Abstract
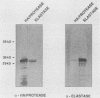
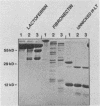
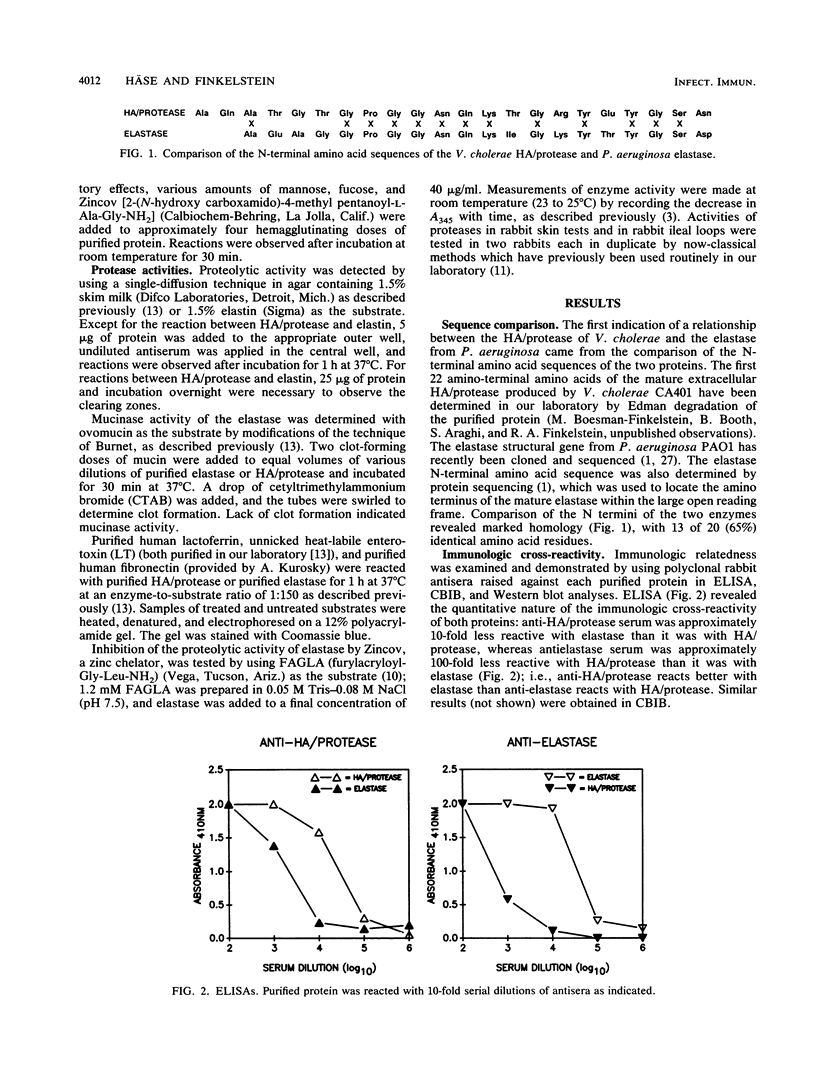
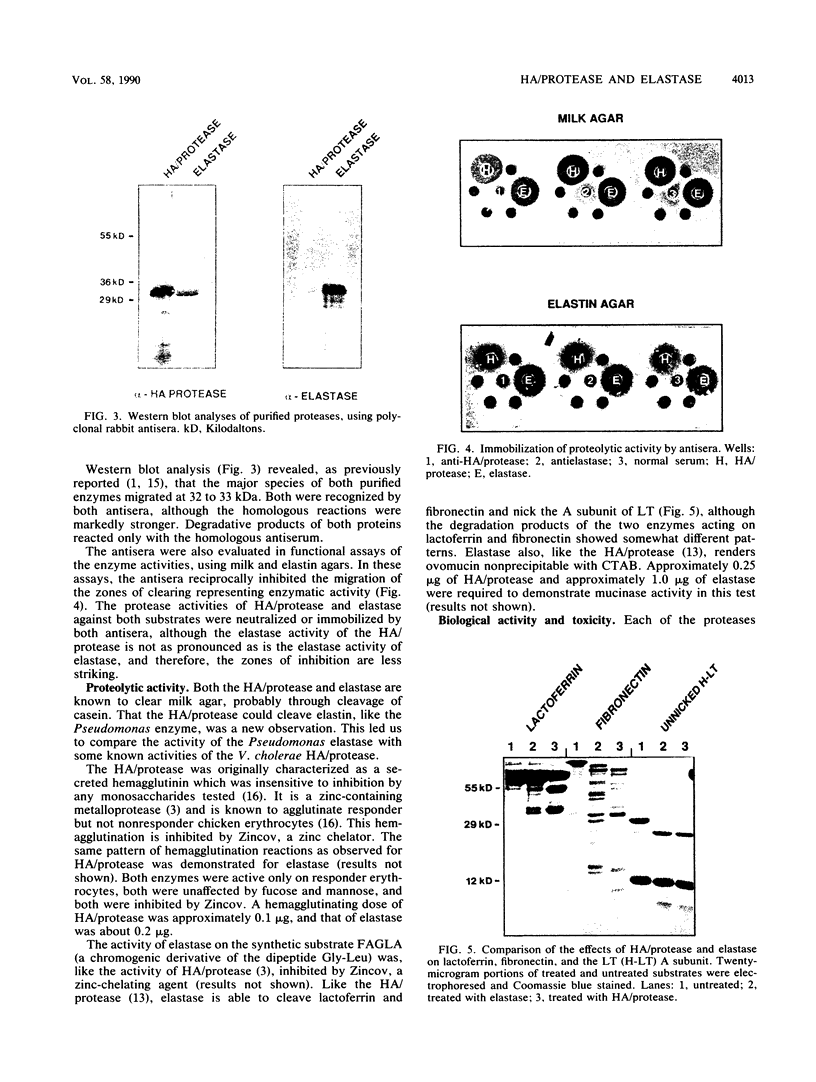
The soluble hemagglutinin/protease (HA/protease) produced by Vibrio cholerae and the elastase of Pseudomonas aeruginosa are both zinc/calcium-dependent proteases. In the present study the two enzymes are compared immunologically and functionally. The N-terminal amino acid sequences of the proteins had 65% identity within the first 20 amino acids. Polyclonal antisera against each purified protein recognized the enzyme of the other species in enzyme-linked immunosorbent assay, checkerboard immunoblot, and Western blot analyses and inhibited the protease activity of both enzymes in milk and elastin agars. Like the HA/protease, the elastase hemagglutinated "responder" but not "nonresponder" chicken erythrocytes, degraded ovomucin, lactoferrin, and fibronectin, and nicked the A subunit of the cholera toxin-related heat-labile enterotoxin from Escherichia coli. Whereas none of the three proteases tested (elastase, HA/protease, or pronase E) had any obvious effect in ileal loop tests in rabbits at doses up to 50 micrograms, all three produced some detectable skin reactions at a dose of 0.1 micrograms and necrosis at a higher dose (i.e., 5 micrograms). We conclude that the V. cholerae HA/protease and the P. aeruginosa elastase are structurally, functionally, and immunologically related.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Quinn F. D., Tompkins L. S. Legionella pneumophila zinc metalloprotease is structurally and functionally homologous to Pseudomonas aeruginosa elastase. J Bacteriol. 1990 May;172(5):2608–2613. doi: 10.1128/jb.172.5.2608-2613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. A., Boesman-Finkelstein M., Finkelstein R. A. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect Immun. 1984 Sep;45(3):558–560. doi: 10.1128/iai.45.3.558-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. A., Boesman-Finkelstein M., Finkelstein R. A. Vibrio cholerae soluble hemagglutinin/protease is a metalloenzyme. Infect Immun. 1983 Nov;42(2):639–644. doi: 10.1128/iai.42.2.639-644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. A., Finkelstein R. A. Presence of hemagglutinin/protease and other potential virulence factors in O1 and non-O1 Vibrio cholerae. J Infect Dis. 1986 Jul;154(1):183–186. doi: 10.1093/infdis/154.1.183. [DOI] [PubMed] [Google Scholar]
