Abstract
The vomeronasal system (VNS) participates in the detection and processing of pheromonal information related to social and sexual behaviors. Within the VNS, two different populations of sensory neurons, with a distinct pattern of distribution, line the epithelium of the vomeronasal organ (VNO) and give rise to segregated sensory projections to the accessory olfactory bulb (AOB). Apical sensory neurons in the VNO project to the anterior AOB (aAOB), while basal neurons project to the posterior AOB (pAOB). In the AOB, the largest population of neurons are inhibitory, the granule and periglomerular cells (GCs and PGs) and remarkably, these neurons are continuously born and functionally integrated in the adult brain, underscoring their role on olfactory function. Here we show that behaviors mediated by the VNS differentially regulate adult neurogenesis across the anterior-posterior axis of the AOB. We used immunohistochemical labeling of newly born cells under different behavioral conditions in mice. Using a resident-intruder aggression paradigm, we found that subordinate mice exhibited increased neurogenesis in the aAOB. In addition, in sexually naive adult females exposed to soiled bedding odorized by adult males, the number of newly born cells was significantly increased in the pAOB; however, neurogenesis was not affected in females exposed to female odors. In addition, we found that at two months of age adult neurogenesis was sexually dimorphic, with male mice exhibiting higher levels of newly born cells than females. Interestingly, adult neurogenesis was greatly reduced with age and this decrease correlated with a decrease in progenitor cells proliferation but not with an increase in cell death in the AOB. These results indicate that the physiological regulation of adult neurogenesis in the AOB by behaviors is both sex and age dependent and suggests an important role of newly born neurons in sex dependent behaviors mediated by the VNS.
Keywords: granule cell, social odors, aggression, aging, vomeronasal system, stress, proliferation
1. INTRODUCTION
The ability to find potential mates and properly identify social status among conspecifics is essential for species survival and largely relies on the detection and recognition of social chemosensory cues by the concerted activity of the main olfactory and vomeronasal systems (Baum and Kelliher, 2009). Sensory neurons in the main olfactory epithelium and in the vomeronasal organ (VNO) send their axons to specific regions of the olfactory bulb (OB), the main and the accessory olfactory bulb (MOB and AOB, respectively), where they establish their first synapse onto principal neurons, the mitral and tufted cells (MCs herein). In addition, in the VNO, sensory neurons exhibit differences in pheromone receptor expression and segregated axonal projection to the AOB, giving rise to anatomical and functional subdivisions in the AOB (Halpern et al., 1995; Jia and Halpern, 1996; Sugai et al., 2006). Thus, neurons in the apical layer of the VNO express V1R receptors and project to the anterior AOB (aAOB), while neurons in the basal layer express V2R receptors and project to the posterior AOB (pAOB, Fig 2A) (Herrada and Dulac, 1997; Rodriguez et al., 1999). This particular arrangement of sensory inputs into the AOB has suggested that these functional subdivisions are involved in the processing of pheromonal information related to species-specific behaviors. For instance, in male mice, presentation of a diestrus female activates neurons in the aAOB (Kumar et al., 1999), whereas male aggressive behavior, exposure of males to females volatile odors, and the exposure of females to male major urinary proteins induces activation of cells in the pAOB (Brennan et al., 1999; Kumar et al., 1999; Yoshikage et al., 2007).
Figure 2.
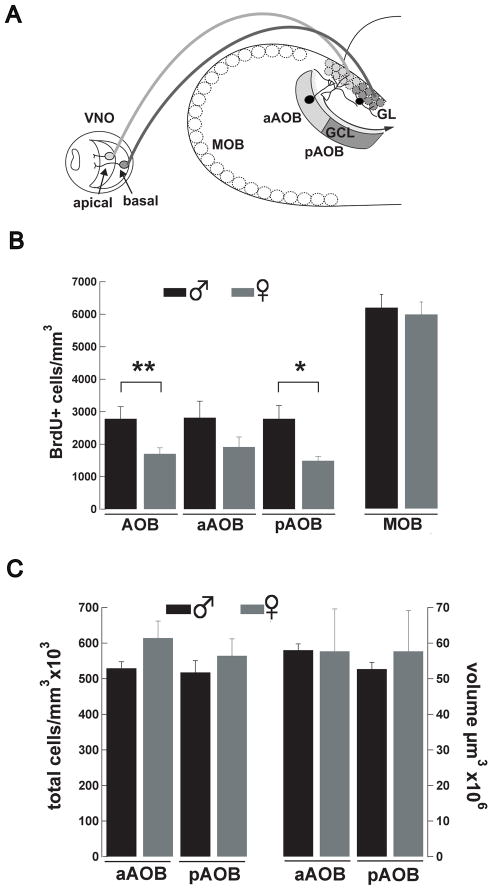
Adult neurogenesis in the AOB is sexually dimorphic. (A) Diagram showing the sensory projections from the vomeronasal organ (VNO) to the AOB. Apical sensory neurons in the VNO (light gray) project their axons to the anterior AOB (aAOB) while basal sensory neurons project their axons to the posterior AOB (pAOB, dark gray); in the AOB sensory neurons make their first synapse onto dendrites of MCs (unfilled cell). Adult-born neurons are found in the GCL and in the GL and they are shown in black. (B) Graph bar showing the total number of BrdU+ cells in the AOB, and its subdivisions, in two-months old male (black) and female (grey) mice. The number of BrdU+ cells is significantly higher in the pAOB of males compared to female mice (t-test,*,p<0.03,**,p<0.02). The total number of BrdU+ cells in the MOB is not different between male and female mice. (C) The total number of cells (cells/mm3) and total tissue volume (μm3) is not different between genders or in the anterior-posterior axis of the AOB.
The most salient physiological mechanism in olfactory processing in the OB is the precise regulation of MCs activity by inhibitory interneurons. These inhibitory neurons are broadly classified as periglomerular and granule cells (PG and GCs, respectively). A large population of these neurons correspond to GCs, which produce recurrent and lateral inhibition of MCs through dendrodendritic synapses, shaping their output and thereby playing a fundamental role in olfactory processing (Arevian et al., 2008; Schoppa and Urban, 2003). Remarkably, unlike most neurons in the adult mammalian brain, these inhibitory neurons are continuously born throughout life in a process known as adult neurogenesis (Altman and Das, 1965b; Lois and Alvarez-Buylla, 1993, 1994). Neurons born during development and in the adult originate from progenitor cells located in the subventricular zone (SVZ) of the brain, from where they migrate to the OB and become functionally integrated within the OB neuronal network. More importantly, the rate of adult neurogenesis can be greatly regulated by a number of factors including olfactory stimuli and the activity of afferent modulatory systems, suggesting that adult neurogenesis is regulated in the context of olfactory behavioral experience (for review see Lledo and Lazarini, 2007).
The Vomeronasal system (VNS) plays an important facilitating role in several social behaviors including mating and aggression (Bean, 1982; Clancy et al., 1984; Leypold et al., 2002; Maruniak et al., 1986; Mugford and Nowell, 1970; Norlin et al., 2003; Stowers et al., 2002). Notably, chemosensory information associated with these behaviors recruits the activity of inhibitory neurons in the AOB, suggesting that these behaviors can regulate adult neurogenesis (Sugai et al., 2006; Yoshikage et al., 2007). Here, we use immunohistochemical labeling of newly born cells to show that social behaviors relying on the activation of the VNS regulate adult neurogenesis in the mouse AOB. We found that adult neurogenesis can be differentially modulated across the anterior-posterior axis of the AOB depending of the type of social stimulus. The induction of aggressive behavior in males, via a resident-intruder paradigm, significantly increased neurogenesis in the aAOB of the intruder. In contrast, naive females exposed to male urine exhibited increased adult neurogenesis in the pAOB. Interestingly, we found that the generation of new neurons in the AOB is sexually dimorphic at one month of age, with males having greater numbers of newly born neurons compared to females. However, this sexual difference is not maintained at later ages and adult neurogenesis dramatically decreases with age in both sexes.
2. EXPERIMENTAL PROCEDURES
2.1 Animals
All experiments were performed on C57/BL6 female and male mice (1–6 moths old) obtained from breeding pairs housed in the animal colony of the Biology Department of the University of Maryland or obtained from Jackson Laboratories (Bar Harbor, MA). For behavioral experiments, male and female mice were housed either singly or in groups of 3–4 and allowed to acclimate for at least one week prior to the behavioral experiments. Animals were kept on a 12 hr light/dark cycle with food and water ad libitum. All experiments were conducted following the guidelines of the IACUC of the University of Maryland, College Park.
