Abstract
An alteration of mitochondrial function can result in disruption of redox homeostasis, and is associated with abnormal cancer cell growth. Manganese superoxide dismutase (SOD2) and glutathione peroxidase 4 (Gpx4) are two of the most important antioxidant defense enzymes that protect cells against oxidative stress. We have previously shown that n-3 polyunsaturated fatty acids (PUFA) promote colonocyte apoptosis, a marker of colon cancer risk, in part by enhancing phospholipid oxidation. To elucidate the mechanisms regulating oxidative stress-induced apoptosis in vivo, we fed heterozygous SOD2Het, Gpx4Het and transgenic Gpx4TG mice diets containing either 15% corn oil by weight (CO, enriched in n-6 PUFA) or 3.5% CO + 11.5% fish oil (FO, enriched in n-3 PUFA) for 4 wk. Our data show that (i) genetic pre-deposition to oxidative stress facilitates apoptosis in the mouse colon (Gpx4Het > SOD2Het > Wt > Gpx4Tg), (ii) dietary n-3 PUFA have an additive effect on the induction of apoptosis in Gpx4Het and SOD2Het mice; and (iii) dietary n-3 PUFA reverse the phenotype in oxidatively protected Gpx4Tg mice by elevating apoptosis to a level observed in wild type (control) animals. Complimentary experiments examining colonic mitochondrial bioenergetic profiles indicate that FO fed mice exhibit a significantly (p<0.05) increased respiration-induced proton leak relative to control CO treatment. This finding is consistent with a loss of membrane potential in response to chronic oxidative stress, and supports the contention that n-3 PUFA alter mitochondrial metabolic activity, thereby enhancing apoptosis and reducing colon cancer risk.
Keywords: apoptosis, n-3 PUFA, oxidation, colon, mitochondria
Introduction
Colorectal cancer is the third most common cause of cancer death in men and women with an estimated incidence in the United States of 142,570 and a mortality rate of 51,370 in 2010 (1). Due to the fact that successful treatment modalities for this malignancy are still limited, significant attention is currently being directed to preventive dietary programs which may interfere with the process of colon carcinogenesis at all stages, thereby improving patient survival.
Colonic epithelial cell homeostasis is maintained by the balance between cell proliferation and apoptosis. Since apoptosis is considered to be a useful marker of colorectal cancer risk (2,3), higher levels are consistent with the enhanced removal of damaged cells, which is thought to prevent clonal expansion and reduce colorectal cancer risk in both animal models (2,4) and humans (5,6). We have shown that animals fed fish oil, enriched in n-3 polyunsaturated fatty acids (n-3 PUFA), e.g., docosahexaenoic acid (DHA), 22:6Δ4,7,10,13,16,19 and eicosapentaenoic acid (EPA), 20:5Δ5,8,11,14,17, trigger the induction of apoptosis in the mouse/rat colon (2,4,7,8). The pro-apoptotic effect of n-3 PUFA has also been observed in humans (9,10).
With respect to molecular targets of n-3 PUFA, mitochondria are key organelles capable of regulating the intrinsic apoptotic pathway and mediating cell death in pathological and stress conditions. We have recently demonstrated that n-3 PUFA promote apoptosis in part via the generation of reactive oxygen species (ROS) such as superoxide/hydrogen peroxide, and in particular, membrane phospholipid hydroperoxides (PLOOH), which disrupt the mitochondrial permeability transition pore (mtPTP), enhance mitochondrial Ca2+ accumulation and trigger the release of soluble intermembrane proteins (11-13). The production of ROS/PLOOH in mitochondria is strictly regulated by multiple anti-oxidant systems, i.e., mitochondrial phospholipid hydroperoxide glutathione peroxidase (Gpx4), classical glutathione peroxidase (cGpx), and Mn-dependent superoxide dismutase (SOD2). Among these mitochondrial antioxidant enzymes, Gpx4 and SOD2 are two of the essential enzymatic defense systems against oxidative damage to membrane constituents (14). Specifically, SOD2 transforms toxic superoxide, a byproduct of the mitochondrial electron transport chain, into hydrogen peroxide and diatomic oxygen. Gpx4 catalyzes the reduction of hydrogen peroxide, organic hydroperoxides, and most specifically, lipid-hydroperoxides.
Due to the fact that oxidative stress is a critical regulator capable of promoting apoptosis in cancer cells (15), we hypothesized that the genetic modification of antioxidant enzymes (SOD2, Gpx4) would alter cell death mediated responses in the colon. Since the potential for diet to modulate oxidative stress-induced apoptosis in the colon has not been clearly defined in vivo, in this study, haploinsufficient heterozygous Gpx4Het (16) and SOD2Het (17,18) mice which exhibit increased mitochondrial oxidative stress, along with Gpx4TG mice which exhibit decreased mitochondrial oxidative stress (19), were utilized in order to further elucidate the mechanisms of oxidative stress-mediated apoptosis induced by n-3 PUFA.
