Abstract
3,4-Methylenedioxymethamphetamine (MDMA)’s O-demethylenated metabolite, 3,4-dihydroxymethamphetamine (HHMA), has been hypothesized to serve as a precursor for the formation of toxic catechol thioether metabolites (e.g. 5-N-acetylcystein-S-yl-HHMA) that mediate 3,4-methylenedioxymethamphetamine (MDMA) neurotoxicity. To further test this hypothesis, HHMA formation was blocked with dextromethorphan (DXM), which competitively inhibits cytochrome P450 enzyme-mediated O-demethylenation of MDMA to HHMA. In particular, rats were randomly assigned to one of four treatment groups (N= 9–12 per group): 1) Saline/MDMA; 2) DXM/MDMA; 3) DXM/Saline; 4) Saline/Saline. During drug exposure, time-concentration profiles of MDMA and its metabolites were determined, along with body temperature. One week later, brain serotonin (5-HT) neuronal markers were measured in the same animals. DXM did not significantly alter core temperature in MDMA-treated animals. A large (greater that 70%) decrease in HHMA formation had no effect on the magnitude of MDMA neurotoxicity. These results cast doubt on the role of HHMA-derived catechol thioether metabolites in the mechanism of MDMA neurotoxicity.
Keywords: Ecstasy, thioether conjugates, cytochrome P450 enzyme, pharmacokinetics
Introduction
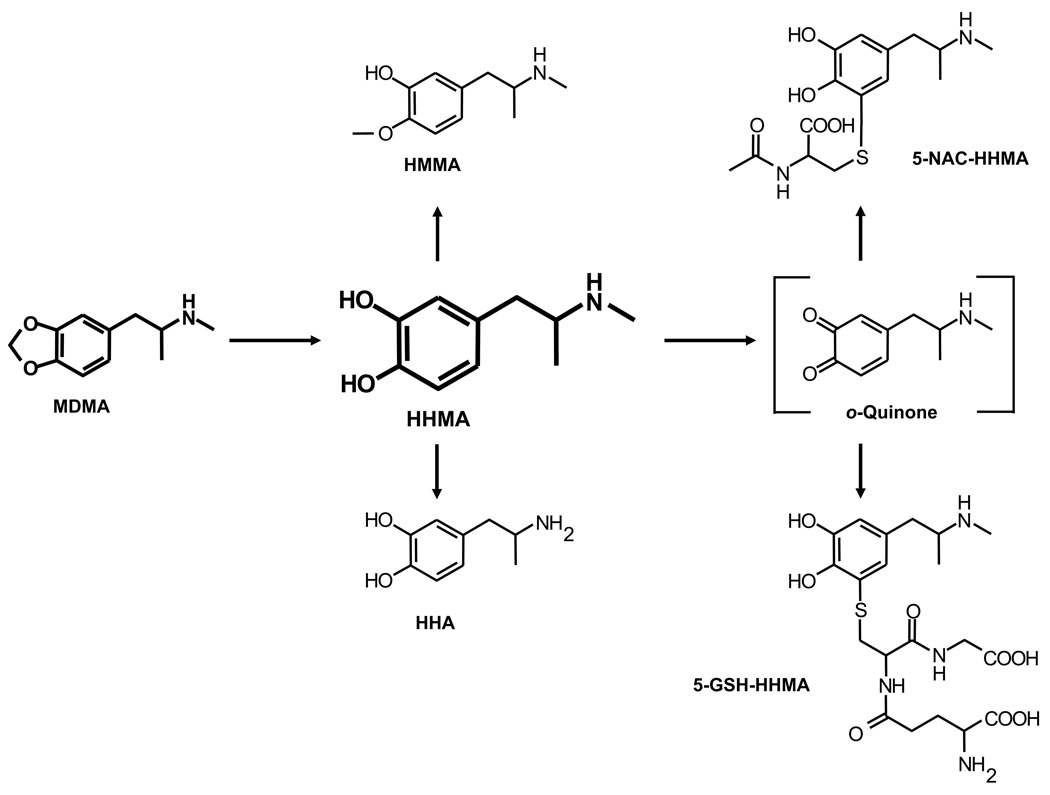
3,4-Methylenedioxymethamphetamine (MDMA, Ecstasy) is a recreational drug of abuse with toxic potential toward brain serotonin (5-HT) neurons (Green et al. 2003; Capela et al. 2009). Despite much research, the mechanism by which MDMA damages brain 5-HT neurons remains unknown. One hypothesis holds that thioether conjugates, derived from O-demethylenated MDMA metabolites, mediate MDMA neurotoxicity (Miller et al. 1997; Bai et al. 1999; Jones et al. 2005). In particular, the MDMA metabolites, 3,4-dihydroxymethamphetamine (HHMA) and 3,4-dihydroxyamphetamine (HHA), have been postulated to serve as precursors for toxic thioether conjugates such as 5-N-acetylcystein-S-yl-HHMA (5-NAC-HHMA) (Fig. 1).