2.2 Bromodeoxyuridine (BrdU) injection and fixation
To visualize mature newly born cells in the OB, adult mice were injected three times intraperitoneally, every two hours, with 5-bromo-2 -deoxyuridine (BrdU, 100 mg/kg) prepared in phosphate-buffered saline (PBS, 10 mg/ml). One month after the BrdU injections, mice were deeply anesthetized with isofluorane (Halocarbon) and the brains fixed by transcardial perfusion with cold PBS, followed by a 4% paraformaldehyde solution (PFA, Electron Microscopy Science) in PBS pH 7.4. To study neuronal proliferation in the SVZ, animals received a single dose of BrdU (100 mg/Kg) and were sacrificed 2 hours later. The brains were dissected carefully to avoid any damage to the olfactory bulbs, post fixed in PFA 4% for 4 hrs at 4 °C, and then cryoprotected overnight with a solution of 30% sucrose in PBS. The brains were then embedded in Tissue-Tek O.C.T. (Electron Microscopy Science) and sagittal sections (20 μm) from a randomly chosen hemisphere were obtained using a Leica CM1850 cryostat. The sections were mounted on frozen tissue slides (Fisher) and stored at −20 °C until use.
2.3 Double labeling immunofluorescence
Sagittal sections of the olfactory bulb containing the accessory and main olfactory bulbs (AOB and MOB, respectively) were used for immunolabeling. First, the slides were warmed at 55 °C for 10 min, hydrated in PBS for 5 min (two times), and rinsed quickly with ddH2O. To denature the DNA, the sections were treated with HCl 2N at 37 °C for 1 hr, followed by neutralization with three washes of Na+ tetraborate buffer (0.1 M, pH 8.5) for 10 minutes. The slides are then washed with PBS, two times for 10 min, and one time with 0.1% Triton X-100 (Sigma Aldrich) in PBS (PBS-T) for 10 minutes. Nonspecific sites were blocked with 10% donkey serum (Sigma Aldrich) in PBS-T for 2 hrs at room temperature (RT). The slices were then incubated with rat anti-BrdU at a dilution of 1:30 (ABD Serotec, MCA2060) or 1:50 (Abcam, ab6325), and mouse anti-NeuN diluted 1:200 (Chemicon International, MAB377) prepared in PBS-T with 2.5% of donkey serum overnight at 4°C. After the incubation with antibodies the samples were washed with PBS-T four times for 10 min and incubated with the secondary antibodies; donkey anti-Rat Alexa-594 (Invitrogen, A-21209) and donkey anti-Mouse Alexa-488 (Invitrogen, A-21202) both diluted at 1:750 in PBS-T with 2.5% of donkey serum, for 2 hrs at RT. The sections were then washed with PBS-T for 10 min followed with three washes with PBS for 10 min. The samples were mounted with Vectashield (Vector Laboratories), visualized with a Leica SP5 X confocal microscope and the images analyzed offline.
2.4 TUNEL Staining
To detect cell apoptosis we performed deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay using the Apoptag fluorescein in situ apoptosis detection kit, following standard protocols (S7110, Millipore, Billerica, MA). The same brains used to quantify the number of BrdU positive cells in the AOB and MOB were used in the TUNEL assay.
2.5 Resident-intruder paradigm
To determine the effect of male aggression on adult neurogenesis we used the resident-intruder paradigm. The resident and control groups of male mice were housed individually, while subordinate mice were housed in groups of four. At day 1, residents and subordinates were injected with BrdU. The bedding of the resident s cage was not changed for at least one week prior to the first presentation of the intruder. Fifteen days after the BrdU injection, a randomly chosen intruder was placed into the resident s cage once a day for 15 min, for five consecutive days. Each day, the intruder was exposed to two different residents within an hour period. A group of control mice was housed individually throughout the 30-day period. The interaction was videotaped and analyzed offline. Biting, tail rattling, chasing, cornering, and tumbling were considered as aggressive behaviors while crouching, retreating and freezing were considered submissive behaviors. The aggressive behavior was quantified using different parameters such as attack and defensive posture frequency.
2.6 Stress by restraint
To determine the effects of stress on adult neurogenesis, male mice were housed individually for at least one week before the BrdU injection. Two weeks after the BrdU injection, a cylindrical acrylic tube was placed in the cage and the animal was allowed to walk inside. Once the mouse was inside, the tube s gate was closed and the animal was confined inside the restrainer for 15 min, once a day, for 5 days. After the 15 min period, the mouse was allowed to back out of the tube. The restrainer allowed back and forth movement but not head to tail turns. As a control we used the same individually housed animals as in the resident-intruder paradigm. Mice exposed to restraint showed increased values of serum corticosterone, which has been shown to correlate with stress (control, 106 ± 17 ng/ml, n = 6; restraint, 208 ± 5 ng/ml, n = 3; t-test, p < 0.006) (Heinrichs and Koob, 2006).
2.7 Exposure of males to social odors
To determine the effect of male social odors on the integration of new neurons, we exposed males to male social odors (urine and bedding). Similar to the resident intruder paradigm (see above) mice were housed individually (“resident or dominant group”) or in a group of four (“intruder or subordinate group”). The resident group was exposed to urine extracted from a group of four different males housed also in a group. This was the urine donor group. A cotton swab was embedded in urine from a different male every day and hung from the lid of the cage for 15 min each day for 5 days. Mice from the intruder group were placed alone for 15 min twice a day (for 5 days) in a cage previously soiled by a mouse housed in isolation for at least one week. Each time the subordinate mouse was exposed to a cage soiled by a different male.
2.8 Exposure of female to social odors
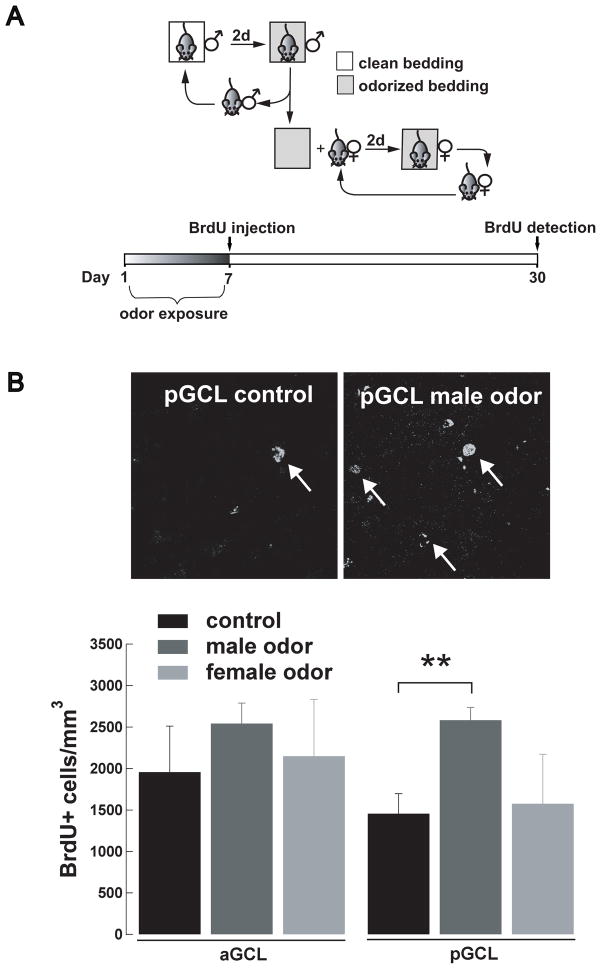
To determine the effect of social odor exposure on adult neurogenesis, a group of female mice was exposed to male odors for 7 days through the use of soiled bedding (Mak et al., 2007). Briefly, two days prior to the beginning of the experiments, a male mouse was placed into a cage with clean bedding and the cage bedding was changed every two days. To expose females to male pheromones, on day 1 the female group was placed into a cage that contained the soiled bedding that had been previously odorized for two days by a male as described above (Fig 4A). Every other day the bedding of the cage housing the females was replaced by the bedding soiled by the same male and this procedure was repeated until the end of day 7; on this day, control and experimental mice were injected with BrdU. The control group consisted of females exposed to clean cage bedding (i.e. not odorized) that was replaced every other day as in the experimental group. Another group of females was exposed to cage bedding soiled by a female, following the same schedule of exposure as the one used for the male odor exposure. Only females that presented regular estrous cycles were chosen for these experiments (18 out of 19 females). The estrus cycle was determined by microscopic inspection of vaginal smears obtained daily between 9 and 11 am.
Figure 4.
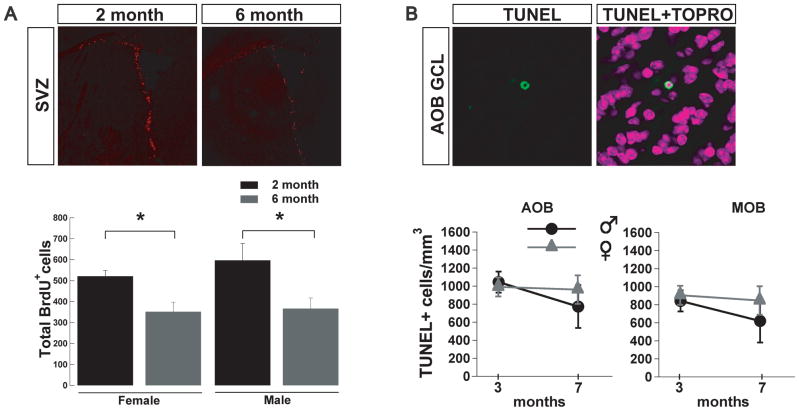
The age-dependent decline in adult neurogenesis is due to a decrease in SVZ proliferation and not an increase cell death in the OB. (A) Top panel, coronal sections immunolabeled with BrdU antibody. The SVZ of older mice (left) exhibited significantly fewer BrdU+ cells than younger mice (right). Bottom panel, the decline in BrdU+ cells was significant in females and males (t-test;*, p<0.05). (B) Top panel, TUNEL staining of an apoptotic cell in the GCL of the AOB. Bottom panel, number of TUNEL+ cells/mm3 in male and female mice at different ages in the AOB and MOB; there was not a significant difference between ages in the OB.