- Feder J., Schuck J. M. Studies on the Bacillus subtilis neutral-protease- and Bacillus thermoproteolyticus thermolysin-catalyzed hydrolysis of dipeptide substrates. Biochemistry. 1970 Jul 7;9(14):2784–2791. doi: 10.1021/bi00816a005. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman-Finkelstein M., Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1092–1095. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanne L. F., Finkelstein R. A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982 Apr;36(1):209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Booth B. A., Boesman-Finkelstein M., Finkelstein R. A. Comparative study of Vibrio cholerae non-O1 protease and soluble hemagglutinin with those of Vibrio cholerae O1. Infect Immun. 1987 Feb;55(2):451–454. doi: 10.1128/iai.55.2.451-454.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaharajo K., Homma J. Y., Aoyama Y., Okada K., Morihara K. Effects of protease and elastase from Pseudomonas aeruginosa on skin. Jpn J Exp Med. 1975 Apr;45(2):79–88. [PubMed] [Google Scholar]
- Kazemi M., Finkelstein R. A. Checkerboard immunoblotting (CBIB): an efficient, rapid, and sensitive method of assaying multiple antigen/antibody cross-reactivities. J Immunol Methods. 1990 Mar 27;128(1):143–146. doi: 10.1016/0022-1759(90)90473-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Lanata C., Sears S., Honda T., Young C. R., Finkelstein R. A. Evaluation in humans of attenuated Vibrio cholerae El Tor Ogawa strain Texas Star-SR as a live oral vaccine. Infect Immun. 1984 Feb;43(2):515–522. doi: 10.1128/iai.43.2.515-522.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Kawaharajo K., Homma J. Y., Aoyama Y., Kubota Y. Studies on the pathogenicity of Pseudomonas aeruginosa by the use of ligated rabbit intestines (the De test). Jpn J Exp Med. 1976 Aug;46(4):245–256. [PubMed] [Google Scholar]
- Peters J. E., Galloway D. R. Purification and characterization of an active fragment of the LasA protein from Pseudomonas aeruginosa: enhancement of elastase activity. J Bacteriol. 1990 May;172(5):2236–2240. doi: 10.1128/jb.172.5.2236-2240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad P. A., Bever R. A., Nicas T. I., Leduc F., Hanne L. F., Iglewski B. H. Cloning and characterization of elastase genes from Pseudomonas aeruginosa. J Bacteriol. 1987 Jun;169(6):2691–2696. doi: 10.1128/jb.169.6.2691-2696.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Isolation and characterization of protease-deficient mutants of vibrio cholerae. J Infect Dis. 1978 Aug;138(2):143–151. doi: 10.1093/infdis/138.2.143. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretlind B., Pavlovskis O. R. Pseudomonas aeruginosa elastase and its role in pseudomonas infections. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S998–1004. doi: 10.1093/clinids/5.supplement_5.s998. [DOI] [PubMed] [Google Scholar]