Abstract
Spiral ganglion Schwann cells (SGSCs) myelinate spiral ganglion neurons (SGNs) and represent a potential source of neurotrophic support for SGNs. Deafening due to loss of hair cells results in gradual degeneration and death of SGNs. Successful efforts to maintain or regenerate a functional auditory nerve may depend on a healthy population of SGSCs, yet the responses of SGSCs to neural injury remain largely unknown. Here we investigate the role of p75NTR in SGSC responses to gradual denervation. Following deafening, SGSCs in the osseous spiral lamina (OSL) and, subsequently, in Rosenthal's canal (RC) expressed elevated p75NTR compared to hearing controls. p75NTR-positive cells co-labeled with S100 and RIP antibodies (Schwann cell markers), but not with anti-neurofilament. The pattern of p75NTR expression mirrored the pattern of neural degeneration, beginning in the OSL of the cochlea base and later extending into the apex. SGSCs expressed sortilin, a p75NTR co-receptor for pro-neurotrophins. Both pro-nerve growth factor (pro-NGF) and pro-brain derived neurotrophic factor (proBDNF) induced apoptosis in cultured SGSCs. Deafened animals exhibited significantly higher levels of SGSC proliferation (as measured by BrdU uptake) compared to hearing animals while total Schwann cell density remained stable, suggesting a tight regulation of SGSC proliferation and cell death. SGSCs undergoing cell division lose p75NTR expression from the cell surface and demonstrate nuclear localization of the intracellular domain (ICD), raising the possibility that p75NTR cleavage and ICD nuclear localization regulate SGSC proliferation. These results suggest that p75NTR contributes to SGSC responses to deafening and neural degeneration.
Keywords: cell cycle, neuron, sortilin, auditory nerve, apoptosis
Introduction
Spiral ganglion neurons (SGNs) conduct auditory information from cochlear hair cells, their sole afferent input, to the brain. Deafening due to loss of hair cells deafferents SGNs, resulting in degeneration of their peripheral axons and their gradual death. In the cochlea, neural degeneration is an obstacle to hearing restoration strategies (Gillespie and Shepherd, 2005; Roehm and Hansen, 2005). Spiral ganglion Schwann cells (SGSCs) myelinate SGNs and are a potential source of neurotrophic support for SGNs (Garbay et al., 2000; Hansen et al., 2001). Understanding the response of SGSCs to deafening will facilitate auditory nerve maintenance and regeneration strategies.
We show here that the neurotrophin receptor p75NTR, normally undetectable in the postnatal spiral ganglion, is expressed post-deafening in spiral ganglion Schwann cells (SGSCs). Because axotomy has previously been shown to result in p75NTR expression in Schwann cells (Provenzano et al., 2008; Syroid et al., 2000; Taniuchi et al., 1988) this SGSC response may be a typical example of Schwann cell responses to loss of axonal contact.
p75NTR function derives from its interaction with a variety of ligands and co-receptors as well as proteolytic processing (DiStefano and Johnson, 1988; Kanning et al., 2003; Roux and Barker, 2002). The p75NTR transmembrane domain can be cleaved by the protease γ-secretase resulting in release of the intracellular domain (ICD) (Kanning et al., 2003; Schor, 2005). Following cleavage, the ICD can translocate to the nucleus where it activates transcription (Parkhurst et al., 2010). p75NTR, interacting with its co-receptor sortilin, binds proneurotrophins, e.g., proBDNF or proNGF, with high affinity, which can trigger apoptosis (Nykjaer et al., 2004; Teng et al., 2005). Indeed, p75NTR−/− mice demonstrate improved survival of sciatic nerve Schwann cells following axotomy (Ferri and Bisby, 1999; Syroid et al., 2000). During this time of acute Schwann cell denervation when p75NTR expression increases, the Schwann cells reenter cell cycle (Reynolds and Woolf, 1993). This raises the possibility that p75NTR contributes to the mitotic as well as apoptotic responses to axotomy in Schwann cells.
Previous work demonstrated increased proBDNF expression in the spiral ganglion after deafening (Tan and Shepherd, 2006). We found that SGSCs upregulate p75NTR after deafening, raising the possibility that p75NTR contributes to SGSC responses following auditory nerve injury, and sought to determine the changes in SGSC behavior that correlated with this increased p75NTR expression. Although treatment with proNGF or proBDNF induced apoptosis of SGSCs in vitro, we found that SGSC numbers remained nearly constant for up to 50 days following deafening in vivo, despite increased p75NTR expression. Rather, we detected reentry of SGSCs into the cell cycle, evident as BrdU uptake. These BrdU-positive SGSCs showed a loss of p75NTR immunoreactivity at the cell surface and accumulation of the p75NTR ICD in the nucleus in vitro and in vivo. These data suggest that loss of p75NTR from the cell surface and concomitant nuclear translocation of the ICD is associated with a shift from apoptotic to mitotic fate in SGSCs.
Materials and Methods
Spiral Ganglion Schwann cell cultures and treatment with proneurotrophins
Spiral ganglion Schwann cell cultures were prepared as previously described (Hansen et al., 2001). Briefly, spiral ganglia were dissected from postnatal day 5 (P5) rat pups, washed in ice cold PBS, and enzymatically digested in 0.2% collagenase (Sigma-Aldrich, St. Louis, MO) and 0.125% typsin with EDTA (Gibco, Carlsbad, CA) for 20 minutes at 37°C in Hanks balanced salt solution free of calcium or magnesium (HBSS −/−) (Gibco). 10% final concentration fetal bovine serum (Gibco, Carlsbad, CA) was used to quench the trypsin, the tissue was washed twice in DMEM (Gibco) and then suspended in DMEM with N2 supplements (Gibco) and 10 μg/ml insulin (Sigma-Aldrich). The cells were dissociated by titration through reduced-bore glass pipettes and cultured on laminin-coated dishes (Labtek, Campbell, California, USA) as previously described (Hegarty et al., 1997). Contaminating fibroblasts were reduced by 3 treatments with AraC (cytarabine, 10 μM, Sigma-Aldrich) in 10% FBS for 48 hr each. Cultures were then maintained in serum-free N2 medium until they reached 70–80% confluency. In the absence of neurotrophic factors and serum less than 5% of the SGNs survive long-term and less than 0.05% of the cells are SGNs (Hansen et al., 2001; Hegarty et al., 1997). Cultures were treated with either 0.1 nM of noncleavable proNGF or proBDNF (provided by Dr. Barbara Hempstead, Cornell Medical School) for 24 hours and then underwent TUNEL staining and immunolabeling. A subset of cultures were treated with N-[N-(3,5-difluorophenacetyl)-L-alanyl]-(S)-phenylglycine t-butyl ester (DAPT, 10 μM, EMD Biosciences, Gibbstown, NJ), a gsecretase inhibitor, that prevents p75NTR cleavage (Frade, 2005).