Materials and Methods
Animals and diets
SOD2Het (B6.129S7-Sod2tm1Leb/J) mice were originally purchased from Jackson Labs (Bar Harbor, ME) and backcrossed to C57BL/6 mice for 10 generations (17). Gpx4Het and Gpx4TG mice were originally generated by Q. Ran at the University of Texas Health Science Center San Antonio, and backcrossed to C57BL/6 mice for 10 generations (16,19). All procedures followed the guidelines approved by Public Health Service and the Institutional Animal Care and Use Committee at Texas A&M University. Mice were genotyped at 3-4 wk of age for SOD2 and Gpx4 status by PCR. Animals enrolled into the study were maintained for 4 wk on a semi-purified defined diet containing 320 (g/kg diet) sucrose, 200 casein, 220 corn starch, 3 DL-methionine, 35 AIN 76 salt mix, 10 AIN 76 mineral mix, 2 choline chloride, 60 cellulose, 150 corn oil (Dyets, Bethlehem, PA) or 115 vacuum deodorized Menhaden fish oil (Omega Protein, Houston, TX) plus 35 corn oil. The major differences regarding the fatty acid compositions of the two lipid sources were the concentrations of EPA (11%) and DHA (8%) in the fish oil diet and the concentration of linoleic acid (18:2Δ9,12) in the corn oil diet (57%). At the end of feeding period, animals were euthanized and the colon was collected. One cm of the distal colon was removed, fixed in 4% paraformaldehyde for 4 h, followed by a series of ethanol washes, then embedded in paraffin. In complementary studies, mucosa was scraped from the colon and mitochondria were isolated and immediately used for metabolic analyses.
Genotyping
Genomic DNA was extracted from 0.5 cm of mouse tail using a Qiagen DNA tissue kit (Qiagen, Inc., Valencia, CA). PCR was performed using a Platinum Taq polymerase kit (Gibco BRL, Gaithersburg, MD). SOD2 mouse genotyping was described previously (8). Gpx4Tg genotype was confirmed by the 299 bp PCR product of human Gpx4 transgene (primers: 5′-GAACTTCACCAAGGTAAGGGGGCTGTG-3′; 5′CCTTCTCTATCACCTGTCGGGGAGG AA-3′). Gpx4Het genotype was confirmed by amplifying the 880 bp PCR product of hypoxanthine-guanine phosphoribosyltransferase (HPRT) in addition to the 310 bp PCR product (internal standard) (primers: 5′-CTACGGTGAGTAGGTAGA-3 ′, 5 ′-GGCCCTGGTTTC TATGTA-3 ′, 5 ′-GTAGGATATGCCCTTGAC T-3′). All primers were synthesized by the Gene Technologies Laboratory at Texas A&M University (College Station, TX).
Phenotyping by Western blot
Colonic mucosa was homogenized in ice-cold lysis buffer containing 0.1% SDS and subjected to polyacrylamide gel electrophoresis in 4-20% precast mini gels as per the method of Laemmli (20). After electrophoresis, proteins were electroblotted onto a PVDF membrane using a Hoefer Mighty Small Transphor Unit (Pharmacia, Piscataway, NJ) at 400 mA for 1.5 h. Following transfer, the membrane was processed and blocked in 4% nonfat dry milk and 0.1% Tween 20 in PBS at room temperature for 1 h with shaking, followed by incubation with shaking overnight at 4°C with primary antibody (rabbit anti-Gpx4 antibody, generated using a 17-amino-acid peptide corresponding to the C terminus of Gpx4 protein as antigen) diluted in PBS containing 4% milk and 0.1% Tween 20. Membranes were washed with PBS containing 0.1% Tween 20 and incubated with secondary antibody (peroxidase-conjugated goat anti-rabbit IgG, Kirkegaard & Perry, Gaithersburg, MD) as per manufacturer’s instructions. Blots were scanned using a Fluor-S Max MultiImager System (Bio-Rad, Hercules, CA).
Measurement of apoptosis
Apoptotic cells were enumerated in paraformaldehyde-fixed sections from the distal colon using a terminal deoxynucleotidyl transferase (TUNEL) labeling kit (Trevigen, Gaithersburg, MD) as we have previously described (8). The number of apoptotic cells was recorded in at least 100 well-oriented crypts per mouse. Data were calculated as percentage of apoptosis index relative to the control (wild type mice fed the control corn oil diet) in the respective transgenic animal models.
Immunohistochemistry
Immunohistochemical staining for 8-hydroxy-2′-deoxyguanosine (8-OHdG) was performed using the avidin-biotin-peroxidase complex (ABC) method as described previously (21). In brief, the specimens embedded in paraffin were cut at 3 μm thickness, stained with hemotoxylin and eosin or used for immunohistochemistry analysis. Representative areas were chosen and cores of 3 mm diameter were punched out from the blocks with a precision instrument (Tissue Microprocessor; Azumaya, Tokyo, Japan). Cores of 24 (6 × 4 array) in a group were embedded in a paraffin block to avoid interspecimen immunostaining condition. Endogenous peroxidase activity was quenched in paraffin embedded tissue sections with 1% H2O2. Antigen was retrieved by pretreatment with citrate buffer (Antigen Unmasking Solution, Vector Laboratories) in a microwave oven at 37°C for 1 h. Non-specific protein-protein interactions were blocked with diluted rabbit serum, the slides were incubated with primary antibody (10 μg/mL for anti-8-OHdG N45.1 antibody), followed by biotinylated rabbit secondary antibody and the ABC complex for 45 min. DAB was used as the chromagen. Slides were washed thoroughly between incubations with PBS. Negative controls were established by replacing the primary antibody with PBS and serum. Positive staining was indicated by the presence of brown-colored precipitate. The colonic epithelial cells in immunostained specimens were evaluated by two registered pathologists (ST and YO) as negative, weak, moderate or intense (0, 1, 2 or 3, respectively). The means of the evaluation of the two pathologists were used for the semi-quantitative analysis.
