Abstract
Fungi and animals constitute sister kingdoms in the eukaryotic domain of life. The major classes of transporters, channels, sensors, and effectors that move and respond to calcium ions were already highly networked in the common ancestor of fungi and animals. Since that time, some key components of the network have been moved, altered, relocalized, lost, or duplicated in the fungal and animal lineages and at the same time some of the regulatory circuitry has been dramatically rewired. Today the calcium transport and signaling networks in fungi provide a fresh perspective on the scene that has emerged from studies of the network in animal cells. This review provides an overview of calcium signaling networks in fungi, particularly the model yeast Saccharomyces cerevisiae, with special attention to the dominant roles of acidic calcium stores in fungal cell physiology.
Calcium Signaling Pathways in the Fungal Cytoplasm
Dozens of genome sequencing projects on phylogenetically diverse fungal species have revealed a basic toolkit of Ca2+-binding proteins and Ca2+ pumps, exchangers, and channels and the remarkable conservation of the signaling network across the fungal kingdom [1]. The central Ca2+ sensor calmodulin can be easily spotted in the genomes of all sequenced fungi. Despite some lineage-specific drift in the amino acid sequence of yeast calmodulins, many targets of Ca2+/calmodulin are also well preserved throughout the kingdom. Examples include two families of serine/threonine protein kinases and a family of serine/threonine protein phosphatases known as calcineurin. As in animal cells, these kinases and phosphatases in the bakers yeast S. cerevisiae and in other fungi become activated upon binding of Ca2+/calmodulin to conserved sequences within their autoregulatory tails and displacement of autoinhibitory motifs from their active sites (reviewed in [2, 3]). Thus, the rise and fall of free Ca2+ concentrations in the cytoplasm can be directly sensed, decoded, and retransmitted to cellular targets through regulated protein phosphorylation and dephosphorylation. Myosins and other well-known targets of calmodulin have also been described in S. cerevisiae and many other fungi. Additionally, the genomes contain a spectrum of conserved proteins that bear EF-hand and C2 domains, which bind Ca2+ and may respond to fluctuation Ca2+ concentrations in their microenvironments. The emerging picture from these accounts is one where a multitude of Ca2+-responsive regulatory pathways exist in fungal cells.
One of the best-studied Ca2+-responsive signaling pathways in fungi (see Fig. 1) involves the calcineurin-dependent dephosphorylation of Crz1, a zinc-finger transcription factor first described in S. cerevisiae [4, 5]. Crz1 is not related to the NFAT family of calcineurin-sensitive transcription factors that famously control many calcineurin-dependent processes in mammals. Similar to the NFAT story, activated calcineurin binds to canonical PxIxIT-like motifs and dephosphorylates several residues in Crz1, resulting in a conformational change that hides a nuclear export signal and exposes a nuclear localization signal (respectively recognized by the β-importins Msn5 and Nmd5) [6–8]. After transport of dephosphorylated Crz1 into the nucleus, the Zn-finger domain binds specific DNA sequences (termed CDREs for Crz1-Dependent Response Elements) present within the promoter regions of target genes and significantly increases expression above the resting basal level [6–12]. Inducible targets of Crz1 include CRZ1 that encodes Crz1 [5], RCN1 and RCN2 that encode positive and negative regulators of calcineurin [13, 14], CMK2 that encodes a Ca2+/calmodulin-dependent protein kinase [15], PMC1, PMR1, and ENA1 that encode several P-type cation pumps responsible for efflux of Ca2+, Mn2+, Na+, and Li+ [5] and 60 to 100 other genes that control other processes [16, 17]. Mutants of S. cerevisiae that lack Crz1 are hypersensitive to high environmental concentrations of these cations as a consequence of failed induction of the cation pumps and they exhibit other phenotypes that can be attributed to failed induction of other targets. Calcineurin-deficient mutants of S. cerevisiae exhibit an even larger set of phenotypes due to defects in the regulation of phosphoproteins other than Crz1. For example, calcineurin-dependent dephosphorylation of Hph1/Hph2 and Slm1/Slm2 protein pairs can alter sensitivity to high pH medium and alter trafficking of secretory and endocytic cargo proteins [18, 19]. Calcineurin-dependent feedback regulation of Ca2+ channels and Ca2+ transporters will be discussed more fully later in this review. An overview of the known and suspected calcineurin targets in S. cerevisiae is given in Figure 1.
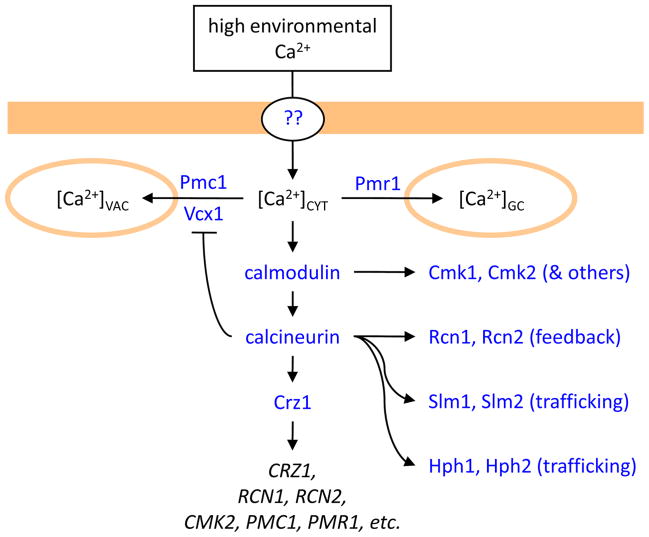
Figure 1. Ca2+ transport and signaling in S. cerevisiae cells exposed to high environmental Ca2+.
Ca2+ enters the cell through unknown pathways and elevates [Ca2+]CYT, which results in activation of calmodulin, calcineurin, and a transcription factor (Crz1) that induces numerous genes. Activated calmodulin and calcineurin also regulate other cellular factors such as protein kinases (Cmk1, Cmk2), feedback regulators of calcineurin (Rcn1, Rcn2), membrane trafficking factors (Slm1, Slm2, Hph1, Hph2), and a vacuolar Ca2+/H+ exchanger (Vcx1). Ca2+ pumps in the vacuole (Pmc1) and the Golgi complex (Pmr1) become up-regulated and help to lower [Ca2+]CYT to non-toxic levels.
In vegetatively growing S. cerevisiae cells, Crz1 is fully phosphorylated, localized to the cytoplasm, and transcriptionally inactive [16, 20]. The simple inference from all these findings is that cytosolic free Ca2+ concentrations are maintained at low non-signaling levels in vegetatively growing S. cerevisiae cells and that [Ca2+]CYT levels rise to levels capable of activating calcineurin and Crz1 in response to specific stimuli or stresses. Thus, cells of S. cerevisiae and its relatives probably behave very much like mammalian cells in their ability to dynamically control [Ca2+]CYT and the downstream signaling pathways. A series of conserved Ca2+ pumps, exchangers, transporters, and channels accomplish that important task in fungal and animal cells. They also control intracellular pools of Ca2+ that have many important functions.
Ca2+ in secretory organelles and store-operated Ca2+ influx
The increasingly acidic lumens of the nuclear envelope, endoplasmic reticulum, Golgi complex, and trans-Golgi/endosomal network contain an array of conserved Ca2+ dependent enzymes that are involved in various aspects of protein secretion (reviewed in [21]). For instance, S. cerevisiae retains homologs of BiP, calnexin, UDP-glucose-glucosyltransferase, glucosidase II, and ERGIC-53 (termed Kar2, Cne1, Kre6, Rot2, and Emp46/47, respectively) in its nuclear envelope and endoplasmic reticulum [22–24]. In S. cerevisiae, many of these enzymes no longer retain an ability to bind Ca2+ and can function independently of Ca2+. Such adaptations are probably related to the loss of SERCA-family Ca2+ pumps that normally supply the endoplasmic reticulum with sufficient Ca2+ for secretory functions. SERCA was probably present in the common ancestor of fungi and animals and subsequently lost several different times independently in the evolution of Ascomycetes (moulds, yeasts), Basidiomycetes (mushrooms, smuts, rusts), and other fungal phyla. A SERCA-family Ca2+ pump is expressed in the endoplasmic reticulum of the mould Neurospora crassa [25] but this enzyme has not yet been characterized biochemically or genetically. Though S. cerevisiae and other budding yeasts do not retain a SERCA-family Ca2+ pump and may have secretory machinery with reduced Ca2+ dependence, Ca2+ starvation of S. cerevisiae cells still causes activation of the so-called Unfolded Protein Response (UPR) signaling pathway that emanates from the endoplasmic reticulum upon its accumulation of misfolded or unassembled secretory proteins [26]. Inhibitors of SERCA elicit similar UPR responses in animal cells. Ca2+ starvation can also decrease the retention of foreign proteins expressed in yeasts [27] and thereby enhance the yield of recombinant protein preparations. Thus, luminal Ca2+ performs important secretory functions in the fungal endoplasmic reticulum, even in S. cerevisiae where both the supply and the demand seem greatly diminished.