2.9 Quantitative and Statistical analysis
One in six serial sagittal sections containing the AOB were used for quantification and three to six sections were counted per animal. The numerical density was calculated using the optical dissector method (Mayhew and Gundersen, 1996). Confocal Z-stack images were captured at 3 μm optical steps using a 63X oil immersion objective. For each section we obtained 2 to 4 Z-stack images of the granule cell and glomerular layers (GCL and GL, respectively) in the AOB and MOB. All images were blindly analyzed and only BrdU positive (BrdU+) cells in which the nucleus was clearly labeled were included in our analysis. For TUNEL quantification, the sections were co-stained with anti-Dioxigenenin-Fluorescein antibody and the nuclear staining TOPRO, and only cells that exhibited colocalized labeling were considered for analysis. For each region (total and subdivisions of the AOB) data are expressed as the mean number of BrdU+ cells normalized to the area of the Z-stack in mm3 (BrdU+ cells/ mm3) ± S.E. (standard error). Total cell density was calculated manually using sections stained with the nuclear dye TOPRO-3 (Invitrogen). The AOB volume was estimated through the Cavalieri principle using the following formulas; Vol(i) = T*ΣAi with A(i) = ΣP*d2 where T= number of slices*t (Mandarim-de-Lacerda, 2003). For these calculations we used low magnification sections (10X) with a thickness (t) of 20 μm. The area (A) was measured by placing over the sections a grid of squares with distance (d) between lines and then counting the total number of points (P, intersections) that fell inside the region of interest. To quantify proliferation in the subventricular zone, four coronal sections were collected from a region of 1420 μm that started at the opening of the left ventricle and continued towards the caudal end. Values shown correspond to the average of all the BrdU+ cells counted in all four sections. To test for statistical significance, we used the t-test and one-way ANOVA, followed by the Bonferroni and Tukey s multiple comparison tests (SPSS 18.0 software). One animal (out of 84) was excluded from the analysis as the value of BrdU+ cells/ mm3 exceeded the value of the mean ± 2SD (standard deviation) of its respective group.
3. RESULTS
3.1 Adult neurogenesis in the accessory olfactory bulb in juvenile mice is sexually dimorphic
Anatomical and physiological evidence indicate that in several species the VNS is sexually dimorphic (Dawley and Crowder, 1995; Herrada and Dulac, 1997; Peretto et al., 2001; Segovia et al., 2006; Segovia et al., 1999), suggesting that adult neurogenesis could be differentially regulated in the AOB of male and female mice. To address this possibility we first determined the level of adult neurogenesis in the AOB of sexually mature and juvenile mice. To this end, one-month old mice were injected with BrdU, a marker that is incorporated into the DNA of dividing cells, and labeled neurons in the AOB were quantified 30 days after the BrdU injection. Previous studies have indicated that after one month, newly born neurons have already differentiated into mature GCs and PGs within the MOB (Belluzzi et al., 2003; Carleton et al., 2003; Lledo et al., 2006; Petreanu and Alvarez-Buylla, 2002).
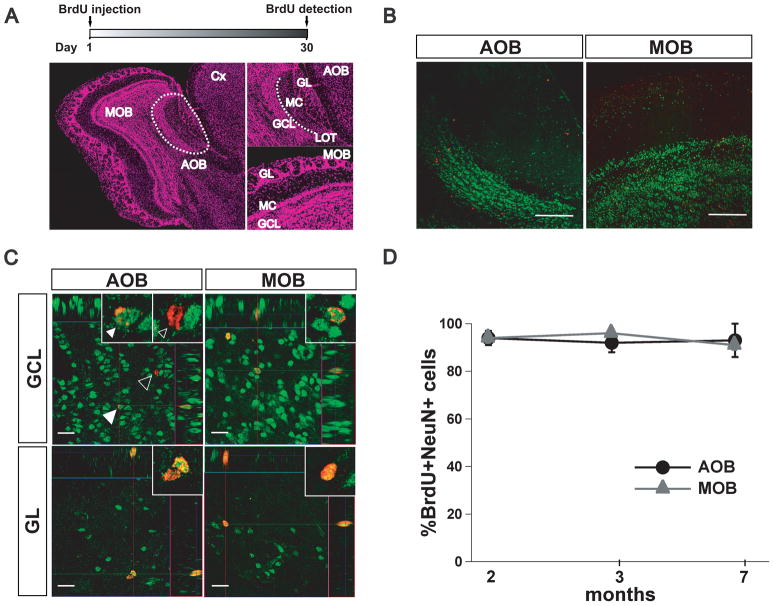
In sagittal sections of the mouse brain, the AOB is located in the dorso-posterior region of the OB and can be clearly distinguished from the MOB (Fig 1A). In the AOB, the mitral/ tufted cell layer (MC) and the GC layer (GCL) are separated by fibers of the lateral olfactory tract (LOT), whereas the glomerular layer (GL) faces the ventral prefrontal cortex (Fig 1A, top right). Mature neurons were recognized by detecting the presence of NeuN, a nuclear marker that is present in post-mitotic and terminally differentiated neurons (Mullen et al., 1992). As shown in Fig 1B we found abundant NeuN staining in the AOB and MOB, especially in the dense GCL. Confocal imaging analysis revealed abundant BrdU positive (BrdU+) cells throughout the GCL and GL in the AOB and MOB (Fig 1C). The pattern of nuclear staining with BrdU was variable; some cells exhibited a punctuated pattern while other cells exhibited a more uniform staining pattern (Fig 1C). Nevertheless, confocal analysis revealed that at all ages the majority of the new cells present in the adult AOB were indeed neurons, since most of the cells that were BrdU+ also stained for NeuN (2, 3 and 7 months; 94 ± 3%; 92 ± 4%, 93± 8%, respectively; n=3 per group, Fig. 1D). Similarly, as previously described most cells in the MOB also exhibited an overlapping staining pattern for BrdU and NeuN at all ages tested (2, 3 and 7 months; 94 ± 2%; 96 ± 1%, 91± 2%, respectively; n=3 per group) as previously reported (Alonso et al., 2006; Mak et al., 2007; Mouret et al., 2009; Rochefort et al., 2002; Veyrac et al., 2009).
Figure 1.
Adult neurogenesis in the AOB. (A) Top, experimental design used to quantify adult neurogenesis; immunoreactive cells were counted in sagittal sections of the olfactory bulb (OB) one month after the BrdU injections (see methods). Left picture, reconstruction of an OB slice stained with TOPRO-3 nuclear staining to show the cellular distribution in the accessory olfactory bulb (AOB) and main olfactory (MOB), using low magnification confocal images (10x). The AOB is located in the dorso-posterior region of the OB (dotted line). Right pictures, higher magnification of the AOB (top) and MOB (bottom) showing the distinct cellular layers analyzed for the presence of BrdU positive (BrdU+) cells (GCL, granule cell layer; GL, glomerular layer). In the AOB the lateral olfactory tract (LOT) separates the mitral/tufted cell (MC) layer from the GCL (dotted line). (B) Low magnification confocal image (10x) of a section double labeled with BrdU (red) and NeuN (green) antibodies (scale bar, 100μm). The AOB and MOB exhibit abundant NeuN labeling in the GCL with scattered BrdU labeling. (C) Orthogonal view of higher magnification images of the GCL and GL in the AOB (left) and in the MOB (right) in double stained sections (63x; scale bar, 20 μm). Left top panel, representative section of the GCL in the AOB showing a cell positive for both BrdU and NeuN (filled white arrow) and a cell positive for BrdU only (empty arrow, see also inset). Similar labeling pattern is observed in the MOB (right panels). (D) Summary graph showing the quantification of the total number of BrdU+ cells that were also positive for NeuN in the AOB and MOB at different ages. At all ages tested, most of the BrdU+ cells are also NeuN positive. In the AOB, the total number were: at 2 months, 104; 3 months, 21 and at 7 months, 8 BrdU+ cells respectively. In the MOB the total number of BrdU+ cells were at 2 months, 360; 3 months, 107; 7 months, 47 BrdU+ cells (n = 3).
