Abstract
We have previously shown that Achilles tendon length is related to walking economy on the flat, presumably because of increased stretch–shortening cycle elastic energy savings. In addition, greater walking economy in African American (AA) women compared to European American (EA) women is explained by longer Achilles tendons in AA women. The purposes of this study were to determine whether economy while walking up a grade and during isometric plantar flexion, two tasks expected to produce proportionately less energy savings from elastic savings are different between AA and EA women. We evaluated walking economy at 4.8 km/h at 0 and 2.5% grade in 48 AA and 48 EA premenopausal women. Plantar flexor muscle metabolic economy (force/ATP) was also evaluated using 31 phosphate magnetic resonance spectroscopy (31P-MRS). AA women walked on the flat more economically (net VO2, AA 8.3 and EA 8.9 ml kg−1 min−1, P = 0.04). No significant ethnic differences were observed while walking up a 2.5% grade or in 31P-MRS determined plantar flexor muscle metabolic economy. These data support our previous study’s suggestion that AA women are more economical while walking on the flat. On the other hand, in activities in which stretch–shortening cycle elastic energy savings would be expected to be reduced (grade walking and isometric force production), no differences in economy during grade walking or isometric force production were observed suggesting that biomechanical, i.e. stretch–shortening cycle elastic energy savings differences rather biochemical differences contribute to the better flat walking economy observed in AA women.
Keywords: Elastic energy, Oxygen uptake, Exercise, Magnetic resonance spectroscopy
Introduction
Storage of elastic energy and increased force-generating potential occurs in the muscle–tendon complex during locomotion (Roberts 2002). During walking and vertical jumping medial gastrocnemius muscle contracts very close to isometrically during the push off phase of the movement (Fukunaga et al. 2001, 2002; Kubo et al. 1999, 2000), allowing more stretch and storage in compliant tendon and increased force generation (Biewener 1998; Fukunaga et al. 2002; Kurokawa et al. 2001; Roberts et al. 1997). Since isometric muscle actions require much less energy compared to concentric muscle actions (Ryschon et al. 1997), more economical locomotion would be expected when the muscle–tendon complex is more compliant. Indeed, in long distance runners greater compliance of the quadriceps tendon is related to improved running economy at different speeds (Adamantios et al. 2006). In addition, using real time ultrasound Fukunaga et al. (2001) have shown that gastrocnemius tendon is stretched during slow walking and that recoil occurs during push-off, potentially reducing the energy needed for fascicle shortening.
We have previously shown that walking economy is related to Achilles tendon length and that differences in walking economy between African American (AA) and European American (EA) women are reduced when adjusted for the longer Achilles tendons of the AA women (McCarthy et al. 2006). This is consistent with the hypothesis that longer tendons may have more potential for elastic energy storage and reuse (Biewener and Roberts 2000). In addition, it is also suggestive that ethnic differences in tendon length (McCarthy et al. 2006) may explain previously reported differences in running (Weston et al. 2000) and walking economy (Bamman 1996) between individuals of African versus European descent. On the other hand, these are strictly observational findings. Therefore, caution concerning cause and effect must be made. It is possible that a number of other factors in muscle including biochemical factors could be causing the improved walking and running economy in AAs.
31P magnetic resonance spectroscopy (31P MRS) can be used to measure intramuscular ATP production rates through the creatine kinase, oxidative phosphorylation, and anaerobic glycolysis pathways (Hunter et al. 2001). ATP production rates and integrated force output (force–time integrals) can be used to calculate muscle metabolic economy (N force/ATP production). This model emphasizes biochemical and de-emphasizes biomechanical aspects of metabolic economy. Comparison of muscle metabolic economy between AA and EA subjects using this model might give insight into whether ethnic differences in muscle biochemistry are likely to contribute to differences in economy during locomotion.
When walking uphill the amount of positive work relative to negative work performed on the center of mass increases, potentially decreasing the elastic stretch and potential reuse of energy (Sawicki and Ferris 2009). If, as our previous work suggests (McCarthy et al. 2006), tendon length is the main factor mediating differences in walking economy between AAs and EAs the difference in walking economy between AAs and EAs should be reduced when walking up a grade.
The purposes of this study were to determine whether differences in grade walking economy exist between AA and EA premenopausal women and determine whether muscle metabolic economy is different between AA and EA women, potentially explaining ethnic differences in walking economy. The results from this study may have value in understanding factors that contribute to variability in economy during locomotion.