The endoplasmic reticulum of S. cerevisiae concentrates Ca2+ approximately 100-fold relative to the cytoplasm largely through the Ca2+ transport activity of Pmr1 [28], the prototypical member of the SPCA-family of Ca2+/Mn2+ pumps that are widely distributed among fungi, animals, and other eukaryotic kingdoms. Pmr1 localizes primarily to the Golgi complex of S. cerevisiae [27, 29], like its homologs in mammals [30], and therefore supplies Ca2+ and Mn2+ to the endoplasmic reticulum during its early biogenesis or through vesicle-mediated trafficking in the retrograde direction from the Golgi complex. Mutants of S. cerevisiae that lack the Pmr1 exhibit a range of secretion defects that can be largely attributed to underperformance of late secretory pathway enzymes. A homolog of the Ca2+-dependent pro-protein convertases or furins (termed Kex2) is highly dependent on Pmr1 function [29, 31]. A Ca2+-dependent lectin-like protein involved in sorting of specific cargo proteins to the lysosome-like vacuole (termed Vps10) also depends on Pmr1 for proper function [32]. The normal retention of foreign secretory proteins that are expressed heterologously in S. cerevisiae also depends on Pmr1 [27, 33]. These defects of Pmr1-deficient mutants can be suppressed by elevating Ca2+ salts in the culture medium or by expressing SERCA in the endoplasmic reticulum, suggesting they are specifically a consequence of luminal Ca2+ insufficiency [32]. On the other hand, defects in N-glycosylation and O-glycosylation of secretory cargo are attributable to Mn2+ insufficiency in the Golgi complex [32]. All these findings are consistent with the hypothesis that the SPCA-family pump Pmr1 supplies the majority of the Ca2+ and Mn2+ that is crucial for normal processing functions in both the endoplasmic reticulum and Golgi complex of S. cerevisiae.
Many cell types in animals are known to employ store-operated Ca2+ entry mechanisms in which Ca2+ influx channels in the plasma membrane become activated in response to depletion of Ca2+ from the lumen of the endoplasmic reticulum. In animals, depletion of Ca2+ stores occurs physiologically through the repetitive activation of IP3-receptors or other Ca2+ channels located in the endoplasmic reticulum. IP3-receptors are not evident in any of the fungal genomes sequenced to date though they are clearly present in animals and amoebas such as Dictyostelium discoideum, which probably diverged before the fungal/animal bifurcation. Moreover, the CRAC/OraI Ca2+ influx channels and the Stim1 Ca2+ sensors in the endoplasmic reticulum that are both essential for store-operated Ca2+ influx in animals are completely absent in the known fungal genomes. In spite of these key differences, the Pmr1-deficient mutants of S. cerevisiae exhibit a much higher rate of Ca2+ influx, elevated [Ca2+]CYT, and activated calcineurin relative to wild-type cells [34], analogous to the situation in animal cells when luminal Ca2+ in the endoplasmic reticulum is depleted.
The mechanism of store-operated Ca2+ influx in S. cerevisiae has been partially unraveled (see Fig. 2). One consequence of Pmr1-deficiency is the up-regulation and mislocalization of Pmc1 [35], a PMCA-type Ca2+ pump located in the vacuole that can partially suppress the secretory defects of Pmr1-deficient mutants [36]. The up-regulation of Pmc1 is a consequence of increased Ca2+ influx through a high-affinity Ca2+ influx system (termed HACS) that activates calcineurin and Crz1 [34]. HACS requires three interacting proteins: Cch1, a homolog of the catalytic α-subunit of voltage-gated Ca2+ channels in animals [37, 38], Mid1, which bears some similarity to the α2δ-subunit of voltage-gated Ca2+ channels in animals [39, 40], and Ecm7, a homolog of regulatory γ-subunits of voltage-gated Ca2+ channels in animals [41]. The Cch1 subunit of HACS becomes phosphorylated in response to depletion of secretory Ca2+ stores in S. cerevisiae by a process that involves activation of a MAP kinase (termed Slt2) and a cascade of upstream protein kinases that couple to rho-type GTPase activation [42]. Though Slt2 is required for HACS activation in these conditions [42], it is not yet clear whether the phosphorylation of Cch1 is necessary or sufficient for HACS activation. Nevertheless, the store-operated Ca2+ entry mechanism in S. cerevisiae is strikingly different from the STIM/Orai mechanism of animal cells.
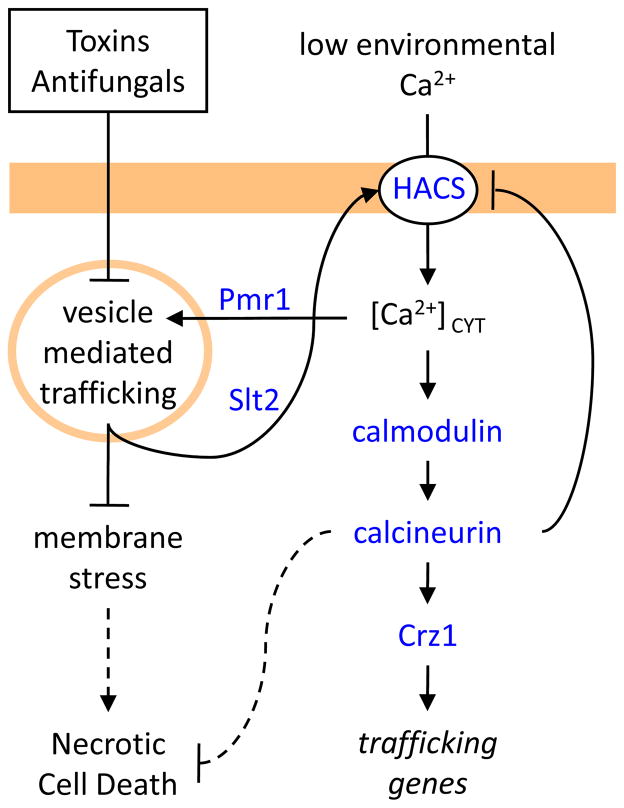
Figure 2. Ca2+ transport and signaling in S. cerevisiae cells exposed to membrane-active toxins and antifungal agents.
Toxins (e.g. tunicamycin) and antifungals (e.g. fluconazole) that disrupt secretory protein biogenesis or membrane biogenesis lead to membrane stresses and activation of a MAP kinase (Slt2) that activates a high-affinity Ca2+ influx system (HACS; Cch1, Mid1, Ecm7). Downstream signaling pathways help to alleviate the stress by boosting secretory performance. Mutants that lack the secretory pathway Ca2+ pump (Pmr1) or other factors important in vesicle-mediated trafficking pathways (e.g. Kex2) result in membrane stresses that constitutively activate Slt2 and HACS. Cells containing calcineurin survive these stresses whereas cells lacking calcineurin die by poorly understood necrosis-like mechanism.
The HACS of S. cerevisiae can also be activated by other deficiencies in the secretory pathway that do not result in Ca2+ depletion. Misfolded proteins in the endoplasmic reticulum can activate Slt2 and HACS by a process that is independent of signaling through the UPR signaling pathway [26]. Defects in an ER-localized phospholipid flipase also promote Ca2+ alterations [43]. Defects in protein trafficking to the vacuole similarly activate HACS [44]. A recent screen of all the viable gene knockout mutants of S. cerevisiae demonstrated HACS can be strongly activated by deficiencies in several dozen different enzymes of the vacuole protein sorting and protein secretion pathways, including the Kex2-deficiency that arises in Pmr1-deficient cells [41]. Thus, a wide variety of stresses in the vesicle mediated trafficking system seem to be capable of activating HACS in S. cerevisiae. Depletion of secretory Ca2+ stores is but one of many ways to generate a HACS-activating stress. The induction of Ca2+ pumps (Pmc1 and Pmr1) by Crz1 and calcineurin is probably one of many compensatory responses controlled by HACS.
Membrane stresses may be common in fungal and animal cells as a consequence of interactions with the environment and toxins that attack them. Microbes often secrete compounds that are toxic to eukaryotic competitors and predators through their effects on key enzymes of secretory pathway. One such compound secreted by Bacillus lysosuperficus is tunicamycin, which enters the eukaryotic cell and potently inhibits an essential enzyme necessary for N-glycan biogenesis [45]. The resulting blockade in protein N-glycosylation in the endoplasmic reticulum of S. cerevisiae causes the accumulation of misfolded and unassembled secretory proteins. While the UPR pathway becomes rapidly activated in response to tunicamycin exposure, activation of the Slt2 MAP kinase cascade, HACS, and calcineurin proceeds much more slowly [26]. The faster response induces molecular chaperones and other factors that repair the damage and help the S. cerevisiae cells adapt and recover from a brief exposure to the toxin. But what are the benefits of the slower response pathway involving Ca2+ ? Those benefits are not completely clear but they seem to be essential to survival of the stressed cell. Mutants of S. cerevisiae and other yeasts that lack HACS or calcineurin rapidly die upon exposure to tunicamycin [15]. In contrast, mutants that lack the UPR pathway remain alive indefinitely in the presence of tunicamycin but cannot repair enough of the damage to proliferate once the compound has been removed [26, 46]. Thus, the calcium signaling pathways of S. cerevisiae may be a life-saving countermeasure against microbial assaults. The invention of novel toxins that target calcineurin, such as FK506 from Streptomyces tsukubaensis [47], may represent a counter-countermeasure in a chemical arms race between bacterial and fungal competitors.
