Abstract
Objective
To investigate whether differences in admixture in African American (AFA) and Hispanic American (HA) adult women are associated with adiposity and adipose distribution.
Design
The proportion of European, sub– Saharan African and Amerindian admixture was estimated for AFA and HA women in the Women's Heath Initiative using 92 ancestry informative markers. Analyses assessed the relationship between admixture and adiposity indices.
Subjects
11712 AFA and 5088 HA self– identified post– menopausal women.
Results
There was a significant positive association between body mass index (BMI) and African admixture when BMI was considered as a continuous variable, and age, education, physical activity, parity, family income and smoking were included covariates (p < 10− 4). A dichotomous model (upper and lower BMI quartiles) showed that African admixture was associated with a high odds ratio [OR = 3.27 (for 100% admixture compared to 0% admixture), 95% confidence interval (CI) 2.08 – 5.15]. For HA there was no association between BMI and admixture. In contrast, when waist to hip ratio (WHR) was used as a measure of adipose distribution, there was no significant association between WHR and admixture in AFA but there was a strong association in HA (p<10− 4; OR Amerindian admixture = 5.93, CI = 3.52 – 9.97).
Conclusion
These studies show that 1) African admixture is associated with BMI in AFA women; 2) Amerindian admixture is associated with WHR but not BMI in HA women; and 3) it may be important to consider different measurements of adiposity and adipose distribution in different ethnic population groups.
Keywords: European admixture, African admixture, Amerindian admixture, post–menopausal women, waist to hip ratio, body mass index
Introduction
Obesity is associated with adverse health outcomes1-4. Health surveys including the California Women's Health Survey II.1 (http://www.cdph.ca.gov/pubsforms/Pubs/OWH–CAWomensHlth_2007.pdf) and other studies5-7 have indicated that African American (AFA) and Hispanic American (HA) women have an increased propensity for this health problem. Multiple indices for adiposity and adipose distribution have been utilized in assessing the impact of obesity on cardiovascular disease and diabetes including body mass index (BMI), waist circumference (WC), hip circumference (HC) and waist to hip circumference ratio (WHR)2, 3, 8-13. Potential differences in these indices of adiposity for different population groups are also suggested by some of these previous studies2, 3. Recent admixture mapping studies in African Americans have suggested that there are several susceptibility loci that are derived from African ancestry14, 15 and at least one derived from European ancestry16.
In some previous studies WHR has been found to be a better predictor of mortality in older people than either BMI or WC17-19 or a better predictor of cardiovascular disease12, 20-22. These findings are not without controversy since other studies have found a stronger correlation with adverse health consequences with either WC or BMI23-26. Although WC has been proposed as an alternative non– invasive indicator of abdominal adiposity27, WC is highly correlated with BMI and may give less information about regional adipose distribution than WHR18. In a large study of Chinese women, WHR appears to be an independent predictor of mortality risk and this finding was observed even in women with lower BMI measurements (<25 kg/m2)19.
Although there is substantial epidemiologic data suggesting differences between populations in body habitus and obesity, few studies have examined differences within particular admixed population groups14-16, 28. In the current study we examine whether differences in adiposity and adipose distribution are influenced by the relative proportion of sub– Saharan African (AFR), Amerindian (AMI) and European (EUR) admixture in a large group of self– identified AFA and Hispanic American (HA) participants in the Woman's Health Initiative (WHI) studies.
Methods
Study Subjects
The current study includes self– identified AFA and HA participants in the WHI. Descriptions of the WHI study and additional details describing this study have been published29-31. All studies were conducted with appropriated informed consent and in agreement with established Human Institutional Review Board procedures. The initial sample size included a total of 19641 AFA and HA women for whom WHI DNA samples were available. Subjects were excluded from analyses based on inadequate DNA samples and/or technical assay failures (1605), failure to meet genotyping quality filtering (<90% complete typing) (788) or unrecognized cryptic relationship (65 pairs of samples), and 138 who did not have complete phenotypic information. The final sample size that was utilized in the phenotypic analyses included 16800 individuals had complete phenotypic information (11712 self– identified AFA and 5088 self– identified HA).
Phenotypes and Covariates
BMI, WHR, HC, WC and all covariates were determined from baseline WHI data. All study participants had their weight, height, and waist and hip circumferences measured at baseline using a calibrated balance, stadiometer, and standard tape measures. BMI was computed as measured weight (kg) divided by the square of measured height (m2). WHR was computed as the ratio of WC (cm) to hip circumference (HC) (cm). For covariates the scales were as developed by WHI with the exception of education for which a modified WHI scale was used. For smoking (smoke years) the following scale was used: 1) <5 pack years; 2) 5 – 9 pack years; 3)10 – 19 pack years; 4) 20 – 29 pack years; 5) 30 – 39 pack years, 6) 40 – 49 pack years; and 7) >50 pack years. For physical activity (MET score) an energy– expenditure score reporting total physical activity as a multiple of basal metabolic expenditure was calculated as per WHI protocol32. Briefly, this protocol applies a standardized classification of the energy expenditure associated with self– report of hours spent on physical activities and the intensity of these activities compared to basal energy expenditures for a given period of time. For parity the following score was used: – 1) never pregnant; 0) never had term pregnancy; 1 to 5 corresponding to the number of full term pregnancies. Income was assessed using family income and the following categorical scores: 1) < $10,000; 2) $10,000 – $19,999; 3) $20,000 – $34,999; 4) $35,000 – $49,999; 5) $50,000 – $74,999; 6) $75,000 – $99,999; 7) $100,000 – $149,999; and 8) >$149,999. For education, we used a modified scale with the following categorical classification: 1) high school or below; 2) vocational; 3) some college (includes associate degree); 4) college degree BA/BS; and 5) beyond college. Caloric intake in kilocalories (kcal) was calculated using the WHI food frequency questionnaire (FFQ) as previously reported33, 34. This method includes detailed questions about 122 food and food groups and uses an established instrument for computing caloric intake. Specific analysis of the effect of specific dietary components was considered beyond the scope of this manuscript. A summary of the included study subject characteristics are provided in Tables 1 and 2.