Methods
Forty-eight AA and 48 EA normal-weight, premenopausal women participated in this study (data available on a subset of 19 EA and 13 AA women for the Achilles tendon and muscle metabolic economy measures). The subjects were selected from a larger study designed to measure metabolic factors that may predispose women to obesity. The study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board. All volunteers were screened and briefed about the experimental protocol, and written informed consent was obtained before testing. Subjects were weight stable for 1 month prior to any tests and a macronutrient-controlled diet (16% protein, 20% fat, and 62% carbohydrate) was provided by the General Clinical Research Center for the 2 weeks preceding testing. Diet was controlled because previous work by Larson et al. (1994) determined that the macronutrient content of the diet can influence the bioenergetic state of skeletal muscle during exercise. Testing was performed in the follicular phase of the menstrual cycle (within 10 days of the start of menses). Net oxygen uptakes for the 4.8 km per hour walk on the flat for 33 of these subjects have previously been reported (McCarthy et al. 2006). The net oxygen uptakes at 4.8 km per hour on the flat are included in this paper only to show that ethnic differences exist between the 48 EA and 48 AA women examined in this study.
Measurement of resting VO2
Subjects spent 23 h in a whole-room respiration calorimeter (3.38 m long, 2.11 m wide, and 2.58 m high). The calorimeter design characteristics and calibration have been previously described (Treuth et al. 1995). Oxygen consumption and carbon dioxide production were continuously measured by a magnetopneumatic differential oxygen analyzer (Magnos 4G) and the NDIR industrial photometer differential carbon dioxide analyzer (Uras 3G, both Hartmann and Braun, Frankfort, Germany). The calorimeter was calibrated before each subject entered the chamber. The zero calibration was carried out simultaneously for both analyzers. The full scale was set for 0–1% for the carbon dioxide analyzer and for 0–2% for the oxygen analyzer.
Each subject entered the calorimeter at 8 a.m. Although metabolic data were collected throughout the 23-h stay, only resting oxygen uptake data are reported here. The subject was awakened at 6:30 a.m. on the second morning in the calorimeter. Resting VO2 was then measured for 30 min prior to the subject leaving the calorimeter at about 7:00 a.m.
Walking economy
Submaximal oxygen uptake (VO2) and heart rate (Polar Beat heart rate monitor, Polar Electra Inc., Woodbury NY) were obtained in the steady state during the third and fourth minutes of walking at 4.84 km h−1 on the flat and at 2.5% grade. Oxygen consumption and carbon dioxide production were measured continuously via open circuit spirometry and analyzed using a Sensor-Medics metabolic cart (Model #2900). Before each test, the gas analyzers were calibrated with certified gases of known standard concentrations. Average VO2 values for the third and fourth minutes were averaged. We have previously shown that AA women have lower resting energy expenditures than matched EA women (Hunter et al. 2000). Therefore, in order to insure that differences in resting VO2 did not influence measures of the oxygen cost of walking, we subtracted resting VO2 from average walking VO2 in the determination of oxygen cost of walking.
MRI and 31P-MRS
1H-magnetic resonance images (MRI) were collected on the right calf muscle using 4.1 T whole body imaging. Subjects were studied on two separate days. A series of resting calf muscle MRIs were collected on the first day to measure cross-sectional area of the gastrocnemius and soleus muscles. Descending from just below the knee, a total of 28 images were measured 0.01 m apart. The images were collected using a torroid coil with the following protocol: repetition time (TR) = 1,000 ms, echo time = 14.5 ms, 256-mm field-of-view, and 5 mm slice thickness with a slice separation of 10 mm. The apex of the fibula was easily identified in all subjects so it was used as a bony landmark reference point. Both heads of the gastrocnemius were confined within the 0.28 m imaged in this study. Therefore, the distance these two muscles extended below the apex of the fibula could be measured. Length of the Achilles tendon was estimated by subtracting respective muscle length from lower leg length. Achilles tendon length was defined as the length of the medial gastrocnemius tendon (shortest muscle of the three muscles attached to the Achilles tendon. All images were analyzed by the same technician who was blinded as to subject identity. Test–retest analysis of Achilles tendon length on five subjects yielded a coefficient of variation of less than 1.1%.