Interestingly, at two months of age the level of adult neurogenesis was significantly different between male and females (Fig 2B). We found that the total number of BrdU+ cells in the AOB was ~40 % higher in males than in females (males, 2782 ± 373, n = 5; females 1704 ± 186, BrdU+ cells/mm3, n = 6; t-test, p < 0.03). Sensory projections from the VNO exhibit a divergent pattern of distribution across the anterior-posterior axis of the AOB, suggesting that these regions may process different chemosensory information (Fig 2A). Therefore, we determined whether the sexual dimorphism in adult neurogenesis observed at this age was also expressed along the anterior-posterior axis. As shown in Fig 2B, two-month old male mice exhibited a significantly higher number of BrdU+ cells in the posterior AOB (pAOB) compared to females (males, 2748 ± 406, n = 6; females, 1493 ± 135 BrdU+ cells/mm3, n = 6; t-test, p < 0.02). The number of newborn neurons found in the anterior AOB (aAOB) was also higher in males compared to females, but this difference did not reach statistical significance within our sample (males, 2817 ± 507; females, 1914 ± 307 BrdU+ cells/mm3; t-test, p < 0.15). In contrast, we did not observe gender differences in the number of BrdU+ cells in the MOB (males, 6171 ± 406, n = 5; females, 5965 ± 380 BrdU+ cells/mm3, n = 6; Fig 2B). The observed gender difference in neurogenesis along the anterior-posterior axis of the AOB could be due to other factors including differences in the number of cells or volume in these two regions. However, as shown in Fig 2C, the total number of cells was not different between males and females (males, aAOB, 529x103 ± 18x103, pAOB, 517x103 ± 32x103 cells/mm3; females, aAOB, 614x103 ± 47x103; pAOB, 564x103 ± 27x103 cells/mm3, n = 3 both). Likewise, the sexual dimorphism in adult neurogenesis is not due to differences in volume between the anterior and posterior subdivisions of the AOB (males, aAOB, 58x106 ± 2x106, pAOB, 52.7x106 ± 2x106; females, aAOB, 57.7x106 ± 12x106; pAOB, 57.7x106 ± 11x106 μm3; n = 3 both).
3.2 Adult neurogenesis in the AOB decreases with age
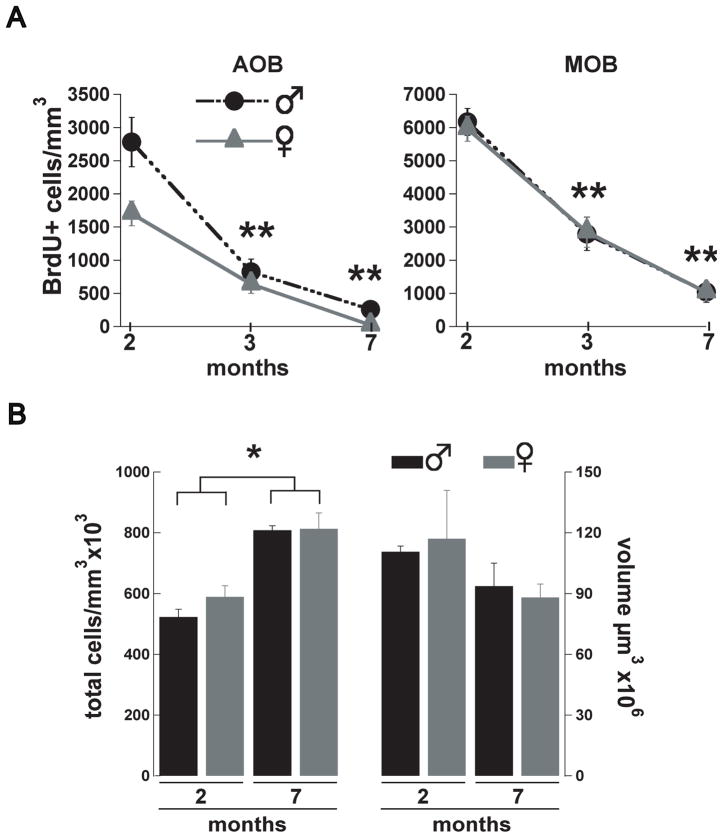
Olfactory dysfunction is a common pathology reported by the elderly population and is one of the first symptoms manifested by people suffering from neurodegenerative diseases (Kovacs, 2004). The decline in the number of new neurons arriving to the MOB has been suggested as a possible mechanism underlying the decrease in olfactory discrimination related with aging (Enwere et al., 2004), yet a similar age-dependent decrease in adult neurogenesis in the AOB has not been examined. Accordingly, we determined the number of BrdU labeled cells at two, three and seven months of age, in male and female mice, injected one-month earlier. As shown in Fig 3A, we found a significant decrease in the number of BrdU+ cells at three and seven months compared to the number of cells at two months. At three months the number of BrdU+ cells in males was decreased by 70%, while at seven months it was decreased by 90% (two months, 2782 ± 373, n = 5; three months, 825 ± 194, n = 6; seven months, 258 ± 102 BrdU+ cells/mm3, n = 5; ANOVA, F(2,13)= 27.9 p < 0.001; Tukey HSD two vs. three months and two vs. seven month p < 0.001). In females, at three months the number of BrdU+ cells was decreased by 63%, while at seven months it was decreased by 99% (two months, 1704 ± 186, n = 6; three months, 640 ± 140, n = 6; seven months, 22 ± 22 BrdU+ cells/mm3, n = 5; ANOVA, F(2,14)= 34.6 p < 0.001; Tukey HSD two vs. three months and two vs. seven month p < 0.001). Nevertheless, despite this age-dependent decrease in neurogenesis, the total cell density in the AOB was higher at seven months than at two months in both males and females (male, two months, 523x103 ± 25x103; seven months, 808x103 ± 14x103 cells/mm3; female, two months 589x103 ± 36x103; seven months, 813x103 ± 52x103 cells/mm3, n = 3 both; t-test, p < 0.025, see methods). However, we did not observe sex differences in the total number of cells at these ages. Similarly, the volume in the AOB was not different at these ages or between sexes (males, two months, 111x106 ± 3x106; seven months, 94x106 ± 11x106 μm3; females, one month, 117 x106 ± 24x106 μm3; seven months, 88x106 ± 7x106 μm3). Interestingly, the sexual dimorphism in adult neurogenesis at two months was not preserved in older animals (Fig 3A), suggesting that sexual dimorphism in juvenile mice could arise from an age-dependent physiological process rather than simple anatomical differences among sexes. Furthermore, in agreement with previous studies, we found an age-dependent decrease in adult neurogenesis in the MOB which affected both genders (males, two months, 6171 ± 406, n = 5, three months, 2796 ± 503, n = 6, seven months, 1041 ± 306 BrdU+ cells/mm3, n = 5, ANOVA, F(2,13)= 34.7 p < 0.001; Tukey HSD two vs. three months and two vs. seven month p < 0.001; females, two months, 5965 ± 380, n = 5, three months, 2848 ± 459, n = 6, seven months, 1036 ± 261 BrdU+ cells/ mm3, n = 5; ANOVA, F(2,14)= 40.3 p < 0.001; Tukey HSD two vs. three months and two vs. seven month p < 0.001). To determine whether the decline in the number of new neurons in the adult AOB was due to a decrease in neuronal integration or a diminished number of neuroblasts being generated in the SVZ, we studied neuronal proliferation at 2 and 6 month of age, which coincides with the time when the animals used in our studies received the BrdU injections. As shown in figure 4A (top panel), we found that the SVZ of 6-month-old mice exhibited a 30–40% decrease in the number of BrdU+ cells compared to the 2 month-old animals. The quantification (Figure 4A, bottom panel) showed a significant decreased in the number of BrdU+ cells in both males (two months, 521 ± 27; six months 352 ± 45 BrdU+ cells; n=4, t-test, p<0.03) and females (two months, 597 ± 80; six months 367 ± 50 BrdU+ cells; n=4, t-test, p<0.05). The age-dependant decreased in adult neurogenesis could also be due to an increase in cell death of cells arriving into the AOB in older mice. To explore this possibility, we quantified the number of apoptotic cells in the OB one month after BrdU injection using the TUNEL assay (Figure 4B, top panel). We found no difference in the number of apoptotic cells between males and females at 3 and 7 months of age in the AOB (males, three months, 1045 ± 118, n = 4, seven months, 774 ± 238, n = 4; females, two months, 992 ± 105, n = 4, seven months, 962 ± 158 TUNEL+ cells/mm3, n = 4) or in the MOB (males, three months, 843 ± 114 cells, n = 4, seven months, 620 ± 261, n = 4; females, two months, 906 ± 334, n = 4, seven months, 847 ± 339 TUNEL+ cells/mm3, n = 4).
Figure 3.
Adult neurogenesis in the AOB decreases with age. (A) Number of new neurons in the AOB and MOB in mice injected at one, two or six months of age and analyzed one month post-injection. In male (black circles) and female (grey triangles) mice the number of BrdU+ cells is significantly decreased at 3 and 7 months compared with 2 months (Females, one-way ANOVA, F(2,14)=34.6 p<0.001; Tukey HSD two vs. three months and two vs. seven month ; males, ANOVA, F(2,13)= 27.9 p<0.001; Tukey HSD two vs. three months and two vs. seven month ;**, P< 0.001). In the MOB the number of BrdU+ cells is not different between male and female mice at any age group. (B) Total number of cells (cells/mm3) and total tissue volume (μm3) in the AOB of male (black bars) and female (gray bars) at 2 and 7 months. The number of cells at 7 months is significantly higher in both male and females (t-test; *, p<0.025) but there is no difference in AOB volume.