Fig. 1.
Shown is scheme of MDMA metabolism. After O-demethylenation to HHMA, metabolism of MDMA can proceed via different routes, including (but not limited to) N-demethylation to HHA, O-methylation to HMMA, and the formation of thioether conjugates, such as 5-NAC-HHMA and 5-glutathion-S-yl-HHMA (5-GSH-HHMA).
Supporting to the notion that thioether conjugates play a role in MDMA neurotoxicity is the report that intrastriatal administration of 5-NAC-HHMA, a thioether conjugate of HHMA, produces lasting depletions of indoles in the rat brain (Jones et al. 2005). However, recent efforts in our laboratory to confirm these findings were unsuccessful (Mueller et al. 2009b). Even doubling the highest dose of 5-NAC-HHMA used by Jones et al. (2005) failed to produce a significant neurotoxic effect. Moreover, results from two other laboratories have shown that altering MDMA metabolism does not impact MDMA neurotoxicity (Schmidt and Taylor 1988; Gollamudi et al. 1989). Although these reports would seem to argue against the hypothesis that metabolites play a role in MDMA neurotoxicity, a recent study found that decreased MDMA metabolism, accomplished by lowering body temperature, led to decreased toxicity (Goni-Allo et al. 2008).
Given these inconsistent reports, the present study was undertaken to further examine the role of HHMA-derived metabolites in MDMA neurotoxicity. In particular, we reasoned that if HHMA-derived metabolites mediate MDMA neurotoxicity, inhibition of HHMA formation should block or attenuate the neurotoxic action of MDMA. To block HHMA formation, we used dextromethorphan (DXM), which is known to be a potent competitive inhibitor of cytochrome P450 (CYP450)-mediated metabolism of MDMA to HHMA (Broly et al. 1990; Ramamoorthy et al. 2002; Heydari et al. 2004).
Materials and Methods
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) weighing 200 – 224 grams were used. Animals were housed three per cage (except during drug treatment when they were housed singly) in standard polypropylene cages (17 inches × 10 inches × 8 inches). Housing temperatures were maintained at 22 ± 2 °C (except during drug treatment, when the ambient temperature was 25 °C), with free access to food and water. Lighting was regulated on a 12:12 h light:dark cycle.
Drugs and Reagents
Racemic MDMA hydrochloride was obtained through the National Institute on Drug Abuse (Rockville, MD, USA). Racemic HHMA hydrochloride and methanolic solutions (1000 mg/l) of racemic MDMA hydrochloride and racemic 3,4-methylenedioxyamphetamine (MDA) hydrochloride were purchased from Lipomed (Cambridge, MA, USA). Methanolic solutions (1000 mg/l) of racemic HMMA and methanolic solutions (100 mg/l) of racemic MDMA-d5 and MDA-d5 were obtained from Cerilliant (Round Rock, TX, USA). 4-Hydroxymethamphetamine (pholedrine), 4-methylcatechol, ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA), and glucuronidase type HP-2 form helix pomatia (glucuronidase activity ≥ 100.000 units/mL and sulfatase activity < 7.500 units/mL) were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Sodium metabisulfite (SMBS) was obtained from E. Merck (Darmstadt, Germany). Perchloric acid (PCA) was obtained from J.T. Baker (Phillipsburg, NJ, USA). DXM was obtained from MP Biomedicals (Solon, OH, USA). The authenticity of the MDMA, HHMA, HMMA, and MDA samples used in the present studies was confirmed using liquid chromatographic/mass spectrometric (LC/MS) methods to determine the corresponding pseudomolecular ions and at least one fragment ion for each compound. Analysis was performed in full scan mode (mass range from 100 – 1000) to check for presence of possible impurities.
Drug treatment
As mentioned above, inhibition of MDMA metabolism was accomplished using DXM, a known inhibitor of CYP450-mediated O-demethylenation of MDMA to HHMA (Broly et al. 1990; Ramamoorthy et al. 2002; Heydari et al. 2004). Rats were randomly assigned to one of four treatment groups (N= 9–12 per group): 1) Saline/MDMA; 2) DXM/MDMA; 3) DXM/Saline; 4) Saline/Saline. A single dose of 20 mg/kg of MDMA (or vehicle) was given orally (by gavage). DXM was given at a dose of 30 mg/kg, intraperitoneally 1 hour and 0.25 hour before and 3 hours after MDMA treatment.
Temperature measurement
Rectal temperature during drug exposure was measured using a BAT-12 thermometer coupled to a RET-2 rat rectal probe (Physiotemp, Inc., Clifton, NJ) at 1 hour before and 1, 2, 4, 8, 9, and 24 hours after treatment.