On the second day women performed a 90-s unilateral, isometric plantar flexion exercises at 45% maximal isometric plantar flexion. A 7-m 1H/31P surface coil, fastened to the underbelly of the calf muscle, was used to collect 2-s time-resolved 31P-MRS data during 60 s of rest, 90 s of exercise, and 7.5 min of recovery. The 31P-MRS data were collected using TR = 2,000 ms, four dummy pulses, one average, and a half-passage adiabatic excitation pulse. The adiabatic pulse increases the signal–noise-ratio and ensures uniform excitation of the muscle volume seen by the coil. An example of the 31P MRS data collected with these parameters in our laboratory has previously been published (Larson-Meyer et al. 2000). Peak areas and positions of the phosphate metabolites were found by time domain fitting, using Fitmasters (Phillips Medical Systems, Inc., Shelton, CT) as previously described (Boska 1994) (Newcomer and Boska 1997). The exercise bench and force collection devices in our laboratory are described elsewhere (Larson-Meyer et al. 2000).
31P MRS is commonly used to measure the intracellular concentrations of phosphocreatine (PCr), inorganic phosphate (PI), and ATP. The intracellular pH is also calculated from the chemical shift difference between PCr and Pi. These pieces of information can be used to quantitatively study the energetics of skeletal muscle during exercise and recovery (Boska 1991, 1994; Newcomer and Boska 1997). A detailed description of the methods and model used for calculating ATP production rates from resolved 31P MR spectra is published elsewhere (Newcomer and Boska 1997). Briefly, the rate of depletion of PCr during exercise is used to measure the ATP production rate from the creatine kinase reaction (Boska 1991, 1994; Newcomer and Boska 1997). The rate of ATP production from anaerobic glycolysis is calculated from the time courses of pH, PCr, and Pi by assuming a H+ stoichiometry of the ATP producing reactions and a buffering capacity of muscle (Boska 1991, 1994; Newcomer and Boska 1997). The rate of PCr increase during the initial part of recovery is used to estimate the ATP production rate from oxidative phosphorylation (Boska 1991, 1994; Newcomer and Boska 1997). Furthermore, the time constant of PCr recovery (TCPCr) and the time constant of ADP (TCADP) (Argov et al. 1996) can be used as surrogate markers of ATP production from oxidative phosphorylation. Muscle exercise economy was calculated as average force across the 90 s of force production divided by ATP production rate from the creatine kinase reaction, anaerobic glycolysis, and oxidative phosphorylation. The sum of these three production rates was defined as the total ATP production rate in this study.
Dual energy X-ray absorptiometry
