over a one year period in either hypertensive or control participants. Post-pre comparisons were uniformly non-significant (p>.3) so further regional comparisons are not reported.
Discussion
The central finding in this study is that alleviation of hypertension and reduction of BP to normotensive levels failed to prevent grey matter loss over one year of treatment. As noted previously, grey matter morphology differences related to hypertension appear to differ from those related to normal aging, e.g. [7,9,17]. It is possible that high BP induces central hemodynamic effects contributing to atrophy of particular grey matter areas. Based on this inference, we expected that reducing BP over the course of a year might arrest or even diminish grey matter loss. Our findings suggest, however, that some aspect of essential hypertension is continuing to influence brain morphology independent of the level of peripheral BP. Of note, another index of brain morphology, cortical thickness, was recently observed to correlate negatively with blood pressure in pre-hypertensive individuals [52]. More generally, a recent review of structural neuroimaging studies of the aging brain [53] concluded that treatment of hypertension reduces, but does not eliminate effects of aging on the brain. As summarized in that review, compared to normotensives, treated hypertensives have more white matter hyperintensities, prefrontal volume shrinkage and reduced hippocampal volume [11,54–56].
We must emphasize though that we only studied two medications over a year of treatment. The literature is not specific enough to determine whether areas specifically related to hypertension show continued or arrested atrophy with longer term treatment. It is possible that early intervention as well as longer duration of intervention would maintain grey matter morphology among treated hypertensive individuals. Alternately, it is conceivable that medication rather than a hypertensive process affects grey matter morphology. We cannot discount this possibility without a placebo control arm to our study, but doubt on this hypothesis is cast by our finding that two medication groups did not differ in their morphology changes despite differences in the mechanism of action of the two medications.
We observed volume loss in nine brain regions over the course of a year of treatment lowering blood pressure. The grey matter regions were chosen for this study specifically because of prior cross-sectional findings of their smaller volume in these areas in hypertensives compared to normotensive controls. Shrinkage of specific grey matter regions after a year of effective anti-hypertensive treatment is in accord with the cross-sectional findings in similar brain areas. The validity of our results rests primarily on this longitudinal verification of cross-sectional results. In the hypertensive group, in seven of the nine examined regions, we observed a mean one-year loss that exceeded shrinkage in the normotensive sample.
Caution is required in interpreting the statistical inferences given the variability of the results in the small comparison sample. A portion of the variability may be due to the use of different scanners at different sites in the comparison sample drawn from the ADNI study. Despite this variability, the utility of the comparison sample was supported by the typical age-related changes in grey matter volume observed. Changes over a year in the percentage of grey matter relative to intracranial volume were similar to those reported in the literature. These changes were the same in women as estimates obtained in large samples by Taki [57] and slightly higher, but well within the range of values for men. Importantly the results do not depend entirely on the comparison sample, as the larger hypertensive sample showed decreases in grey matter volume across all of the nine areas examined, albeit varying in degree of decrease. Exploratory analyses suggested that longitudinal changes in treated hypertensives was largely confined to the areas defined a priori, although gyrus rectus was identified as decreasing more in hypertensives relative to controls in these exploratory analyses. The gyrus rectus has not been well studied specifically in hypertensives, but its grey matter has been shown to decrease with age [58] and to correlate with diagnosis of unipolar depression [59], age-related differences in the olfaction [60], and performance on executive function tasks [61].
Although some of the examined regions were identified in prior work as hemisphere- or gender-specific, our conservative strategy was to test areas bilaterally and across genders in an attempt to avoid spurious findings. Despite this, we did find accentuated grey matter loss among hypertensives in BA 21, BA28, and thalamus extending prior findings [8,9,12,13]. Follow-up analyses showed that the loss in BA21 was greater in women than in men. Medial frontal and hippocampal areas showed directional effects, but were not separately significant discriminators between hypertensives and controls [6,10–12,14,17], although our follow-up analysis showed hypertensive women showing hippocampal loss that was greater than that among men. Occipital and inferior parietal areas showed no losses among hypertensives relative to controls over the year, despite some evidence of such loss longitudinally in occipital cortex [7] and cross-sectionally in inferior parietal cortex [13]. We have no explanation for this. Aging has typically been shown to influence the occipital areas less than the other areas we examined, although the parietal lobe is typically shown to be influenced by age [62–64]. An increase in cerebellar cerebrospinal fluid with hypertension was previously reported by Strassburger et al. [15], but we did not replicate this finding and found no substantial differences in white matter or cerebrospinal fluid volumes over the year of treatment. As noted earlier, functional correlations of grey matter change have not been widely investigated. We examined this question but found no strong correlations between neuropsychological function and the decrease in grey matter volume over a year. Neuropsychological deficits related to hypertension are small and the current sample size as well as the relatively short (one year) time frame may have obscured any ongoing functional changes related to grey matter volume loss.