Mitochondrial bioenergetic analysis
For these studies, mice were euthanized at the end of a 4 week feeding period, colon mucosa removed, and mitochondria immediately isolated using a Mitochondrial Fractionation Kit (Active Motif, Carlsbad, CA). Mitochondrial bioenergetic profiles were immediately measured using a Seahorse XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA) as described by Wu et al (22) with some modifications. Briefly, mitochondria were resuspended in mitochondrial assay buffer (MAS-1 buffer) containing 70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, 0.2% FA-free BSA, and pH to 7.4 using KOH. Mitochondria were aliquoted into Seahorse XF 24-well cell culture plates (10 μg/50 μL/well), centrifuged at 2,000 x g at 4°C for 10 min to facilitate mitochondria attachment to the plates. Four hundred and fifty μL of 1.1 X initiation assay buffer (MAS-1 buffer supplemented with 5.5 mM succinate and 2.2 μM rotenone) was added to each well immediately following centrifugation. Culture plates (containing attached mitochondria) were subsequently incubated in a non-CO2 environment at 37°C for 8 min. Following incubation, XF 24-well culture plates were transferred to the XF24 Extracellular Flux Analyzer. Hydrated cartridges containing mitochondrial mediators, ADP [1 mM], Oligomycin [2 μM], FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone) [4 μM], and antimycin A [4 μM] were layered over the 24-well plates. The compounds were injected at timed intervals into the samples, and the oxygen consumption rates were monitored continuously.
Cardiolipin peroxidation
10-N-nonyl-acridine orange (NAO; Molecular Probes, Eugene, OR) binding to mitochondria was used to estimate cardiolipin (CL) peroxidation as previously described (23), with some modifications. Freshly isolated mitochondria from colon mucosa was resuspended in a mixture (1:9, v/v) of MAS-1 buffer and buffer A (125 mM KCl, 10 mM Hepes, 5 mM MgCl2, and 2 mM K2HPO4, pH 7.4). One hundred and fifty μL of 20 μM NAO was then added to each mitochondrial sample (10 μg/50 μL), gently mixed and incubated for 10 min at 25°C, followed by centrifugation at 20,000 x g for 10 min at 4°C. Free dye in the supernatant was determined by measuring absorbance at 495 nm, and the NAO bound to mitochondria was calculated as the total minus free NAO.
Statistics
The Brown-Forsythe’s test of homogeneity of variance was conducted to ensure that the variances between the treatment groups were equal. A two-way ANOVA was used for analysis, followed by the least-squares means test for multiple comparisons. Data are expressed as means ± SE. Statistical significance was set at p<0.05.
Results
Genotyping and functional status of Gpx4 in transgenic and heterozygous mice
To confirm that Gpx4 targeted deletion resulted in the anticipated reduction in Gpx4 protein expression, lysates from colonic mucosa were probed using antibodies recognizing Gpx4. As shown in Supplemental Figure 1, Gpx4Tg mice expressed higher, while Gpx4Het mice exhibited lower (almost non-detectable) Gpx4 expression in both the colon and liver compared to their respective wild type siblings. The effect of SOD2 haploinsufficiency on colonic mucosa in SOD2Het mice has been previously described (8). There was a significant main effect of diet on animal body weights, with FO mice weighing significantly (P=0.01) less than CO fed mice (5.8 vs 10.4 g, n=20).
Genetic modification of antioxidant enzymes alter the induction of apoptosis in mouse colon
Regardless of dietary treatment, genetic modification alone modulated the levels of colonic apoptosis. As shown in Figure 1, both SOD2Het and Gpx4Het mice exhibited significantly (p<0.05) higher levels of apoptosis relative to control wild type mice. In contrast, Gpx4Tg mice had significantly (p<0.05) lower levels of apoptosis compared to their wild type siblings.
Figure 1. Effect of genetic modification of antioxidant enzymes on the induction of apoptosis in the colon.
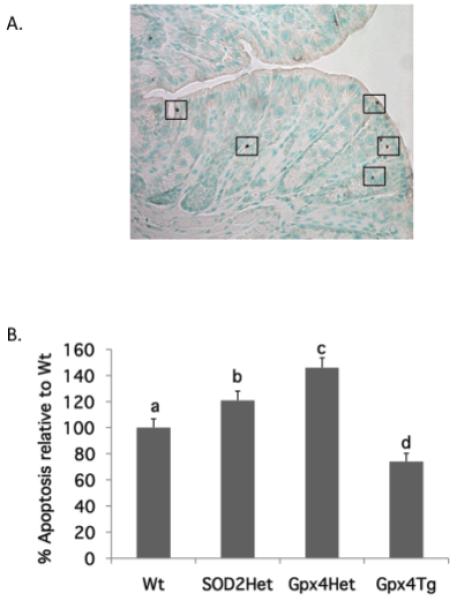
SOD2Het, Gpx4Het, GPx4Tg and respective Wt siblings were fed CO diet (control, enriched in n-6 PUFA) or FO diet (enriched in n-3 PUFA) for 4 weeks. Apoptosis in the distal colon was measured using the TUNEL assay. (A) Representative TUNEL micrograph. DAB stained (brown positive) spots indicate apoptotic cells. (B) Apoptotic index was calculated as the total number of apoptotic cells per 100 crypts. Data are presented as % apoptotic index relative to respective Wt siblings (mean ± SE, all diet groups pooled, n=28-42). Bars not sharing the same letters are significantly different, p<0.05.