Lean and fat mass were determined by DXA (DPX-L: Lunar Radiation Corp. Madison, WI). The scans were analyzed by using the adult software (version 1.33). Lower leg length was determined from the skeletal X-ray planogram generated by DXA and the Lunar software as previously described (McCarthy et al. 2006).
Statistics
T tests were used to determine differences in all variables between AA and EA women. Pearson product correlations were run between Achilles tendon length and exercise economy for the 3 tasks. Alpha was set at 0.05 and the Statistical Packages for the Social Sciences (SPSS)-windows 8.0 (SPSS INC., Chicago, IL) was used in all analyzes.
Results
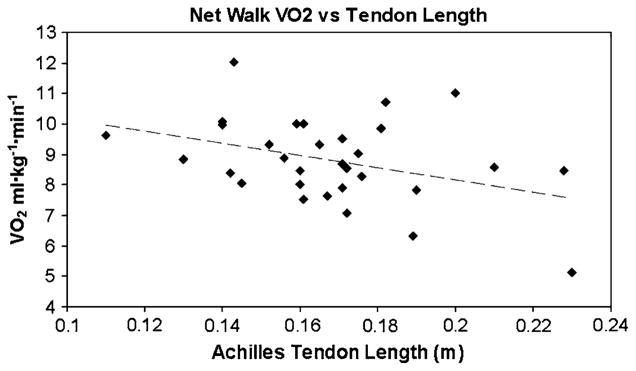
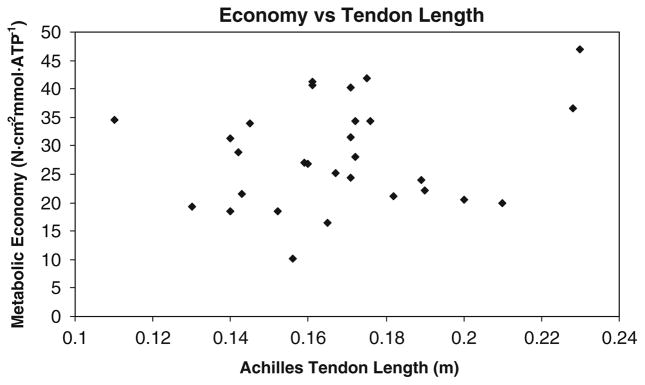
No significant differences in descriptive variables height, weight, age, or percent fat were found between the AA and EA women (Table 1 contains all study contrasts). However, the AA women had a lower VO2max and longer Achilles tendons. Achilles tendon length was significantly related to level walking net VO2 (r = −0.36, P = 0.04, Fig. 1) but not significantly related to grade walking net VO2 (r = 0.06, P = 0.77, Fig. 2) or muscle metabolic economy (r = 0.16, P = 0.37, Fig. 3). AA women had significantly lower level walking VO2 than EA women (11.4 ± 1.6 vs. 12.3 ± 1.5 ml kg−1 min−1, P = 0.01). AA women had a significantly lower level walking net VO2 than the EA women (8.3 ± 1.5 vs. 8.9 ± 1.5 ml kg−1 min−1, P < 0.03). The grade walking VO2 (14.0 ± 1.6 vs. 14.3 ± 1.5 ml kg−1 min−1, P = 0.41) was not significantly different between the AA and EA women nor was the almost identical grade walking net VO2 (11.0 ± 1.4 vs. 11.1 ± 1.4 ml kg−1 min−1, P = 0.84). 31P MRS muscle metabolic economy was also not significantly different between the AA and EA women (26.5 ± 8.0 vs. 26.9 ± 9.8 N cm−2 mmol ATP−1, P = 0.84).
Table 1.
Descriptive and study variables for 48 AA and 48 EA premenopausal women
| Variables | AA | EA | Probability |
|---|---|---|---|
| Height (m) | 1.641 ± 0.080 | 165.0 ± 0.054 | 0.52 |
| Body mass (kg) | 62.6 ± 7.8 | 63.9 ± 5.2 | 0.51 |
| Age (years) | 33.7 ± 5.5 | 34.1 ± 6.2 | 0.72 |
| Max VO2 (ml kg−1 min−1) | 29.3 ± 4.7 | 33.6 ± 5.5 | 0.01 |
| % Fat | 32.1 ± 4.2 | 32.6 ± 4.2 | 0.62 |
| Level Walking VO2 (ml kg−1 min−1) | 11.4 ± 1.6 | 12.3 ± 1.5 | 0.02 |
| Level Walking net VO2 (ml kg−1 min−1) | 8.3 ± 1.5 | 8.9 ± 1.5 | 0.04 |
| Grade Walking VO2 (ml kg−1 min−1) | 14.0 ± 1.6 | 14.3 ± 1.5 | 0.41 |
| Grade Walking net VO2 (ml kg−1 min−1) | 11.0 ± 1.4 | 11.1 ± 1.4 | 0.84 |
| Achilles tendon length (m) | 0.182 ± 0.026 | 0.159 ± 0.022 | 0.01 |
| Muscle metabolic economy (N cm−2 mmol ATP−1) | 26.5 ± 8.0 | 26.9 ± 9.8 | 0.84 |
Fig. 1.
Graph of the relationship between Level Walking net VO2 and Achilles Tendon Length, r = −0.36, P = 0.04
Fig. 2.
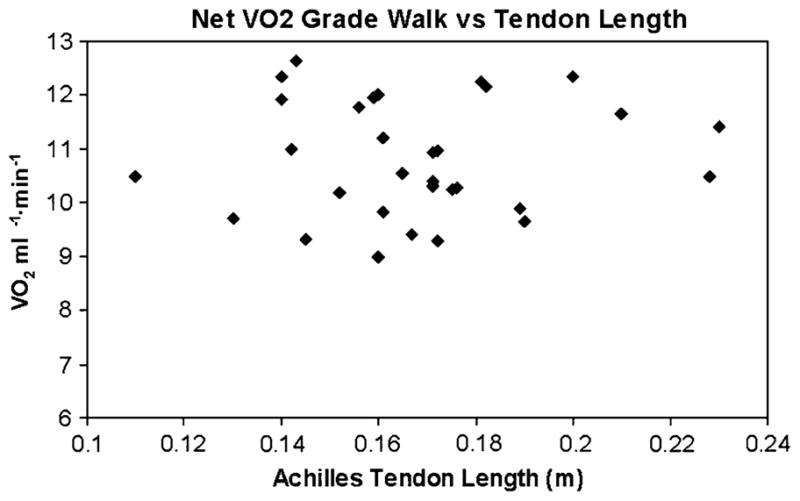
Graph of the relationship between Grade Walking net VO2 and Achilles Tendon Length, r = 0.06, P = 0.77
Fig. 3.