Blood sampling and plasma preparation
Blood was sampled at 1, 2, 4, 8, 9, and 24 hours after MDMA administration. At each time point, approximately 0.2 ml of blood was collected by means of retro-orbital bleeding. Blood samples were dispensed into 2 ml BD Vacutainer hematology tubes (Becton-Dickinson, Franklin Lakes, NJ, USA), and stored on ice for up to 30 min, until centrifuged. Plasma was processed and stored as previously described (Mueller et al. 2009b).
Measurement of plasma MDMA and metabolite concentrations
Plasma MDMA, 3,4-methylenedioxyamphetamine (MDA), HHMA, and 4-hydroxy-3-methoxymethamphetamine (HMMA) concentrations were determined as recently described using liquid chromatography coupled with mass spectrometry methods (Mueller et al. 2007). Total amounts (conjugated and free) of HHMA and HMMA were determined. The procedure employed for cleavage of conjugates in rat plasma has been optimized and has been found to be reproducible (Mueller et al. 2009a). In addition, the method has been re-validated for the use of rat plasma (previously assay validation was conducted using squirrel monkeys plasma) with the following results: First, selectivity was shown for all analytes. Recoveries, combining tests for extraction efficiencies and possible matrix effects, ranged from 79.2 –105.5%. Linearity of the method ranged from 10–500ng/mL for MDA and from 25–1000ng/mL for MDMA, HHMA, and HMMA. Data for accuracy, in terms of bias, were all within the acceptance limits, namely ±15% of the nominal values. The criteria for repeatability (within-day precision) and time-different intermediate precision (combined within-day and between-day effects) were ≤15% RSD for all analytes. No instability was observed after repeated freezing or in processed samples.
Calculation of pharmacokinetic parameters
Peak plasma concentrations (Cmax) and areas under the concentration-time curve (AUC) for each analyte were obtained using the pharmacokinetic functions for Microsoft Excel (developed by Usansky et al., http://www.boomer.org/pkin/xcel/pkf/pkf.doc). AUC was calculated using the linear trapezoidal rule starting at time zero and finishing at the last quantifiable point.