3.3 Adult neurogenesis in the AOB can be differentially regulated by behavior
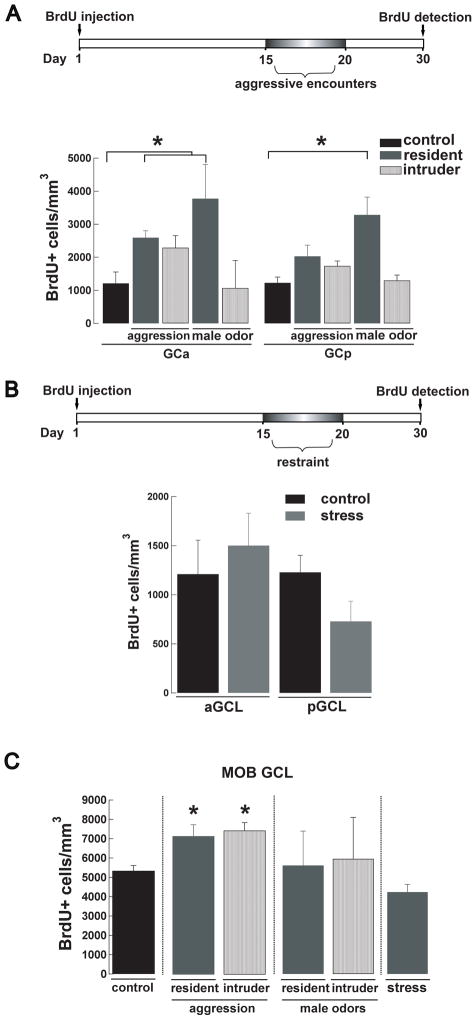
The activity of the VNS is crucial for social behaviors such as identifying and attracting mates and discerning the social status of conspecifics (Dulac and Torello, 2003). Several of these behaviors involve olfactory learning, which is thought to result from synaptic plasticity within the neuronal network of the OB, and adult neurogenesis provides an exciting mechanism for this plasticity (Keverne, 1995; Lledo and Lazarini, 2007). To explore the possibility that activation of the AOB circuitry during aggressive behaviors could regulate integration of adult-born neurons, we subjected male mice to a resident-intruder paradigm two weeks post injection of BrdU (Figure 5A, top panel). Offline analysis of the videotaped interactions indicated that all mice exhibited the expected dominant (resident) and subordinate (intruder) behavior throughout the entire experiment. However, as previously described (Mitra et al., 2006; Rodriguez-Alarcon et al., 2007), resident mice showed a significant decrease in the attack frequency over the 5 days of the trials (day 1, 13 ± 2; day 5, 8 ± 2; n = 6, t-test, p < 0.05), while the intruders showed a decrease in defensive posture frequency (day 1, 13 ± 3. s; day 5, 6 ± 2 s; n = 4, t-test, p < 0.05). Nevertheless, we found that male mice exposed to aggression exhibited a region-specific increase in neurogenesis that was limited to the aAOB. As shown in Fig 4A, male intruders had a significant increase in the number of new neurons in the aGCL compared to individually-housed controls (intruder, 2594 ± 209, n = 4; control, 1210 ± 346, BrdU+ cells/ mm3, n = 4; t-test, p<0.015). The number of new neurons in the aGCL of the residents was higher than in control mice, but within the sample analyzed this difference was not statistically significant (resident, 2289 ± 365 BrdU+ cells/ mm3, n = 6; t-test p < 0.08). Surprisingly, the resident-intruder paradigm also induced changes in neurogenesis in the MOB (Fig 5C). Likewise the AOB, the increase in neurogenesis was circumscribed to the GCL, however, unlike the AOB, both subordinates and dominant males exhibited a significant increase in neurogenesis (control, 5340 ± 268, n = 4; resident, 7158 ± 595, n = 6; intruder, 7448 ± 409 BrdU+ cells/ mm3, n = 4; t-test control vs. intruder p < 0.05, control vs. resident p < 0.05). Recently it has been shown that a protein component in urine promotes male-male aggression (Chamero et al., 2007), therefore we wondered whether the exposure of males to male odors such as male bedding or urine would be a sufficient stimulus to promote adult neurogenesis in the AOB. To examine this possibility we exposed intruders to bedding soiled by a dominant male and resident males to urine collected from group-housed males. Interestingly, we found that in the intruder mice, but not the resident, these odor stimuli increased the integration of new neurons in both regions of the AOB GCL compared to controls (intruder, aGC, 3777 ± 1032;n=3, t-test, p<0.04; pGC, 3286 ± 534 BrdU+ cells/ mm3; n=3, t-test, p<0.009; resident, aGC 1070 ± 837; n=3, t-test, p=0.87; pGC, 1301 ± 163 BrdU+ cells/ mm3, n=3; t-test, p=0.78). Surprisingly, the same stimulus did not affect the integration of newly born neurons in the MOB of the resident or the intruder mice (intruder, 5955 ± 2146;n=3, t-test, p=0.075; resident, 5613± 1775 BrdU+ cells/ mm3; n=3, t-test, p=0.87).
Figure 5.
Aggression and male odors, but not stress increases adult neurogenesis in the AOB of male mice. (A) Top panel, experimental design used for the resident-intruder aggression paradigm. Male mice are injected with BrdU at day 0 and from day 15 to day 20 the resident is exposed to a daily encounter of 15 minutes with an intruder. Neurogenesis is quantified 10 days later. A control group of male mice is singly housed but not exposed to any intruder. Bottom panel, number of BrdU+ cells in the GCL of the AOB in control (black), resident (dark grey) and intruder males exposed to aggression or male odors. The intruder showed a significant higher number of BrdU+ cells in the anterior GCL (aGCL) when exposed to aggression and throughout the GCL when exposed to bedding soiled by a male (t-test;*, p<0.05). (B) Top panel, experimental design used for the stress by restraint paradigm. At day 0 male mice were injected with BrdU and from day 15 to day 20, each animal was restrained daily for 15 min and neurogenesis was quantified at day 30. Bottom panel, stress by restraint did not produce a significant change in the number of BrdU+ cells in the GCL of the AOB (anterior and posterior). (C) Males exposed to aggression (resident and intruders) showed an increased number of BrdU+ cells in the GCL of the MOB (t-test *, p < 0.05). This increase in BrdU+ cells was not observed in resident or intruders exposed to male odors. As in the AOB animals exposed to stress showed no significant difference between the control and treated group.
The resident-intruder paradigm has also been used to elicit stress in mice (Yap et al, 2006), therefore stress could have affected adult neurogenesis in our aggressive behavior paradigm. To explore this possibility, male mice were exposed to stress by restraint, using a similar exposure regime as was used in the aggression protocol (Fig 5B, top panel). Noticeably, the stress paradigm did not influence adult neurogenesis in the GCL of the AOB (Fig 5B bottom; control, aGCL, 1210 ± 346, pGCL, 1228 ± 173 BrdU+ cells/ mm3, n = 4; stressed, aGCL, 1502 ± 329, pGCL, 730 ± 204 BrdU+ cells/ mm3, n = 6). Similarly, there was no difference in adult neurogenesis in the GCL of the MOB in mice subjected to the stress paradigm (Fig 5C; control, 5340 ± 268, n = 4; stressed, 4241 ± 397 BrdU+ cells/ mm3, n = 6).
Hormonal changes associated with pregnancy and male odor exposure, regulate the proliferation of new neurons in the SVZ (Larsen et al., 2008; Mak et al., 2007; Segovia et al., 1999; Shingo et al., 2003). Thus, to further support our hypothesis that social odors known to influence the activity of the AOB can regulate adult neurogenesis, by increasing proliferation, we exposed naïve females to male odors. Females were exposed to soiled bedding odorized by a male (Fig 6A, top) for seven days, and subsequently injected with BrdU. A group of control females was exposed only to clean bedding. After the odor-exposure paradigm, animals were injected with BrdU and BrdU+ cells were quantified at day 30 (Fig 6A, bottom). We found that a seven days exposure to male odors greatly enhanced neurogenesis in the AOB of females in a region specific manner. As shown in Fig 6B, females exposed to male odors exhibited a differential increase in the number of new neurons in the GCL of the pAOB compared to control females (odor-exposed, 2583 ± 154, n = 8; control, 1456 ± 240 BrdU+ cells/ mm3, n = 7; ANOVA pGCL F(2,15)= 8.23 p < 0.005; Dunnett p < 0.004). In contrast, the increase in neurogenesis was not present in females exposed to female odors, suggesting that this effect was gender specific (Fig 5B; 1576 ± 597 BrdU+ cells/ mm3, n = 3). Interestingly, as previously shown (Mak et al., 2007) a similar effect was observed in the MOB, where females exposed to male but not to female odors exhibited an increase in the number of BrdU+ cells in the GCL (control, 6563 ± 574, n = 7; females exposed to male odors, 8463 ± 252, n = 8; females exposed to female odors, 7710 ± 1213 BrdU+ cells/ mm3, n = 3; one-way ANOVA MOB GCL F(2,15)= 4.99 p < 0.03; Dunnett p < 0.02).