Deafening, BrdU labeling and tissue sectioning
Sprague Dawley rats (Harlan Sprague Dawley Inc, Indianapolis, IN) were housed at the animal care facilities and used according to the University of Iowa animal care regulations with the animal care review board approving all protocols. Rat pups were injected with kanamycin (400 mg/kg) daily P8–P16 and hearing loss confirmed by lack of auditory brainstem responses (ABR) at P16 as previously described (Alam et al., 2007). In addition to ABR testing, cochlear sections from deafened animals were immunostained using anti-Myosin VIIA antibodies (1:200, Proteus Biosciences, Inc, Ramona, CA) to confirm hair cell loss throughout all cochlear turns. Deafening was performed at age P8–P16 since it is difficult to reliably eliminate hair cells in all turns of the cochlea using aminoglycosides in older animals and we have extensively characterized the pattern of neural degeneration following this protocol (Alam et al., 2007). Age matched hearing littermates served as controls. Prior to sacrifice, deaf and control animals received four injections of BrdU (5-bromo-2'-deoxy-uridine) (50 μg/gm per injection) over 24 hr. The rats were euthanized at P20, P30 and P60. Intraperitoneal 0.1 mg/kg ketamine (Hospira, Lake Forest, IL, USA) and 0.1 mg/kg xylazine (Phoenix Scientific, St. Joseph, MO, USA) were used to anesthetize the animals followed by sevofluorane inhalation (Abbott, Abbott Park, IL, USA). A sternotomy was performed, the animals were given intracardiac infusion with 5 ml PBS (Gibco, Carlsbad, CA, USA) followed by 5 ml 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) and then decapitated. The temporal bone was removed and decalcified in 120 mM EDTA over 7 days with daily changes of EDTA solution. The bones were then equilibrated in sucrose gradients (10%, 20% and 30%) for 30 min each and placed in TBS Tissue Freezing Media (Triangle Biomedical sciences, Durham, NC, USA) for 1 week at room temperature. Midmodiolar cryosections 6–7 μm thick were placed on glass slides and stored at −80°C until immunostaining.
Western blotting
P32 and P60 deafened and undeafened control rats were deeply anesthetized, euthanized by decapitation, and the temporal bones removed. The cochleae were isolated, weighed and an equal volume of 4× sample buffer (final: 50 mM Tris, pH 6.8, 2% SDS, 5% β-mercaptoethanol, 50 mM EDTA) added with 2× volume of ddH2O, phosphatase inhibitor cocktail (Roche, Indianapolis, IN) and protease inhibitor cocktail (Roche), heated to 100°C for 5 min, centrifuged and the pellets discarded. Samples (15 μl) were run on 10% SDS-PAGE gels and blotted to ECL nitrocellulose membranes (Amersham, General Electric Healthcare, United Kingdom). Blots were probed overnight with rabbit anti-p75NTR ICD (9992, provided by Dr. Moses Chao) diluted 1:6,000 and mouse monoclonal anti-GAPDH (6c5, Abcam cat# Ab8245) diluted 1:5000. The blots were washed, incubated with HRP-conjugated goat ant-rabbit IgG and anti-mouse IgG (Sigma) antibodies diluted 1:25,000 and using a GE ECL Plus western blotting kit (cat# RPN2132). The blots were performed in duplicate.
Immunostaining
Cochlear sections and cell cultures were permeabilized with 0.2% Triton X-100 in PBS followed by blocking buffer (2% BSA, 5% goat serum, 0.1% Triton X-100 in PBS). The following antibodies were diluted in blocking buffer: anti- S100 (1:800 v/v) (Sigma-Aldrich), anti-sortilin (1:1000 v/v) (Abcam, Cambridge, England), anti-p75NTR intracellular domain (1:800 v/v), anti-p75NTR extracellular domain (1:1000 v/v) (both p75NTR antibodies provided by Moses Chao, New York University Medical School), anti-2',3'-cyclic nucleotide 3'-phosphodiesterase (“RIP” 1:800 v/v, Developmental Studies Hybridoma Bank) and anti-neurofilament 200 (NF200, 1:800 v/v) (Sigma-Aldrich). The efficacy and specificity of these anti-p75NTR antibodies have been previously described (Donovan et al., 1994; Donovan et al., 1993; Huber and Chao, 1995) and they have been used to show p75NTR labeling in inner pillar cells in neonatal animals (Pirvola et al., 2002). Finally, the anti-p75NTR antibodies detect a single band of the correct molecular weight on western blots (Figure 6D) further confirming specificity. Following three washes, fluorescently labeled secondary antibodies (Invitrogen, Carlsbad, CA) diluted in blocking buffer (1:800 v/v) were applied at 37°C for one hour. Nuclei were stained with Hoechst 3342 (Sigma-Aldrich) 10 μg/ml in PBS for 10 minutes at room temperature. 5-bromo-2'-deoxy-uridine (BrdU) staining was performed on cell cultures by growing the cells in their standard media and then adding 5-bromo-2'-deoxy-uridine (final concentration 10 μM) for 24 hours. Cochlear section and cell cultures were then permeabilized and treated with 2N HCl for 30 minutes at room temperature. Anti-BrdU antibody (clone G3G4, University of Iowa Hybridoma Core) (1:800 v/v) was diluted in blocking buffer and applied for 2 hours at 37°C then washed with PBS. Secondary antibody (1:800 v/v) was applied and the cells were washed in PBS. Digital fluorescent images were captured on a Leica DMIRE2 or a Zeiss Axioplan microscope equipped with epifluorescent filters and a CCD camera, or a Leica SP5 confocal microscope. Composite images were created using Adobe Photoshop CS2 (Adobe, San Jose, CA, USA). In vitro experiments were repeated at least three times. Cochlear sections were performed on minimum of four animals per group with multiple sections through each cochlea.
Figure 6.
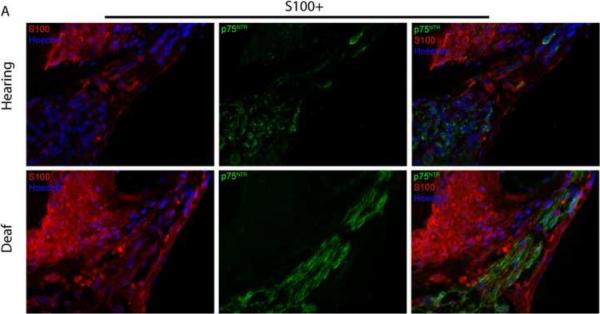
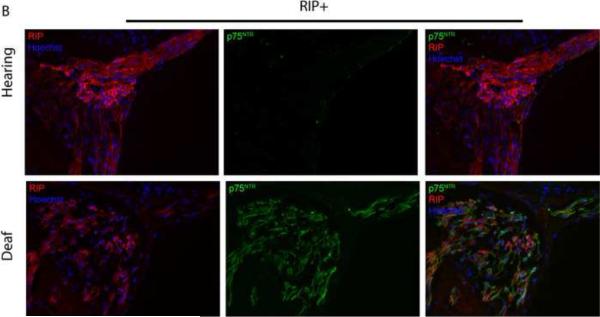
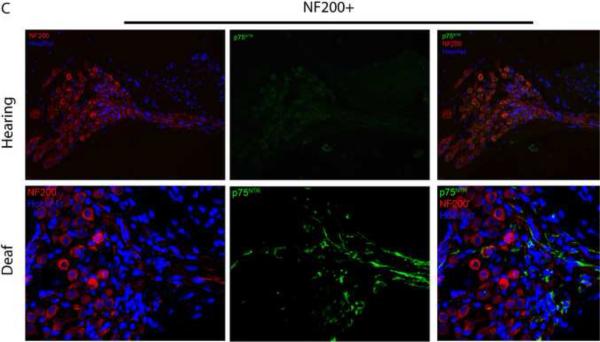
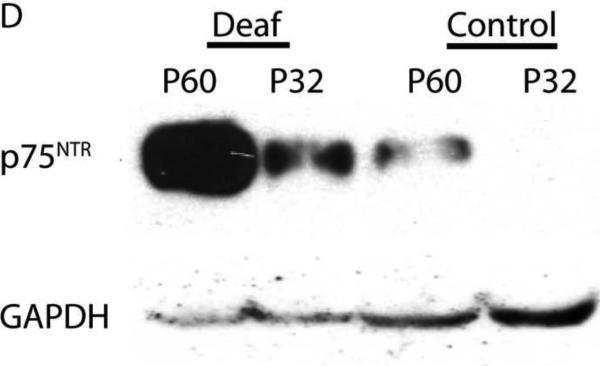
Deafening increases p75NTR expression in SGSCs. Mid-modiolar cryosections from hearing and deafened cochleae were immunostained. (A) Anti-S100 (red), anti- p75NTR (green) and Hoechst (blue). Compared to hearing animals, deafened animals demonstrated increased levels of p75NTR in Rosenthal's canal (RC) and the osseous spiral lamina (OSL). Increased p75NTR expression was restricted to S100-positive cells. Images from hearing and deafened cochleae are from the same time post-deafening and are representative of data from all time points. (B) Hearing and deafened cochleae immunostained with RIP (red) and anti- p75NTR (green) and Hoechst (blue) to further confirm p75NTR localization to Schwann cells. RIP-positive cells demonstrate increased p75NTR immunoreactivity in deafened cochleae. (C) Immnostaining with anti-NF200 (red), anti- p75NTR (green) and Hoechst (blue); scale bar = 10 μm. NF200 expression appeared throughout RC and the OSL in hearing cochleae whereas there was a significance loss of NF200-positive SGNs and their peripheral axons in RC and the OSL, respectively, in deafened cochleae. Increased p75NTR expression was not associated with NF200-positive neural elements in RC or the OSL, and was evident in the OSL devoid of SGN axons. (D) Western blots of spiral ganglion lysates from deafened and hearing animals at P32 and P60 probed with anti-p75NTR ICD. Deafened animals demonstrated a markedly higher level of p75NTR compared to hearing counterparts. GAPDH was used for a loading control. There was a low level of p75NTR expression in lysates from P60 hearing animals consistent with the immunohistochemistry findings.