The proapoptotic effect of SOD2 or Gpx4 deletion is enhanced by dietary n-3 PUFA
Both allelic ablation of SOD2 (p=0.04) and dietary n-3 PUFA (p=0.021) enhanced apoptosis in the colon (Figure 2A). Specifically, FO-fed mice exhibited a 22% increase in apoptosis relative to CO-fed mice. Similarly, apoptosis was increased by 19% in heterozygous compared with wild type mice. Overall, the effects of genotype and diet were additive, with SOD2Het/FO mice exhibiting the highest level of apoptosis, 46% higher than Wt/CO mice. A similar overall profile with more pronounced effects were observed in Gpx4Het mice, with both allelic ablation of Gpx4 (p=0.0001) and dietary n-3 PUFA (p=0.001) increasing apoptosis in the colon (Figure 2B). Specifically, FO-fed mice exhibited a 29% increase in the number of apoptotic cells relative to CO-fed mice; while apoptosis was increased by 48% in heterozygous compared with wild type mice. The overall effects of genotype and diet were additive, with Gpx4Het/FO mice exhibiting the highest level of apoptosis, 89% higher than Wt/CO mice.
Figure 2. The proapoptotic effect of SOD2 or Gpx4 deletion is enhanced by dietary n-3 PUFA.
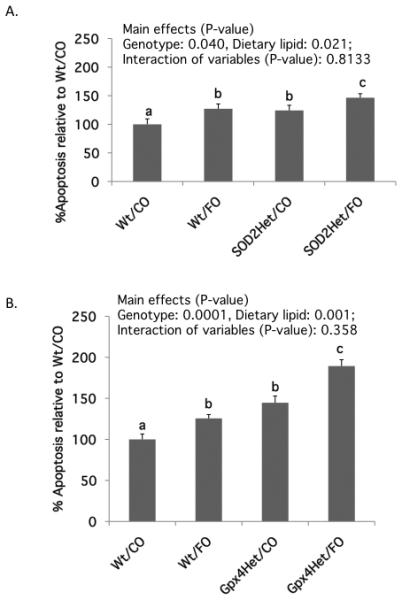
Data are presented as % apoptotic index relative to control Wt mice fed CO diet (mean ± SE, n=14-16). A. SOD2Het mice; B. Gpx4Het mice. Values not sharing the same letter indicate a significant difference (p<0.05). Refer to Figure 1 for legend details.
The anti-apoptotic effect of Gpx4 overexpression is abolished by dietary n-3 PUFA
Overexpression of Gpx4 reduced apoptosis (p=0.0002), while dietary n-3 PUFA enhanced (p=0.002) apoptosis in the colon (Figure 3). Specifically, Wt/FO-fed mice exhibited a 26% increase in apoptosis relative to Wt/CO-fed mice. However, apoptosis was decreased by 25% following Gpx4Tg overexpression compared to wild type mice. Overall, the effects of genotype and diet were antagonistic, with Gpx4Tg/FO mice exhibiting a similar level of apoptosis compared to Wt/CO mice.
Figure 3. Gpx4 over expression suppresses apoptosis in the colon.
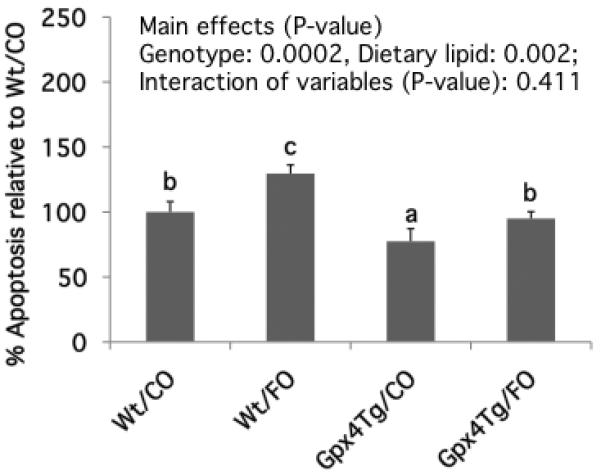
Data are presented as % apoptotic index relative to control Wt mice fed CO diet (mean ± SE, n=19-23). Values not sharing the same letter indicate a significant difference (p<0.05). Refer to Figure 1 for legend details.
Gpx4 overexpression suppresses dietary n-3 PUFA induced oxidative DNA damage
Since persistent oxidative stress can damage DNA (24), we examined the effect of diet and Gpx4 status on colonic 8-hydroxydeoxyguanosine (8-OHdG) levels. Dietary n-3 PUFA increased 8-OHdG DNA adduct levels in Wt/FO mice by 64% compared to the control Wt/CO (n-6 PUFA-enriched) group (Figure 4). However, the promotive effect of n-3 PUFA was diminished in Gpx4Tg mice, with both CO- and FO-fed Gpx4Tg mice exhibiting comparable levels of 8-OHdG.
Figure 4. Gpx4 over expression suppresses oxidative DNA damage induced by dietary n-3 PUFA.