Common antifungal antibiotics use to combat fungal infections in humans seem to mimic the effects of the natural toxins described above. For example, the antifungals that target sterol biosynthetic enzymes in the endoplasmic reticulum of fungal pathogens (fluconazole, miconazole, terbinafine, fenpropimorph, and several others) probably activate essential functions of HACS and calcineurin because the co-administration of these agents together with either FK506 or cyclosporine (another potent inhibitor of calcineurin) usually results in potent fungicidal effects rather than simple fungistatic effects when used alone [26, 48–54]. Inhibitors of sterol biosynthesis are not known to activate or inhibit the UPR, so they probably trigger membrane stresses akin to those produced by tunicamycin or mutations in the non-essential genes of the secretory pathway described earlier. The fungicidal synergism of calcineurin inhibitors and sterol biosynthesis inhibitors is broadly conserved in diverse fungal pathogens (reviewed in [55, 56]), which suggests that calcineurin activation in response to membrane stresses may be a widespread occurrence in the fungal kingdom.
To summarize the key differences between fungi and animals with regard to Ca2+ in the secretory pathway, animals possess a unique store-operated Ca2+ entry mechanism that replenishes the endoplasmic reticulum and other secretory compartments after IP3 signals their depletion. Fungi completely lack the IP3 receptors, luminal Ca2+ sensors, and Ca2+ influx channels that constitute the core of this system. On the other hand, depletion of Ca2+ from secretory organelles in fungi can produce membrane stress and activation of a MAP kinase cascade that promotes Ca2+ influx through HACS-type channels. A wide array of toxins, mutations, and antifungal drugs may create similar types of membrane stress and similar effects on HACS. The resulting rise in [Ca2+]CYT not only resupplies the secretory organelles but signals through calmodulin, calcineurin, and Crz1 to regulate processes that may restore luminal Ca2+, mitigate membrane stress, and promote cell survival.
The vacuole and Ca2+ sequestration/release mechanisms
Organelles of the secretory pathway in fungi, like those of mammalian cells, are expected to become increasingly acidic as a consequence of increasing activity of H+-pumping V-ATPases. Fungi also contain very acidic organelles termed vacuoles that are acidified by V-ATPases and, like lysosomes in animals, are constructed using well-conserved vesicle-mediated trafficking pathways [57]. The bread mould Neurospora crassa contains several different types of vacuoles that can be distinguished by morphological and compositional criteria [25]. It is not yet clear if those vacuoles represent different intermediates in a pathway of vacuole maturation or different endpoints. The yeast S. cerevisiae contains one to several vacuoles that can undergo cycles of invagination, fusion, and fission in response to cell cycle and environmental triggers. A remarkable feature of fungal vacuoles is their ability to sequester large amounts of Ca2+ and release it in response to particular stimuli (see Fig. 3).
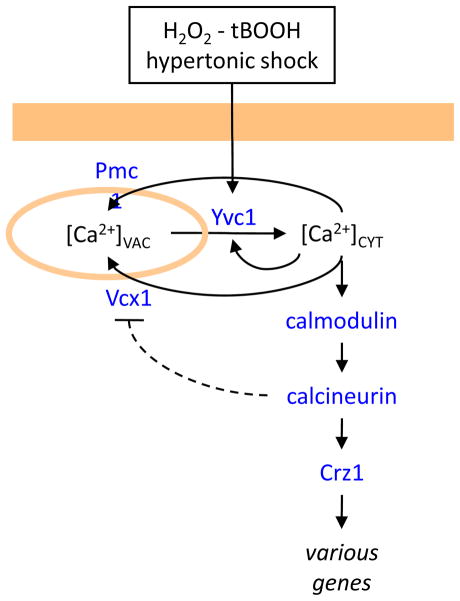
Figure 3. Ca2+ release from acidic vacuoles in S. cerevisiae cells.
The vacuolar TRPC-family ion channel (Yvc1) becomes rapidly activated in response to hydrogen peroxide (H2O2) or tert-butylhydroperoxide (tBOOH). Hypertonic shock (e.g. high salt or sugar in the environment) triggers a slower activation of Yvc1, possibly through stretching of the vacuole membrane. Both stimuli result in transient elevation of [Ca2+]CYT that is reversed by the actions of vacuolar Ca2+ transporters (Pmc1, Vcx1). The activation of downstream signaling pathways has unknown functions.
In the yeast S. cerevisiae, more than 90% of total cell-associated Ca2+ is immobilized in the vacuole largely in complexes with inorganic polyphosphate [58]. The vacuole probably receives a small amount of Ca2+ through the fusion of vesicles derived from the Golgi complex that received its Ca2+ directly from SPCAs or indirectly from the environment through endocytosis. A large majority of vacuolar Ca2+ comes directly from the cytoplasm through the action of Ca2+ pumps and Ca2+/H+ exchangers that are specifically localized to the vacuole membrane.
The vacuolar Ca2+ pump of S. cerevisiae (termed Pmc1) is the major contributor of vacuolar Ca2+ [36]. Pmc1 is conserved in nearly all fungi and is closely related to the PMCA-family of plasma membrane Ca2+ ATPases found in animals, plants, and other kingdoms of eukaryotes. However, Pmc1 has not been observed in the plasma membrane of S. cerevisiae and only has been observed in the vacuole membrane, though Pmr1-deficient mutants induce Pmc1 expression and accumulate some fraction in earlier secretory compartments [35]. Overexpression of Pmc1 can partially suppress the secretory defects associated with Pmr1-deficient mutants [36], suggesting some ability to transport Ca2+ while en route to the vacuole. The simultaneous loss of both Pmc1 and Pmr1 is lethal to S. cerevisiae, though lethality can be avoided in conditions that increase activity of the vacuolar Ca2+/H+ exchanger (Vcx1) [59]. The loss of Pmc1 alone is not lethal in ordinary growth conditions, though vacuoles contain only ~10% of the normal level of Ca2+. Pmc1-deficient mutants of S. cerevisiae are strongly hypersensitive to supplemental Ca2+ salts in the culture medium. Wild-type S. cerevisiae respond to high Ca2+ environments with a large induction of Pmc1 expression and a small induction of Pmr1 expression, both mediated by the activation of calcineurin and Crz1 [4, 5]. When taken altogether, these findings suggest that Pmc1 and the vacuole play a major role in the detoxification of cytoplasmic Ca2+ and perhaps a minor role in supplying the secretory pathway, where Pmr1 normally predominates.
A vacuolar Ca2+/H+ exchanger of S. cerevisiae (termed Vcx1) was identified genetically based on its ability to confer tolerance to high Ca2+ and high Mn2+ salts in the culture medium when the enzyme is overexpressed [59, 60]. Earlier experiments had predicted that Vcx1 was responsible for most Ca2+ transport into the vacuole in vivo in normal growth conditions [58]. However, Vcx1-deficient knockout mutants exhibited wild-type levels of vacuolar Ca2+ and wild-type tolerance to supplemental Ca2+ salts in the culture medium [59, 60], in striking contrast to the aforementioned Pmc1-deficient mutants. On the other hand, the contributions of Vcx1 to vacuolar Ca2+ uptake and to Ca2+ tolerance are markedly increased when calcineurin is inactivated by either mutations or inhibitors [59]. These findings suggest that calcineurin effectively blocks Vcx1 activity in vivo. Consistent with this view, several different single amino-acid substitutions in Vcx1 have been recovered that greatly increase its activity even when calcineurin is functioning [59, 61, 62]. Such hyperactive variants of Vcx1 can often transport Mn2+ in addition to Ca2+ and compete with Pmr1 in the Golgi complex for substrates, thus depriving the secretory pathway of essential minerals and triggering membrane stress described in the previous section [59]. The mechanism by which calcineurin inhibits Vcx1 function is not yet known. Crz1 and the other known targets of calcineurin are not required for the calcineurin-dependent inhibition of Vcx1. Vcx1 abundance and gel mobility also do not change in a calcineurin-dependent fashion. Therefore the regulation of Vcx1 may be indirect, for example through a calcineurin-dependent inhibition of the V-ATPase or some other factor that impinges on Vcx1 but not Pmc1. It will be important to define the molecular basis of this interaction because Vcx1 also inhibits calcineurin by removing Ca2+ from the cytoplasm that is necessary for calcineurin activation by calmodulin. Thus, Vcx1 and calcineurin form a double-negative feedback interaction. Double-negative feedback loops are often capable of producing bi-stability, a phenomenon where the network switches rapidly between two stable states but does not significantly populate the intermediate states [63, 64]. For instance, the Vcx1-on/calcineurin-off state may rapidly switch to the Vcx1-off/calcineurin-on state, and vice versa, which might then contribute to the dramatic “spikes” of [Ca2+]CYT elevation and “bursts” of Crz1 localization to the nucleus that have been recently observed in S. cerevisiae cells [20]. For several minutes following exposure to high Ca2+ salts, Vcx1 remains very effective at lowering [Ca2+]CYT [65], but what happens after calcineurin activation has not been thoroughly investigated. Obviously, more work needs to be completed before the roles of Vcx1 in Ca2+ homeostasis and signaling are fully understood.
Vcx1 is a member of the CAX family of Ca2+/H+ exchangers, which are not found in animals but are expressed widely in plants and many other eukaryotes [66]. Vcx1 also retains some weak Na+/H+ exchange activity, at least in vitro [67]. The VNX family of cation exchangers (formerly called type II CAX exchangers) from S. cerevisiae and the zebrafish Danio rerio function as Na+/H+ exchangers in the vacuole membrane [68, 69]. Fungi lack members of NCX and NCKX families of Ca2+/Na+ exchangers that have been well studied in animal cells [70]. Fungi also express members of the CCX family of cation/cation exchangers that are expressed in animals, but very poorly characterized. The sole CCX protein of mammals (termed NCLX or NCKX6) functions as a Ca2+/Na+ exchanger in the plasma membrane and mitochondria [71–73]. No fungal CCX proteins have been characterized to date, so their potential contributions to Ca2+ efflux, storage, and signaling are wholly unknown at present.