TUNEL (Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) staining
TUNEL was performed as previously described (Provenzano et al., 2008). After immunolabeling, cultures were washed for 10 minutes at room temperature in reaction buffer (25 mM Tris-HCl, 200 mM sodium cacodylate, 0.25 mg/ml BSA, 1 mM cobalt chloride), treated with 2 U/ml terminal transferase (Roche, Indianapolis, IN, USA) and with 0.9 μM Biotin-16-dUTP and 0.9 μM unlabeled dUTP (Roche, Indianapolis, IN, USA) for two hours at 45°C. Slides were washed in stopping buffer (300 mM NaCl, 30 mM sodium citrate), rinsed in PBS, incubated with Streptavidin Alexa Fluor 568 conjugate (Molecular Probes, Carlsbad, CA, USA) in PBS (1:2000) for 30 minutes at room temperature then washed in PBS.
Quantification of spiral ganglion Schwann cell apoptosis
To determine the percentage of apoptotic cells in culture, 6–7 randomly selected fields from each condition were digitally photographed. Criteria for scoring apoptotic nuclei were a TUNEL-positive nucleus with condensed nuclear morphology in a S100-positive cell. The percent of apoptotic SCs was expressed as a fold difference compared to the control condition. Each condition was performed in duplicate and repeated in at least three times. The mean was calculated and a two tail Student's t-test was performed to determine statistical significance.
Schwann Cell counting
Mid-modiolar 6 μm-thick sections of cochleae from hearing and deafened rats prepared as previously described (Alam, 2007) were immunostained with rabbit anti-p75NTR, and mouse anti-NF200 (Sigma) to label neurons or mouse RIP (Developmental Studies Hybridoma Bank) to detect Schwann cells, and Hoechst 33258 bisbenzamide to label nuclei. We refer to S100-positive glial cells in the osseous spiral lamina and Rosenthal's canal as Schwann cells. RIP specificity for cochlear Schwann cells was confirmed by co-localization of RIP immunofluorescence with immunofluorescence of rabbit anti-S100 and rabbit anti-connexin 29. Alexa Fluor secondary antibodies (Molecular Probes) were used to visualize the labeling on a Zeiss Axioplan microscope equipped for epifluorescence and DIC (Nomarski) optics. Fluorescence and brightfield DIC images were captured of axons and spiral ganglion Schwann cells (SGSCs) in the habenula perforata traversing the osseous spiral lamina (OSL) and of SGNs and SGSCs in the ganglion within Rosenthal's canal (RC) in apical, middle and basal turns. In each image, the area of the OSL or RC was measured in ImageJ software (http://rsbweb.nih.gov/ij/index.html) using combined DIC and fluorescence images to distinguish the periosteum and neural tissue. The number of animals per experimental group is listed in Supplemental Table 2.
Counts were obtained in Adobe Photoshop for each image of the total number of nuclei (Hoechst 33258 fluorescence) and the total number of neuronal nuclei (NF-200-immunofluorescent cells containing a nucleus in the plane of the section). The number of Schwann cell nuclei was determined indirectly by subtracting the number of neuronal nuclei from the total number of nuclei and confirmed by double-labeling with Hoechst 33258 and RIP. The number of p75NTR-positive Schwann cells was determined by counting nuclei with colocalized p75NTR. Colocalized RIP immunoreactivity was used to confirm that these cells were Schwann cells. Clearly distinguishable p75NTR-positive neurons (colocalized NF-200 and p75NTR immunoreactivity) were not detected. Some neurons with p75NTR immunofluorescence at their perimeters were detected but in these cases the p75NTR immunofluorescence colocalized with RIP immunofluorescence of satellite cells surrounding the neuronal somata. Statistical analysis was performed using InStat (Graphpad) software.
Quantification of BrdU-positive Schwann cells in cochlear sections
Mid-modilar cochlear sections were stained with anti-BrdU and either anti-S100 or anti-p75NTR antibodies as described above. BrdU-positive Schwann cell (S100-positive) nuclei were counted in both RC and the OSL for hearing and deafened animals for all three time points. BrdU staining was confirmed for each section by examining cells contained in blood vessels within the section. Each time point and treatment group consisted of 5 animals and at least 3 sections were scored per cochlea. The average number of BrdU-positive cells were determined for each age cohort and treatment group. A two tail Student's t-test was then performed to determine a statistically significant difference (p < 0.05) between deafened and hearing animals at each time point with an ANOVA on ranks and a post hoc Dunn's method used to calculate the statistical difference between the three time points for deaf and hearing animals.
Results
proNGF and proBDNF promote apoptosis in cultured spiral ganglion Schwann cells
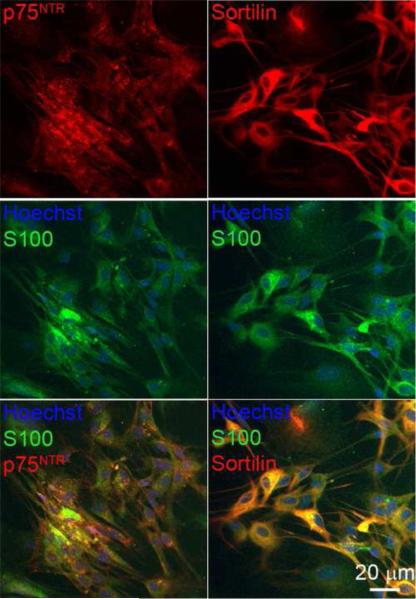
Deafness due to aminoglycoside toxicity leads to proBDNF expression in the spiral ganglion (Tan and Shepherd, 2006). Since proneurotrophins promote sciatic nerve SC apoptosis following nerve injury in p75NTR-dependent manner, we determined the response of cultured SGSCs to proneurotrophins. To verify expression of p75NTR and sortilin in cultured SGSCs, fixed cultures were immunostained with either anti-p75NTR intracellular domain (ICD) or anti-sortilin antibodies and anti-S100 antibody. As shown in Fig. 1, SGSCs, determined by S100 labeling, expressed both p75NTR and sortilin. p75NTR labeling predominantly localized to the cell surface while sortilin localized to the cytoplasm and the cell surface.