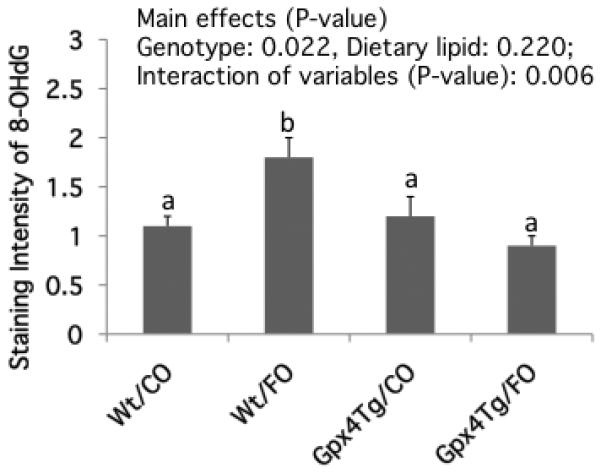
Immunohistochemical staining of 8-OHdG was performed using 4% paraformaldehyde fixed colonic tissue as described in the Materials and Methods. Data are presented as mean staining intensity of 8-OHdG (mean ± SE, n=5 mice per treatment) in Gpx4Tg and Wt mice fed CO or FO diets. Values not sharing the same letter indicate a significant difference (p<0.05).
n-3 PUFA enhances colonocyte mitochondrial proton leak
A representative quantitative measure of colonocyte mitochondrial function using the Seahorse Bioscience Extracellular Flux Analyzer is shown in Supplemental Figure 2. In this analytical system, the proton leak curve is defined as the oxygen consumption rate (OCR) attributed to all processes contributing to ion movement across the mitochondrial inner membrane (25). Hence, the proton leak rate represents proton “escape” into the mitochondrial matrix contributing to the dissipation of mitochondrial membrane potential in state 4 (without ADP). Compared with the CO control (wild type animals only) group, FO treatment significantly (p=0.019) enhanced the proton leak-related OCR by 54% (Figure 5). In contrast, there was no effect of Gpx4 haploinsufficiency or overexpression on the proton leak level (Supplemental Table 1). Additional respiration parameters (State II, State III respiration and respiratory control ratio) related to mitochondria function are shown in Supplemental Table 1.
Figure 5. Mitochondrial respiration associated proton leak is enhanced by dietary n-3 PUFA.
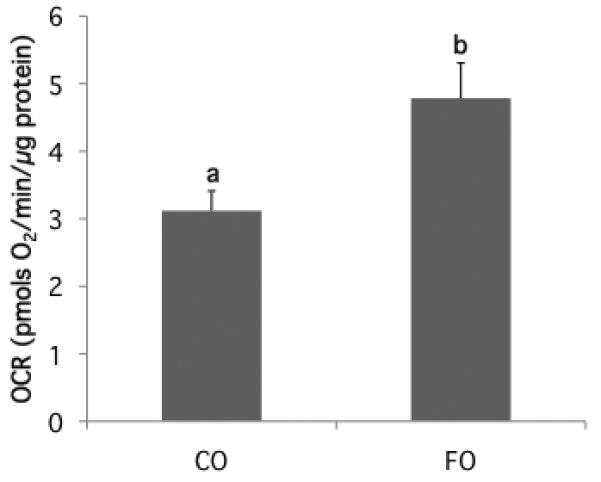
The colonic mitochondrial bioenergetic profile was measured using a Seahorse XF24 Analyzer as described in the Materials and Methods. The proton leak curve is defined as the oxygen consumption rate (OCR) attributed to all processes which allow ion movement across the mitochondrial inner membrane. Proton leak data represent the difference in OCR following oligomycin and antimycin A challenge in mice fed CO or FO diet (mean ± SE, all transgenic and wt groups pooled, n=26-28). Values not sharing the same letter indicate a significant difference (p<0.05).
Gpx4 haploinsufficiency enhances mitochondrial cardiolipin peroxidation
NAO binds to CL with high affinity, and the fluorochrome loses its affinity for peroxidized CL (26). Assuming that the total CL mass in the colon is not altered by diet (27) or genetic modification (23), a reduction in bound NAO indirectly indicates an elevation in CL peroxidation. Figure 6 summarizes the significant (p=0.035) effect of genotype, with Gpx4Het mice exhibiting the lowest level of NAO binding, indicating an increased level of CL peroxidation relative to wild type and Gpx4Tg animals. The separate effects of diet and Gpx4 status are shown in Supplemental Table 2.
Figure 6. Colonic mitochondrial cardiolipin peroxidation is enhanced in Gpx4 haploinsufficient mice.
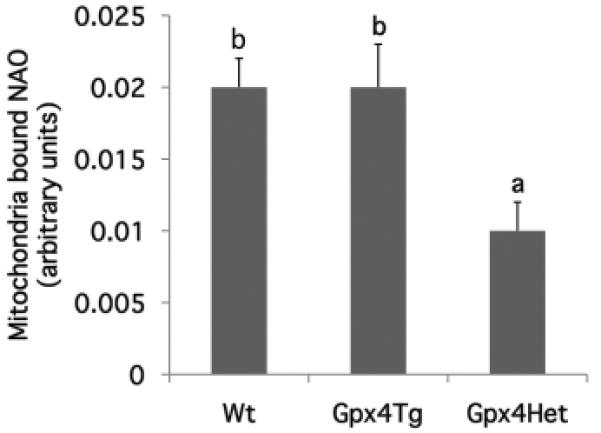
Cardiolipin peroxidation was determined by measuring bound 10-N-nonyl-acridine orange (NAO) in freshly isolated colonic mitochondria from Gpx4Tg, Gpx4Het and Wt mice as described in the Materials and Methods (mean ± SE, all diet groups pooled, n=8-16). Values not sharing the same letter indicate a significant difference (p<0.05).