Table 1. Adiposity and Adipose Distribution in African American and Hispanic WHI Participants' Studieda.
| Populationb | Sample Size | Mean +/− SD All | Mean +/− SD Top 25% | Range Top 25% | Mean +/− SD Bottom 25% | Range Bottom 25% |
|---|---|---|---|---|---|---|
| BMI | ||||||
| ALL | 16800 | 30.45 +/− 6.30 | 39.02 +/− 4.65 | 34.00 – 61.98 | 23.47 +/− 1.91 | 15.18 – 25.97 |
| AFA | 11712 | 31.10 +/− 6.50 | 39.95 +/− 4.70 | 34.79 – 61.98 | 23.87 +/− 2.05 | 15.18 – 26.56 |
| AFA subgroup | 10854 | 31.13 +/− 6.49 | 39.97 +/− 4.69 | 34.83 – 61.98 | 23.89 +/− 2.06 | 15.18 – 26.58 |
| HA | 5088 | 28.93 +/− 5.53 | 36.43 +/− 4.13 | 32.02 – 60.56 | 22.79 +/− 1.65 | 15.54 – 25.00 |
| HA subgroup | 1977 | 28.99 +/− 5.43 | 36.36 +/− 3.81 | 32.25 – 53.46 | 22.90 +/− 1.71 | 16.54 – 25.09 |
| WHR | ||||||
| ALL | 16800 | 0.82 +/− 0.08 | 0.92 +/− 0.05 | 0.88 – 1.45 | 0.74 +/− 0.04 | 0.28 – 0.77 |
| AFA | 11712 | 0.82 +/− 0.08 | 0.93 +/− 0.05 | 0.88 – 1.42 | 0.73 +/− 0.04 | 0.28 – 0.77 |
| AFA subgroup | 10854 | 0.82 +/− 0.08 | 0.93 +/− 0.05 | 0.88 – 1.42 | 0.73 +/− 0.04 | 0.28 – 0.77 |
| HA | 5088 | 0.82 +/− 0.07 | 0.91 +/− 0.05 | 0.87 – 1.45 | 0.74 +/− 0.04 | 0.33 – 0.77 |
| HA subgroup | 1977 | 0.82 +/− 0.07 | 0.92 +/− 0.06 | 0.88 – 1.45 | 0.74 +/− 0.03 | 0.61 – 0.77 |
| WC | ||||||
| ALL | 16800 | 90.3 +/− 13.6 | 109.1 +/− 8.5 | 99.1 – 191.6 | 74.32 +/− 4.9 | 35.0 – 80.5 |
| AFA | 11712 | 91.8 +/− 13.8 | 110.3 +/− 8.6 | 100.3 – 191.6 | 75.77 +/− 5.1 | 35.5 – 82.0 |
| AFA subgroup | 10854 | 91.8 +/− 13.8 | 110.4 +/− 8.5 | 100.6 – 191.6 | 75.79 +/− 5.1 | 35.5 – 82.0 |
| HA | 5088 | 86.8 +/− 12.5 | 103.6 +/− 8.0 | 94.6 – 158.0 | 72.66 +/− 4.5 | 35.0 – 78.0 |
| HA subgroup | 1977 | 87.3 +/− 12.5 | 104.5 +/− 7.9 | 95.5 – 158.0 | 72.75 +/− 4.3 | 56.0 – 78.0 |
| HC | ||||||
| ALL | 16800 | 109.8 +/− 12.9 | 127.8 +/− 9.3 | 117.1 – 178.0 | 95.7 +/− 4.4 | 77.5 – 101.0 |
| AFA | 11712 | 111.4 +/− 13.2 | 129.8 +/− 9.3 | 119.2 – 178.0 | 96.7 +/− 4.5 | 77.5 – 102.0 |
| AFA subgroup | 10854 | 111.5 +/− 13.2 | 129.7 +/− 9.2 | 119.2 – 178.0 | 96.7 +/− 4.5 | 77.5 – 102.0 |
| HA | 5088 | 106.1 +/− 11.5 | 121.8 +/− 8.7 | 112.1 – 170.0 | 93.5 +/− 3.7 | 79.0 – 98.0 |
| HA subgroup | 1977 | 105.9 +/− 11.5 | 121.7 +/− 8.6 | 112.1 – 170.0 | 93.5 +/− 3.8 | 79.0 – 98.0 |
The mean values for body mass index (BMI), waist hip ratio (WHR), waist circumference (WC), and hip circumference (HC) is shown for all study participants with admixture information.
ALL consists of all self– identified African American (AFA) and Hispanic Americans (HA) participants; AFA consists of self– identified AFA participants; AFA subgroup consists of the self– identified AFA with >0.2 AFR and <0.05 AMI admixture; HA consists of self– identified HA participants; HA subgroup consists of the self– identified HA with >0.1 AMI and <0.1 AFR admixture.
Table 2. Covariates for Subject Groups.
| Population Groupa | ||||||
|---|---|---|---|---|---|---|
| Scaleb | ALL | AFA | AFA subgroup | HA | HA subgroup | |
| Age (Mean +/− SD) | 61.2 +/− 7.1 | 61.7 +/− 7.1 | 61.7 +/− 7.1 | 60.2 +/− 6.8 | 59.9 +/− 6.9 | |
| Physical Activity (Mean +/− SD) | 9.87 +/− 13.02 | 9.62 +/− 12.71 | 9.58 +/− 12.65 | 10.48 +/− 13.71 | 10.19 +/− 13.23 | |
| Energy intake (Mean +/− SD) | 1.45 +/− 0.89 | 1.44 +/− 0. 89 | 1.44+/− 0.89 | 1.48 +/− 0.90 | 1.498 +/− 0.89 | |
| Education (number, %) | 1 | 5156 (31.1) | 2980 (25.8) | 2757 (25.7) | 2176 (43.5) | 894 (45.9) |
| 2 | 2069 (12.5) | 1458 (12.6) | 1360 (12.7) | 611 (12.2) | 243 (12.4) | |
| 3 | 4181 (25.3) | 3042 (26.3) | 2813 (26.3) | 1139 (22.8) | 454 (23.3) | |
| 4 | 1381 (8.3) | 1004 (8.7) | 927 (8.7) | 377 (7.5) | 121 (6.2) | |
| 5 | 3778 (22.8) | 3077 (26.6) | 2855 (26.6) | 701 (14.0) | 238 (12.2) | |
| Parity (number, %) | − 1 | 1294 (7.8) | 869 (7.5) | 800 (7.5) | 425 (8.5) | 169 (8.7) |
| 0 | 827 (4.9) | 685 (5.9) | 632 (5.9) | 142 (2.8) | 52 (2.7) | |
| 1 | 2188 (13.2) | 1751 (15.1) | 1614 (15.0) | 437 (8.7) | 145 (7.4) | |
| 2 | 3643 (22.0) | 2632 (22.7) | 2448 (22.8) | 1011 (20.2) | 361 (18.5) | |
| 3 | 3134 (18.9) | 2110 (18.2) | 1940 (18.1) | 1024 (20.4) | 389 (19.9) | |
| 4 | 2261 (13.6) | 1449 (12.5) | 1343 (12.5) | 812 (16.2) | 311 (15.9) | |
| 5 | 3247 (19.6) | 2088 (18.1) | 1958 (18.2) | 1159 (23.2) | 525 (26.9) | |
| Smoke years (number, %) | 0 | 9394 (55.9) | 6033 (51.5) | 5602 (51.6) | 3361 (66.1) | 1299 (65.7) |
| 1 | 1091 (6.5) | 685 (5.8) | 633 (5.8) | 406 (8.0) | 172 (8.7) | |
| 2 | 672 (4.0) | 477 (4.1) | 446 (4.1) | 195 (3.8) | 99 (5.0) | |
| 3 | 1604 (9.6) | 1238 (10.6) | 1135 (10.5) | 366 (7.2) | 130 (6.6) | |
| 4 | 1754 (10.4) | 1378 (11.8) | 1266 (11.7) | 376 (7.4) | 134 (6.8) | |
| 5 | 1382 (8.2) | 1144 (9.8) | 1072 (9.9) | 238 (4.7) | 86 (4.3) | |
| 6 | 690 (4.1) | 580 (4.9) | 535 (4.9) | 110 (2.1) | 42 (2.1) | |
| 7 | 213 (1.3) | 177 (1.5) | 165 (1.5) | 36 (0.7) | 15 (0.8) | |
| Income (number, %) | 1 | 2159 (14.1) | 1356 (12.5) | 1251 (12.5) | 803 (17.8) | 319 (18.2) |
| 2 | 2818 (18.4) | 1898 (17.5) | 1771 (17.7) | 920 (20.4) | 358 (20.4) | |
| 3 | 3709 (24.2) | 2644 (24.4) | 2441 (24.3) | 1065 (23.6) | 408 (23.3) | |
| 4 | 2722 (17.7) | 1982 (18.3) | 1839 (18.3) | 740 (16.4) | 275 (15.7) | |
| 5 | 2400 (15.6) | 1809 (16.7) | 1665 (16.6) | 591 (13.1) | 244 (13.9) | |
| 6 | 879 (5.7) | 656 (6.1) | 607 (6.1) | 223 (4.9) | 88 (5.0) | |
| 7 | 504 (3.3) | 386 (3.6) | 358 (3.6) | 118 (2.6) | 43 (2.4) | |
| 8 | 146 (1.0) | 95 (0.9) | 92 (0.9) | 51 (1.2) | 19 (1.1) | |
ALL consists of all self– identified African American (AFA) and Hispanic Americans (HA) participants; AFA consists of self– identified AFA participants; AFA subgroup consists of the self– identified AFA with >0.2 AFR and <0.05 AMI admixture; HA consists of self– identified HA participants; HA subgroup consists of the self– identified HA with >0.1 AMI and <0.1 AFR admixture.