Graph of the relationship between Muscle metabolic economy and Achilles Tendon Length, r = 0.16, P = 0.37
Discussion
Previously we have shown that increased Achilles tendon length is related to reduced net oxygen uptake (increased economy) while walking on the flat (McCarthy et al. 2006). In addition, longer Achilles tendon lengths explained greater walking economy of AA women compared to EA women (McCarthy et al. 2006), suggesting AA women are able to obtain more elastic energy reuse from their longer tendons, walk with less muscle contractile activity and thus use less energy while walking.
These results were only observational so caution must be made in attributing cause and effect between tendon length and reduced walking economy. It can not be ruled out that differences in walking economy between AA and EA women are due to other factors, such as biochemical differences in the muscle. For example, it has previously been shown that Type I muscle fiber type is positively related to muscle metabolic economy, walking economy (Hunter et al. 2001) and cycling economy (Coyle et al. 1992). In addition, individuals of African descent may have a lower percentage of Type I muscle fiber (Ama et al. 1986; Kohn et al. 2007; Tanner et al. 2002). However, if the AA women in this study were to have lower Type I muscle fibers, lower not higher walking economy would be expected. So it is unlikely that differences in muscle fiber type explain the ethnic differences walking economy. In this study we also show that there is no difference in muscle metabolic economy of force production in the plantar flexor muscles of AA and EA women (in fact although there are no significant differences, the EA women show a trend toward more economical muscle force production). This would suggest that biomechanic differences may contribute to the ethnic differences in walking economy.
The kinetics of walking were not evaluated in this study, so only speculation can be made concerning potential biomechanic differences. However, it is consistent with the presented data to speculate that tendon length may be involved in any biomechanic differences. We show that the difference between the two ethnic groups is greatly truncated (with no significant difference observed between the two ethnic groups with 48 EA and 48 AA women) when walking up a 2.5% grade, a condition that has been previously shown to increase the proportion of energy from plantar flexor muscle shortening and reduce the proportion of elastic energy used in walking (Sawicki and Ferris 2009). Finally no significant relationship was observed between grade walking or muscle metabolic economy and tendon length. Taken together these results further support the contention that ethnic differences in Achilles tendon length may be mediating at least some of the increased economy observed in AA women during level walking.
The reduction in the relationship between grade walking economy, rather than flat walking and tendon length would be expected. Sawicki and Ferris (2009) found that the apparent efficiency acquired during their studies using a robotic ankle exoskeleton decreased as grade increased suggesting that plantar flexor muscle shortening provides a larger fraction of total muscle–tendon positive work. In addition, increases in work at the hip and knee but not the ankle during grade walking decreased the proportion of energy expended at the ankle during grade walking (Sawicki and Ferris 2009). Both these factors would proportionately decrease the importance of elastic stretch and reuse of energy by the Achilles tendon during grade walking. Consistent with a proportional decreased energy savings from elastic energy storage during grade locomotion, Roberts et al. (1997) have previously shown that in turkeys, muscle shortening and EMG activity of the lateral gastrocnemius increase while running up an incline as compared to running with no grade. If proportional decrease in elastic energy storage and reuse does occur with grade walking the relationship between walking economy and tendon length should be reduced during grade walking. Further, if tendon length is the main factor mediating differences in walking economy between AAs and EAs the difference in walking economy between AAs and EAs should be reduced when walking up a grade. Our results showing no difference in walking economy between AA and EA women while walking up a 2.5% grade are thus consistent with the hypothesis that a major contributor to differences in walking economy between AA and EA women is increased use of elastic energy storage and reuse with longer Achilles tendons.
Isometric contractions by muscle in the un-stretched position such as used in our 31P muscle metabolic economy model would be expected to obtain little if any stretch shortening cycle elastic energy savings. Therefore, any differences in metabolic economy of force production for these isometric contractions would be thought to be primarily due to biochemical processes within the muscle. The lack of a relationship between muscle metabolic economy and Achilles tendon length for the isometric plantar flexions used in this study would support this supposition. In addition, the lack of a difference between the two ethnic groups also supports the hypothesis that the differences in walking economy between AA and EA women is not due to biochemical or bioenergetic differences between the ethnic groups but is probably biomechanical, with tendon length contributing to these biomechanical differences.