Discussion
Oxidative stress and gene-environment interactions play a significant role in the development of colon cancer (28). Oxidative stress results from an imbalance in the production of ROS and the cell’s antioxidant defenses. Although oxidative stress has been traditionally considered as a toxic by-product of cell metabolism, cooperating with inflammatory/oncogenic signaling in cellular transformation (29), under certain circumstances it can also trigger apoptosis and play an important inhibitory role in tumor initiation (30,31). From a mechanistic perspective, oxidative stress regulates a broad array of signal transduction pathways that link the endoplasmic reticulum, mitochondrial function and apoptosis (32,33).
Colorectal cancer continues to pose a serious health problem in the U.S. In view of safety concerns surrounding the use of pharmaceutical agents such as non-steroidal anti-inflammatory drugs and tamoxifen as cancer chemopreventive agents, potentially innocuous dietary chemopreventive agents are now considered attractive alternatives (34). In this respect, there is substantial experimental, epidemiological and clinical evidence indicating that fish oil-containing diets rich in n-3 polyunsaturated fatty acids (PUFA) are protective against colon tumorigenesis (2,9,10,35-38). In a major recent finding, it was demonstrated that EPA reduced rectal polyp number and size in patients with familial adenomatous polyposis (FAP) (39). Most impressive was the fact that fish oil derived n-3 PUFA suppressed FAP to a degree similar to the selective COX-2 inhibitor celecoxib. Collectively, these data indicate that n-3 PUFA hold promise as a chemoprevention agent for FAP and sporadic colon cancer. From a mechanistic standpoint, we have shown that n-3 PUFA promote colonic mitochondrial oxidative stress and apoptosis (a critical marker of colorectal cancer risk) in both animal and cell culture models (4,8,12,13,40). In order to further elucidate the mechanisms regulating oxidative stress-induced apoptosis in vivo, we fed SOD2Het, Gpx4Het, Gpx4Tg and their respective wild type siblings, diets containing either n-3 or n-6 PUFA (control)-enriched diets. Overall, the data support our hypothesis linking the genetic pre-deposition to oxidative stress (e.g., SOD2 and Gpx4 haploinsufficiency) to the enhancement of apoptosis in the colon. Consistent with this outcome, the genetic enhancement of a critical mitochondrial antioxidant defense system (e.g., Gpx4 over-expression), decreased apoptosis. It is noteworthy that the level of apoptosis was higher in Gpx4Het relative to SOD2Het mice, suggesting a primary role of GPx4 in mediating oxidative stress-induced apoptosis in the colon. Interestingly, among the mitochondrial antioxidant enzymes, Gpx4 is unique because it directly reduces peroxidized phospholipids in membranes (41,42). Therefore, Gpx4 is considered to be the primary enzymatic defense system against oxidative damage to cellular membranes (43).
Since EPA and DHA are oxidatively susceptible lipids due to their high degree of unsaturation (44), we anticipated that FO feeding would magnify the proapoptotic phenotype observed in SOD2+/− and Gpx4+/− mice. As predicted, an additive (diet + genetic deletion) proapoptotic effect was observed. The combinatorial effect of dietary n-3 PUFA and genetic pro-oxidative modification (SOD2 or Gpx4 haploinsufficiency) on the induction of colonic apoptosis, and the antagonist effect of dietary n-3 PUFA and genetic anti-oxidant modification (n-3 PUFA plus Gpx4 over expression), further indicate the important role of oxidative stress in modulating colonic apoptosis.
As aforementioned, when the cellular level of oxidative species exceeds the capacity of the antioxidant defense system(s), this scenario can generate oxidative stress, ultimately translating into DNA damage (24). Since 8-hydroxy-2′-deoxyguanosine (8-OHdG) is the most abundant oxidative DNA adduct, we determined the combined effects of dietary n-3 PUFA and anti-oxidant modification (Gpx4 over expression) on the level of oxidative DNA damage within the colonic crypt. As expected, Gpx4Tg mice endowed with an enhanced mitochondrial specific antioxidant capability (19), exhibited a reduction (p<0.05) in the levels of DNA damage compared to wild type siblings. In contrast, the inclusion of FO in the diet enhanced 8-OHdG levels in wild type mice. Interestingly, we have previously reported that FO feeding lowers intestinal 8-OHdG following the initiation of an acute wounding/inflammatory episode using dextran sodium sulfate (4,45). This apparent discrepancy may be explained by the level of oxidative stress generated in the target tissue, i.e., acute “pathological” inflammatory agent versus chronic “physiological” dietary effect. Since oxidative stress cannot be defined in universal terms, we propose in future studies to meticulously document the colonocyte intracellular environment following diet manipulation. This will help clarify the role of products of oxidative stress in terms of essential signals for the execution of the apoptotic program. Our overall goal is to delineate the impact of the source and severity of oxidative stress on colonocyte biology.
It has been reported that oxidative stress in mitochondria triggers an increase in proton leak rates resulting in the depletion of membrane potential (18,46). This is noteworthy, because mitochondrial proton leak can contribute directly to the induction of apoptosis (47,48). Hence, we were interested in examining colonocyte mitochondrial bioenergetic profiles in Gpx4Het, Gpx4Tg and wild type sibling mice. Interestingly, although there was no effect of Gpx4 haploinsufficiency or overexpression per se on proton leak levels (Supplemental Table 1), FO feeding significantly (P<0.05) increased proton leak across the inner mitochondrial membrane in the colon (Figure 5). The higher level of proton leak in all FO groups is consistent with the loss of membrane potential, which corroborates previous data indicating that colonocytes from FO-fed animals exhibit a decreased mitochondrial membrane potential, resulting in higher caspase 3 activity and the induction of apoptosis (49).