Determination of brain 5-HT and 5-HIAA concentrations
One week after drug treatment, animals were sacrificed for regional brain 5-HT axonal markers using methods previously described (Mechan et al. 2006).
Statistics
The significance of differences between means was determined using student’s t-test and one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. Statistical analyses were performed using Prism, Version 3.02 (GraphPad Software, Inc. San Diego, CA, USA). Differences were considered significant if p < 0.05.
Results
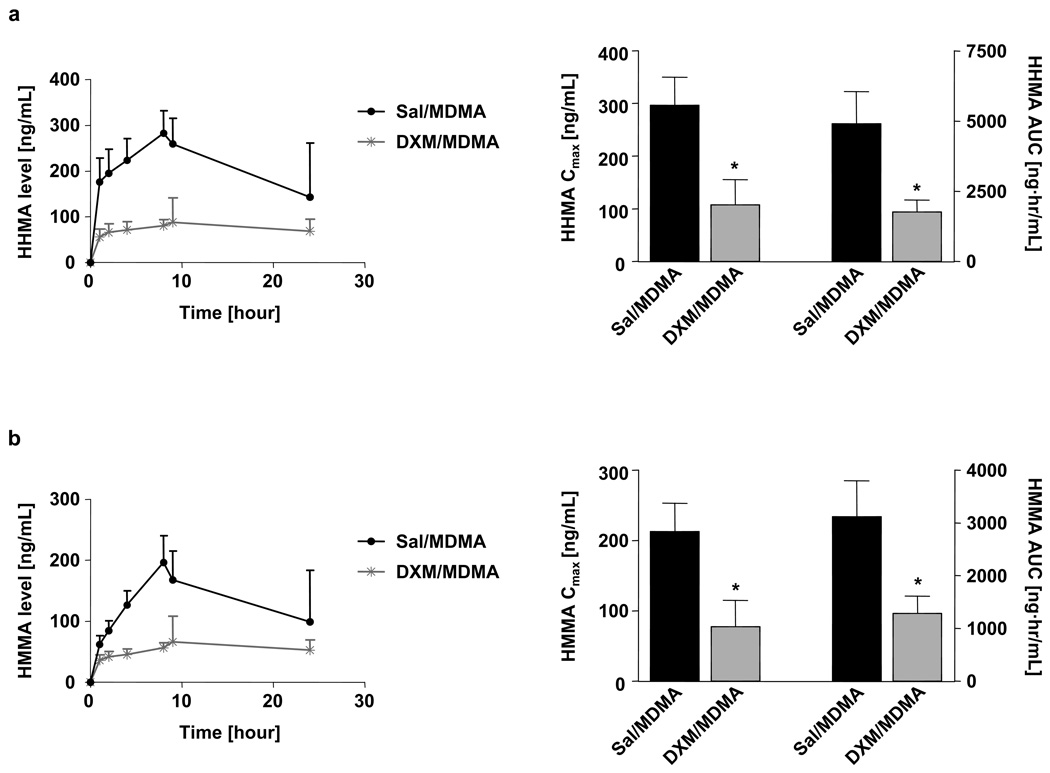
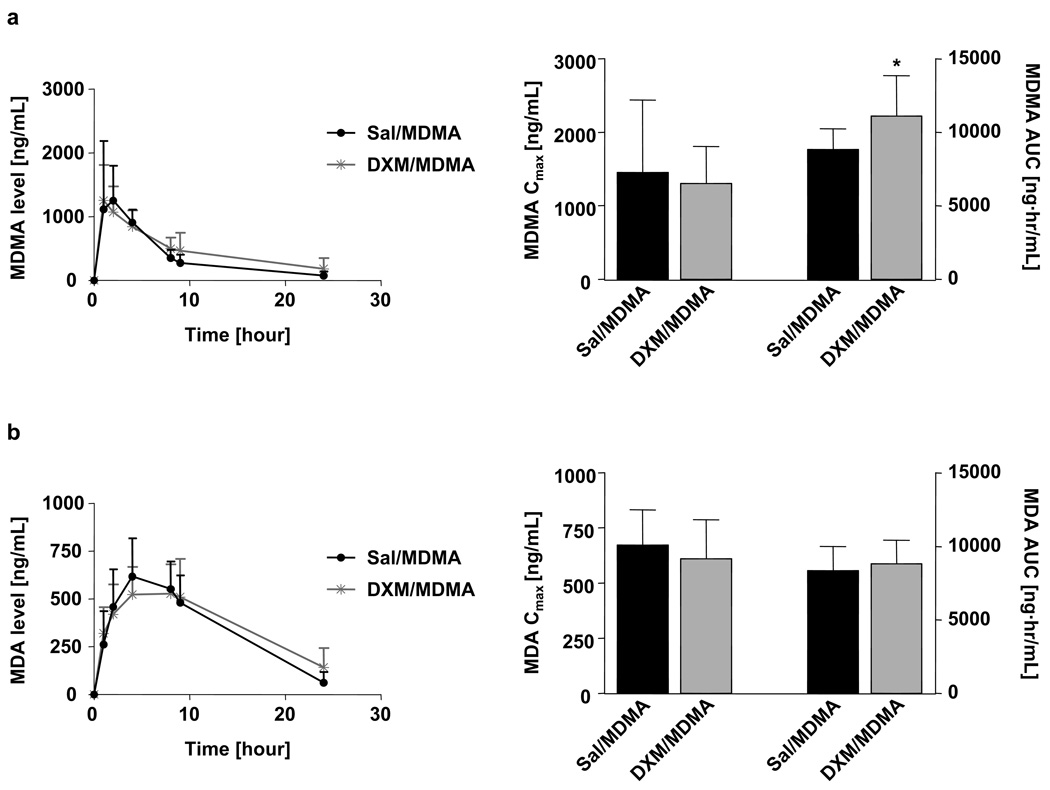
DXM markedly inhibited metabolism of MDMA to HHMA and other downstream products (e.g., HMMA) (Fig. 2). In particular, both the Cmax and AUC of HMMA and HHMA were markedly decreased in animals receiving MDMA and DXM (Fig. 2a and b). DXM produced a modest but significant increase in the AUC of MDMA, but no significant effect on its Cmax (Fig. 3a). DXM had no effect on the pharmacokinetics of MDA (Fig. 3b).
Fig. 2.
Plasma time-concentration profiles of HHMA (a) and HMMA (b) are shown on the left; corresponding pharmacokinetic parameters (Cmax and AUC) are shown on the right. Animals received an oral dose of 20 mg/kg MDMA. One group of rats received DXM (30 mg/kg i.p.) 1 and 0.25 hours before and 3 hours after MDMA; the other group received saline at the same time points. Values represent the mean ±SD (N=9–12 per group). Student’s t-test was performed and significance assumed when p<0.05.
Fig. 3.
Plasma time-concentration profiles of MDMA (a) and MDA (b) and the corresponding pharmacokinetic parameters Cmax and AUC. All animals received an oral dose of 20 mg/kg MDMA. One group of rats received DXM (30 mg/kg i.p.) 1 and 0.25 hours before and 3 hours after MDMA was administered, while the second group of rats received saline as co-treatment. Values represent the mean ±SD (N=9–12 per group). Student’s t-test was performed and significance assumed when p<0.05.