A limitation in this study is that kinetics of walking was not examined. It is possible if not probable that biomechanics of walking were different between the AA and EA women. These potential biomechanical differences may or may not have been influenced by differences in tendon length. However, it is beyond the scope of this study to address those potential differences.
By showing that muscle metabolic economy and grade walking economy are not different but flat walking is different between AA and EA women we support our previous study’s suggestion that longer gastrocnemius tendon length may contribute to greater elastic energy savings in AA women during level walking. Hopefully this work will stimulate further research into the role tendon plays in generating force in human locomotion. It should be apparent that this understanding has important implications for understanding human performance, both during sport and in everyday life.
Acknowledgments
We thank Paul Zuckerman, Betty Darnell, Harry Vaughn, Kathy Landers, Nancy Davis, Dr. David Fields, and Robert Petri for diligent assistance in all aspects of data acquisition. Sources of support: NIH grants R01 DK 49779 and R01 DK51684, DRR General Clinical Research Center grant RR-32, Clinical Nutrition Research Unit grant P30-DK56336, UAB Unversity-wide Clinical Nutrition Research Center grant, and Stouffer’s Lean Cuisine entrees used for control of dietary intake were kindly provided by the Nestle Food Co., Solon, OH.
Footnotes
Communicated by Jean-René Lacour.
Contributor Information
Gary R. Hunter, Email: Ghunter@uab.edu, Departments of Human Studies, University of Alabama at Birmingham, Room 205 Education Building, Birmingham 35294, AL, USA
John P. McCarthy, Departments of Physical Therapy, University of Alabama at Birmingham, Birmingham 35294, AL, USA
Marcas M. Bamman, Departments of Physiology and Biophysics, University of Alabama at Birmingham, B irmingham 35294, AL, USA
D. Enette Larson-Meyer, Family and Consumer Sciences, University of Wyoming, Laramie 82070, WY, USA.
Gordon Fisher, Departments of Nutrition Sciences, University of Alabama at Birmingham, Birmingham 35294, AL, USA.
Bradley R. Newcomer, Critical Care and Diagnostic Care, University of Alabama at Birmingham, Birmingham 35294, AL, USA
References
- Adamantios A, De Monte G, Karamanidis K, Morey-Klapsing G, Stafilidis S, Bruggemann G. Influence of the muscle-tendon unit’s mechanical and morphological properties on running economy. J Exp Biol. 2006;209:3345–3357. doi: 10.1242/jeb.02340. [DOI] [PubMed] [Google Scholar]
- Ama PFM, Simoneau JA, Boulay MR, Serresse O, Theriault G, Bouchard C. Skeletal muscle characteristics in sedentary Black and Caucasian males. J Appl Physiol. 1986;61:1758–1761. doi: 10.1152/jappl.1986.61.5.1758. [DOI] [PubMed] [Google Scholar]
- Argov Z, De Stefano N, Arnold DL. ADP recovery after a brief ischemic exercise in normal and diseased human muscle—a 31P MRS study. NMR Biomed. 1996;9:165–172. doi: 10.1002/(SICI)1099-1492(199606)9:4<165::AID-NBM408>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bamman MM. PhD. University of Florida; 1996. Effects of bed rest and resistance exercise on locomotor muscle. [Google Scholar]
- Biewener AA. IMuscle function in vivo: a comparison of muscles used for elastic energy savings vs. muscles used to generate mechanical power. Am Zool. 1998;38:703–717. [Google Scholar]
- Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic savings: a comparative perspective. Exerc Sports Sci Rev. 2000;28:99–107. [PubMed] [Google Scholar]
- Boska M. Estimating the ATP cost of force production in the human gastrocnemius/soleus muscle group using P MRS and H MRI. NMR Biomed. 1991;4:173–181. doi: 10.1002/nbm.1940040404. [DOI] [PubMed] [Google Scholar]
- Boska M. ATP production rates as a function of force level in the human gastrocnemius/soleus using P MRS. MRM. 1994;32:1–10. doi: 10.1002/mrm.1910320102. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sports Exerc. 1992;24:782–788. [PubMed] [Google Scholar]