Figure 1.
Cultured spiral ganglion Schwann cell (SGSC) express p75NTR and sortilin. Immunostaining with anti-S100 showed a homogenous population (approximately 98% Schwann cell) free of fibroblasts and neurons. S100-positive cells also express p75NTR and sortilin. SGSCs were immunostained with anti-S100 (green) and anti- p75NTR (red) and nuclei were stained with Hoechst (blue). Parallel SGSC cultures were immunostained with anti-S100 (green) and anti-sortilin (red) and Hoechst (blue). Scale bar = 20 μm.
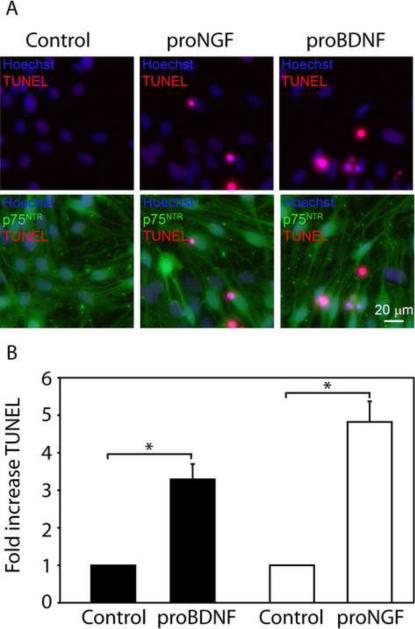
Next, we sought to determine if proNGF and proBDNF induce apoptosis in SGSCs. SGSC cultures, plated at equal density (approximately 70% confluent), were treated with either proNGF (0.1 nM) or proBDNF (0.1 nM) for 24 hr while cultures treated with artificial cerebral spinal fluid served as a control. After treatment the cells were immunostained with anti- p75NTR ICD antibody followed by labeling with TUNEL. Nuclei were detected with Hoescht. proBDNF resulted in a 3 fold (3.3 ± 0.4, mean ± SEM) increase in the percent TUNEL positive SGSCs compared to control (p<0.05) while proNGF treatment showed a 5 fold (4.8 ± 0.5, mean ± SEM) increase in the percent TUNEL positive SGSCs (p<0.05) (Fig. 2). The experiment was performed in duplicate and repeated three times.
Figure 2.
proBDNF or proNGF promote SGSC apoptosis. (A) SGSC cultures were treated with proNGF (0.1 nM), proBDNF (0.1 nM), or artificial cerebrospinal fluid (control) for 24 hours and stained for p75NTR ECD (green), Hoechst (blue) or TUNEL (red). Scale bar = 20 μm. The control group had few TUNEL-positive cells indicating a low basal level of apoptosis. proNGF and proBDNF both promoted apoptosis evidenced by the increase in TUNEL staining within condensed nuclei. p75NTR was expressed in apoptotic and non apoptotic cells in control and treated conditions. (B) The percent of TUNEL-positive cells were quantified and expressed as a fold increase in apoptosis compared to control. Treatment with proBDNF resulted in a 3 fold (3.3 ± 0.4, mean ± SEM) increase in TUNEL-positive Schwann cell (p<0.05) while proNGF treatment showed a 5 fold (4.8 ± 0.5, mean ± SEM) increase in TUNEL-positive Schwann cell compared to control (p<0.05).
The p75NTR ICD localizes to the nucleus in BrdU positive SGSCs in vitro
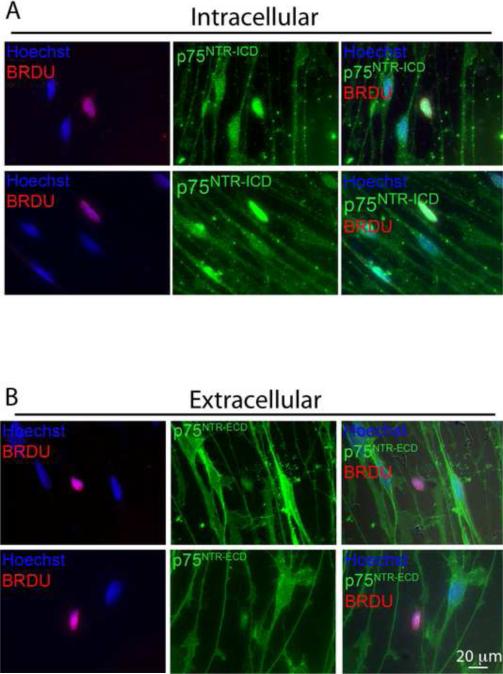
Since increased p75NTR expression in denervated sciatic and facial nerve SCs correlates with both proliferation and apoptosis, we sought to determine if there was any difference in p75NTR expression in cultured SGSCs undergoing cell division compared with non-mitotic SGSCs. SGSC cultures were labeled with BrdU (10 μM) for 24 hr and then stained with anti-BrdU antibodies and antibodies directed towards either the extracellular domain (ECD) or ICD of p75NTR. Anti-p75NTR ECD immunostaining revealed a uniform distribution of p75NTR in BrdU-negative cells (Fig. 3), comparable to anti-p75NTR ICD staining (Figs. 1 and 3). However, BrdU-positive cells exhibited an absence of p75NTR ECD labeling. Bright field microscopy verified that the loss of p75NTR ECD labeling occurred in SCs with normal cytoplasmic processes and was not due to contraction of the cell. This lack of p75NTR ECD immunoreactivity could be due to a loss of p75NTR expression in proliferating SGSCs or to proteolytic cleavage with shedding of the ECD. To distinguish these possibilities, we immunostained BrdU-labeled SGSCs with the anti-p75NTR ICD antibody. Staining with anti-p75NTR ICD antibody showed high levels of p75NTR ICD immunoreactivity within the nucleus in BrdU-positive SGSCs and an absence of p75NTR ICD expression on the cell surface (Fig. 3). These results demonstrate that cleavage of p75NTR with nuclear localization of p75NTR ICD highly correlates with entry into the cell cycle.
Figure 3.
BrdU-positive SGSCs demonstrate loss cell surface p75NTR expression and nuclear localization the intracellular domain (ICD). SCSC cultures were labeled with BrdU (10 μM) and immunostained with antibodies directed towards p75NTR extracellular domain (ECD) or ICD (A) Anti-p75NTR ICD (green), Hoechst (blue) and anti-BrdU (red). p75NTR ICD localized to the nuclei in BrdU-positive cells and remained on the cell surface in BrdU-negative cells. (B) Anti-p75NTR ECD (green), Hoechst (blue) and anti-BrdU (red); scale bar = 20 μm. Immunofluorescence demonstrates a loss of p75NTR ECD immunoreactivity in BrdU-positive cells while BrdU-negative cells retain p75NTR ECD on the cell surface.