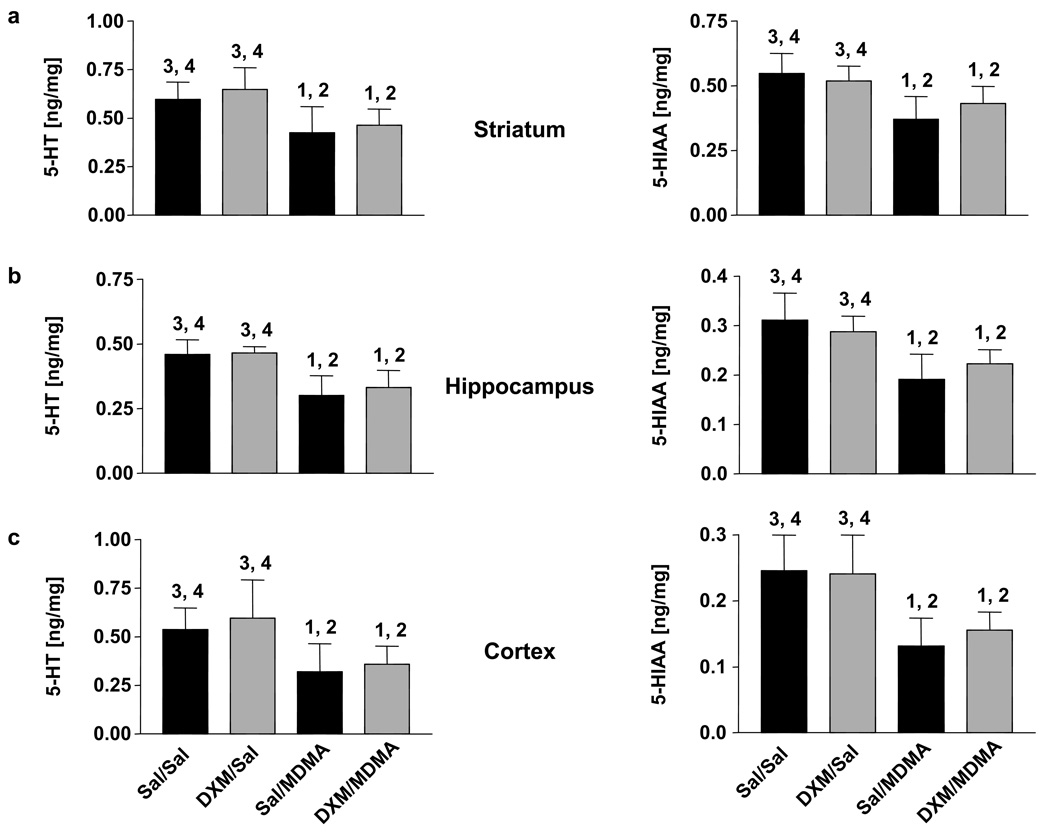
Although DXM markedly inhibited HHMA formation, it did not decrease the lasting effects of MDMA on 5-HT and 5-hydroxyindolacetic acid (5-HIAA) concentrations across brain regions (Fig. 4a–c). When given alone, DXM treatment alone did not alter 5-HT or 5-HIAA levels (Fig. 4a–c).
Fig. 4.
5-HT levels (left panels) and 5-HIAA levels (right panels) in striatum (a), hippocampus (b), and cerebral cortex (c) of rats one week after treatment with MDMA alone or in combination with DXM. MDMA was given orally at a dose of 20 mg/kg. DXM was given i.p. at a dose of 30 mg/kg, three times (1 and 0.25 hours before and 3 hours after MDMA administration). Values represent the mean ±SD (N=9–12 for MDMA groups and N=9 for saline groups). One-way ANOVA followed by Tukey's multiple comparison test was performed and differences regarded as significant when p<0.05. 1 Indicates significant different from saline+saline group; 2 Indicates significant different from DXM+saline group; 3 Indicates significant different from saline+MDMA group; 4 Indicates significant different from DXM+MDMA group.