In S. cerevisiae cells grown in standard conditions, vacuolar free Ca2+ concentration ([Ca2+]VAC) has been estimated at ~30 μM whereas total vacuolar Ca2+ has been estimated at ~3 mM [58], suggesting that 99% of vacuolar Ca2+ is buffered by inorganic polyphosphate. In spite of complete releasability of vacuolar Ca2+ by the ionophore A23187, little or no vacuolar Ca2+ is released into the cytoplasm or the culture medium when S. cerevisiae cells are cultivated in standard culture medium [36, 58]. Therefore, very little Ca2+ is released from the vacuole during proliferation in normal conditions. The possibility that Ca2+ stored in the vacuole can be released and reutilized during Ca2+ starvation has not been explored. In conditions of Mg2+ starvation, Ca2+ influx via unknown transporters in the plasma membrane is dramatically increased [74] and the resulting increases in vacuolar Ca2+ probably serve to displace Mg2+ from binding sites on inorganic polyphosphate and promote Mg2+ reutilization elsewhere in the cell [75]. Vacuolar H+ probably affects this process too. The possibility that Vcx1 releases Ca2+ from the vacuole in some conditions also has not been explored fully. Vcx1 and the other cation/cation exchangers are generally reversible if the substrate concentrations are favorable on both sides of the membrane. A sudden loss of vacuole acidity (or uncoupling) will not only prevent movement of Ca2+ through Vcx1 into the vacuole but will potentially increase the reverse-mode transport activity of Vcx1, effectively allowing Ca2+ release and reutilization [76]. To date, the clearest examples of regulated Ca2+ release from the vacuole occurs via via TRPC-family ion channels (termed Yvc1).
Yvc1 is well conserved among fungi and the family is most closely related to the TRPC-family of Ca2+ channels that are well characterized in animals [77]. Yvc1 is localized almost exclusively to the vacuole membrane of S. cerevisiae [78]. Yvc1-dependent Ca2+ release occurs within ~2 seconds of injecting hydrogen peroxide or tert-butylhydroperoxide into the culture medium [79]. These compounds are membrane permeable oxidants that, over time, cause production of reactive oxygen species and a range of oxidative damage, in addition to compensatory responses. Yvc1 is detrimental to S. cerevisiae cell growth in the presence of hydrogen peroxide or tert-butylhydroperoxide [79], which suggests that the release of vacuolar Ca2+ or other cations is somehow toxic in the presence of these oxidants.
Yvc1-dependent Ca2+ release also occurs with much slower and more prolonged kinetics after exposure of S. cerevisiae cells to hypertonic conditions such as high salinity and high sugar [78]. A broad survey of genes that alter Yvc1 function in S. cerevisiae did not reveal any candidates for direct regulators of Yvc1 [80]. The screen revealed instead a broad correlation between vacuolar Ca2+ content and the amount of Ca2+ released upon hyperosmotic shock. However, a recent study identified Fab1, a PI(3)P 5-kinase that synthesizes phosphatidylinositol-3,5-bisphosphate, as essential for Yvc1 activation in response to hyperosmotic shock [81]. Electrophysiological studies of Yvc1 in isolated vacuoles show that channel gating can be activated by membrane stretch, suggesting Yvc1 is directly mechanosensitive [82]. Extensive electrophysiological characterizations also suggest activation by reducing agents and an ability to pass Na+ and K+ in vitro in addition to Ca2+ [77, 82–85]. These properties suggest that Yvc1 may be capable of rapidly releasing cationic osmolytes into the cytoplasm upon hypertonic shock, which may provide temporary relief against cytoplasmic dehydration and osmotic imbalance. This prediction has not yet been tested with viability experiments, so it remains possible that Yvc1 activation is neutral or even harmful in these conditions. Clearly, much remains to be learned about the physiological roles of Yvc1 in S. cerevisiae and other fungi.
An interesting, yet counterintuitive, property of Yvc1 is its dependence on cytosolic Ca2+ for maximal gating (reviewed in [86]). This finding may indicate a role for auto-activation in Yvc1 physiology by a process commonly known as Ca2+-induced Ca2+ release. In animals, Ca2+-induced Ca2+ release contributes to the coordinated release of Ca2+ from the endoplasmic or sarcoplasmic reticulum for the purpose of generating coherent waves of [Ca2+]CYT elevation. The existence of this phenomenon in S. cerevisiae is surprising because of the well-established role of the vacuole in detoxifying Ca2+ after exposure to high Ca2+ salts in the environment. If Yvc1 becomes activated by [Ca2+]CYT elevation and remains active in the vacuole membrane for long periods of time, it is difficult to imagine how Pmc1 and Vcx1 can effectively diminish and detoxify [Ca2+]CYT. Strains of S. cerevisiae that lack Yvc1 are not detectably hypersensitive to high Ca2+ environments and do not exhibit an increased activation of calcineurin. However, strains that overexpress Yvc1 exhibit hypersensitivity to environmental Ca2+ [78] but the potential deleterious effects of Na+ and K+ release have not been discriminated from Ca2+ release in this instance. Thus, similar to the confusing state of affairs with Vcx1, the potential feedback regulation of Yvc1 is both fascinating and crucial for understanding global Ca2+ homeostasis and signaling in fungi.
Ca2+ has been shown to leak from purified vacuoles at a defined step in the process of homotypic (vacuole to vacuole) fusion [87–90], which is one of several processes that dynamically synthesize, reshape, and redistribute vacuoles in S. cerevisiae. Originally this Ca2+ leak was thought to be important for triggering the formation of protein complexes and fusion pores near the end of the homotypic fusion process through effects on calmodulin. However, subsequent work showed that the Ca2+ binding sites in calmodulin were not required for homotypic fusion of vacuoles [90] and that Ca2+ chelators blocked homotypic fusion in vitro not by buffering Ca2+ but by changing the ionic strength of the reaction buffer [91]. Furthermore, vacuolar Ca2+ was not required for homotypic fusion because vacuoles that lack both Pmc1 and Vcx1 fuse as efficiently as normal vacuoles [88]. The release of Ca2+ correlates with the formation of inter-vacuolar SNARE complexes [90] but does not require Yvc1 [77]. Though it remains possible that some other vacuolar Ca2+ channel or transporter and some other targets of Ca2+ in the cytoplasm will be discovered during the process, the evidence currently favors a model where Ca2+ release from vacuoles during homotypic fusion is coincidental rather than purposeful.
Finally, there has been a report of inositol-1,4,5-trisphosphate (IP3)-dependent release of Ca2+ from purified S. cerevisiae vacuoles [92]. The IP3-sensitive channel or transporter has not yet been identified and, as mentioned earlier, homologs of the known IP3-receptors found in animals and amoebas are not evident in fungi. Yvc1 also fails to respond to IP3 [77]. Evidence for IP3-sensitive Ca2+ influx pathways, on the other hand, is growing.
IP3-sensitive Ca2+ influx
Two different experimental regimens have been shown to trigger Ca2+ influx into S. cerevisiae cells in a fashion that depends on phospholipase C (see Fig. 4). The first regimen involves the use of protonophores (carbonyl cyanide m-chlorophenylhydrazone [CCCP]), which are thought to promote influx of H+ in acidic media but may also uncouple vacuolar and mitochondrial membranes. Within seconds of CCCP addition, wild-type S. cerevisiae cells exhibit robust Ca2+ influx and elevation of [Ca2+]CYT [93]. Extracellular Ca2+ and phospholipase C (termed Plc1) were absolutely required for these effects. Additionally, the rise of [Ca2+]CYT is augmented by elimination of an IP3-kinase (termed Arg82) that consumes IP3. These findings suggest H+ influx may activate Plc1 and the resulting rise of IP3 production may activate Ca2+ influx pathways. Within a minute of [Ca2+]CYT elevation, the plasma membrane H+-ATPase (termed Pma1) primarily responsible for maintenance of cytoplasmic pH becomes significantly activated [93]. Consistently, Pma1 activation in response to protonophores is dampened by the activities of Arg82 (IP3-kinase) and Pmc1 (vacuolar Ca2+ pump) and is dependent on the activities of Plc1 and Pkc1 (protein kinase C) which may directly phoshorylate the C-terminus of Pma1 [93]. It is not yet known how H+ influx might activate Plc1. Identification of the proposed IP3-sensitive Ca2+ influx channel or transporter will be a key advance in this field.
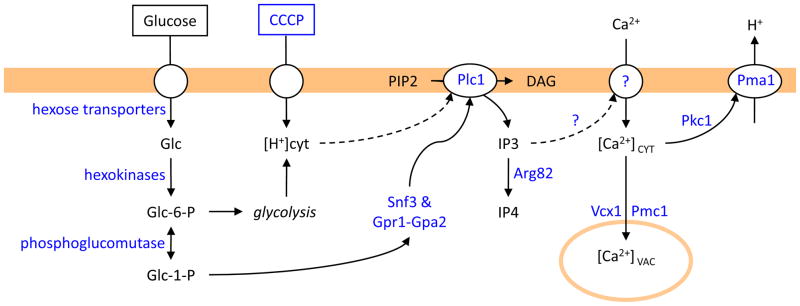
Figure 4. IP3-dependent Ca2+ influx and signaling in S. cerevisiae cells in response to protonophores and sugar refeeding.