The scales were as follows. Age is provided in years. For physical activity (metabolic equivalents): exercise per week was calculated from self– report of hours spent at various activities as per WHI protocol. Energy intake was measured in kilocalories as calculated using the WHI food frequency questionnaire (FFQ). For education, we used a modified scale with the following categorical classification: 1) high school or below; 2) vocational; 3) some college (includes associate degree); 4) college degree BA/BS; and 5) beyond college. For parity the following scale was applied: – 1) never pregnant; 0) never had term pregnancy; 1 to 5 corresponding to the number of full term pregnancies. Income (family income/year): 1) < $10,000; 2) $10,000 – $19,999; 3) $20,000 – $34,999; 4) $35,000 – $49,999; 5) $50,000 – $74,999; 6) $75,000 – $99,999; 7) $100,000 – $149,999; and 8) >$149,999. For Smoking (smoke years): 1) <5 pack years; 2) 5 – 9 pack years; 3)10 – 19 pack years; 4) 20 – 29 pack years; 5) 30 to 39 pack years, 6) 40 – 49 pack years; and 7) >50 pack years.
Ancestry Informative Markers (AIMs)
The AIMs were chosen based on our previous studies35, 36 and performance in the TaqMan® OpenArray® assay. The final marker set included 92 SNPs that demonstrated large differences in allele frequency between populations derived from three different continents: Europe; sub– Saharan Africa, and America. The marker set was chosen to exclude SNPs that show differences in allele frequency within different continental groups35. As previously characterized these markers distinguish between European, sub– Saharan African and Amerindian groups and show very similar results for several different populations within each continental group35, 36. The current study does not address differences in population substructure within the continental contribution. The AIMs and population allele frequencies are provided in Supplemental Table 1.
Genotyping Methods
Genotyping was performed using the TaqMan® OpenArrays® system (https://products.appliedbiosystems.com/ab/en/US/adirect/ab?cmd=catNavigate2&catID=605783) (ABI) (Foster City, CA 94404 USA). Genotypes were scored using the OpenArray® SNP Genotyping Analysis Software provided by the manufacturer. All assays were validated by concordant results with standard ABI TaqMan® assays (>98% concordance) for >48 assays. All AIM SNPs had >93% call rate and showed >98% concordance in 5% duplicate assays. All individuals had data for >75% of each of the AIM SNPs and >90% call rate when technical exclusions were considered. The mean call rate for the included subjects was 97.1%. All AIM SNPs were in Hardy Weinberg equilibrium (p>0.005) in parental populations.
Admixture Assessment
The 16800 individuals were assessed for AFR, AMI, and EUR contribution using STRUCTURE analyses of genotyping results with AIMs as previously described35, 36. The analyses were performed using representatives of the three parental populations for analyses under the assumption of three populations (K = 3) and with representatives of the two parental populations (K = 2) under the assumption of two populations. The samples used to represent the parental population groups included: 128 European Americans from the New York Cancer Project and 60 CEPH Europeans (CEU) for EUR; 56 Yoruban African (YRI), 19 Bini West African, and 23 Kanuri West African for AFR; and 50 Mayan Amerindians, 26 Quechuan Amerindians, and 29 Nahua Amerindians for AMI as previously described35. We performed three separate analyses of population structure for each group or subgroup using STRUCTURE v2.3.337, 38 parameters and AIMs previously described35. The results were most consistent and reproducible using prior parental population assignment with < 0.02 difference between each of the separate analyses. An example of the admixture assessment results is shown in Figure 1. The African American subject set showed the following admixture contributions [mean +/− standard deviation (SD)]: EUR, 0.225 +/− 0.147; AFR, 0.757 +/− 0.150; and AMI, 0.019 +/− 0.025. The Hispanic American subject set showed the following admixture contributions: EUR, 0.597 +/− 0.189; AFR, 0.135 +/− 0.121; and AMI 0.267 +/− 0.186.
Figure 1. Estimation of individual admixture in WHI African American and Hispanic American participants.
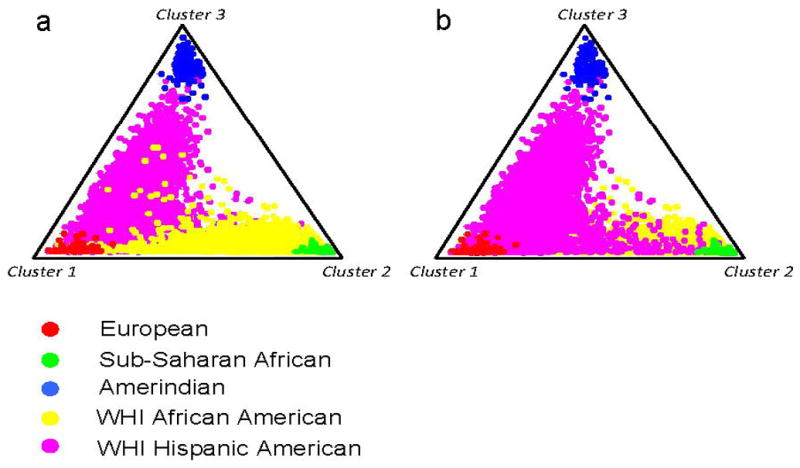
The triangle plots show the distribution of individual WHI participants and reference population representative when admixture was assessed using 92 ancestry informative markers (AIMs). The population groups European, sub– Saharan African, Amerindian, WHI African American (AFA), and WHI Hispanic American (HA) are as indicated by color coded symbols in the key. The admixture was assessed under a K=3 model (assuming 3 parental population groups) using the STRUCTURE program that applies a Bayesian clustering algorithm. Panels a and b show two separate analyses under the same conditions with either the AFA participants in the foreground (panel a) or the HA participants in the foreground (panel b).