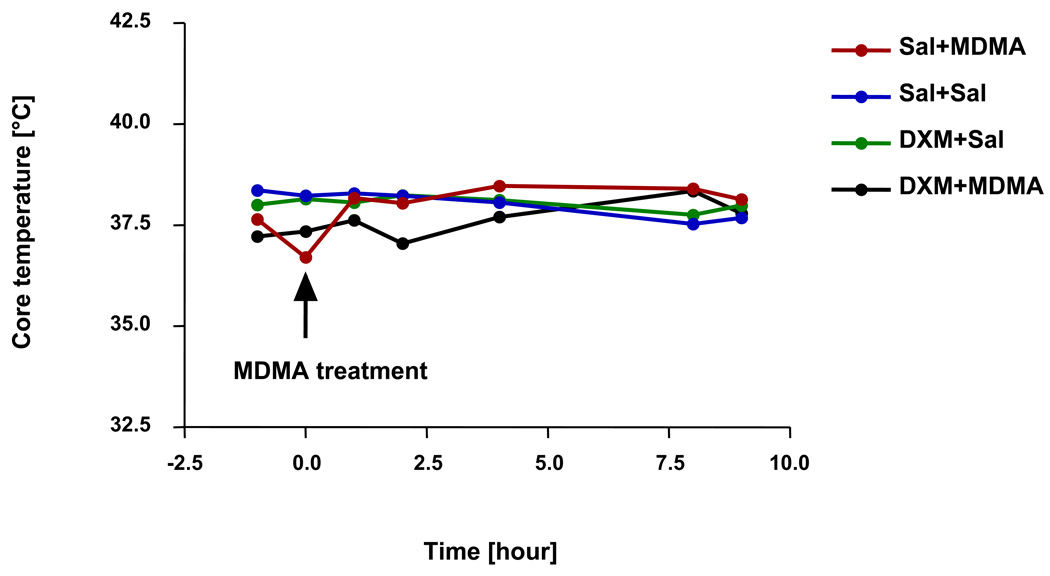
Treatment with MDMA caused an initial drop in body temperature, followed by increase of core temperature (Fig. 5a). The combination of MDMA and DXM caused a longer lasting decrease in temperature, which was followed by an increase in core temperature (Fig. 5). Peak temperatures were lower in the MDMA/DXM group than in the group receiving MDMA/Saline, but the difference did not achieve statistical significance.
Fig. 5.
Body temperature in rats treated with either MDMA (20 mg/kg, p.o.) or saline (0.3 mL p.o.), alone or in combination with either DXM (30 mg/kg i.p.) or saline (0.3 mL i.p.). Values represent the mean (N= 9–12). Temperature differences among treatment groups were compared using one-way ANOVA. None were significant.
Discussion
The major finding of the present study is that inhibition of MDMA metabolism that results in a marked decrease into HHMA formation had no effect on MDMA-induced 5-HT neurotoxicity. In particular, decreased formation of HHMA and other downstream metabolites (e.g. HMMA) did not block or attenuate lasting effects of MDMA on 5-HT neuronal markers (5-HT and 5-HIAA). Bai and colleagues (2001) have postulated that plasma HHMA serves as precursor for thioether conjugates, such as 5-NAC-HHMA, that are formed peripherally, then cross the blood brain barrier and produce 5-HT neurotoxic effects. If this hypothesis is correct, diminished availability of peripheral HHMA should result in decreased peripheral formation of 5-NAC-HHMA and other thioether conjugates and this, in turn would be expected to result in attenuated neurotoxicity. However, despite a greater than 70% reduction in plasma HHMA concentration, no effect on the magnitude of MDMA neurotoxicity was observed. This observation, coupled with our recent finding that direct administration of the thioether conjugate, 5-NAC-HHMA, does not produce lasting effects on brain 5-HT axonal markers (Mueller et al. 2009b), casts doubt on the hypothesis that HHMA-derived catechol-thioether conjugates mediate MDMA neurotoxicity.
Notably, inhibition of MDMA metabolism led to only a modest increase in the AUC of MDMA and no change in its peak concentration (Cmax) (Fig. 3a). At first glance, this finding may seem surprising, because inhibition of metabolic degradation of MDMA might be expected to result in higher MDMA levels. However, the lack of change in the peak concentration of MDMA presently observed is likely related to the fact that that O-demethylenation and N-demethylation operate at comparable rates in rat, and N-demethylation is not inhibited by DXM.
The fact that a lack of change in peak MDMA concentrations was associated with no change in the severity of MDMA neurotoxicity favors the view that the parent compound (MDMA), rather than a HHMA-derived metabolite (e.g., one of the catechol thioether conjugates), is more closely linked to the neurotoxic process (Mueller et al. 2009b). However, data from previous studies indicate that, after central administration, MDMA does not produce toxic effects (Paris and Cunningham 1992; Esteban et al. 2001; Escobedo et al. 2005). While this would seem to argue against the notion that parent compound (MDMA) is directly responsible for neurotoxicity, it is possible that peripheral pharmacological effects of MDMA, effects not reproduced by central MDMA administration (e.g. hyperthermia), are required for the expression of neurotoxicity. Further, precise levels and duration of action of centrally administered MDMA may need to be appropriately adjusted for centrally administered MDMA to produce neurotoxic effects.