Protonphores (CCCP) stimulate H+ influx and subsequent Ca2+ influx via unknown pathways that depend on phospholipase C (Plc1) and are sensitive to IP3-kinase (Arg82). Sugar (glucose) refeeding to starved cells induces similar Plc1-dependent Ca2+ influx that also depends on glucose transporters, hexokinases, phosphoglucomutases, and glucose sensors (Snf3; Gpr1-Gpa2). Both stimuli result in transient elevation of [Ca2+]CYT and activation of a plasma membrane H+ pump (Pma1) through a process that may depend on protein kinase C (Pkc1).
The second regimen that triggers Plc1-dependent Ca2+ influx and Pma1 activation in S. cerevisiae cells is sugar starvation and refeeding [94–96]. Glucose refeeding to starved cells induces a rise of [Ca2+]CYT within ten seconds and an increase of Pma1 activity within minutes. Hexose transporters, hexokinases/glucokinases, and phosphoglucomutases are all required for the [Ca2+]CYT elevation upon glucose refeeding [65, 95, 97], indicating that elevated concentrations of glucose-1-phosphate (or a derivative) may be crucial for the effect. The glucose metabolite may be sensed by receptors (termed Snf3 and Gpr1), the latter of which couples to a heterotrimeric G-protein (termed Gpa2) that may somehow activate Plc1 [95, 98]. Alternatively, glucose metabolites may trigger H+ influx similar to the effects of protonophores described earlier or glucose metabolism in general may acidify the cytoplasm directly. In any case, Plc1 is required for waves of IP3 production and the subsequent [Ca2+]CYT rise whereas Arg82 dampens these effects [96]. Though the molecular targets of intracellular IP3 is not yet known, observations suggest that HACS plays either a large role [97] or a small role [98] in the Ca2+ influx that occurs after glucose refeeding. The resulting rise of [Ca2+]CYT is more strongly dissipated by Vcx1 than by Pmc1 [99]. Though glucose refeeding can activate calcineurin and Crz1 [100], the primary role of the [Ca2+]CYT elevation may be the activation of Pma1 and the maintenance of cellular pH during bursts of glycolytic activity [94].
Metabolic engineering experiments that cause constitutively high levels of glucose-1-phosphate accumulation cause long-term increases in Ca2+ influx, [Ca2+]CYT, and calcineurin signaling [65, 97, 101–103]. Again, the relevant sensors of intracellular glucose metabolites have not yet been identified. The regulatory network operating in these conditions may be more challenging to unravel because the growth conditions (galactose utilization in phosphoglucomutase-deficient cells) also generate stress in the endoplasmic reticulum, which activates the UPR signaling pathway [103] and probably activates HACS via the MAP kinase cascade described earlier. Because Pmc1 contributes to the stress in these long-term experiments [103], it seems possible that the constitutively elevated glucose-1-phosphate somehow diminishes Pmr1 function or enhances Ca2+ efflux from secretory compartments, thus generating secretory stresses. Therefore, the acute accumulation of glucose-1-phosphate through glucose refeeding and the chronic accumulation through metabolic engineering may utilize distinct mechanisms in the coupling to Ca2+ influx and signaling.
Much remains to be learned about the linkages between glucose metabolism, H+ influx, IP3 production, Ca2+ influx, sequestration, and signaling in S. cerevisiae and other fungi. The identification of the IP3 sensors, the glucose-1-phosphate sensors, and the relevant ion channels will be novel and critical for determining a much more realistic picture of this signaling network, which can then be compared and contrasted to related networks that exist in animal cells.
Ca2+ signaling during the mating response
Haploid cells of S. cerevisiae and many other fungi that are of complimentary mating types often undergo developmental programs that allow for their union and formation of stable diploid cells or heterokaryons. These programs are generally referred to as mating. In S. cerevisiae each of the two mating cell types secretes a peptide pheromone that arouses and attracts members of the other cell type and coordinates a process of pair-wise mate selection that culminates with cell and nuclear fusion. The pheromones, receptors, and response pathways in this model fungus are extremely well characterized as a consequence of several decades of intensive study. In terms of Ca2+ influx and signaling during mating, it is first necessary to distinguish the cells that successfully mate from those that respond to mating pheromones and fail to mate.
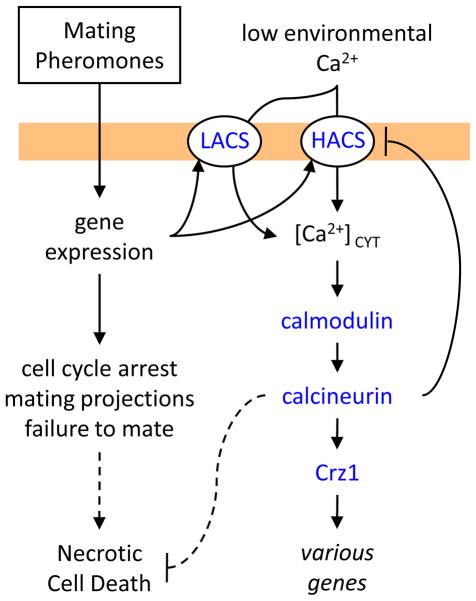
Different concentrations of mating pheromones are known to evoke different developmental programs in the responding cells. For example, low concentrations cause a cell-cycle arrest whereas moderate concentrations induce polarized growth and changes in cell morphology. Moderate concentrations of mating pheromones are required to stimulate Ca2+ influx via HACS (involving Cch1, Mid1, and Ecm7 proteins) and to elevate [Ca2+]CYT [37–39, 104, 105]. High concentrations of mating pheromones are required to stimulate a low-affinity Ca2+ influx system (LACS) that involves Fig 1, a member of the claudin superfamily of four-spanner transmembrane proteins that includes γ-subunits of voltage-gated Ca2+ channels as well as regulatory subunits of ionotropic glutamate receptors in animals [106]. As depicted in Figure 5, HACS and LACS function independently of each other but the potential for cross regulation becomes evident when considering that calcineurin may dephosphorylate Cch1 and specifically inhibit HACS in vivo [34]. Regardless of the system utilized in any particular condition, the influx of extracellular Ca2+ and the elevation of [Ca2+]CYT begin to occur after 45–60 minutes of pheromone exposure, which is a period of time that is sufficient to allow changes in cell morphology. It is not yet known whether these slow responses involve glucose-1-phosphate, IP3, membrane stresses, H+ fluxes, or other possible mediators. Nevertheless, they do result in the eventual activation of calcineurin and expression of Crz1-dependent target genes. Mutants that lack Crz1 seem to survive as long as wild-type S. cerevisiae cells in the continuous presence of mating pheromones [4, 5]. In contrast, mutants that lack calcineurin, calmodulin, or the upstream Ca2+ channels slowly die when continuously exposed to mating pheromones in the absence of mates [39, 104, 107, 108]. The manner of cell death was originally proposed as apoptosis [109] but subsequent work refutes apoptosis and suggests a necrosis-like process instead [110]. Thus, calcineurin activation is essential for long-term survival of cells that are responding to mating pheromones but are unable to find mating partners or mate.
Figure 5. Ca2+ transport and signaling in S. cerevisiae cells responding to mating pheromones in the absence of mates.
Mating pheromones elicit mating responser in haploid S. cerevisiae cells that include induction of a low-affinity Ca2+ influx system (LACS) that depends on Fig 1 and a high-affinity Ca2+ influx system (HACS) that depends on Cch1, Mid1, and Ecm7. The delayed elevation of [Ca2+]CYT results in activation of downstream signaling pathways that are essential for long-term survival of cells that do not successfully mate. Rapidly mated cells (not shown) become desensitized to mating pheromones and do not require LACS, HACS, calmodulin, or calcineurin for efficient mating.
In mixtures of the two haploid mating types of S. cerevisiae, nearly all the cells respond to secreted mating pheromones but only a small fraction of them successfully fuse to form a diploid zygote (that becomes insensitive to both mating pheromones). Removal of extracellular Ca2+ has little effect on mating efficiency, but some fusion-incompetent mutants of S. cerevisiae become more fertile when environmental Ca2+ is elevated [111]. Quantitative assays of mating efficiency show that calcineurin and calmodulin are not required in either cell type for efficient mating [107, 112, 113], so most mating is probably completed prior to the time at which the calcineurin-deficient mutant cells typically die from pheromone exposure. Mutants that lack Pmc1, Vcx1, or Yvc1 also seem to mate efficiently, suggesting the vacuolar Ca2+ stores are not critical for the mating process. Mutants that lack Pmr1 mate poorly because one of the mating pheromones, α-factor, depends on the Ca2+-dependent protease Kex2 in the Golgi complex for proteolytic activation [29]. Mutants that lack the high- or low-affinity Ca2+ channels also mate efficiently in standard conditions, but the zygotes sometimes fail to remove some of the cell wall material at the septum between mating partners [41, 106, 114]. These studies have revealed little or no role for intracellular Ca2+ elevation in the process of mating, if indeed such elevations actually occur in mating cells. No direct observations of either [Ca2+]CYT increases or calcineurin activation have been reported on mating S. cerevisiae cells. Toward this goal, it may be possible to adapt the GFP-based sensors of [Ca2+]CYT and calineurin activity used in the analysis of spike and burst phenomena in single S. cerevisiae cells [20] to S. cerevisiae cells engaged in mating. Until real-time assays can be applied, we will not know with certainty if [Ca2+]CYT regulates the mating process or if it just promotes survival of the cells that cannot find mates. How acidic Ca2+ stores of S. cerevisiae and other fungi shape developmental processes such as this is yet another important question to be addressed.