For some of the analyses we examined subgroups of the self– identified population group affiliations. For AFA, we considered the subgroup with >20% AFR and < 5% AMI admixture containing 10854 participants of the total 11712 in self identified AFA group in an effort to minimize potential database or laboratory errors that cause miss– assignment. This definition excludes only extreme outliers (> 2 SD) in the self– AFA admixture analyses. For this AFA subgroup the admixture was then re– evaluated using K = 2 in which the contribution of only AFR and EUR was considered. For the HA, we also examined the subset of individuals with > 10% AMI and <10%AFR admixture to further assess the possible differences between AMI and EUR admixture (1977 of the 5088 self– identified HA). Sample sizes for other subsets included <1000 participants and were not considered in separate analyses.
Statistical Analyses
Analyses were carried out for the following population groups: ALL (self– identified AFA and HA participants), AFA (self– identified AFA participants), AFA subgroup (self– identified AFA participants with >20% AFR and < 5% AMI admixture), HA (self– identified HA participants), HA subgroup (self– identified HA participants with > 10% AMI and <10%AFR admixture). The effect size of admixture was estimated as the proportion of the SD of either BMI or WHR using models incorporating appropriate covariates. This allows the standardizing of the effect proportional on the amount of variation thus enabling comparison of the effects on different measurement scales. Natural log transformations of the BMI and WHR were adopted to obtain a normal distribution of these traits. The correlation statistic (r) was determined using the standard Pearson product– moment correlation coefficient and the two tailed p values determined from the t– statistic. Linear regression was performed to study the admixture effect on the traits, in which the admixture effect was assessed as a continuous variable on the standardized continuous dependent variable [ln (BMI) or ln (WHR)], adjusting for age at entry, smoking, education, income, parity and physical activity. Logistic regression was conducted to obtain the odds ratios and 95% confidence intervals. To estimate odds ratios for the effect of admixture we defined the phenotypic parameters (BMI and WHR) as dichotomous traits using the top and bottom quartiles (values shown in Table 1). A unit increase in admixture is defined as the effect of the 100% admixture compared to no admixture of the particular continental population. Comparing the effects of 100% vs. 0% admixture corresponds to comparing one parental population to another parental population.
Results
Adiposity Measurements in African American and Hispanic American Women
When BMI, WC or HC were used as a measure of adiposity there was a marked difference between the self– identified AFA and HA women (Table 1). The mean BMI, HC and WC of AFA were significantly higher than that of the HA subjects with or without correction for age, education, smoking, parity, physical activity and income covariates (p <0.0001 for both). In contrast there was no difference between AFA and HA when WHR was considered (Table 1).
Very similar results were observed when subsets of subjects from these groups were considered. For the AFA subgroup, the mean BMI, WC, HC and WHR were almost identical to the entire self– identified AFA group. Also, the HA subgroup enriched for AMI admixture with limited AFR admixture (<0.1) showed a nearly identical BMI, WC, HC and WHR to the entire self– identified HA group (Table 1).
Correlation of Adiposity Characteristics with Admixture
To examine the effect of admixture on indices of adiposity we first examined the correlation without considering covariates. Natural log transformations of the BMI and WHR resulted in a normal distribution of these traits. Admixture proportions were then ascertained for each individual and mean admixture proportions for the normally distributed intervals were plotted (Fig. 2). In ALL, the complete dataset including both AFA and HA, when each individual measurement (BMI and AFR admixture) was considered, there was a significant correlation between BMI and AFR admixture (r = 0.18, p<0.0001). This relationship was also observed for the AFA subgroup with a significant correlation between BMI and AFR admixture (r = 0.095. p < 0.0001). When comparing normally distributed BMI groups there was a high correlation between these groups and AFR admixture (Fig. 2 panel c, r = 0.96 for this ecological correlation). As expected based on the mean population group results, both AMI and EUR admixture showed inverse correlations with BMI in ALL (AMI, r = − 0.10, p < 0.0001; EUR, r = − 0.16, p <0.0001). For BMI in HA there were no significant correlations either in the self– identified HA or the HA subgroups.
Figure 2. Histograms showing correlation between continental admixture and adiposity and adipose distribution.
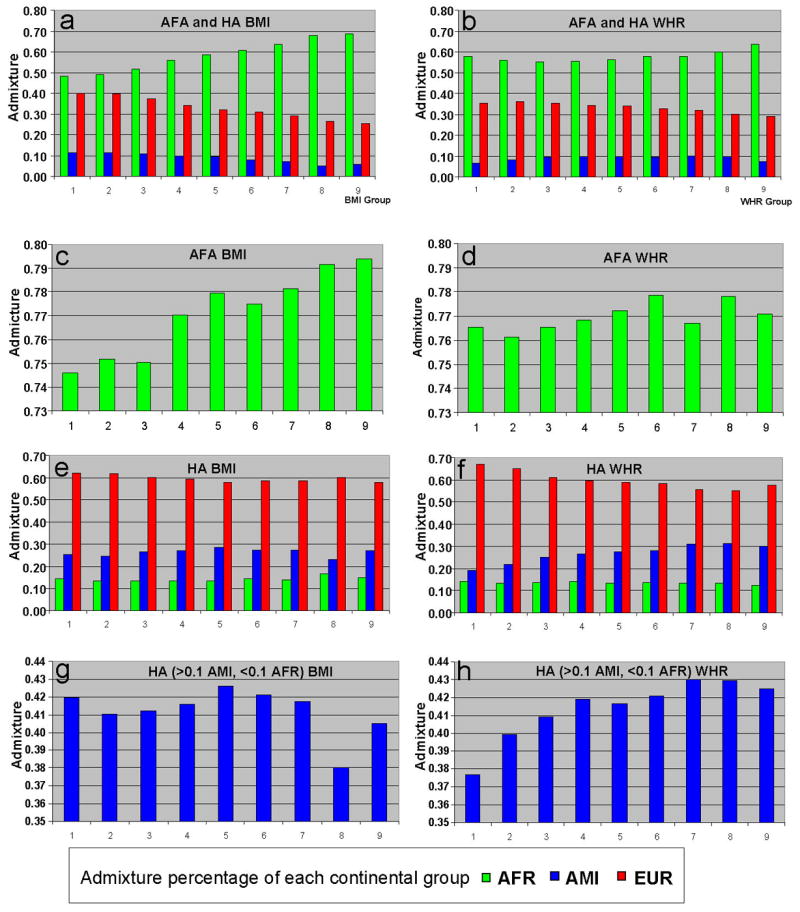
For both the left side [body mass index (BMI)] and the right side panels [(waste hip ratio (WHR)], the admixture corresponding to normally distributed groups (abscissa) is shown by the color coded continental contribution (ordinate). For both BMI and WHR, natural log transformations were used to achieve normal distributions. The BMI and WHR groups corresponded to the following ranges: 1) < 22.20, <0.71; 2) 22.20 – 24.53, 0.71 – 0.74; 3) 24.54 – 27.11, 0.75 – 0.77; 4) 27.12 – 29.96, 0.78 – 0.81; 5) 29.97 – 33.11, 0.82 – 0.84; 6) 33.12 – 33.59, 0.85 – 0.88; 7) 33.60 – 40.44, 0.89 – 0.92; 8) 40.45 – 44.69, 0.93 – 0.97; and 9) >44.69, > 0.98. Panels a and b show the entire participants [African American (AFA) and Hispanic Americans (HA) included in the analyses. Panels c and d show the self identified AFA participants (AFR > 0.2, AMI <0.1). Panels e and f show the self identified HA participants. Panels g and h show the subsets of HA participants with >0.1 AMI and < 0.1 AFR. For panels a, b, e, and f, the admixture was determined using 3 population clusters corresponding to AFR, AMI and EUR. For panels c and d the admixture was determined using two population clusters corresponding to AFR and EUR (only AFR shown). For panels g and h, the admixture was determined using two population clusters corresponding to AMI and EUR.