Because body temperature can markedly influence MDMA neurotoxicity [higher temperatures cause greater toxicity, lower temperatures afford neuroprotection (Malberg and Seiden 1998)], core temperature was monitored during the period of drug exposure, to guard for possible confounding effects related to DXM effects on body temperature. As shown in Fig. 5, DXM had no significant effect on body temperature, either alone or in combination with MDMA. If anything, DXM slightly lowered body temperature in MDMA-treated animals. If one assumes that this small hypothermic effect was functionally (even if not statistically) significant, the expectation would be decreased neurotoxicity, and this would have made it impossible to tell if the reduced toxicity was related to decreased HHMA formation or decreased body temperature. However, as no change in toxicity was observed, it does not appear that the small (statistically insignificant) temperature effect of DXM confounds the present conclusion HHMA-derived conjugates do not play a crucial role in MDMA neurotoxicity.
Potential limitations of this study should be acknowledged. First, not all demethylenated metabolites of MDMA were measured. In particular, HHA, the O-demethylenated metabolite of MDA, was not included in our assay of various MDMA metabolites. Unfortunately, HHA of appropriate purity for quantitative analyses is not commercially available. Also, it is important to note that HHA is formed from MDA via the same O-demethylenation pathway as HHMA, and would therefore be expected to be decreased to a comparable extent as HMMA following DXM treatment. A second limitation of the present study is that levels of the HHMA-derived thioether conjugates were not directly determined, making it difficult to state with certainty that DXM treatment led to decreased thioether conjugate levels. However, given that DXM administration reduced formation of HHMA by 70%, it seems reasonable to infer that marked reductions in HHMA availability translate into decreased levels of 5-NAC-HHMA (and other thioether conjugates). Further, as noted above, direct central administration of 5-NAC-HHMA does not produce neurotoxic effects (Mueller et al. 2009b). One could argue that the formation of thioether conjugates might be saturated at low substrate (HHMA) concentrations, in which case, reduced HHMA levels would not necessarily result in decreased conjugate levels. Nonetheless, if saturation occurred at low HHMA concentrations (and if HHMA conjugates are, in fact, responsible for MDMA neurotoxicity), one would not expect to see a dose-related neurotoxic effect of MDMA (because conjugate formation would be saturated after low MDMA doses, and higher doses of MDMA would not give rise to higher concentrations of toxic HHMA conjugates). Given that MDMA neurotoxic effects are known to be dose-related (with higher doses causing greater neurotoxicity), occurrence of saturation seems unlikely. Third, effects of DXM other than its property as CYP2D6 inhibitor could have potentially confounded the neurotoxic outcome. In particular, DXM is also a 5-HT uptake inhibitor and a N-methyl D-aspartate (NMDA) receptor antagonist (Church et al. 1989; Henderson and Fuller 1992; Gillman 2005; Werling et al. 2007), and these effects could, theoretically, have afforded neuroprotection. The fact that no change in neurotoxicity was observed suggests that none of these confounds was operant. Additionally, if any of these potentially confounding effects had been at play, the result would have been decreased (rather than unchanged) neurotoxicity.
In summary, the present results cast further doubt on the role of the O-demethylenated metabolite HHMA and its thiother adducts in MDMA-induced 5-HT neurotoxicity in rats. Rather, they tend to favor the view that the parent compound is more closely linked to the neurotoxic process. Additional research is needed to determine whether MDMA, in fact, triggers the neurotoxic cascade that results in 5-HT axon loss, and to clarify why central MDMA administration has, thus far, failed to produce neurotoxic effects.
Acknowledgments
This work was supported by NIH grants DA 05707 and DA01796401 (GR).