Acidic Ca2+ stores in fungi, animals, and beyond
The discoveries of Pmc1, Vcx1, and Yvc1 as major Ca2+ importers and exporters in the acidic vacuoles of S. cerevisiae has opened new frontiers in the study of acidic Ca2+ stores in other fungi and eukaryotes. The few studies of these proteins in other fungi are generally consistent with the S. cerevisiae paradigms: localization to the vacuole membrane, regulation by calcineurin, and contributions to Ca2+ homeostasis and signaling. But the other fungal species allow investigations of acidic Ca2+ stores in wholly new contexts. For example, the human pathogen Cryptococcus neoformans requires Vcx1 in its vacuole for full virulence in a mouse model of inhalation cryptococcosis [115]. The human pathogen Candida albicans utilizes Pmc1 to promote hypersensitivity to common antifungal medications [116] in a process that can be studied in S. cerevisiae. The plant pathogen Magnaporthe oryzae employs homologs of both Pmc1 and Yvc1 for virulence in rice and wheat and for several developmental processes [117]. These effects of vacuolar Ca2+ transporters and channels on pathogenicity may result from their impacts on signaling by calcineurin or other sensors of [Ca2+]CYT. Alternatively, the stored Ca2+ itself may contribute in unknown ways to the function of acidic compartments and their roles in fungal virulence.
The sequenced animal genomes so far indicate a complete absence of CAX-type Ca2+/H+ exchangers (Vcx1 homologs) and a re-localization of PMCA-type Ca2+ pumps and TRPC-type Ca2+ channels (Pmc1 and Yvc1 homologs) to the plasma membranes of most cell types. The acidic Ca2+ stores of animal cells may instead be supplied by SPCA-type Ca2+ pumps with some additional contributions by PMCA- and SERCA-type pumps and may instead be tapped by TPC-type Ca2+ channels, which are not found in the available fungal genomes. A Golgi-localized V-type H+ pump may also facilitate Ca2+ uptake and release through coupling effects on Na+/H+ and Ca2+/Na+ exchangers in animals. Such differences between fungi and animals probably reflect the somewhat distinct functions of the major acidic organelles in fungi and animals. Fungal vacuoles seem larger and more oriented toward storage of nutrients and metabolites than animal lysosomes, which seem more specialized for digestive and degradative functions given their smaller size and much greater acidity. Such rationalizations should be taken with due skepticism, however, especially in light of the growing recognition that these organelles exist as a spectrum of vacuole-like and lysosome-like structures even within a single cell type.
From the more distant branches of the tree of life, the core toolkit responsible for Ca2+ influx, sequestration, release, and signaling become even more apparent. The CAX-type Ca2+/H+ exchangers are common in plants, apicomplexan parasites, and other protozoans that can impact human health and wellbeing. Given their prevalence, why would this useful class of Ca2+ transporter have been lost in the earliest animals only to be replaced by more elaborate systems? The answers to questions like this one may provide a very useful framework for viewing and understanding the acidic Ca2+ stores of humans.
Acknowledgments
The author thanks his many colleagues who have contributed to this exciting field of research and also thanks Hyemin Kim, Tovah Aronin, Chris Stefan, and Adam Kim for critical comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Zelter A, Bencina M, Bowman BJ, Yarden O, Read ND. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet Biol. 2004;41:827–841. doi: 10.1016/j.fgb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Cyert MS. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu Rev Genet. 2001;35:647–672. doi: 10.1146/annurev.genet.35.102401.091302. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham KW. Calcium Signaling Networks in Yeast. In: Putney JW, editor. Calcium Signaling. 2. CRC Press; 2005. p. 536. [Google Scholar]
- 4.Stathopoulos AM, Cyert MS. Calcineurin acts through the CRZ1/TCN1 encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matheos DP, Kingsbury TJ, Ahsan US, Cunningham KW. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alepuz PM, Matheos DP, Cunningham KW, Estruch F. The Saccharomyces cerevisiae RanGTP binding protein Msn5p is involved in different signal transduction pathways. Genetics. 1999;153:1219–1231. doi: 10.1093/genetics/153.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boustany LM, Cyert MS. Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev. 2002;16:608–619. doi: 10.1101/gad.967602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polizotto RS, Cyert MS. Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p. J Cell Biol. 2001;154:951–960. doi: 10.1083/jcb.200104078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stathopoulos-Gerontides A, Guo JJ, Cyert MS. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 1999;13:798–803. doi: 10.1101/gad.13.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kafadar KA, Zhu H, Snyder M, Cyert MS. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev. 2003;17:2698–2708. doi: 10.1101/gad.1140603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy J, Li H, Hogan P, Cyert M. A conserved docking site modulates substrate affinity for calcineurin, signaling output, and in vivo function. Mol Cell. 2007;25:889–901. doi: 10.1016/j.molcel.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez A, Roy J, Martinez-Martinez S, Lopez-Maderuelo MD, Nino-Moreno P, Orti L, Pantoja-Uceda D, Pineda-Lucena A, Cyert MS, Redondo JM. A conserved docking surface on calcineurin mediates interaction with substrates and immunosuppressants. Mol Cell. 2009;33:616–626. doi: 10.1016/j.molcel.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;13:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta S, Li H, Hogan PG, Cunningham KW. Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast. Mol Cell Biol. 2009;29:2777–2793. doi: 10.1128/MCB.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudgeon DD, Zhang N, Ositelu OO, Kim H, Cunningham KW. Nonapoptotic death of Saccharomyces cerevisiae cells that is stimulated by Hsp90 and inhibited by calcineurin and Cmk2 in response to endoplasmic reticulum stresses. Eukaryot Cell. 2008;7:2037–2051. doi: 10.1128/EC.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimoto H, Saltsman K, Gasch AP, Li HX, Ogawa N, Botstein D, Brown PO, Cyert MS. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J Biol Chem. 2002;277:31079–31088. doi: 10.1074/jbc.M202718200. [DOI] [PubMed] [Google Scholar]
- 17.Hilioti Z, Gallagher DA, Low-Nam ST, Ramaswamy P, Gajer P, Kingsbury TJ, Birchwood CJ, Levchenko A, Cunningham KW. GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev. 2004;18:35–47. doi: 10.1101/gad.1159204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heath VL, Shaw SL, Roy S, Cyert MS. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryot Cell. 2004;3:695–704. doi: 10.1128/EC.3.3.695-704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bultynck G, Heath V, Majeed A, Galan J, Haguenauer-Tsapis R, Cyert M. Slm1 and slm2 are novel substrates of the calcineurin phosphatase required for heat stress-induced endocytosis of the yeast uracil permease. Mol Cell Biol. 2006;26:4729–4745. doi: 10.1128/MCB.01973-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai L, Dalal CK, Elowitz MB. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature. 2008;455:485–490. doi: 10.1038/nature07292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 22.Rose MD, Misra LM, Vogel JP. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 23.de Virgilio C, Burckert N, Neuhaus JM, Boller T, Wiemken A. CNE1, a Saccharomyces cerevisiae homologue of the genes encoding mammalian calnexin and calreticulin. Yeast. 1993;9:185–188. doi: 10.1002/yea.320090209. [DOI] [PubMed] [Google Scholar]
- 24.Parlati F, Dominguez M, Bergeron JJ, Thomas DY. Saccharomyces cerevisiae CNE1 encodes an endoplasmic reticulum (ER) membrane protein with sequence similarity to calnexin and calreticulin and functions as a constituent of the ER quality control apparatus. J Biol Chem. 1995;270:244–253. doi: 10.1074/jbc.270.1.244. [DOI] [PubMed] [Google Scholar]
- 25.Bowman BJ, Draskovic M, Freitag M, Bowman EJ. Structure and distribution of organelles and cellular location of calcium transporters in Neurospora crassa. Eukaryot Cell. 2009;8:1845–1855. doi: 10.1128/EC.00174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. Embo J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao JI, Moir DT. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 28.Strayle J, Pozzan T, Rudolph HK. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 μM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 1999;18:4733–4743. doi: 10.1093/emboj/18.17.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antebi A, Fink GR. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burk SE, Lytton J, MacLennan DH, Shull GE. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. J Biol Chem. 1989;264:18561–18568. [PubMed] [Google Scholar]