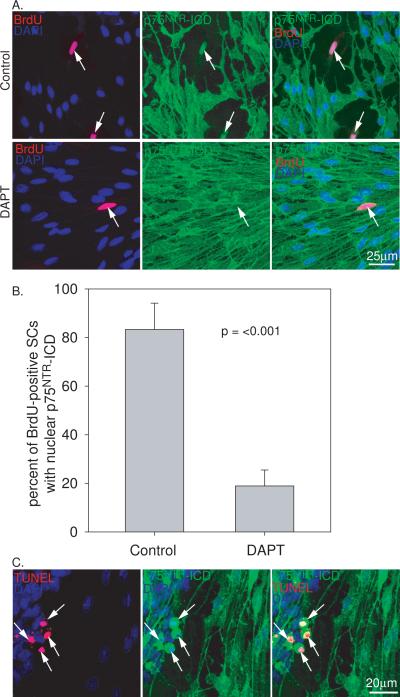
To confirm that the loss of p75NTR cell surface expression and nuclear localization of the ICD was due to proteolytic cleavage, we treated cultures with DAPT (10 mM), a g-secretase inhibitor that prevents p75NTR cleavage (Frade, 2005). The percent of BrdU-positive SGSCs exhibiting nuclear localization of the ICD was determined from three separate cultures. Treatment with DAPT prevented the loss of p75NTR cell surface expression and significantly reduced the percent of BrdU-positive SGSCs with nuclear localized ICD (p<0.001, Fig. 4). As shown above, treatment of the cultures with proNGF induced SGSC apoptosis. In these apoptotic SGSCs there was condensation of cytoplasmic and nuclear cellular components with parallel condensation of p75NTR labeling. In these cells, it was difficult to exclude nuclear localization of the ICD. However, at least some of the staining appeared extranuclear (Fig. 4). Additionally, non-apoptotic SGSCs retained p75NTR cell surface expression following treatment with proNGF (Figs. 2 and 4). Taken together, these results confirm that loss of p75NTR expression at the cell surface and localization of p75NTR ICD to the nucleus results from proteolytic cleavage.
Figure 4.
Treatment with the protease inhibitor DAPT prevents p75NTR cleavage and intracellular domain localization to the nucleus. (A) SG cultures immunostained with anti-BrdU (red), anti- p75NTR ICD (green) antibodies and DAPI (blue). Control cells demonstrate p75NTR cleavage and ICD localization in BrdU-positive cells. Treatment with DAPT prevents cleavage of p75NTR and loss of ICD nuclear localization. scale bar = 25 μm (B) Percent of BrdU-positive SGSCs with nuclear localized p75NTR ICD determined for control and DAPT-treated cultures averaged from 3 separate cultures. Cultures treated with DAPT demonstrate lower percentage of BrdU-positive with nuclear localized p75NTR ICD (p<0.001, two tail Student's t-test) compared to control cultures. (C) Anti-p75NTR ICD immunostaining (green) of SGSC cultures treated with proNGF and co-labeled with TUNEL (red) and DAPI (blue). p75NTR remains on the cell surface of non-apoptotic cells and demonstrates cytoplasmic and nuclear condensation in TUNEL-positive apoptotic cells. scale bar = 20 μm.
p75NTR expression increases in Spiral Ganglion Schwann Cells after deafening
To begin to understand the role of p75NTR in degeneration of the SG following deafening, we first assessed whether p75NTR levels were changing in the SG of deafened animals compared to their control counterparts. Deafening was confirmed with both ABR testing and immunostaining which confirmed hair cell loss throughout the cochlear turns (Fig. 5).
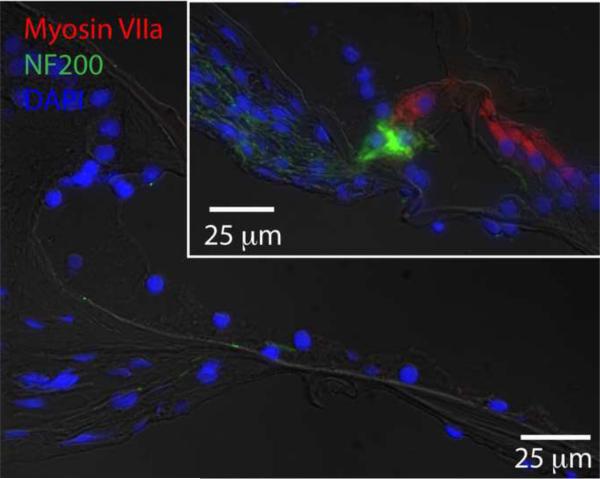
Figure 5.
Deafening with kanamycin eliminates Myosin VIIA-positive hair cells from the organ of Corti. Cochlear sections from p60 hearing (inset – right, upper panel) or deafened (left, lower panel) animals were immunostained with anti-Myosin VIIA (red) and anti-NF200 (greed) antibodies. Nuclei were stained with DAPI (blue). Scale bar=25 μm for upper, inset panel. Scale bar=25 μm for left panel.
Figure 6 shows representative images of p75NTR immunoreactivity in cochlear sections from deafened and hearing rats. Immunostaining of control neonatal cochlear sections with anti-p75NTR has previously demonstrated specificity for the pillar cells in the organ of Corti (Pirvola et al., 2002). Images for both treatment groups are from the same time point and are representative of the other time points (data not shown). Deafening led to a significant increase in p75NTR expression in RC and the OSL compared to hearing animals. Western blots of cochlear lysates confirmed a robust increase in p75NTR expression following deafening (Fig. 6D).
To determine whether the increased p75NTR expression occurred in SGNs and/or SGSCs, we co-labeled a subset of cochlear sections with anti-p75NTR antibody and with either anti-S100, RIP, or anti-neurofilament 200 (NF200) antibodies. The increase in p75NTR immunoreactivity in RC and the OSL was restricted to S100-positive cells and did not correspond to neurofilament staining (Fig. 6) implying that p75NTR expression is in the glia and not the neurons. Indeed, the absence of NF200 immunostaining in the OSL not only confirms the expected degeneration of the peripheral axons following deafening but also verifies that the increase in p75NTR expression in the OSL occurs in the remaining SCs. The cells with increased p75NTR also co-labeled with RIP further confirming that the p75NTR-positive cells are glia.
Schwann cell densities are initially preserved following deafening
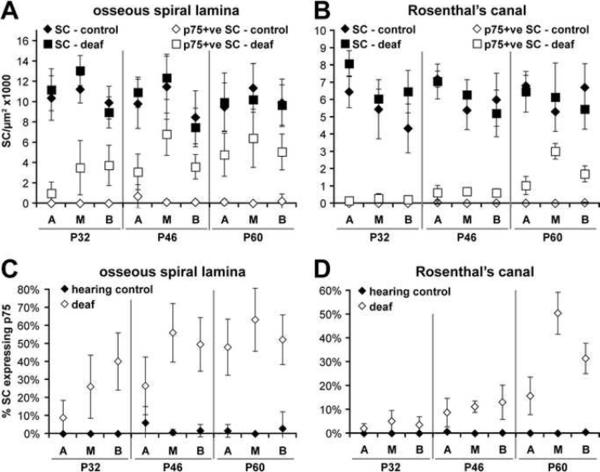
To determine whether deafening and subsequent neural degeneration results in a loss of Schwann cells in the cochlea, we counted the number of Schwann cells per μm2 in the apex, middle and base of hearing and deafened rat cochleae. Separate counts were performed for RC and the OSL. Counts occurred at three time points: p32, p46 and p60. Total Schwann cell and p75NTR-positive Schwann cells were recorded separately and are displayed in Figure 7. Statistical analysis is contained in Supplemental Table 1 and the number of animals analyzed at each time point is presented in Supplemental Table 2. Total Schwann cells were not significantly different when comparing deafened and hearing animals across all time points, position along the cochlear turns, or regions of the cochlea (i.e. RC or the OSL). Although the total number of Schwann cells did not significantly change across groups, the percentage of p75NTR-positive Schwann cells significantly increased after deafening for all time points and at all locations along the cochlea (Fig. 7)(p<0.05 for each time-point). The percentage of p75NTR-positive Schwann cells was generally higher in the middle and basal turns of the cochlea compared to the apex. p75NTR-immunoreactivity also increased with time and was more pronounced in the OSL compared to RC. These results demonstrate a preservation of SGSCs for at least 45 days following deafening supporting the notion that they are capable of providing a local source of trophic support to limit the rate and extent of SGN loss.