These analyses driven by prior literature were supplemented by a post hoc examination of BP and grey matter volume in the current sample. Using pretreatment and control participant BP we found relations between systolic BP and superior orbital frontal cortex, lateral orbital cortex, gyrus rectus, cerebellum, and BA 11. Note that areas detected did not include all the areas assessed in our longitudinal analysis; more information is clearly needed on the time course of morphological changes. Slow, incremental change would not have been detected in our one year study. We did not detect interactions with age or gender (overall), but it is possible that morphological changes are accentuated in older sample or cluster around menopausal periods in women.
Mechanisms accounting for the precise pattern of grey matter change in hypertension are unclear. General changes in brain morphology have been related to small vessel arteriosclerosis and lipohyalinosis as well as rarefaction, white matter lesions, oxidative stress, neuronal shrinkage, apoptosis, local ischemia, and beta amyloid deposition [65–68]. It remains unclear how hypertension accelerates these factors, as these reviews indicate, and it is equally unclear at present why these mechanisms would be specifically active in the regions identified in our study. Areas showing specific loss with hypertension were subsumed within larger regions typically associated with aging, e.g., temporal lobe. Our analyses directed at areas know to show changes with aging suggested accelerated aging in the temporal lobe of hypertensives although other areas seemed to show equivalent aging effects between hypertensive and control participants. Given this, we cannot determine at this point whether hypertension accelerates processes related to normal aging in specific areas or introduces new factors independent of normal aging process. Overall, temporal and thalamic changes appeared the most robust in the current sample. Future work with fine-grained analyses will be required to clarify the possible mechanisms.
Our study has several limitations. The most prominent is we were unable to find closer matching between the groups. The members of a small sample obtained form LONI ADNI database differed from our hypertensive participants in age and BMI. This comparison provides only initial data suggesting the observed losses in the hypertensive patients were not due to normal aging. This limitation is emphasized by the gains in grey matter volume over a year apparent in the mean data from the small comparison group; such an effect is possible, but larger sample sizes would be required to decrease the likelihood of variation due to statistical and methodological ‘noise’. The ADNI sample did, however, appear to represent normal aging appropriately. Moreover, because hypertensive group was younger, there was an a priori lesser likelihood of finding brain shrinkage in that group rather than in older controls. This reduces a threat to validity of our conclusions. A strength of this study is that the results were not confounded by prior pharmacological treatment. However, we observed the reduction of BP with only two of the many possible medications for treating BP. Thus, generalization to patients who were exposed to various pharmacological regimens and those who were treated with different anti-hypertensive agents must be made with caution.
In summary, in cortical regions previously identified as vulnerable to hypertension, newly treated hypertensive individuals continue to show grey matter shrinkage over a year of successful treatment. The continued atrophy in the presence of successful reduction of BP suggests that essential hypertension may directly affect the brain via factors that are not mediated by increased BP. This observation contributes to accumulating research suggesting that essential hypertension from its onset may be a disease of the brain as well as of the vasculature [69].
Table 4.
Summary Table
| What is known about this topic? |
|
| What this study adds |
|
Acknowledgements
We thank Megan Nable and Adrienne Soehner for assistance with the scoring and NIH grant NHLBI HL57529 for support of this research and for further support of the investigators P.J.G. HL-R01-089850; MH-K01-070616, R37 AG011230 (NR). Normotensive comparison data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI; Principal Investigator: Michael Weiner; NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and through generous contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer's Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the U.S. Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Footnotes
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI investigators include (complete listing available at www.loni.ucla.edu/ADNI/Collaboration/ADNI_Citatation.shtml).
Disclosure Statement
None of the authors have any actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) their work. The work reported was reviewed and approved for the appropriate conduct of human research by the Internal Review Board of the University of Pittsburgh.