For WHR, AFR admixture had a much weaker correlation in the AFA subgroup (r = 0.029, p = 0.0024). In contrast to the results with BMI, AMI admixture was significantly correlated in the HA individuals (r = 0.14, p< 0.0001). Similarly, correlations with AMI admixture were observed in the HA subgroup (r = 0.084, p = 0.0001) with WHR.
Estimation of the Effect of Admixture on Adiposity
To more precisely determine the effect of admixture a linear regression model was examined with covariates for age at entry, education, smoking, parity, physical activity and income. When BMI is used as a measurement for adiposity AFR admixture showed a large positive effect size in the total group and in the AFA subgroup (Table 3). AMI and EUR continental admixture showed significant negative effect size for BMI in the total group. EUR admixture also showed a negative relationship in the AFA subgroup. In the HA there was also a positive effect from AFR contribution.
Table 3. Effect of Admixture on Adiposity and Adipose Distribution in African American and Hispanic American Women.
| BMI | WHR | ||||||
|---|---|---|---|---|---|---|---|
| Groupa | Admixtureb | Estimatec | SEM | p– value | Estimate | SEM | p– value |
| ALL | AFR | 0.58 | 0.03 | <.00001 | 0.09 | 0.03 | 0.001 |
| EUR | − 0.68 | 0.03 | <0.0001 | − 0.22 | 0.04 | <0.0001 | |
| AMI | − 0.88 | 0.06 | <0.0001 | 0.14 | 0.06 | 0.010 | |
| AFA | AFR | 0.34 | 0.06 | <0.0001 | 0.02 | 0.07 | 0.762 |
| EUR | − 0.35 | 0.07 | <0.0001 | − 0.03 | 0.07 | 0.707 | |
| AMI | − 0.21 | 0.37 | 0.566 | 0.14 | 0.38 | 0.700 | |
| AFA subgroup | AFR | 0.42 | 0.08 | <0.0001 | 0.01 | 0.08 | 0.860 |
| HA | AFR | 0.30 | 0.12 | 0.017 | 0.05 | 0.12 | 0.696 |
| EUR | − 0.15 | 0.08 | 0.060 | − 0.67 | 0.08 | <0.0001 | |
| AMI | 0.03 | 0.08 | 0.741 | 0.68 | 0.08 | <0.0001 | |
| HA subgroup | AMI | 0.05 | 0.23 | 0.816 | 0.74 | 0.23 | 0.001 |
ALL consists of all self– identified African American (AFA) and Hispanic Americans (HA) participants; AFA consists of self– identified AFA participants; AFA subgroup consists of the self– identified AFA with >0.2 AFR and <0.05 AMI admixture; HA consists of self– identified HA participants; HA subgroup consists of the self– identified HA with >0.1 AMI and <0.1 AFR admixture.
Analysis was performed using the following covariates: age at entry, smoking, education, income, parity and physical activity.
The estimate is the proportion of the SD attributed to admixture from each continent based on complete (100%) admixture.
In contrast, when WHR is used as a measurement for adipose distribution, AMI admixture showed a strong effect in the HA with an increase in this index comparable to the effect size of AFR for BMI in AFA (Table 3). This effect was also seen in the HA subgroup.
We also examined both HC and WC (Table 4). For both HC and WC, the results were similar to BMI in that AFR admixture showed a significant positive association in the ALL, AFA and AFA subgroup. However, the effect size was smaller in AFA and the AFA subgroup. The AMI admixture showed a significant negative association with HC and a marginal positive association with WC. Both HC and WC show strong correlations with BMI for each of the groups analyzed, where as WHR was poorly correlated with these other measurements (Supplemental Table 2).
Table 4. Effect of Continental Admixture on Hip and Waist Circumference in African American and Hispanic American.
| Hip Circumference | Waist Circumfirence | ||||||
|---|---|---|---|---|---|---|---|
| Groupa | Admixtureb | Estimatec | SEM | p value | Estimate | SEM | p value |
| ALL | AFR | 0.63 | 0.03 | <0.0001 | 0.54 | 0.03 | <0.0001 |
| EUR | − 0.66 | 0.03 | <0.0001 | − 0.65 | 0.03 | <0.0001 | |
| AMI | − 1.15 | 0.06 | <0.0001 | − 0.80 | 0.05 | <0.0001 | |
| AFA | AFR | 0.21 | 0.07 | 0.002 | 0.18 | 0.06 | 0.006 |
| EUR | − 0.22 | 0.07 | 0.001 | − 0.19 | 0.07 | 0.005 | |
| AMI | 0.04 | 0.38 | 0.991 | 0.10 | 0.37 | 0.794 | |
| AFA subgroup | AFR | 0.28 | 0.08 | 0.0003 | 0.23 | 0.08 | 0.002 |
| HA | AFR | 0.32 | 0.12 | 0.011 | 0.27 | 0.12 | 0.032 |
| EUR | 0.18 | 0.08 | 0.031 | − 0.28 | 0.08 | 0.001 | |
| AMI | − 0.33 | 0.08 | <0.0001 | 0.17 | 0.08 | 0.036 | |
| HA subgroup | AMI | − 0.40 | 0.23 | 0.079 | 0.15 | 0.23 | 0.508 |
ALL consists of all self– identified African American (AFA) and Hispanic Americans (HA) participants; AFA consists of self– identified AFA participants; AFA subgroup consists of the self– identified AFA with >0.2 AFR and <0.05 AMI admixture; HA consists of self– identified HA participants; HA subgroup consists of the self– identified HA with >0.1 AMI and <0.1 AFR admixture.