- 31.Fuller RS, Brake A, Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. PMCID: PMC15103. 1989;86:1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dürr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moir DT, Davidow LS. Production of proteins by secretion from yeast. Methods Enzymol. 1991;194:491–507. doi: 10.1016/0076-6879(91)94037-d. [DOI] [PubMed] [Google Scholar]
- 34.Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol Cell Biol. 2000;20:6686–6694. doi: 10.1128/mcb.20.18.6686-6694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchi V, Sorin A, Wei Y, Rao R. Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett. 1999;454:181–186. doi: 10.1016/s0014-5793(99)00803-0. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham KW, Fink GR. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer M, Schnell N, Chattaway J, Davies P, Dixon G, Sanders D. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett. 1997;419:259–262. doi: 10.1016/s0014-5793(97)01466-x. [DOI] [PubMed] [Google Scholar]
- 38.Paidhungat M, Garrett S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol Cell Biol. 1997;17:6339–6347. doi: 10.1128/mcb.17.11.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iida H, Nakamura H, Ono T, Okumura MS, Anraku Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol Cell Biol. 1994;14:8259–8271. doi: 10.1128/mcb.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iida K, Tada T, Iida H. Molecular cloning in yeast by in vivo homologous recombination of the yeast putative alpha1 subunit of the voltage-gated calcium channel. FEBS Lett. 2004;576:291–296. doi: 10.1016/j.febslet.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Martin DC, Kim H, Mackin NA, Maldonado-Báez L, Evangelista CC, Jr, Beaudry V, Dudgeon DD, Naiman DQ, Erdman SE, Cunningham KW. New subunits and regulators of a high affinity Ca2+ influx system (HACS) revealed through a genome-wide screen in yeast. J Biol Chem. doi: 10.1074/jbc.M110.177451. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonilla M, Cunningham KW. MAP kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell. 2003;14:4296–4305. doi: 10.1091/mbc.E03-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cronin SR, Rao R, Hampton RY. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J Cell Biol. 2002;157:1017–1028. doi: 10.1083/jcb.200203052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miseta A, Fu L, Kellermayer R, Buckley J, Bedwell DM. The Golgi apparatus plays a significant role in the maintenance of Ca2+ homeostasis in the vps33Δ vacuolar biogenesis mutant of Saccharomyces cerevisiae. J Biol Chem. 1999;274:5939–5947. doi: 10.1074/jbc.274.9.5939. [DOI] [PubMed] [Google Scholar]
- 45.Lehle L, Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976;72:167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, Shi G, Le QT, Brown JM, Koong AC. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Cancer Res. 2005;3:669–677. doi: 10.1158/1541-7786.MCR-05-0181. [DOI] [PubMed] [Google Scholar]
- 47.Goto T, Kino T, Hatanaka H, Nishiyama M, Okuhara M, Kohsaka M, Aoki H, Imanaka H. Discovery of FK-506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplant Proc. 1987;19:4–8. [PubMed] [Google Scholar]
- 48.Marchetti O, Entenza JM, Sanglard D, Bille J, Glauser MP, Moreillon P. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob Agents Chemother. 2000;44:2932–2938. doi: 10.1128/aac.44.11.2932-2938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother. 2000;44:2373–2381. doi: 10.1128/aac.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, Perfect JR, McCusker JH, Heitman J. Calcineurin is essential for survival during membrane stress in Candida albicans. Embo J. 2002;21:546–559. doi: 10.1093/emboj/21.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edlind T, Smith L, Henry K, Katiyar S, Nickels J. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signalling. Mol Microbiol. 2002;46:257–268. doi: 10.1046/j.1365-2958.2002.03165.x. [DOI] [PubMed] [Google Scholar]
- 52.Onyewu C, Blankenship JR, Del Poeta M, Heitman J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother. 2003;47:956–964. doi: 10.1128/AAC.47.3.956-964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur R, Castano I, Cormack BP. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob Agents Chemother. 2004;48:1600–1613. doi: 10.1128/AAC.48.5.1600-1613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinbach WJ, Schell WA, Blankenship JR, Onyewu C, Heitman J, Perfect JR. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob Agents Chemother. 2004;48:1664–1669. doi: 10.1128/AAC.48.5.1664-1669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blankenship JR, Steinbach WJ, Perfect JR, Heitman J. Teaching old drugs new tricks: reincarnating immunosuppressants as antifungal drugs. Curr Opin Investig Drugs. 2003;4:192–199. [PubMed] [Google Scholar]
- 56.Bastidas RJ, Reedy JL, Morales-Johansson H, Heitman J, Cardenas ME. Signaling cascades as drug targets in model and pathogenic fungi. Curr Opin Investig Drugs. 2008;9:856–864. [PMC free article] [PubMed] [Google Scholar]
- 57.Bowers K, Stevens TH. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2005;1744:438–454. doi: 10.1016/j.bbamcr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Dunn T, Gable K, Beeler T. Regulation of cellular Ca2+ by yeast vacuoles. J Biol Chem. 1994;269:7273–7278. [PubMed] [Google Scholar]
- 59.Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pozos TC, Sekler I, Cyert MS. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol Cell Biol. 1996;16:3730–3741. doi: 10.1128/mcb.16.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.del Pozo L, Osaba L, Corchero J, Jimenez A. A single nucleotide change in the MNR1 (VCX1/HUM1) gene determines resistance to manganese in Saccharomyces cerevisiae. Yeast. 1999;15:371–375. doi: 10.1002/(sici)1097-0061(19990330)15:5<371::aid-yea380>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 62.Pittman JK, Cheng NH, Shigaki T, Kunta M, Hirschi KD. Functional dependence on calcineurin by variants of the Saccharomyces cerevisiae vacuolar Ca2+/H+ exchanger Vcx1p. Mol Microbiol. 2004;54:1104–1116. doi: 10.1111/j.1365-2958.2004.04332.x. [DOI] [PubMed] [Google Scholar]
- 63.Ferrell JE., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 64.Tsai TY, Choi YS, Ma W, Pomerening JR, Tang C, Ferrell JE., Jr Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science. 2008;321:126–129. doi: 10.1126/science.1156951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu L, Miseta A, Hunton D, Marchase RB, Bedwell DM. Loss of the major isoform of phosphoglucomutase results in altered calcium homeostasis in Saccharomyces cerevisiae. J Biol Chem. 2000;275:5431–5440. doi: 10.1074/jbc.275.8.5431. [DOI] [PubMed] [Google Scholar]
- 66.Shigaki T, Rees I, Nakhleh L, Hirschi KD. Identification of three distinct phylogenetic groups of CAX cation/proton antiporters. J Mol Evol. 2006;63:815–825. doi: 10.1007/s00239-006-0048-4. [DOI] [PubMed] [Google Scholar]
- 67.Cagnac O, Aranda-Sicilia MN, Leterrier M, Rodriguez-Rosales MP, Venema K. Vacuolar cation/H+ antiporters of Saccharomyces cerevisiae. J Biol Chem. 2010;285:33914–33922. doi: 10.1074/jbc.M110.116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cagnac O, Leterrier M, Yeager M, Blumwald E. Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J Biol Chem. 2007;282:24284–24293. doi: 10.1074/jbc.M703116200. [DOI] [PubMed] [Google Scholar]
- 69.Manohar M, Mei H, Franklin AJ, Sweet EM, Shigaki T, Riley BB, Macdiarmid CW, Hirschi K. Zebrafish (Danio rerio) endomembrane antiporter similar to a yeast cation/H+ transporter is required for neural crest development. Biochemistry. 2010;49:6557–6566. doi: 10.1021/bi100362k. [DOI] [PubMed] [Google Scholar]
- 70.Lytton J. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J. 2007;406:365–382. doi: 10.1042/BJ20070619. [DOI] [PubMed] [Google Scholar]
- 71.Cai X, Lytton J. Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J Biol Chem. 2004;279:5867–5876. doi: 10.1074/jbc.M310908200. [DOI] [PubMed] [Google Scholar]
- 72.Palty R, Ohana E, Hershfinkel M, Volokita M, Elgazar V, Beharier O, Silverman WF, Argaman M, Sekler I. Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J Biol Chem. 2004;279:25234–25240. doi: 10.1074/jbc.M401229200. [DOI] [PubMed] [Google Scholar]
- 73.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiesenberger G, Steinleitner K, Malli R, Graier WF, Vormann J, Schweyen RJ, Stadler JA. Mg2+ deprivation elicits rapid Ca2+ uptake and activates Ca2+/calcineurin signaling in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6:592–599. doi: 10.1128/EC.00382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beeler T, Bruce K, Dunn T. Regulation of cellular Mg2+ by Saccharomyces cerevisiae. Biochim Biophys Acta. 1997;1323:310–318. doi: 10.1016/s0005-2736(96)00199-x. [DOI] [PubMed] [Google Scholar]
- 76.Forster C, Kane PM. Cytosolic Ca2+ homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J Biol Chem. 2000 doi: 10.1074/jbc.M006650200. [DOI] [PubMed] [Google Scholar]
- 77.Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. PMCID: PMC15103. 2001;98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Denis V, Cyert MS. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Popa CV, Dumitru I, Ruta LL, Danet AF, Farcasanu IC. Exogenous oxidative stress induces Ca2+ release in the yeast Saccharomyces cerevisiae. FEBS J. 2010;277:4027–4038. doi: 10.1111/j.1742-4658.2010.07794.x. [DOI] [PubMed] [Google Scholar]