Figure 6.
Adult neurogenesis in the AOB increases in females exposed to male odors. (A) Diagram of the experimental paradigm used for odor exposure. Female mice are placed for seven days in a cage containing bedding previously soiled by a male. Every other day the soiled bedding is changed for new soiled bedding from the same male to continuously expose females to male odors. Mice are injected with BrdU on the last day of the odor exposure (day 7) and neurogenesis quantified at day 30. (B) Representative confocal images of BrdU+ cells in the GCL of the pAOB. Female mice exposed to male odors (right) show a larger number of BrdU+ cells in the GCL of the pAOB (white arrows) than control females that were exposed only to clean bedding (left). (C) Number of BrdU+ cells in the GCL of the aAOB and pAOB in female exposed to male (dark grey) or female odors (light gray). A group of control females was exposed only to clean bedding (back). Female mice exposed to male-soiled bedding exhibit a significantly greater number of BrdU+ cells in the anterior GCL (ANOVA pGCL F(2,15)=8.23 p<0.005; Dunnett,**, p<0.004).
4. DISCUSSION
The generation of new neurons in the adult persists throughout life in two areas of the brain, the OB and the hippocampus (Altman and Das, 1965a; Lledo et al., 2006; Lois and Alvarez-Buylla, 1994; Ming and Song, 2005). In the MOB, the generation of neurons in the adult plays an important role in olfactory-dependent behaviors, although the mechanisms underlying the replacement of neurons has not been fully elucidated (Gheusi et al., 2009; Lledo et al., 2008; Lledo and Saghatelyan, 2005; Zhao et al., 2008). Adult neurogenesis is regulated by several physiological conditions and behaviors, including odor enrichment, mate choice, pregnancy and aging (Bovetti et al., 2009; Lemasson et al., 2005; Mak et al., 2007; Rochefort et al., 2002; Shingo et al., 2003). Here we show that adult neurogenesis is regulated by social behaviors in the AOB, a region of the OB involved in pheromonal communication. Aggressive behavior in males and exposure of females to male odors increased adult neurogenesis in a zone-specific pattern in the AOB. In addition, we found that neurogenesis is sexually dimorphic in juvenile mice; male mice exhibit higher levels of newly born cells in the AOB than females. However, in addition to a gradual age-dependent decrease in neurogenesis, this sexual dimorphism was not maintained in older animals. These results indicate that adult neurogenesis in the AOB is influenced by behaviors and it can be differentially regulated in a sex and age-dependent manner.
Neuroblasts born in the SVZ migrate tangentially and reach the OB within a week, where they begin to migrate radially to specific layers within the OB. Within a month they have already matured into GC and PG neurons (Petreanu and Alvarez-Buylla, 2002). Accordingly, four weeks after the BrdU injections we identified abundant labeled cells in the GCL and GL of the AOB and most of these cells corresponded to mature GCs and PGs as they were also positive for NeuN. Furthermore, two-month old male mice exhibited a larger number of adult born cells than females in the AOB, and this difference was mainly attributed to increased neurogenesis in the pAOB. The observed difference in neurogenesis was not due to differences in cell density or volume along the anterior axis of the AOB ruling out the possibility that these factors contributed to the sex differences observed. Thus, it is possible that the difference in the number of BrdU+ cells between male and female, might not be large enough to significantly alter the much larger number of total cells in the AOB. The physiological relevance of this sexual dimorphism is unknown but it is tempting to speculate that a larger availability of newly born neurons in juvenile males may correlate with an increase in neuronal activity in the AOB. For example, at this age male mice begin to establish dominance hierarchy and engage in sexual activity for which the circuitry of the AOB is actively recruited. The sexual dimorphism in neurogenesis reported here is in agreement with previous studies showing anatomical sexual dimorphism in the AOB and other regions of the VNS (Segovia et al., 2006; Segovia et al., 1999; Suarez and Mpodozis, 2009) and with a study showing sexual dimorphism in neurogenesis in rat AOB (Peretto et al., 2001). Interestingly, a recent report by the latter group did not find significant differences in neurogenesis between sexes in mice (Oboti et al., 2009). The nature of the difference between the present work and that of Oboti et al (2009) is not known; however, we note that the strain of mice used in these versus the present study is different (BL/C57 vs. CD-1), and significant differences in adult neurogenesis have been reported for different strains of mice in the adult hippocampus (Kempermann et al., 1997). Nevertheless, the density of newly born neurons in the adult AOB reported here is comparable to those recently reported for mice and rat (Oboti et al., 2009; Peretto et al., 2001). Intriguingly, the sexual dimorphism in neurogenesis observed in younger mice was not preserved in older animals; instead we observed a steady decline in neurogenesis with age (see below). Thus, the hormonal and/or behavioral influences that lead to marked differences in basal neurogenesis in juvenile mice of different sex are not pronounced at later ages.
We found a consistent decline, with similar time course, in the number of newly born neurons in the AOB and MOB with aging. Furthermore, this decline in OB neurogenesis corresponded with a reduction in the number of proliferating neuroblasts in the SVZ as we found a significant decrease in the number of labeled cells in the SVZ of older mice after an acute injection of BrdU. In addition, the decline in adult neurogenesis was not due to an increase in cell death, since no difference in the number of apoptotic cells was found between sexes and, more importantly, across ages. The latter results are in agreement with studies showing that the number of newly born neurons in the MOB greatly decreases within the first two months postnatally and at older ages, resulting mainly from a decrease in SVZ proliferation and not from an increase in cell death (Ahlenius et al., 2009; Enwere et al., 2004; Lemasson et al., 2005; Mirich et al., 2002). The decline in MOB neurogenesis has been correlated with the decline in olfactory function with aging, suggesting that a similar decline in function may occur in the AOB (Enwere et al., 2004). In the MOB, newly arrived neurons integrate into the existing neuronal network and participate in inhibition, an essential component of olfactory processing (Belluzzi et al., 2003; Carleton et al., 2003; Schoppa and Urban, 2003). Consequently, the generation of new neurons and their programmed death in the MOB greatly influences olfactory processes, including odor discrimination and odor learning (Alonso et al., 2006; Moreno et al., 2009; Mouret et al., 2008; Mouret et al., 2009; So et al., 2008). Further studies are necessary to determine if the decline in neurogenesis affects these processes in the AOB.
Interestingly, and in accordance with other studies (Oboti et al., 2009; Peretto et al., 2001), the density of adult born neurons in the AOB was lower than in the MOB at all ages tested. The lower number of new cells in the AOB is not due to differences in total number of cells compared to the MOB, suggesting that adult neurogenesis of inhibitory neurons in these two regions is under different regulation and/or has a different physiological role. In the VNS, sensory axons synapse onto multiple glomeruli and MCs send primary dendrites to multiple glomeruli and have shorter lateral dendrites. This anatomical arrangement suggests that lateral and recurrent inhibition by GCs and PGs in the AOB may have a different function. For example, it has been proposed that the connectivity in the AOB is more suited for the analysis of blends of social odors (Wagner et al., 2006). Thus, neurogenesis of inhibitory neurons in these two regions could be under different regulatory mechanisms of proliferation and/or integration, resulting in different turnover rates. In agreement with this possibility, recent studies indicated that the neurogenic pool in the SVZ that gives rise to cells in the MOB is heterogeneous (for review see Lledo et al. (2008). For example, neuronal stem cells located in the dorsal regions of the SVZ give rise to PGs that contain tyrosine hydroxylase and to GCs that integrate into the superficial layers of the MOB. On the other hand, neuronal progenitors located in the ventral SVZ give rise to PGs that contain calbindin and to GCs that integrate within deep layers of the GCL (Merkle et al., 2007). In addition, the complexity of the neurogenic pool is greater than expected because it can give rise to excitatory cells as has recently been described in the MOB (Brill et al., 2009). Thus, different pool of neuronal progenitors in the SVZ could give rise to neurons to the AOB and MOB.