Analysis was performed using the following covariates: age at entry, smoking, education, parity, exercise and income.
The estimate is the proportion of the SD attributed to admixture from each continent based on complete (100%) admixture.
Admixture Risk for Adiposity Indices
As another measure of the relationship between admixture and adiposity indices we examined the odds ratios (OR) using the upper and lower quartile of adiposity measurements as a dichotomous measure of adiposity (see Methods and Table 1 for mean and range of dichotomous groups). The OR and 95% confidence interval were calculated using age at entry, smoking, education, income, parity and physical activity as covariates (Table 5). When BMI is used as a measurement for adiposity, AFR admixture showed a strong effect on increased BMI in the ALL group, AFA, and in the AFA subgroup with significant p– values (OR = 4.91, p < 0.0001; OR= 2.63, p < 0.0001; OR = 3.27, p < 0.0001, respectively) (Table 5). On the other hand, AMI and EUR admixture showed an opposite strong effect on decreased BMI in the ALL group (OR = 0.08, p < 0.0001; OR = 0.15, p < 0.0001, respectively). Together with the previous results these findings further supported the strong association of AFR admixture with increased BMI in the ALL group, AFA group and the AFA subgroup. However, AFR admixture was not significantly associated with BMI in the HA group (p = 0.22) (Table 5).
Table 5. Admixture Odds Ratios for Adiposity and Adipose Distribution in African American and Hispanic American Women.
| BMI | WHR | ||||||
|---|---|---|---|---|---|---|---|
| Groupa | Admixture | ORb | 95% CI | P value | OR | 95% CI | p value |
| ALL | AFR | 4.91 | 4.16 – 5.79 | <0.0001 | 1.27 | 1.08 – 1.48 | 0.004 |
| EUR | 0.15 | 0.12 – 0.19 | <0.0001 | 0.57 | 0.46 – 0.70 | <0.0001 | |
| AMI | 0.08 | 0.06 – 0.12 | <0.0001 | 1.54 | 1.09 – 2.18 | 0.014 | |
| AFA | AFR | 2.63 | 1.82 – 3.80 | <0.0001 | 0.92 | 0.63 – 1.36 | 0.681 |
| EUR | 0.38 | 0.26 – 0.56 | <0.0001 | 1.06 | 0.72 – 1.58 | 0.761 | |
| AMI | 0.23 | 0.03 – 1.84 | 0.166 | 1.99 | 0.23 – 17.34 | 0.534 | |
| AFA subgroup | AFR | 3.27 | 2.08 – 5.15 | <0.0001 | 0.93 | 0.57 – 1.46 | 0.740 |
| HA | AFR | 1.57 | 0.77 – 3.22 | 0.217 | 1.29 | 0.62 – 2.68 | 0.500 |
| EUR | 0.56 | 0.34 – 0.92 | 0.022 | 0.17 | 0.10 – 0.28 | <0.0001 | |
| AMI | 1.46 | 0.89 – 2.42 | 0.137 | 5.93 | 3.52 – 9.97 | <0.0001 | |
| HA subgroup | AMI | 1.39 | 0.35 – 5.53 | 0.640 | 7.15 | 1.83 – 27.90 | 0.005 |
ALL consists of all self– identified African American (AFA) and Hispanic Americans (HA) participants; AFA consists of self– identified AFA participants; AFA subgroup consists of the self– identified AFA with >0.2 AFR and <0.05 AMI admixture; HA consists of self– identified HA participants; HA subgroup consists of the self– identified HA with >0.1 AMI and <0.1 AFR admixture.
The odds ratio (OR) attributed to a unit (0 to 100%) increase in admixture. For these analyses the top and bottom quartiles were compared and the OR reflects the increased or decreased risk for higher adiposity measurement attributed to admixture of the indicated continental contribution. Analysis was performed using the following covariates: age at entry, smoking, education, income, parity and physical activity.
Similar to the continuous model, the results were very different when WHR is used as a measurement for adiposity. AMI admixture showed higher OR values in the HA (OR = 5.93) and HA subgroup (OR = 7.15) (Table 5) than that in the ALL group (OR = 1.54). Thus, in contrast to the effect of AMI admixture in BMI (minimal effect or negative effect), AMI admixture shows a strong association with increased WHR in the HA and the HA subgroup.
Discussion
In the current study similar to previous studies in AFA, BMI was associated with AFR admixture14, 28. Our results extend this finding to adult postmenopausal women. The data also demonstrate an association of WHR but not BMI with AMI admixture in HA postmenopausal women. Several potential explanations and limitations of our study are considered below. It should be noted that diabetic individuals may have changed both diet and weight as a consequence of their treatments, and many other unknowable confounders may affect results of studies in elderly population groups. When analyses were performed using only non-diabetic participants the results were nearly identical (Supplemental Table 3), however, it is not possible to exclude all confounders. Nevertheless, differences in relative adiposity in these population groups are associated with admixture profiles and may have important implications for future genetic studies as well as therapeutic intervention and assessment.
For BMI in HA, in contrast to the results in AFA, there was only a minimal effect size from admixture. AFR, EUR or AMI admixture all showed insignificant ORs for BMI (Table 5). In a previous study in Mexican Americans, BMI was modestly lower in individuals with higher AMI admixture39 further suggesting very different results than what we observed with our analyses WHR; in HA, our WHR results showed a significant and strong association with AMI admixture. Thus, the main finding in the current study is the difference in results between BMI and WHR among AFA and HA participants. Specifically, in AFA, AFR admixture is associated with BMI but not WHR while in HA, AMI admixture is associated with WHR but not BMI. The difference with respect to AFA is consistent with findings from studies segmenting body composition from CT scans that show that AFA have less visceral fat than other self– reported ethnic groups40. Therefore although WHR may be not a useful measurement in AFA, our findings in HA suggest that WHR may be an important measure in populations with substantial AMI admixture.
In contrast to many of the previous studies, our results were adjusted for multiple covariates. These covariates included the available measures of socio– economic status including self reported family income and education level. Education may serve as a surrogate for socio– economic status during adolescence and early adulthood and has been used as a general surrogate for socio– economic status41. It should be noted that the level of education for participants from the WHI study is in general higher than similarly aged women and that the available information may not fully account for socio– economic effects. When the socio– economic covariates were not included in the analysis we did observe a higher effect from AFR admixture (OR = 4.73 compared with OR =3.27 for BMI in the AFA subgroup) (Supplemental Table 4). This result is consistent with barriers and access to health care explaining a substantial proportion of the admixture effect. Although these measurements may not fully account for socio– economic and other cultural factors, they support the conclusion that a component of association with admixture reflects genetic differences. Previous studies identifying specific regions containing risk alleles of AFR origin in admixture mapping studies also support a genetic explanation14, 15.