References
- Bai F, Lau SS, Monks TJ. Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol. 1999;12:1150–1157. doi: 10.1021/tx990084t. [DOI] [PubMed] [Google Scholar]
- Broly F, Libersa C, Lhermitte M, Dupuis B. Inhibitory studies of mexiletine and dextromethorphan oxidation in human liver microsomes. Biochem Pharmacol. 1990;39:1045–1053. doi: 10.1016/0006-2952(90)90283-q. [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Church J, Jones MG, Davies SN, Lodge D. Antitussive agents as N-methylaspartate antagonists: further studies. Can J Physiol Pharmacol. 1989;67:561–567. doi: 10.1139/y89-090. [DOI] [PubMed] [Google Scholar]
- Escobedo I, O'Shea E, Orio L, Sanchez V, Segura M, de la Torre R, Farre M, Green AR, Colado MI. A comparative study on the acute and long-term effects of MDMA and 3,4-dihydroxymethamphetamine (HHMA) on brain monoamine levels after i.p. or striatal administration in mice. Br J Pharmacol. 2005;144:231–241. doi: 10.1038/sj.bjp.0706071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban B, O'Shea E, Camarero J, Sanchez V, Green AR, Colado MI. 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacology (Berl) 2001;154:251–260. doi: 10.1007/s002130000645. [DOI] [PubMed] [Google Scholar]
- Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95:434–441. doi: 10.1093/bja/aei210. [DOI] [PubMed] [Google Scholar]
- Gollamudi R, Ali SF, Lipe G, Newport G, Webb P, Lopez M, Leakey JE, Kolta M, Slikker W., Jr Influence of inducers and inhibitors on the metabolism in vitro and neurochemical effects in vivo of MDMA. Neurotoxicology. 1989;10:455–466. [PubMed] [Google Scholar]
- Goni-Allo B, O Mathuna B, Segura M, Puerta E, Lasheras B, de la Torre R, Aguirre N. The relationship between core body temperature and 3,4-methylenedioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology (Berl) 2008;197:263–278. doi: 10.1007/s00213-007-1027-1. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Henderson MG, Fuller RW. Dextromethorphan antagonizes the acute depletion of brain serotonin by p-chloroamphetamine and H75/12 in rats. Brain Res. 1992;594:323–326. doi: 10.1016/0006-8993(92)91144-4. [DOI] [PubMed] [Google Scholar]
- Heydari A, Yeo KR, Lennard MS, Ellis SW, Tucker GT, Rostami-Hodjegan A. Mechanism-based inactivation of CYP2D6 by methylenedioxymethamphetamine. Drug Metab Dispos. 2004;32:1213–1217. doi: 10.1124/dmd.104.001180. [DOI] [PubMed] [Google Scholar]
- Jones DC, Duvauchelle C, Ikegami A, Olsen CM, Lau SS, de la Torre R, Monks TJ. Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J Pharmacol Exp Ther. 2005;313:422–431. doi: 10.1124/jpet.104.077628. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechan A, Yuan J, Hatzidimitriou G, Irvine RJ, McCann UD, Ricaurte GA. Pharmacokinetic profile of single and repeated oral doses of MDMA in squirrel monkeys: relationship to lasting effects on brain serotonin neurons. Neuropsychopharmacology. 2006;31:339–350. doi: 10.1038/sj.npp.1300808. [DOI] [PubMed] [Google Scholar]
- Miller RT, Lau SS, Monks TJ. 2,5-Bis-(glutathion-S-yl)-alpha-methyldopamine, a putative metabolite of (+/−)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations. Eur J Pharmacol. 1997;323:173–180. doi: 10.1016/s0014-2999(97)00044-7. [DOI] [PubMed] [Google Scholar]
- Mueller M, Kolbrich-Spargo EA, Peters FT, Huestis MA, Ricaurte GA, Maurer HH. Hydrolysis of 3,4-methylenedioxymethamphetamine (MDMA) metabolite conjugates in human, squirrel monkey, and rat plasma. Anal Bioanal Chem. 2009a doi: 10.1007/s00216-009-2607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Yuan J, Felim A, Neudorffer A, Peters FT, Maurer HH, McCann UD, Largeron M, Ricaurte GA. Further studies on the role of metabolites in (+/−)-3,4-methylenedioxymethamphetamine-induced serotonergic neurotoxicity. Drug Metab Dispos. 2009b;37:2079–2086. doi: 10.1124/dmd.109.028340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Ricaurte GA, Maurer HH. Validated liquid chromatographic-electrospray ionization mass spectrometric assay for simultaneous determination of 3,4-methylenedioxymethamphetamine and its metabolites 3,4-methylenedioxyamphetamine, 3,4-dihydroxymethamphetamine, and 4-hydroxy-3-methoxymethamphetamine in squirrel monkey plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;855:262–270. doi: 10.1016/j.jchromb.2007.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JM, Cunningham KA. Lack of serotonin neurotoxicity after intraraphe microinjection of (+)-3,4-methylenedioxymethamphetamine (MDMA) Brain Res Bull. 1992;28:115–119. doi: 10.1016/0361-9230(92)90237-r. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy Y, Yu AM, Suh N, Haining RL, Tyndale RF, Sellers EM. Reduced (+/−)-3,4-methylenedioxymethamphetamine ("Ecstasy") metabolism with cytochrome P450 2D6 inhibitors and pharmacogenetic variants in vitro. Biochem Pharmacol. 2002;63:2111–2119. doi: 10.1016/s0006-2952(02)01028-6. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Taylor VL. Direct central effects of acute methylenedioxymethamphetamine on serotonergic neurons. Eur J Pharmacol. 1988;156:121–131. doi: 10.1016/0014-2999(88)90154-9. [DOI] [PubMed] [Google Scholar]
- Werling LL, Lauterbach EC, Calef U. Dextromethorphan as a potential neuroprotective agent with unique mechanisms of action. Neurologist. 2007;13:272–293. doi: 10.1097/NRL.0b013e3180f60bd8. [DOI] [PubMed] [Google Scholar]