A critical function of Gpx4 is to repair oxidative damage in biological membranes, which are enriched in phospholipids. We have previously reported that n-3 PUFA promote colonocyte apoptosis in part via the generation of membrane phospholipid hydroperoxides (11). When the level of ROS/PLOOH exceeds the detoxification capacity of the mitochondria, the resulting chronic oxidative stress, e.g., oxidized cardiolipin, can directly trigger the release of proapoptotic factors from mitochondria into the cytosol (50). Since there is strong evidence that incorporation of EPA and DHA into mitochondrial membranes increases susceptibility to oxidative stress (11,27), we used the NAO binding method to indirectly measure the level of cardiolipin peroxidation in Gpx4 genetically modified mice fed CO or FO diets. To our surprise, no dietary effect was observed in any of the mouse models (Wt, GPx4Tg and Gpx4Het) (Supplemental Table 2), which suggests that the effect of n-3 PUFA on phospholipid peroxidation does not directly target intestinal cardiolipin. However, we did observe elevated cardiolipin peroxidation (lower NAO bound) in Gpx4Het mice, which is consistent with the antioxidant function of Gpx4 on membrane phospholipids. These data support our observation that genetic predeposition to oxidative stress promotes colonocyte apoptosis in a cardiolipin-dependent manner.
In summary, we have addressed for the first time the in vivo apoptogenic effect of dietary fat composition in oxidatively stressed animals. We demonstrate that oxidative stress promotes apoptosis in the colon. In addition, fish oil derived n-3 PUFA promote an oxidation-reduction imbalance in the intestine, increasing proton leak across the mitochondrial inner membrane, contributing to a permissive environment for apoptosis. We conclude that both genetic and dietary-induced oxidative stress enhance apoptosis in the mouse colon via complementary, overlapping mechanisms. Given the critical nature of apoptosis in colon cancer prevention, and the fact that inhibition of apoptosis is an integral component in the genesis of colon cancer, it is imperative to elucidate the precise mechanisms by which n-3 PUFA promote apoptosis in the colon.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants CA59034, CA129444, P30ES09106, and USDA 2008-34402-19195, “Designing Foods for Health” through the Vegetable & Fruit Improvement Center.
References
- 1.American Cancer Society 2010 http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/most-requested-tables-figures-2010.
- 2.Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–30. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 3.Koornstra JJ, de Jong S, Hollema H, de Vries EG, Kleibeuker JH. Changes in apoptosis during the development of colorectal cancer: a systematic review of the literature. Crit Rev Oncol Hematol. 2003;45:37–53. doi: 10.1016/s1040-8428(01)00228-1. [DOI] [PubMed] [Google Scholar]
- 4.Hong MY, Bancroft LK, Turner ND, et al. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutr Cancer. 2005;52:166–75. doi: 10.1207/s15327914nc5202_7. [DOI] [PubMed] [Google Scholar]
- 5.Bedi A, Pasricha PJ, Alchtar AJ, Barber JP, Bedi GC, Giairdiello FM, Zehnbauer BA, Hamiltion SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–816. [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Davidson LA, Nguyen DV, Hokanson RM, et al. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan YY, Zhan Y, Aukema HM, et al. Proapoptotic effects of dietary (n-3) fatty acids are enhanced in colonocytes of manganese-dependent superoxide dismutase knockout mice. J Nutr. 2009;139:1328–32. doi: 10.3945/jn.109.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J, Ogawa K, Kuriki K, Yokoyama Y, Kamiya T, Seno K, et al. Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Lett. 2003;193:17–24. doi: 10.1016/s0304383502007176. [DOI] [PubMed] [Google Scholar]
- 10.Courtney ED, Matthews S, Finlayson C, et al. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22:765–76. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 11.Ng Y, Barhoumi R, Tjalkens RB, et al. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–21. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolar SS, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–8. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- 13.Kolar SN, Barhoumi R, Callaway ES, Fan YY, Wang N, Lupton JR, et al. Synergy between docosahexaenoic acid and butyrate elicits p53-dependent apoptosis via mitochondrial Ca2+ accumulation in colonocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G935–43. doi: 10.1152/ajpgi.00312.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stowe DF, Camara AKS. Mitochondrial reactive oxygen species production in excitable cells: Modulators of mitochondrial and cell function. Antioxidants Redox Sig. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cvorovic J, Tramer F, Granzotto M, Candussio L, Decorti G, Passamonti S. Oxidative stress-based cytotoxicity of delphinidin and cyanidin in colon cancer cells. Arch Biochem Biophys. 2010;501:151–7. doi: 10.1016/j.abb.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Yant LJ, Ran Q, Rao L, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 17.Lebovitz RM, Zhang H, Vogel H, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci. 1996;93:9782–7. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci. 2001;98:2278–83. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ran Q, Liang H, Gu M, et al. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004;279:55137–46. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–74. [PubMed] [Google Scholar]