Other covariates used in our analyses (smoking, physical activity, age and parity) have been suggested by previous analyses as potential predictors of obesity42-52. These covariates, in contrast to socio– economic covariates, had only modest effects in the current study with the exception of very high parity (data not shown). In addition, we also examined whether self– reported food frequency questionnaire (FFQ) data, in particular daily energy intake, might explain the admixture– BMI association. Energy intake did not alter the results and energy intake had an OR for BMI very close to 1.0 in all groups examined. Interestingly, calorie counts were significantly higher (p < 0.0001) in HA (1.48 Kcal +/− 0.90) than AFA (1.44 Kcal +/− 0.89) which is opposite to what might be expected from the BMI population results (Tables 1 and 2). These results show that self reported energy intake does not account for the observed admixture association. However, previous analysis of this self– reported dietary intake instrument (WHI FFQ) has suggested that it underestimates energy intake especially in AFA34 suggesting that interpretation of the dietary intake results requires some caution.
Multiple studies have used BMI as a predictor for adverse health outcomes due to obesity8-11; however, this measurement does not differentiate between body fat and lean mass. Dual– emission X– ray absorption (DXA) is generally considered to be a more accurate method for distinguishing between body fat and lean mass, although variation in tissue hydration can lead to potential errors in assessment of body fat53. DXA measurements of truncal body fat were available for less than 15% of the subjects in the current study thus precluding a full analysis of DXA calculated body fat. However, the DXA results from those subjects with measurements of BMI showed a very strong correlation between truncal fat and BMI for the each of the subject subsets (Supplemental Table 2) suggesting that BMI is probably a good surrogate for body fat in our study similar to another recent study54.
As noted in the introduction, there are multiple contradictory studies with regards to the importance of BMI, waist circumference and WHR as predictive indices for adverse health consequences12, 17, 20-26. Our results suggest that both BMI and WHR should be considered as separate variables and that additional study is warranted to determine the importance of these factors in health consequences in different ethnic groups. WC and HC were more similar to BMI consistent with other studies18, 55, 56 and in our study also correlated with DXA truncal fat (Supplemental Table 2),
It should be emphasized that this study is specifically relevant to adult post– menopausal women and that the mean weight of WHI participants' is greater than the general age matched population. Differences in hormonal patterns may have a profound effect on the deposition and distribution of body fat and changes at menopause have been linked to increased abdominal and visceral adipose tissue accumulation57, 58. Whether hormonal or other metabolic pathways differ in populations of different ethnicity is unclear. The thrifty gene hypothesis states that certain genes may have been selected as advantageous in hunter– gatherer populations, especially in child bearing women59. Although attractive, this hypothesis has been challenged60, 61. Insulin resistance in response to food deprivation has been advanced more recently to explain possible differences in the propensity to obesity and might explain the AMI admixture association with abdominal adiposity observed in HA61. Interestingly, a previous study has suggested associations between increased levels of a variety of adipocytokines and higher EUR admixture in AFA62. Clearly additional studies are necessary to determine the underlying mechanisms.
In summary, our study found that differences in both adiposity and adipose distribution are associated with continental admixture in adult post– menopausal women and provide additional support to the hypothesis that differences in ethnic origins may be a critical component for etiologic and therapeutic studies. The results also emphasize that different indices of adiposity and adipose distribution should be carefully assessed in genetic and epidemiologic studies.
Supplementary Material
Acknowledgments
The authors thank the participants of the WHI and acknowledge the contributions of WHI investigators for the development of study materials (Supplemental Acknowledgements). This work was supported by National Institutes of Health NHLBI BAA Contract No. HHSN268200764319C. The study design was approved by the NHLBI as part of a BAA for the Women's Health Initiative. The Women's Health Initiative provided access to clinical data and DNA samples under appropriate Institutional Review Board approval. The Women's Health Initiative Publication and Presentation Committee reviewed and approved the manuscript for submission. The NHLBI was not otherwise involved in the design and conduct of the study, or in the analysis of data or preparation of the manuscript.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356(3):213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81(2):409–15. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 3.Escobedo J, Buitron LV, Velasco MF, Ramirez JC, Hernandez R, Macchia A, et al. High prevalence of diabetes and impaired fasting glucose in urban Latin America: the CARMELA Study. Diabet Med. 2009;26(9):864–71. doi: 10.1111/j.1464-5491.2009.02795.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, Jr, Clark LT, Cooper RS, Daniels SR, Kumanyika SK, Ofili E, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation. 2005;111(10):e134–9. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 6.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89(6):2590–4. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 8.Kim KS, Owen WL, Williams D, Adams-Campbell LL. A comparison between BMI and Conicity index on predicting coronary heart disease: the Framingham Heart Study. Ann Epidemiol. 2000;10(7):424–31. doi: 10.1016/s1047-2797(00)00065-x. [DOI] [PubMed] [Google Scholar]
- 9.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128(2):81–8. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 12.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–6. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CY, Kao WH, Patterson N, Tandon A, Haiman CA, Harris TB, et al. Admixture mapping of 15,280 African Americans identifies obesity susceptibility loci on chromosomes 5 and X. PLoS Genet. 2009;5(5):e1000490. doi: 10.1371/journal.pgen.1000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng CY, Reich D, Coresh J, Boerwinkle E, Patterson N, Li M, et al. Admixture mapping of obesity-related traits in African Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Obesity (Silver Spring) 18(3):563–72. doi: 10.1038/oby.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu A, Tang H, Arnett D, Gu CC, Mosley T, Kardia S, et al. Admixture mapping of quantitative trait loci for BMI in African Americans: evidence for loci on chromosomes 3q, 5q, and 15q. Obesity (Silver Spring) 2009;17(6):1226–31. doi: 10.1038/oby.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84(2):449–60. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 18.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Arch Intern Med. 2000;160(14):2117–28. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Shu XO, Yang G, Li H, Cai H, Gao YT, et al. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 2007;167(9):886–92. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 21.Dagenais GR, Yi Q, Mann JF, Bosch J, Pogue J, Yusuf S. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149(1):54–60. doi: 10.1016/j.ahj.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Noble RE. Waist-to-hip ratio versus BMI as predictors of cardiac risk in obese adult women. West J Med. 2001;174(4):240–1. doi: 10.1136/ewjm.174.4.240-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord. 2001;25(5):652–61. doi: 10.1038/sj.ijo.0801582. [DOI] [PubMed] [Google Scholar]