The detection and recognition of pheromones by the VNS is crucial for the proper execution of aggressive behaviors in mice. Removal of the VNO dramatically abolishes aggressive behavior in males (Clancy et al., 1984; Maruniak et al., 1986). Mice lacking the TRPC2 channel, which is thought to be a critical component of signal transduction in the VNO, show impaired male-male aggression (Leypold et al., 2002; Stowers et al., 2002). The proliferation of progenitor cells in the SVZ and the differentiation and integration into the circuitry of the MOB are regulated by social behaviors or social stimuli in several species, suggesting that the activity of newly integrated neurons is recruited in the processing of relevant odors that mediate these social encounters (Gheusi et al., 2009). Aggressive behavior in male mice increased the number of new neurons in the aAOB of the intruder in a resident-intruder behavioral assay. Similarly, the number of cells in the aAOB was also higher in the resident, although this value did not reach significance; therefore, we cannot rule out the possibility that both groups of mice, if exposed for longer times to aggression, could exhibit increased neurogenesis in the AOB. Nevertheless, the localized increase in newly born neurons in the aAOB suggests the involvement of activity of sensory neurons in the apical layer of the VNO, which projects to this region. Aggression in males is triggered mainly by cues present in urine, which are detected by the VNO. Two fractions of urine, one of low and one of high molecular weight (LMW and HMW), can be isolated and produce localized activation in the AOB. Noteworthy, the HMW fraction has been recently identified as a member of the major urinary protein (MUP) family, for a long time suspected to mediate pheromonal effects in the VNS (Chamero et al., 2007; Mugford and Nowell, 1970). Surprisingly, both the HMW and LMW fractions activate the aAOB, while the pAOB is activated by the HMW fractions only (Brennan et al., 1999; Papes et al.), however, these observations are in agreement with previous studies showing that chemosensory cues hastening aggression can activate either of these two regions of the AOB (Kumar et al., 1999; Sugai et al., 2006). Furthermore, in agreement with our findings, mice lacking Gαi2, a G protein subunit expressed by apical neurons in the VNO which project to the aAOB, also show diminished aggressive behavior (Norlin et al., 2003). Together, these findings suggest that complementary chemosensory cues (i.e. of LMW and HMW) can trigger the expression of aggressive behaviors by producing activation of both subdivisions of the AOB, which in turn could promote the integration of newly born neurons. Interestingly, we found that exposure to only the bedding soiled by the resident was sufficient to increase integration of neurons in the intruder, suggesting that that physical contact or the presence of the antagonistic male was not necessary and that the resident odor was sufficient stimulus. Moreover, this stimulus increased in integration throughout the whole GCL of the AOB. This can be beneficial in the wild, where subordinate males needs to detect dominant males perimeters even when they are not physically present in a determine area. Resident mice on the other hand, failed to show upregulation of adult neurogenesis when they were presented with male urine, indicating that perhaps this stimulus needs a physical contact. These findings indicate that the regulation of adult neurogenesis in the resident AOB requires the physical act of aggression, perhaps acute hormonal changes triggered by aggression. It is also possible that pheromones from other secretions (i.e. lacrimal or salivary gland) might also add to promote neurogenesis in the context of aggressive behaviors (Thompson et al., 2007).
Our results also indicate that the increase in the integration of new neurons induced in the resident-intruder assay was not due to the stress resulting from social conflict generated between the males (Heinrichs and Koob, 2006; Koolhaas et al., 1997). Mice subjected to stress did not exhibit a significant change in adult neurogenesis in the AOB or in the MOB. These results are in contrast to observations in the hippocampus where social stress, induced by a similar paradigm to that used in our studies, decreased the rate of adult neurogenesis (Mitra et al., 2006; Yap et al., 2006). Thus, our data provides further evidence to the notion that neurogenesis can be differentially regulated by behavior and/or physiological conditions in different parts of the brain (Fowler et al., 2002; Kaneko et al., 2006; Larsen et al., 2008).
Several studies have indicated that pheromonal cues from males can influence adult neurogenesis in females. For example, estrous induction in females by exposure to males increases cell proliferation in the SVZ of prairie voles (Smith et al., 2001). In female mice, exposure for 7 days to male odors prior to BrdU injections (Larsen et al., 2008; Mak et al., 2007), also promotes an increase in the number if BrdU+ cells in the SVZ and in the MOB. In agreement with these observations, we found that exposure to social odors for the same period of time increased adult neurogenesis in the AOB; females exposed to male but not female odors had a greater density of labeled cells in the pAOB. Interestingly, exposure to male odors produce changes in prolactin levels in the female mice, and these changes in prolactin can be related to an increase in neuronal proliferation in the SVZ (Larsen et al., 2008; Mak et al., 2007). Thus, hormonal changes driven by a particular social stimulus, could affect the proliferation rate of a specific neurogenic pool in the SVZ (see above), which may lead to the integration of neurons in a particular region of the AOB. Alternatively, selective activation of sensory neurons in the basal layer of VNO, during the stimulation period by the signals from the male, could induce hormonal changes that induce a delayed increase in the number of newly born neurons in the pAOB. Future experiments are needed to determine the mechanisms by which male odors induce differential increases in the anterior-posterior axis of the AOB.
The differential increase in neurogenesis between the AOB sub-regions observed with male aggression and male odor exposure in females is consistent with the prevalent notion that segregated projections from the sensory neurons in the VNO allow for chemosensory stimuli to differentially regulate the activity of the aAOB and pAOB in the VNS pathway (Tirindelli et al., 2009). However, the primary region of the AOB activated by sexual stimuli remains controversial; activation of the aAOB, pAOB or both has been described in rodents (Brennan et al., 1999; Dudley and Moss, 1999; Halem et al., 2001; Inamura et al., 1999; Kimoto and Touhara, 2005; Kumar et al., 1999; Sugai et al., 2006; Yamaguchi et al., 2000; Yoshikage et al., 2007). In addition, a recent study indicated that exposure of female mice to male odors increased the number of new neurons in both subdivisions of the AOB (Oboti et al., 2009). As suggested above, the differences between these and our studies could be due to differences in the strain, age and/or BrdU injection protocol. In addition, a recent report indicated that behavior can be greatly influenced by environmental conditions, making comparison across behavioral studies more difficult (Oliva et al., 2010). Nevertheless, further studies are necessary to determine whether activation of V2R sensory neurons in the VNO by social odors and concomitant activation of the pAOB promotes neurogenesis. It should be noted, however, that an increase in the number of new neurons in a particular region does not need to result from a local increase in neuronal activity; instead, other regulatory mechanisms can trigger the increase in integration/survival of new neurons, a possibility that needs to be further explored.
Surprisingly, in addition to changes in the level of neurogenesis in the AOB, induced by behavior, adult neurogenesis was also increased in the MOB of males, irrespective of their hierarchical status in the resident-intruder assay, and in females exposed to male odors. One possibility is that the large quantity of chemicals found in urine may provoke a general activation in the MOB leading to increased neurogenesis, which has been extensively shown to be affected by olfactory enrichment in the MOB (Bovetti et al., 2009; Moreno et al., 2009; Rochefort et al., 2002; Veyrac et al., 2009). The dual activation of these regions of the bulb during olfactory-guided behaviors has been only occasionally described (Fiber and Swann, 1996; Guo et al., 1997; Huang and Bittman, 2002; Martel and Baum, 2007; Xu et al., 2005); however, our results are in agreement with several pieces of evidence that support the emerging notion of a complementary role for the main olfactory system (MOS) and the VNS in mate recognition and aggression in rodents (Baum and Kelliher, 2009; Keller et al., 2009; Tirindelli et al., 2009). In females, activation of the VNS is necessary for the proper execution of behaviors elicited by pheromonal cues such as acceleration of puberty (Vandenbergh, 1975), estrous induction (Whitten, 1959) and pregnancy block (Bruce, 1959), while activation of the MOS is necessary for the processing of chemosensory information that conveys social attraction and social recognition (Baum and Kelliher, 2009; Keller et al., 2009; Spehr et al., 2006). Thus, removal of the VNO in male mice elicited normal sexual discrimination among conspecifics but decreased male preference for the urine of estrous females (Pankevich et al., 2004), while ablation of the main olfactory epithelium (MOE) reduced females sexual behavior and olfactory investigation (Keller et al, 2006). Similarly, genetic modifications of sensory neurons in the MOE also support the critical role of the MOS in social behaviors. Mice deficient in CNGA2, a cyclic nucleotide-gated channel that participates in signal transduction, exhibit impaired individual recognition (Mandiyan et al., 2005). In addition, male mice lacking the adenylate cyclase type 3 (AC3), another component of the canonical transduction pathway in the MOS, exhibit impaired sexual and male-male aggressive behaviors (Wang et al., 2006). Furthermore, integration of olfactory information at the level of the amygdala, principally through reciprocal connections with olfactory cortices, greatly contributes to the complementary role between the MOS and VNS (Dulac and Torello, 2003; Kang et al., 2009; Keller et al., 2009). Further studies are necessary to determine the function of newborn neurons in the adult OB within the context of complementary olfactory cues and the contribution of adult neurogenesis in the generation, maintenance and extinction of olfactory-guided social behaviors. Differential regulation of adult neurogenesis in the AOB along the anterior-posterior axis may be crucial for social and sexual behaviors that require the generation of selective odor memories that are necessary to maintain an adequate social behavior.
Acknowledgments
We wish to thank the members of the Araneda lab for their helpful comments on the manuscript.
GRANTS
This work was partially funded by National Institute of Deafness and Other Communicational Disorders Grant DC RO1-DC-009817 to R.C. Araneda and a doctoral fellowship from the Chilean government to Alexia Nunez-Parra.