Figure 7.
Schwann cell densities remain stable in the cochlea following deafening. Schwann cell density counts (Schwann cells/μm2× 1000) were performed on hearing (open and closed diamonds) and deafened animals (open and closed squares). The percent of p75NTR-positive cells was also determined. Separate counts were performed for the apex (A), middle (M) and basal (B) turns of the cochlea for animals at the P32, P46 and P60 time points. Supplemental Table 1 presents the statistical analysis for all turns, time points and conditions and Supplemental Table 2 presents the number of animals analyzed at each time point. (A) Schwann cell density counts in the OSL. Total Schwann cell density remained stable across cochlear location and almost all time points for both hearing and deafened animals. Total Schwann cells were statistically different at the apex when comparing P32 and P60 animals (p<0.001). There were also noted differences between various turn of the cochlea within the same time point. There was no difference between hearing and deafened animals with regards to total Schwann cells. Deafened animals did have a significantly higher level of p75NTR-positive cells compared to hearing controls for all time points (p<0.05). (B) Schwann cell density counts in RC. Total Schwann cells remained unchanged when comparing hearing and deafened animals. Deafened animals demonstrated significantly higher densities of p75NTR-positive cells compared to controls for all time points (p<0.05). (C) The percent of p75NTR-positive cells in the OSL. p75NTR-positive cells were highest in the basal turn of the cochlea and increased with animal age. The percent of p75NTR-positive cells was significantly higher in deafened animals at all time points and cochlear locations except at the middle turn in the P32 animals (p<0.05). (D) The percent of p75NTR-positive cells in RC. The percent of p75NTR-positive cells in deafened animals was significantly higher compared to hearing counterparts for all locations and time points except the apex and middle turns in the P32 animals (p<0.05).
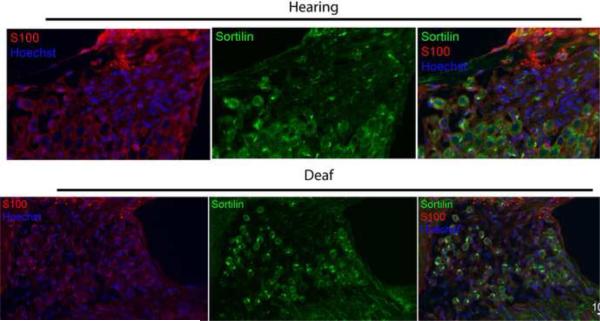
Sortilin staining remains stable following deafening
Staining with an anti-sortilin and anti-S100 antibodies was also performed on the deafened and hearing animals to determine if sortilin, a necessary co-receptor for proneurotrophin-mediated apoptosis, was expressed in the SGSCs in vivo (Fig. 8). Sortilin was expressed in SGSCs at all time points in both hearing and deafened animals. There was no appreciable difference in sortilin immunoreactivity between deafened and hearing animals at any of the three time points nor were there any appreciable differences when comparing animals of the same treatment group at the different time points.
Figure 8.
Sortilin expression in RC and OSL appear unchanged before and after deafening. Midmodiolar cryosections from hearing and deafened cochleae were immunostained with anti-sortilin (green) and anti-S100 (red) antibodies and Hoechst (blue); scale bar = 10 μm. Schwann cells are seen wrapping around soma. Images from hearing and deafened cochleae were taken from the same time point and are representative of results at all three time points. Sortilin immunostaining was apparent in S100-positive cells in all treatment groups. Deafening appeared to have no effect on sortilin expression.
Deafening increases proliferation of SGSCs with BrdU-positive cells exhibiting nuclear localization of p75NTR ICD
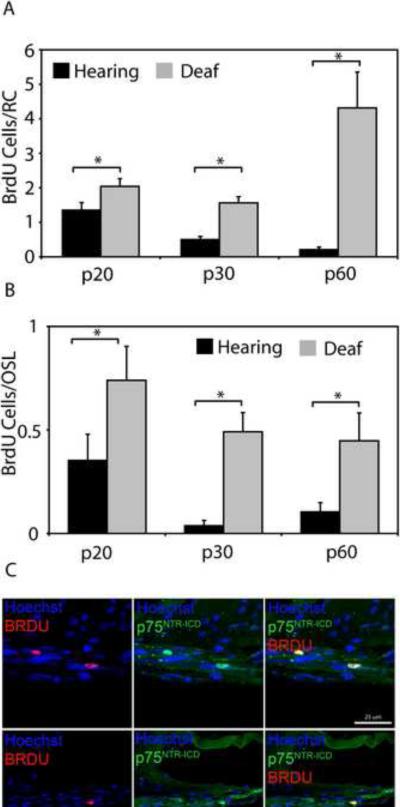
The known proliferative response of peripheral nerve SCs to neural injury led us to inquire if deafening results in a similar increase in SGSC proliferation. We first sought to determine if there was a difference in the number of BrdU-positive Schwann cells in RC and the OSL of deafened animals versus their hearing counterparts (Fig. 9). There was a significant increase in the number of BrdU-positive Schwann cells in RC and the OSL of deafened animals compared to their hearing controls for p20, p30 and p60 (all p<0.05). Thus, deafening significantly increases SC proliferation in RC and the OSL. Statistical analysis with an ANOVA on ranks and a post hoc Dunn's method showed that there was no significant difference between deafened animals at the three time points for either RC or the OSL.
Figure 9.
Deafening increases SGSC proliferation and leads to nuclear localization of p75NTR ICD in BrdU-positive SGSCs. The mean number of BrdU-positive SGSCs in both RC (A) and the OSL (B) was significantly greater in deafened compared to hearing cochleae for each age group (p<0.05). There were no significant differences in the mean number of BrdU-positive SGSCs between the different time points for hearing or deafened cochleae (p>0.05, ANOVA on ranks and a post hoc Dunn's method). (C) Immunostaining of deafened and hearing cochleae with anti-BrdU (red) and anti-p75NTR ICD (green) antibodies and Hoechst (blue). Scale bar = 25 μm. The p75NTR ICD localized to the nuclei of BrdU-positive cells as was previously observed in cultured SGSCs.
Given the observations of increased p75NTR after deafening and the localization of p75NTR ICD to the nucleus in BrdU-positive SGSCs in vitro, we asked whether reentry into cell cycle in vivo correlates with movement of p75NTR ICD into the nucleus. We immunostained cochlear sections from deafened animals with anti-BrdU and anti-p75NTR ICD antibodies (Fig. 9). As was the case in vitro, anti-p75NTR ICD staining localized within the nuclei of BrdU-positive Schwann cells while p75NTR ICD staining intensity appeared to be uniform throughout the cell in BrdU-negative cells. Within the OSL, 89.6% ± 8.3% of BrdU-positive SCs demonstrated nuclear localization of p75NTR ICD while in RC, 78.1% ± 7.8% of BrdU-positive SCs demonstrated nuclear localization of p75NTR ICD. In both cases, the percent of BrdU-positive SCs with nuclear-localized p75NTR ICD was significantly greater than the percent of BrdU-positive SCs without p75NTR ICD nuclear localization (p<0.001, chi-square). We did not observe nuclear localization of p75NTR ICD in BrdU-negative SCs. This correlation between movement of p75NTR ICD into the nucleus and reentry of SGSCs into cell cycle raises the possibility that cleavage of p75NTR and nuclear localization of the ICD contributes to cell cycle regulation in SGSCs.