- 22.Wu M, Neilson A, Swift AL, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–36. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 23.Liang H, Ran Q, Jang YC, et al. Glutathione peroxidase 4 differentially regulates the release of apoptogenic proteins from mitochondria. Free Radic Bio Med. 2009;47:312–20. doi: 10.1016/j.freeradbiomed.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helbock HJ, Beckman KB, Ames BN. 8-hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of DNA damage. Meth Enzymol. 1999;300:156–66. doi: 10.1016/s0076-6879(99)00123-8. [DOI] [PubMed] [Google Scholar]
- 25.Diers AR, Higdon AN, Ricart KC, et al. Mitochondrial targeting of the electrophilic lipid 15-deoxy-Delta12,14-prostaglandin J2 increases apoptotic efficacy via redox cell signalling mechanisms. Biochem J. 2010;426:31–41. doi: 10.1042/BJ20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkinezos IG, Bacman SR, Hemandez D, et al. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25:164–72. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapkin RS, Hong MY, Fan YY, et al. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–9. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 28.Acharya A, Das I, Chandhok D, Saha T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartsch H, Nair J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutation Res. 2005;591:34–44. doi: 10.1016/j.mrfmmm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Engel RH, Evens AM. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci. 2006;11:300–12. doi: 10.2741/1798. [DOI] [PubMed] [Google Scholar]
- 31.Sarotra P, Sharma G, Kansal S, et al. Chemopreventive effect of different ratios of fish oil and corn oil in experimental colon carcinogenesis. Lipids. 2010;45:785–98. doi: 10.1007/s11745-010-3459-3. [DOI] [PubMed] [Google Scholar]
- 32.Storz P. Mitochondriall ROS-radical detoxification, mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Simmen T, Lynes EM, Gesson K, Thomas G. Oxidative protein folding in the endoplasmic reticulum: Tight links to the mitochondria-associated membrane (MAM) Biochim. Biophys. Acta. 2010;1798:1465–1473. doi: 10.1016/j.bbamem.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott EN, Gescher AJ, Steward WP, Brown K. Development of dietary phytochemical chemopreventive agents: Biomarkers and choice of dose for early clinical trials. Cancer Prev Res. 2009;2:525–30. doi: 10.1158/1940-6207.CAPR-08-0223. [DOI] [PubMed] [Google Scholar]
- 35.Anti M, Armelao F, Marra G, Percesepe A, Bartoli GM, Palozza P, et al. Effects of Different doses of fish Oil on Rectal Cell Proliferation in Patients with Sporadic Colonic Adenomas. Gastroenterology. 1994;107:1709–18. doi: 10.1016/0016-5085(94)90811-7. [DOI] [PubMed] [Google Scholar]
- 36.Bartram HP, Gostner A, Scheppach W, Reddy BS, Rao CV, Dusel G, et al. Effects of Fish Oil on Rectal Cell Proliferation, Mucosal Fatty Acids, and Prostaglandin E2 Release in Healthy Subjects. Gastroenterology. 1993;105:1317–22. doi: 10.1016/0016-5085(93)90135-y. [DOI] [PubMed] [Google Scholar]
- 37.Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17:1136–43. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pot GK, Majsak-Newman G, Geelen A, et al. Fish consumption and markers of colorectal cancer risk: a multicenter randomized controlled trial. Am J Clin Nutr. 2009;90:354–61. doi: 10.3945/ajcn.2009.27630. [DOI] [PubMed] [Google Scholar]
- 39.West NJ, Clark SK, Phillips RK, Hutchinson JM, Leicester RJ, Belluzzi A, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–25. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 40.Sanders LM, Henderson CE, Hong MY, et al. Enhancement of reactive oxygen species by dietary fish oil and attenuation of antioxidant defenses by dietary pectin coordinately heightens apoptosis in rat. J Nutr. 2004;134:3233–8. doi: 10.1093/jn/134.12.3233. [DOI] [PubMed] [Google Scholar]
- 41.Weitzel F, Ursini F, Wendel A. Phospholipid hydroperoxide glutathione peroxidase in various mouse organs during selenium deficiency and repletion. Biochim Biophys Acta. 1990;1036:88–94. doi: 10.1016/0304-4165(90)90018-r. [DOI] [PubMed] [Google Scholar]
- 42.Arai M, Imai H, Koumura T, Yoshida M, Emoto K, Umeda M, et al. Mitochondrial phospholipid hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J Biol Chem. 1999;274:4924–33. doi: 10.1074/jbc.274.8.4924. [DOI] [PubMed] [Google Scholar]
- 43.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–83. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 44.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Rad Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 45.Bancroft LK, Lupton JR, Davidson LA, Taddeo SS, Murphy ME, Carroll RJ, Chapkin RS. Dietary fish oil reduces oxidative DNA damage in rat colonocytes. Free Rad Biol Med. 2003;35:149–159. doi: 10.1016/s0891-5849(03)00240-5. [DOI] [PubMed] [Google Scholar]
- 46.Puche JE, Garcia-Fernandez M, Muntane J, Roija J, Gonzalez-Baron S, Cortazar IC. Low doses of insulin-like growth factor-I induce mitochondrial protection in aging rats. Endocrinol. 2008;149:2620–7. doi: 10.1210/en.2007-1563. [DOI] [PubMed] [Google Scholar]
- 47.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–22. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 48.Ran Q, Liang H, Ikeno Y, et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci. 2007;62:932–42. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 49.Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, et al. Fish oil feeding increases the unsaturation index in mitochondrial phospholipids, enhancing reactive oxygen species generation and initiating apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–25. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- 50.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chem Biol. 2005;1:223–32. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