References
- 1.Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Research. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgmans S, van Boxtel MP, Gronenschild EH, Vuurman EF, Hofman P, Uylings HB, et al. Multiple indicators of age-related differences in cerebral white matter and the modifying effects of hypertension. NeuroImage. 2010;49:2083–2093. doi: 10.1016/j.neuroimage.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change, and blood pressure. J Neurol. 2007;254:713–721. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology. 2002;59:713–719. doi: 10.1212/wnl.59.5.713. [DOI] [PubMed] [Google Scholar]
- 5.Longstreth WT, Jr., Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 6.Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: Insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann NY Acad Sci. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- 8.Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. NeuroImage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 10.den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64:263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- 11.Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: The Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 12.Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging. 2004;25:455–463. doi: 10.1016/j.neurobiolaging.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Wen W, Anstey KJ, Sachdev PS. Effects of cerebrovascular risk factors on gray matter volume in adults aged 60-64 years: A voxel-based morphometric study. Psychiatry Res. 2006;147:105–114. doi: 10.1016/j.pscychresns.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, et al. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28:1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- 16.Waldstein SR, Katzel LI. Hypertension and cognitive function. In: Waldstein SR, Katzel LI, Elias MF, editors. Neuropsychology of Cardiovascular Disease. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2001. pp. 15–36. [Google Scholar]
- 17.Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabez R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. Oxford, UK: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- 18.Baumbach GL, Chillon JM. Effects of angiotensin-converting enzyme inhibitors on cerebral vascular structure in chronic hypertension. J Hypertens Suppl. 2000;18:S7–S11. [PubMed] [Google Scholar]
- 19.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–1659. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 20.Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmichael OT, Aizenstein HA, Davis SW, Becker JT, Thompson PM, Meltzer CC, et al. Atlas-based hippocampus segmentation in Alzheimer’s disease and mild cognitive impairment. NeuroImage. 2005;27:979–990. doi: 10.1016/j.neuroimage.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Jennings JR, Muldoon MF, Price JC, Christie IC, Meltzer CC. Cerebrovascular support for cognitive processing in hypertensive patients is altered by blood pressure treatment. Hypertension. 2008;52:65–71. doi: 10.1161/HYPERTENSIONAHA.108.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR, Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, et al. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- 26.Bastianello S, Bozzao A, Paolillo A, Giugni E, Gasperini C, Koudriavtseva T, et al. Fast spin-echo and fast fluid-attenuated inversion-recovery versus conventional spin-echo sequences for MR quantification of multiple sclerosis lesions. Amer J Neuroradiol. 1997;18:699–704. [PMC free article] [PubMed] [Google Scholar]
- 27.Jennings JR, Muldoon MF, Whyte EM, Scanlon J, Price J, Meltzer CC. Brain imaging findings predict blood pressure response to pharmacological treatment. Hypertension. 2008;52:1113–1119. doi: 10.1161/HYPERTENSIONAHA.108.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreescu C, Butters MA, Meng S, Begley A, Rajji T, Wu M, et al. Gray matter changes in late life depression: A structural MRI analysis. Neuropsychopharmacology. 2008;33:256–257. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM. Fast robust automated brain extraction. Hum Brain Map. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soreca I, Rosano C, Jennings JR, Sheu LK, Kuller LH, Matthews KA, et al. Gain in adiposity across 15 years is associated with reduced gray matter volume in healthy women. Psychosom Med. 2009;71:485–490. doi: 10.1097/PSY.0b013e3181a5429d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thirion JP. Image matching as a diffusion process: An analogy with Maxwell's demons. Med Image Anal. 1998;2:243–260. doi: 10.1016/s1361-8415(98)80022-4. [DOI] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 34.Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Map. 2006;27:747–754. doi: 10.1002/hbm.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu M, Rosano C, Aizenstein HJ. A morphological method to improve skull stripping of MR brain images. 11th Annual Meeting of the Organization for Human Brain Mapping; Chicago, Illinois. 2005. [Google Scholar]