Discussion
Spiral ganglion Schwann cells as a local source of neurotrophic support for SGNs
The gradual loss of SGNs following acute loss of hair cells in the organ of Corti and evidence of sustained prosurvival intracellular signaling in SGNs in the absence of hair cells suggest that there are sources of neurotrophic support other than hair cells and these alternative sources are sufficient to keep many SGNs alive for several months following deafening (Alam et al., 2007; Dodson and Mohuiddin, 2000). In humans, SGN loss occurs even more slowly following hair cell loss suggesting ongoing neurotrophic support by cells outside of the organ of Corti and allowing functional stimulation of SGNs with implanted electrodes for many years after the onset of deafness (Gomaa et al., 2003; Khan et al., 2005; Linthicum and Fayad, 2009). Paracrine support by SGSCs represents one potential source of trophic support for SGNs. Indeed, SGSCs express BDNF and neurotrophin-3 (NT-3) (Hansen et al., 2001), both of which support SGN survival in vitro and in vivo (Hegarty et al., 1997; Roehm and Hansen, 2005; Shepherd et al., 2008). Our observation of persistence of SGSCs following aminoglycoside-induced deafness is consistent with a hypothesis that SGNs and SGSCs reciprocally support each other (Hansen et al., 2001).
Supporting cells in the organ of Corti represent another potential source of neurotrophic support following hair cell loss (Stankovic et al., 2004; Sugawara et al., 2005; Xiang et al., 2003). However, SGN peripheral axons degenerate after hair cell loss limiting access of the SGNs to trophic factors in the organ of Corti.
Deafening induces p75NTR expression in Schwann cells
Here we show p75NTR expression in SGSCs post-deafening rises within two weeks after deafening and remains elevated for at least two months thereafter. We confirm a previous report of p75NTR expression post-deafening (Tan and Shepherd, 2006) but, by co-labeling with anti-p75NTR and both neuronal and two different glial markers, we show that p75NTR immunoreactivity colocalizes only with Schwann cell and not with neuronal markers. These observations indicate that p75NTR expression is glial and not neuronal as had been previously hypothesized. Similarly, increased p75NTR expression has been previously reported in sciatic and facial nerve Schwann cells following acute axotomy (Taniuchi et al., 1986). In this case, p75NTR expression in the Schwann cells is due to their loss of contact with axons because p75NTR expression disappears as axons regenerate and Schwann cells regain contact with axons (Ferri and Bisby, 1999). Because gradual neural degeneration is occurring when p75NTR is expressed in SGSCs, it is plausible that, as in axotomized peripheral nerves, p75NTR expression in SGSCs is a result of loss of contact with SGN axons or cells bodies.
The spatiotemporal pattern of p75NTR expression supports the idea that increased p75NTR expression in SGSCs results from losing contact with SGNs. In rodents, hair cell loss results in degeneration of the SGN peripheral axons with gradual degeneration of the SGNs themselves (Bichler et al., 1983; Leake and Hradek, 1988; Nadol, 1990; Spoendlin, 1975, 1984). In neonatally-deafened rats, such as those used in this study, loss of SGN somata in the spiral ganglion first becomes evident only at P32 with loss of most SGNs occurring later (Alam et al., 2007). p75NTR expression in SGSCs corresponds to this pattern, appearing first in the osseous spiral lamina where SGN peripheral axons are degenerating and only later in the ganglion, within Rosenthal's canal, when the number of neuronal somata is declining significantly. Moreover, neuronal degeneration and death due to aminoglycoside-induced hair cell death begins in the cochlear base and later extends to the apex. Similarly, p75NTR expression in SGSCs is first evident in the basal turn and only later appears in the apical turn – and the percentage of p75NTR-expressing SGSCs is lowest in the apex at all time points – paralleling the pattern of neural degeneration. Thus, p75NTR expression in SGSCs follows axon and soma degeneration in the cochlea spatially and temporally and is likely a consequence of loss of neural contact by the SGSCs.
Function of p75NTR in SGSCs post-deafening
We also asked what is the function of p75NTR expression in SGSCs postdeafening. One function associated with p75NTR after axotomy is induction of Schwann cell apoptosis (Syroid et al., 2000). We therefore considered the possibility that p75NTR expression drives apoptosis in SGSCs, which constitutively express the co-receptor sortilin. In the long term, deafening leads to loss of most neurons and Schwann cells in the SG (Alam et al., 2007; Leake and Hradek, 1988; Webster and Webster, 1981). ProBDNF and proNGF, at subnanomolar concentrations, promote SGSC apoptosis in vitro. ProBDNF, along with cleavage products, is found in hearing and aminoglycoside deafened cochleae (Tan and Shepherd, 2006). With both sortilin and proBDNF present, the appearance of p75NTR would be sufficient to induce SGSC apoptosis. Because SGSCs can provide neurotrophic support to SGNs (Hansen et al., 2001), SGSC apoptosis could contribute to SGN apoptosis late in the cell death period post-deafening.
While an apoptotic function of p75NTR in SGSCs is evident in vitro and plausible in vivo, we find, unexpectedly, that SGSC numbers are maintained for at least 6–7 weeks after deafening, even while p75NTR is expressed on a large fraction of SGSCs. During this period, there are many mitotic SGSCs, evidenced by nuclear BrdU immunoreactivity. Schwann cell mitosis has also been observed in other cases of acute or chronic nerve injury (Gupta and Steward, 2003; Muller and Stoll, 1998). This raises the possibility that, even if SGSC death occurs after deafening, a proliferative response allows for replenishment of dying Schwann cells.
Both apoptotic and mitotic responses to neurodegeneration may be regulated by p75NTR expression. Shared cellular regulatory mechanisms for Schwann cell proliferation and death have been previously observed; for example, Krox-20 prevents both proliferation and death in Schwann cells (Parkinson et al., 2004). Our data show that the number of BrdU-positive SGSCs significantly increases in RC and in the OSL of deaf animals relative to age-matched hearing littermates, correlated with p75NTR expression.