- 80.Loukin S, Zhou X, Kung C, Saimi Y. A genome-wide survey suggests an osmoprotective role for vacuolar Ca2+ release in cell wall-compromised yeast. FASEB J. 2008;22:2405–2415. doi: 10.1096/fj.07-101410. [DOI] [PubMed] [Google Scholar]
- 81.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) Controls Membrane Traffic by Direct Activation of Mucolipin Ca Release Channels in the Endolysosome. Nat Commun. 2010;1 doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou XL, Batiza AF, Loukin SH, Palmer CP, Kung C, Saimi Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc Natl Acad Sci U S A. 2003;100:7105–7110. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wada Y, Ohsumi Y, Tanifuji M, Kasai M, Anraku Y. Vacuolar ion channel of the yeast Saccharomyces cerevisiae. J Biol Chem. 1987;262:17260–17263. [PubMed] [Google Scholar]
- 84.Tanifuji M, Sato M, Wada Y, Anraku Y, Kasai M. Gating behaviors of a voltage-dependent and Ca2+-activated cation channel of yeast vacuolar membrane incorporated into planar lipid bilayer. J Membr Biol. 1988;106:47–55. doi: 10.1007/BF01871766. [DOI] [PubMed] [Google Scholar]
- 85.Bertl A, Slayman CL. Cation-selective channels in the vacuolar membrane of Saccharomyces: dependence on calcium, redox state, and voltage. PMCID: PMC15103. 1990;87:7824–7828. doi: 10.1073/pnas.87.20.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang Y, Schlenstedt G, Flockerzi V, Beck A. Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett. 2010;584:2028–2032. doi: 10.1016/j.febslet.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 87.Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 88.Ungermann C, Wickner W, Xu Z. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. PMCID: PMC15103. 1999;96:11194–11199. doi: 10.1073/pnas.96.20.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 2001;409:581–588. doi: 10.1038/35054500. [DOI] [PubMed] [Google Scholar]
- 90.Merz AJ, Wickner WT. trans-SNARE interactions elicit Ca2+ efflux from the yeast vacuole lumen. J Cell Biol. 2004;164:195–206. doi: 10.1083/jcb.200310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Starai VJ, Thorngren N, Fratti RA, Wickner W. Ion regulation of homotypic vacuole fusion in Saccharomyces cerevisiae. J Biol Chem. 2005;280:16754–16762. doi: 10.1074/jbc.M500421200. [DOI] [PubMed] [Google Scholar]
- 92.Belde PJ, Vossen JH, Borst-Pauwels GW, Theuvenet AP. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of Saccharomyces cerevisiae. FEBS Lett. 1993;323:113–118. doi: 10.1016/0014-5793(93)81460-h. [DOI] [PubMed] [Google Scholar]
- 93.Pereira MB, Tisi R, Fietto LG, Cardoso AS, Franca MM, Carvalho FM, Tropia MJ, Martegani E, Castro IM, Brandao RL. Carbonyl cyanide m-chlorophenylhydrazone induced calcium signaling and activation of plasma membrane H+-ATPase in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2008;8:622–630. doi: 10.1111/j.1567-1364.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 94.Coccetti P, Tisi R, Martegani E, Souza Teixeira L, Lopes Brandao R, de Miranda Castro I, Thevelein JM. The PLC1 encoded phospholipase C in the yeast Saccharomyces cerevisiae is essential for glucose-induced phosphatidylinositol turnover and activation of plasma membrane H+-ATPase. Biochim Biophys Acta. 1998;1405:147–154. doi: 10.1016/s0167-4889(98)00099-8. [DOI] [PubMed] [Google Scholar]
- 95.Tisi R, Baldassa S, Belotti F, Martegani E. Phospholipase C is required for glucose-induced calcium influx in budding yeast. FEBS Lett. 2002;520:133–138. doi: 10.1016/s0014-5793(02)02806-5. [DOI] [PubMed] [Google Scholar]
- 96.Tisi R, Belotti F, Wera S, Winderickx J, Thevelein JM, Martegani E. Evidence for inositol triphosphate as a second messenger for glucose-induced calcium signalling in budding yeast. Curr Genet. 2004;45:83–89. doi: 10.1007/s00294-003-0465-5. [DOI] [PubMed] [Google Scholar]
- 97.Tokes-Fuzesi M, Bedwell DM, Repa I, Sipos K, Sumegi B, Rab A, Miseta A. Hexose phosphorylation and the putative calcium channel component Mid1p are required for the hexose-induced transient elevation of cytosolic calcium response in Saccharomyces cerevisiae. Mol Microbiol. 2002;44:1299–1308. doi: 10.1046/j.1365-2958.2002.02956.x. [DOI] [PubMed] [Google Scholar]
- 98.Tropia MJ, Cardoso AS, Tisi R, Fietto LG, Fietto JL, Martegani E, Castro IM, Brandao RL. Calcium signaling and sugar-induced activation of plasma membrane H+-ATPase in Saccharomyces cerevisiae cells. Biochem Biophys Res Commun. 2006;343:1234–1243. doi: 10.1016/j.bbrc.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 99.Kellermayer R, Szigeti R, Kellermayer M, Miseta A. The intracellular dissipation of cytosolic calcium following glucose re-addition to carbohydrate depleted Saccharomyces cerevisiae. FEBS Lett. 2004;571:55–60. doi: 10.1016/j.febslet.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 100.Tisi R, Groppi S, Belotti F, Palardi C, Martegani E. Glucose activates calcineurin in S. cerevisiae. Yeast Genetics and Molecular Biology Meeting; Toronto. 2008. p. Abstract 179B. [Google Scholar]
- 101.Aiello DP, Fu L, Miseta A, Bedwell DM. Intracellular Glucose-1-Phosphate and Glucose-6-Phosphate Levels Modulate Ca2+ Homeostasis in Saccharomyces cerevisiae. J Biol Chem. 2002;277:45751–45758. doi: 10.1074/jbc.M208748200. [DOI] [PubMed] [Google Scholar]
- 102.Miseta A, Tokes-Fuzesi M, Aiello DP, Bedwell DM. A Saccharomyces cerevisiae mutant unable to convert glucose to glucose-6-phosphate accumulates excessive glucose in the endoplasmic reticulum due to core oligosaccharide trimming. Eukaryot Cell. 2003;2:534–541. doi: 10.1128/EC.2.3.534-541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aiello DP, Fu L, Miseta A, Sipos K, Bedwell DM. The Ca2+ homeostasis defects in a pgm2Δ strain of Saccharomyces cerevisiae are caused by excessive vacuolar Ca2+ uptake mediated by the Ca2+-ATPase Pmc1p. J Biol Chem. 2004;279:38495–38502. doi: 10.1074/jbc.M400833200. [DOI] [PubMed] [Google Scholar]
- 104.Iida H, Yagawa Y, Anraku Y. Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. A study of [Ca2+]i in single Saccharomyces cerevisiae cells with imaging of fura-2. J Biol Chem. 1990;265:13391–13399. [PubMed] [Google Scholar]
- 105.Muller EM, Locke EG, Cunningham KW. Differential regulation of two Ca2+ influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics. 2001;159:1527–1538. doi: 10.1093/genetics/159.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muller EM, Mackin NA, Erdman SE, Cunningham KW. Fig 1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J Biol Chem. 2003;278:38461–38469. doi: 10.1074/jbc.M304089200. [DOI] [PubMed] [Google Scholar]
- 107.Geiser JR, van Tuinen D, Brockerhoff SE, Neff MM, Davis TN. Can calmodulin function without binding calcium? Cell. 1991;65:949–959. doi: 10.1016/0092-8674(91)90547-c. [DOI] [PubMed] [Google Scholar]
- 108.Moser MJ, Geiser JR, Davis TN. Ca2+-calmodulin promotes survival of pheromone-induced growth arrest by activation of calcineurin and Ca2+-calmodulin-dependent protein kinase. Mol Cell Biol. 1996;16:4824–4831. doi: 10.1128/mcb.16.9.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Severin FF, Hyman AA. Pheromone induces programmed cell death in S. cerevisiae. Curr Biol. 2002;12:R233–235. doi: 10.1016/s0960-9822(02)00776-5. [DOI] [PubMed] [Google Scholar]
- 110.Zhang NN, Dudgeon DD, Paliwal S, Levchenko A, Grote E, Cunningham KW. Multiple signaling pathways regulate yeast cell death during the response to mating pheromones. Mol Biol Cell. 2006;17:3409–3422. doi: 10.1091/mbc.E06-03-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aguilar PS, Engel A, Walter P. The plasma membrane proteins Prm1 and Fig 1 ascertain fidelity of membrane fusion during yeast mating. Mol Biol Cell. 2007;18:547–556. doi: 10.1091/mbc.E06-09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cyert MS, Kunisawa R, Kaim D, Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. PMCID: PMC15103. 1991;88:7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cyert MS, Thorner J. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol Cell Biol. 1992;12:3460–3469. doi: 10.1128/mcb.12.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Erdman S, Lin L, Malczynski M, Snyder M. Pheromone-regulated genes required for yeast mating differentiation. J Cell Biol. 1998;140:461–483. doi: 10.1083/jcb.140.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kmetzsch L, Staats CC, Simon E, Fonseca FL, de Oliveira DL, Sobrino L, Rodrigues J, Leal AL, Nimrichter L, Rodrigues ML, Schrank A, Vainstein MH. The Vacuolar Ca2+ Exchanger Vcx1 Is Involved in Calcineurin-Dependent Ca2+ Tolerance and Virulence in Cryptococcus neoformans. Eukaryot Cell. 2010;9:1798–1805. doi: 10.1128/EC.00114-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol. 2003;48:959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- 117.Nguyen QB, Kadotani N, Kasahara S, Tosa Y, Mayama S, Nakayashiki H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol Microbiol. 2008;68:1348–1365. doi: 10.1111/j.1365-2958.2008.06242.x. [DOI] [PubMed] [Google Scholar]