- 36.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comp Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 37.Chen M. 3-D deformable registration using a statistical atlas with applications in medicine. Pittsburgh, PA: Carnegie Mellon University; 1999. [Google Scholar]
- 38.Yoo T. Insight into Images: Principles and Practice for Segmentation, Registration, and Image Analysis. Wellesey, MA: AK Peters Ltd.; 2004. [Google Scholar]
- 39.Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 40.Lezak MD. Neuropsychological Assessment. 3rd Edition. New York: University Press; 1995. [Google Scholar]
- 41.Kaplan E, Fein D, Morris R, Delis D. WAIS-R as a Neurospychological Instrument. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 42.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 43.Thompson LI, Heaton RK. Comparison of different versions of the Boston Naming Test. Clin Neuropsychol. 1989;3:184–192. [Google Scholar]
- 44.Morrow LA, Ryan C. Normative data for a working memory test: The four word short-term memory test. Clin Neuropsychol. 2002;16:373–380. doi: 10.1076/clin.16.3.373.13850. [DOI] [PubMed] [Google Scholar]
- 45.Lewis R, Kupke T. Intermanual differences on skilled and unskilled motor tasks in nonlateralized brain dysfunction. Clin Neuropsychol. 1992;6:374–382. [Google Scholar]
- 46.Reitan RM. Validity of the trailmaking test as an indicator of organic brain damage. Percep Motor Skills. 1958;8:271–276. [Google Scholar]
- 47.Golden CJ. Stroop Color and Word Test. Chicago: Stoelting; 1978. [Google Scholar]
- 48.Aaronson D, Watts B. Extensions of Grier's computational formulas for A" and B" to belowchance performance. Psychol Bull. 1987;102:439–442. [PubMed] [Google Scholar]
- 49.Grier J. Nonparametric indexes for sensitivity and bias: Computing formulas. Psychol Bull. 1971;75:424–429. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- 50.Milliken GA, Johnson DE. Analysis of Messy Data. New York: Van Nostrand Reinhold; 1984. [Google Scholar]
- 51.Miller GM, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 52.Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.10.050. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: Cardiovascular determinants of dementia study. Hypertension. 2004;44:625–630. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- 55.Kraut MA, Beason-Held LL, Elkins WD, Resnick SM. The impact of magnetic resonance imaging detected white matter hyperintensities on longitudinal changes in regional cerebral blood flow. J Cereb Blood Flow Metab. 2008;28:190–197. doi: 10.1038/sj.jcbfm.9600512. [DOI] [PubMed] [Google Scholar]
- 56.Nobili F, Rodriguez G, Marenco S, De Carli F, Gambaro M, Castello C, et al. Regional cerebral blood flow in chronic hypertension. A correlative study. Stroke. 1993;24:1148–1153. doi: 10.1161/01.str.24.8.1148. [DOI] [PubMed] [Google Scholar]
- 57.Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.003. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Meier-Ruge W, Ulrich J, Brühlmann M, Meier E. Age-related white matter atrophy in the human brain. Ann N Y Acad Sci. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- 59.Elderkin-Thompson V, Hellemann G, Pham D, Kumar A. Prefrontal brain morphology and executive function in healthy and depressed elderly. Int J Geriatr Psychiatry. 2009;24:459–468. doi: 10.1002/gps.2137. [DOI] [PubMed] [Google Scholar]
- 60.Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD. Functional anatomy of human odor sensation, discrimination, and identification in health and again. Neuropsychology. 2003;17:482–495. doi: 10.1037/0894-4105.17.3.482. [DOI] [PubMed] [Google Scholar]
- 61.Elderkin-Thompson V, Ballmaier M, Hellemann G, Pham D, Kumar A. Executive function and MRI prefrontal volumes among healthy older adults. Neuropsychology. 2008;22:626–637. doi: 10.1037/0894-4105.22.5.626. [DOI] [PubMed] [Google Scholar]
- 62.Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 63.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 65.Akiyama H, Meyer JS, Mortel KF, Terayama Y, Thornby JI, Konno S. Normal human aging: Factors contributing to cerebral atrophy. J Neurol Sci. 1997;152:39–49. doi: 10.1016/s0022-510x(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 66.Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008;4:363–381. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyer JS, Rauch GM, Rauch RA, Haque A, Crawford K. Cardiovascular and other risk factors for Alzheimer's disease and vascular dementia. Ann N Y Acad Sci. 2000;903:411–443. doi: 10.1111/j.1749-6632.2000.tb06393.x. [DOI] [PubMed] [Google Scholar]
- 68.Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: Hypotension as a key point. Vas Health Risk Manag. 2008;4:395–402. doi: 10.2147/vhrm.s2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jennings JR, Zanstra Y. Is the brain the essential in hypertension? NeuroImage. 2009;47:914–921. doi: 10.1016/j.neuroimage.2009.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]