The data further suggest a mechanism by which p75NTR can alternatively promote apoptosis or mitosis. Labeling with antibodies directed towards either the ICD or ECD of p75NTR reveals a dramatic change in p75NTR localization associated with mitosis. In most p75NTR-positive SGSCs, both ECD and ICD immunoreactivity appear at the cell surface. In BrdU-positive SGSCs, p75NTR ECD immunoreactivity is absent and p75NTR ICD immunoreactivity is nuclear in vitro and in vivo. These data suggest that cleavage of p75NTR with loss of the ECD and translocation of the ICD to the nucleus corresponds to a shift from proapoptotic to mitogenic signaling. Because apoptotic signaling initiated by p75NTR involves regulation of the membrane-associated small GTPase Rac (Harrington et al., 2002), loss of p75NTR from the plasma membrane may curtail its ability to generate proapoptotic signaling. Conversely, accumulation of p75NTR ICD in the nucleus may contribute to mitogenic signaling and cell cycle progression. Significantly, the p75NTR ICD has been shown to localize to the nucleus in PC12 cells and bind the promoter of at least one gene involved in cell cycle progression, cyclin E1 (Parkhurst et al., 2010). Also, consistent with a role for the p75NTR ICD in promoting glial proliferation, generation of the ICD in malignant glioma cells results in more aggressive behavior (Wang et al., 2008). Thus, proteolytic processing of p75NTR may switch it from an initiator of proapoptotic to an initiator of mitogenic signaling.
p75NTR expression as a protective response to neurodegeneration in the cochlea
Our study demonstrates that the response of SGSCs to gradual neurodegeneration mirrors that of acutely denervated Schwann cells with regards to p75NTR expression and reentry into cell cycle. The proliferative response tightly correlates with movement of p75NTR ICD into the nucleus raising the possibility that p75NTR not only mediates an apoptotic response in SCs, but also promotes cell cycle progression. Proliferation of SGSCs following deafness allows for preservation of a nearly normal complement of glial cells; this can contribute to neurotrophic support of deafferented SGNs following acute hair cell loss. Thus, in the case of SC proliferation after deafening, p75NTR expression may constitute an adaptive response to neural trauma, contributing to the ability of SCs to support deafferented neurons. Maintaining SGNs and their peripheral axons is important in efforts to improve the efficacy of cochlear implants in deaf individuals (Roehm and Hansen, 2005); viable, functional SGSCs may be valuable in this regard with p75NTR playing a significant role. In addition to providing neurotrophic support, Schwann cell functions in axon guidance and myelination will likely prove critical to efforts to regenerate the auditory nerve.
Conclusion
Upon deafening and loss of hair cells in the cochlea, SGSCs reenter a tightly regulated system of cell proliferation and apoptosis; p75NTR appears to participate in both of these cellular responses. p75NTR expression increases after deafening, is isolated to SGSCs and mirrors the loss of neurons. Expression of p75NTR and subsequent apoptosis correlates with its demonstrated role in pro-neurotrophin mediated apoptosis. We have also demonstrated that a subpopulation of SGSCs lose p75NTR expression at the cell surface and segregate the intracellular domain of p75NTR into the nucleus in cells undergoing proliferation. Our findings suggest that p75NTR may be vital in maintaining a population of SGSCs necessary for functioning auditory nerves after deafening.
Supplementary Material
Acknowledgements
The authors thank Moses Chao, PhD for providing the anti-p75NTR antibodies and Barbara Hempstead MD, PhD for the proNGF and proBDNF protein and plasmids.
Support This work was supported by grants from NIH grants R01 DC009801 (MRH), RO1 DC002961 (SHG), P30 DC010362 and DOD grant NF050193 (MRH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. 2007;503:832–852. doi: 10.1002/cne.21430. [DOI] [PubMed] [Google Scholar]
- Bichler E, Spoendlin H, Rauchegger H. Degeneration of cochlear neurons after amikacin intoxication in the rat. Arch Otorhinolaryngol. 1983;237:201–208. doi: 10.1007/BF00453725. [DOI] [PubMed] [Google Scholar]
- DiStefano PS, Johnson EM., Jr. Identification of a truncated form of the nerve growth factor receptor. Proc Natl Acad Sci U S A. 1988;85:270–274. doi: 10.1073/pnas.85.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. Response of spiral ganglion neurones to cochlear hair cell destruction in the guinea pig. J Neurocytol. 2000;29:525–537. doi: 10.1023/a:1007201913730. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Hempstead B, Huber LJ, Kaplan D, Tsoulfas P, Chao M, Parada L, Schofield D. Identification of the neurotrophin receptors p75 and trk in a series of Wilms' tumors. Am J Pathol. 1994;145:792–801. [PMC free article] [PubMed] [Google Scholar]
- Donovan MJ, Hempstead BL, Horvath C, Chao MV, Schofield D. Immunohistochemical localization of Trk receptor protein in pediatric small round blue cell tumors. Am J Pathol. 1993;143:1560–1567. [PMC free article] [PubMed] [Google Scholar]
- Ferri CC, Bisby MA. Improved survival of injured sciatic nerve Schwann cells in mice lacking the p75 receptor. Neurosci Lett. 1999;272:191–194. doi: 10.1016/s0304-3940(99)00618-7. [DOI] [PubMed] [Google Scholar]
- Frade JM. Nuclear translocation of the p75 neurotrophin receptor cytoplasmic domain in response to neurotrophin binding. J Neurosci. 2005;25:1407–1411. doi: 10.1523/JNEUROSCI.3798-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbay B, Heape AM, Sargueil F, Cassagne C. Myelin synthesis in the peripheral nervous system. Prog Neurobiol. 2000;61:267–304. doi: 10.1016/s0301-0082(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22:2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa NA, Rubinstein JT, Lowder MW, Tyler RS, Gantz BJ. Residual speech perception and cochlear implant performance in postlingually deafened adults. Ear Hear. 2003;24:539–544. doi: 10.1097/01.AUD.0000100208.26628.2D. [DOI] [PubMed] [Google Scholar]
- Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol. 2003;461:174–186. doi: 10.1002/cne.10692. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001;161:87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Developmental biology. 1995;167:227–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB., Jr. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115:672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Jr., Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–422. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HW, Stoll G. Nerve injury and regeneration: basic insights and therapeutic interventions. Curr Opin Neurol. 1998;11:557–562. doi: 10.1097/00019052-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr. Degeneration of cochlear neurons as seen in the spiral ganglion of man. Hear Res. 1990;49:141–154. doi: 10.1016/0378-5955(90)90101-t. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Zampieri N, Chao MV. Nuclear localization of the p75 neurotrophin receptor intracellular domain. The Journal of biological chemistry. 2010;285:5361–5368. doi: 10.1074/jbc.M109.045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D'Antonio M, Mirsky R, Jessen KR. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164:385–394. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Provenzano MJ, Xu N, Ver Meer MR, Clark JJ, Hansen MR. p75NTR and sortilin increase after facial nerve injury. Laryngoscope. 2008;118:87–93. doi: 10.1097/MLG.0b013e31814b8d9f. [DOI] [PubMed] [Google Scholar]
- Reynolds ML, Woolf CJ. Reciprocal Schwann cell-axon interactions. Curr Opin Neurobiol. 1993;3:683–693. doi: 10.1016/0959-4388(93)90139-p. [DOI] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg. 2005;13:294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Schor NF. The p75 neurotrophin receptor in human development and disease. Prog Neurobiol. 2005;77:201–214. doi: 10.1016/j.pneurobio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242:100–109. doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol Suppl. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syroid DE, Maycox PJ, Soilu-Hanninen M, Petratos S, Bucci T, Burrola P, Murray S, Cheema S, Lee KF, Lemke G, Kilpatrick TJ. Induction of postnatal schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. J Neurosci. 2000;20:5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Shepherd RK. Aminoglycoside-induced degeneration of adult spiral ganglion neurons involves differential modulation of tyrosine kinase B and p75 neurotrophin receptor signaling. Am J Pathol. 2006;169:528–543. doi: 10.2353/ajpath.2006.060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Johnson EM., Jr. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Schweitzer JB, Johnson EM., Jr. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Rahn JJ, Lun X, Sun B, Kelly JJ, Weiss S, Robbins SM, Forsyth PA, Senger DL. Gamma-secretase represents a therapeutic target for the treatment of invasive glioma mediated by the p75 neurotrophin receptor. PLoS Biol. 2008;6:e289. doi: 10.1371/journal.pbio.0060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M, Webster DB. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.