- 24.Ketel IJ, Volman MN, Seidell JC, Stehouwer CD, Twisk JW, Lambalk CB. Superiority of skinfold measurements and waist over waist-to-hip ratio for determination of body fat distribution in a population-based cohort of Caucasian Dutch adults. Eur J Endocrinol. 2007;156(6):655–61. doi: 10.1530/EJE-06-0730. [DOI] [PubMed] [Google Scholar]
- 25.Picon PX, Leitao CB, Gerchman F, Azevedo MJ, Silveiro SP, Gross JL, et al. Waist measure and waist-to-hip ratio and identification of clinical conditions of cardiovascular risk: multicentric study in type 2 diabetes mellitus patients. Arq Bras Endocrinol Metabol. 2007;51(3):443–9. doi: 10.1590/s0004-27302007000300013. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555–63. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 27.Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness--a critical review. Int J Obes Relat Metab Disord. 1998;22(8):719–27. doi: 10.1038/sj.ijo.0800660. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez JR, Shriver MD, Beasley TM, Rafla-Demetrious N, Parra E, Albu J, et al. Association of African genetic admixture with resting metabolic rate and obesity among women. Obes Res. 2003;11(7):904–11. doi: 10.1038/oby.2003.124. [DOI] [PubMed] [Google Scholar]
- 29.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 30.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 31.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 32.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 33.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 34.Hebert JR, Patterson RE, Gorfine M, Ebbeling CB, St Jeor ST, Chlebowski RT. Differences between estimated caloric requirements and self-reported caloric intake in the women's health initiative. Ann Epidemiol. 2003;13(9):629–37. doi: 10.1016/S1047-2797(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 35.Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, et al. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30(1):69–78. doi: 10.1002/humu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nassir R, Kosoy R, Tian C, White PA, Butler LM, Silva G, et al. An ancestry informative marker set for determining continental origin: validation and extension using human genome diversity panels. BMC Genet. 2009;10:39. doi: 10.1186/1471-2156-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–87. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Jorgenson E, Gadde M, Kardia SL, Rao DC, Zhu X, et al. Racial admixture and its impact on BMI and blood pressure in African and Mexican Americans. Hum Genet. 2006;119(6):624–33. doi: 10.1007/s00439-006-0175-4. [DOI] [PubMed] [Google Scholar]
- 40.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13(8):1458–65. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 41.Liao Y, McGee DL, Kaufman JS, Cao G, Cooper RS. Socioeconomic status and morbidity in the last years of life. Am J Public Health. 1999;89(4):569–72. doi: 10.2105/ajph.89.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visser M, Launer LJ, Deurenberg P, Deeg DJ. Past and current smoking in relation to body fat distribution in older men and women. J Gerontol A Biol Sci Med Sci. 1999;54(6):M293–8. doi: 10.1093/gerona/54.6.m293. [DOI] [PubMed] [Google Scholar]
- 43.Canoy D, Wareham N, Luben R, Welch A, Bingham S, Day N, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obes Res. 2005;13(8):1466–75. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 44.Bamia C, Trichopoulou A, Lenas D, Trichopoulos D. Tobacco smoking in relation to body fat mass and distribution in a general population sample. Int J Obes Relat Metab Disord. 2004;28(8):1091–6. doi: 10.1038/sj.ijo.0802697. [DOI] [PubMed] [Google Scholar]
- 45.King GA, Fitzhugh EC, Bassett DR, Jr, McLaughlin JE, Strath SJ, Swartz AM, et al. Relationship of leisure-time physical activity and occupational activity to the prevalence of obesity. Int J Obes Relat Metab Disord. 2001;25(5):606–12. doi: 10.1038/sj.ijo.0801583. [DOI] [PubMed] [Google Scholar]
- 46.Heitmann BL, Kaprio J, Harris JR, Rissanen A, Korkeila M, Koskenvuo M. Are genetic determinants of weight gain modified by leisure-time physical activity? A prospective study of Finnish twins. Am J Clin Nutr. 1997;66(3):672–8. doi: 10.1093/ajcn/66.3.672. [DOI] [PubMed] [Google Scholar]
- 47.Bjorkelund C, Lissner L, Andersson S, Lapidus L, Bengtsson C. Reproductive history in relation to relative weight and fat distribution. Int J Obes Relat Metab Disord. 1996;20(3):213–9. [PubMed] [Google Scholar]
- 48.Wen W, Gao YT, Shu XO, Yang G, Li HL, Jin F, et al. Sociodemographic, behavioral, and reproductive factors associated with weight gain in Chinese women. Int J Obes Relat Metab Disord. 2003;27(8):933–40. doi: 10.1038/sj.ijo.0802318. [DOI] [PubMed] [Google Scholar]
- 49.Newby PK, Dickman PW, Adami HO, Wolk A. Early anthropometric measures and reproductive factors as predictors of body mass index and obesity among older women. Int J Obes (Lond) 2005;29(9):1084–92. doi: 10.1038/sj.ijo.0802996. [DOI] [PubMed] [Google Scholar]
- 50.Arroyo P, Avila-Rosas H, Fernandez V, Casanueva E, Galvan D. Parity and the prevalence of overweight. Int J Gynaecol Obstet. 1995;48(3):269–72. doi: 10.1016/0020-7292(94)02284-6. [DOI] [PubMed] [Google Scholar]
- 51.Koch E, Bogado M, Araya F, Romero T, Diaz C, Manriquez L, et al. Impact of parity on anthropometric measures of obesity controlling by multiple confounders: a cross-sectional study in Chilean women. J Epidemiol Community Health. 2008;62(5):461–70. doi: 10.1136/jech.2007.062240. [DOI] [PubMed] [Google Scholar]
- 52.Mansour AA, Ajeel NA. Parity is associated with increased waist circumference and other anthropometric indices of obesity. Eat Weight Disord. 2009;14(2-3):e50–5. doi: 10.1007/BF03327800. [DOI] [PubMed] [Google Scholar]
- 53.St-Onge MP, Wang Z, Horlick M, Wang J, Heymsfield SB. Dual-energy X-ray absorptiometry lean soft tissue hydration: independent contributions of intra- and extracellular water. Am J Physiol Endocrinol Metab. 2004;287(5):E842–7. doi: 10.1152/ajpendo.00361.2003. [DOI] [PubMed] [Google Scholar]
- 54.Sun Q, van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol. 172(12):1442–54. doi: 10.1093/aje/kwq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Hoy WE. Waist circumference, body mass index, hip circumference and waist-to-hip ratio as predictors of cardiovascular disease in Aboriginal people. Eur J Clin Nutr. 2004;58(6):888–93. doi: 10.1038/sj.ejcn.1601891. [DOI] [PubMed] [Google Scholar]
- 56.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. Bmj. 1995;311(6998):158–61. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32(6):949–58. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10(2):154–67. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 59.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 60.Speakman JR. A nonadaptive scenario explaining the genetic predisposition to obesity: the “predation release” hypothesis. Cell Metab. 2007;6(1):5–12. doi: 10.1016/j.cmet.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Watve MG, Yajnik CS. Evolutionary origins of insulin resistance: a behavioral switch hypothesis. BMC Evol Biol. 2007;7:61. doi: 10.1186/1471-2148-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wassel Fyr CL, Kanaya AM, Cummings SR, Reich D, Hsueh WC, Reiner AP, et al. Genetic admixture, adipocytokines, and adiposity in Black Americans: the Health, Aging, and Body Composition study. Hum Genet. 2007;121(5):615–24. doi: 10.1007/s00439-007-0353-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