- Fukunaga T, Kubo K, Kawakami Y, Kanehisa H, Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc Roy Soc Lond-Ser B Biol Sci. 2001;268:229–233. doi: 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga T, Kawakami Y, Kubo K, Kanehisa H. Muscle and tendon interaction during human movements. Exerc Sports Sci Rev. 2002;30:106–110. doi: 10.1097/00003677-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Weinsier RL, Darnell BE, Zuckerman PA, Goran MI. Racial differences in energy expenditure and aerobic fitness in premenopausal women. Am J Clin Nutrit. 2000;71:500–506. doi: 10.1093/ajcn/71.2.500. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve. 2001;24:654–661. doi: 10.1002/mus.1051. [DOI] [PubMed] [Google Scholar]
- Kohn TA, Essen-Gustavsson B, Myburgh KH. Do skeletal muscle phenotypic characteristics of Xhosa and Caucasian endurance runners differ when matched for training and racing distances. J Appl Physiol. 2007;103:932–940. doi: 10.1152/japplphysiol.01221.2006. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kawakami Y, Fukunaga T. Influence of elastic properties of tendon structures on jump performance in humans. J Appl Physiol. 1999;87:2090–2096. doi: 10.1152/jappl.1999.87.6.2090. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Takeshita D, Kawakami Y, Fukashiro S, Fukunaga T. In vivo dynamics of human medial gastrocnemius muscle-tendon complex during stretch-shortening cycle exercise. Acta Physiol Scand. 2000;170:127–135. doi: 10.1046/j.1365-201x.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Kurokawa S, Fukunaga T, Fukashiro S. Behavior of fascicles and tendinous structures of human gastrocnemius during vertical jumping. J Appl Physiol. 2001;90:1349–1358. doi: 10.1152/jappl.2001.90.4.1349. [DOI] [PubMed] [Google Scholar]
- Larson DE, Hesslink RL, Hrovat MI, Fishman RS, Systrom DM. Dietary effects on exercising muscle metabolism and performance by P-MRS. J Appl Physiol. 1994;77:1108–1115. doi: 10.1152/jappl.1994.77.3.1108. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Hunter GR, Hetherington HP, Weinsier R. 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed. 2000;13:14–17. doi: 10.1002/(sici)1099-1492(200002)13:1<14::aid-nbm605>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- McCarthy JP, Hunter GR, Weinsier RLP, Larson-Meyer DE, Bamman MM, Landers KA, Newcomer BR. Ethnic differences in triceps surae muscle-tendon complex and walking economy. Str Cond Res. 2006;20:511–518. doi: 10.1519/17395.1. [DOI] [PubMed] [Google Scholar]
- Newcomer BR, Boska M. Adenosine triphosphate production rates, metabolic economy calculations, ph, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve. 1997;20:336–346. doi: 10.1002/(SICI)1097-4598(199703)20:3<336::AID-MUS11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Roberts TJ. The integrated function of muscles and tendons during locomotion. Comp Biochem Physiol. 2002;133A:1087–1099. doi: 10.1016/s1095-6433(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyland PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Ryschon TW, Fowler MD, Wysong RE, Anthony AP, Balaban RS. Efficiency of human skeletal muscle in vivo—comparison of isometric, concentric muscle action. J Appl Physiol. 1997;83:867–874. doi: 10.1152/jappl.1997.83.3.867. [DOI] [PubMed] [Google Scholar]
- Sawicki GS, Ferris DP. Mechanics and energetics of incline walking with robotic ankle exoskeletons. J Exp Biol. 2009;212:32–41. doi: 10.1242/jeb.017277. [DOI] [PubMed] [Google Scholar]
- Tanner CJ, Barakat HA, Dohn GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JR. Muscle fiber type is associated with obesity and weight loss. Am J Physiol. 2002;282:E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- Treuth MS, Hunter GR, Weinsier RL, Kell S. Energy expenditure and substrate utilization in older women after strength training: 24 hour metabolic chamber. J Appl Physiol. 1995;78:2140–2146. doi: 10.1152/jappl.1995.78.6.2140. [DOI] [PubMed] [Google Scholar]
- Weston AR, Mbambo Z, Myburgh KH. Running economy of African and Caucasian distance runners. Med Sci Sports Exerc. 2000;32:1130–1134. doi: 10.1097/00005768-200006000-00015. [DOI] [PubMed] [Google Scholar]