Abbreviations
- AOB
accessory olfactory bulb
- aAOB
anterior accessory olfactory bulb
- aGCL
anterior granule cell layer
- BrdU
5-bromo-2 -deoxyuridine
- GC
granule cell
- GCL
granule cell layer
- HMW
High molecular weight
- LMW
Low molecular weight
- LOT
lateral olfactory tract
- MC
mitral/tufted cell
- MOB
main olfactory bulb
- OB
olfactory bulb
- PBS
phosphate-buffer-saline
- pAOB
posterior accessory olfactory bulb
- PFA
paraformaldehyde
- PG
periglomerular cell
- SVZ
subventricular zone
- VNO
vomeronasal organ
- VNS
vomeronasal system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Viollet C, Gabellec MM, Meas-Yedid V, Olivo-Marin JC, Lledo PM. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965a;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965b;207:953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- Arevian AC, Kapoor V, Urban NN. Activity-dependent gating of lateral inhibition in the mouse olfactory bulb. Nat Neurosci. 2008;11:80–87. doi: 10.1038/nn2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–160. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Bean NJ. Olfactory and vomeronasal mediation of ultrasonic vocalizations in male mice. Physiol Behav. 1982;28:31–37. doi: 10.1016/0031-9384(82)90097-x. [DOI] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S, Veyrac A, Peretto P, Fasolo A, De Marchis S. Olfactory enrichment influences adult neurogenesis modulating GAD67 and plasticity-related molecules expression in newborn cells of the olfactory bulb. PLoS One. 2009;4:e6359. doi: 10.1371/journal.pone.0006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Schellinck HM, Keverne EB. Patterns of expression of the immediate-early gene egr-1 in the accessory olfactory bulb of female mice exposed to pheromonal constituents of male urine. Neuroscience. 1999;90:1463–1470. doi: 10.1016/s0306-4522(98)00556-9. [DOI] [PubMed] [Google Scholar]
- Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascon S, Erdelyi F, Szabo G, Parras C, Guillemot F, Frotscher M, Berninger B, Hevner RF, Raineteau O, Gotz M. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci. 2009;12:1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Coquelin A, Macrides F, Gorski RA, Noble EP. Sexual behavior and aggression in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2222–2229. doi: 10.1523/JNEUROSCI.04-09-02222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawley EM, Crowder J. Sexual and seasonal differences in the vomeronasal epithelium of the red-backed salamander (Plethodon cinereus) J Comp Neurol. 1995;359:382–390. doi: 10.1002/cne.903590303. [DOI] [PubMed] [Google Scholar]
- Dudley CA, Moss RL. Activation of an anatomically distinct subpopulation of accessory olfactory bulb neurons by chemosensory stimulation. Neuroscience. 1999;91:1549–1556. doi: 10.1016/s0306-4522(98)00711-8. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiber JM, Swann JM. Testosterone differentially influences sex-specific pheromone-stimulated Fos expression in limbic regions of Syrian hamsters. Horm Behav. 1996;30:455–473. doi: 10.1006/hbeh.1996.0050. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo PM. A niche for adult neurogenesis in social behavior. Behav Brain Res. 2009;200:315–322. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Guo J, Zhou A, Moss RL. Urine and urine-derived compounds induce c-fos mRNA expression in accessory olfactory bulb. Neuroreport. 1997;8:1679–1683. doi: 10.1097/00001756-199705060-00024. [DOI] [PubMed] [Google Scholar]
- Halem HA, Baum MJ, Cherry JA. Sex difference and steroid modulation of pheromone-induced immediate early genes in the two zones of the mouse accessory olfactory system. J Neurosci. 2001;21:2474–2480. doi: 10.1523/JNEUROSCI.21-07-02474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M, Shapiro LS, Jia C. Differential localization of G proteins in the opossum vomeronasal system. Brain Res. 1995;677:157–161. doi: 10.1016/0006-8993(95)00159-n. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Curr Protoc Neurosci. Unit8. Chapter 8. 2006. Application of experimental stressors in laboratory rodents; p. 4. [DOI] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Huang L, Bittman EL. Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Horm Behav. 2002;41:343–350. doi: 10.1006/hbeh.2002.1767. [DOI] [PubMed] [Google Scholar]
- Inamura K, Kashiwayanagi M, Kurihara K. Regionalization of Fos immunostaining in rat accessory olfactory bulb when the vomeronasal organ was exposed to urine. Eur J Neurosci. 1999;11:2254–2260. doi: 10.1046/j.1460-9568.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- Jia C, Halpern M. Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Gi alpha 2 and G(o alpha)) and segregated projections to the accessory olfactory bulb. Brain Res. 1996;719:117–128. doi: 10.1016/0006-8993(96)00110-2. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 2006;11:1145–1159. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the 'vomeronasal' amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB. Olfactory learning. Curr Opin Neurobiol. 1995;5:482–488. doi: 10.1016/0959-4388(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Touhara K. Induction of c-Fos expression in mouse vomeronasal neurons by sex-specific non-volatile pheromone(s) Chem Senses. 2005;30(Suppl 1):i146–147. doi: 10.1093/chemse/bjh156. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Kovacs T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 2004;3:215–232. doi: 10.1016/j.arr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dudley CA, Moss RL. Functional dichotomy within the vomeronasal system: distinct zones of neuronal activity in the accessory olfactory bulb correlate with sex-specific behaviors. J Neurosci. 1999;19:RC32. doi: 10.1523/JNEUROSCI.19-20-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Kokay IC, Grattan DR. Male pheromones initiate prolactin-induced neurogenesis and advance maternal behavior in female mice. Horm Behav. 2008;53:509–517. doi: 10.1016/j.yhbeh.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Lemasson M, Saghatelyan A, Olivo-Marin JC, Lledo PM. Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J Neurosci. 2005;25:6816–6825. doi: 10.1523/JNEUROSCI.1114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Lazarini F. Neuronal replacement in microcircuits of the adult olfactory system. C R Biol. 2007;330:510–520. doi: 10.1016/j.crvi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An Acad Bras Cienc. 2003;75:469–486. doi: 10.1590/s0001-37652003000400006. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci. 2007;26:463–475. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruniak JA, Wysocki CJ, Taylor JA. Mediation of male mouse urine marking and aggression by the vomeronasal organ. Physiol Behav. 1986;37:655–657. doi: 10.1016/0031-9384(86)90300-8. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. If you assume, you can make an ass out of u and me': a decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188 ( Pt 1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. J Comp Neurol. 2002;454:361–372. doi: 10.1002/cne.10426. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sundlass K, Parker KJ, Schatzberg AF, Lyons DM. Social stress-related behavior affects hippocampal cell proliferation in mice. Physiol Behav. 2006;89:123–127. doi: 10.1016/j.physbeh.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci U S A. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret A, Gheusi G, Gabellec MM, de Chaumont F, Olivo-Marin JC, Lledo PM. Learning and survival of newly generated neurons: when time matters. J Neurosci. 2008;28:11511–11516. doi: 10.1523/JNEUROSCI.2954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM. Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci. 2009;29:12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford RA, Nowell NW. Pheromones and their effect on aggression in mice. Nature. 1970;226:967–968. doi: 10.1038/226967a0. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Norlin EM, Gussing F, Berghard A. Vomeronasal phenotype and behavioral alterations in G alpha i2 mutant mice. Curr Biol. 2003;13:1214–1219. doi: 10.1016/s0960-9822(03)00452-4. [DOI] [PubMed] [Google Scholar]
- Oboti L, Savalli G, Giachino C, De Marchis S, Panzica GC, Fasolo A, Peretto P. Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. Eur J Neurosci. 2009;29:679–692. doi: 10.1111/j.1460-9568.2009.06614.x. [DOI] [PubMed] [Google Scholar]
- Oliva AM, Salcedo E, Hellier JL, Ly X, Koka K, Tollin DJ, Restrepo D. Toward a mouse neuroethology in the laboratory environment. PLoS One. 2010;5:e11359. doi: 10.1371/journal.pone.0011359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto P, Giachino C, Panzica GC, Fasolo A. Sexually dimorphic neurogenesis is topographically matched with the anterior accessory olfactory bulb of the adult rat. Cell Tissue Res. 2001;306:385–389. doi: 10.1007/s00441-001-0471-1. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Alarcon G, Canales JJ, Salvador A. Rewarding effects of 3,4-methylenedioxymethamphetamine ("Ecstasy") in dominant and subordinate OF-1 mice in the place preference conditioning paradigm. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:191–199. doi: 10.1016/j.pnpbp.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Feinstein P, Mombaerts P. Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell. 1999;97:199–208. doi: 10.1016/s0092-8674(00)80730-8. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- Segovia S, Garcia-Falgueras A, Carrillo B, Collado P, Pinos H, Perez-Laso C, Vinader-Caerols C, Beyer C, Guillamon A. Sexual dimorphism in the vomeronasal system of the rabbit. Brain Res. 2006;1102:52–62. doi: 10.1016/j.brainres.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Segovia S, Guillamon A, del Cerro MC, Ortega E, Perez-Laso C, Rodriguez-Zafra M, Beyer C. The development of brain sex differences: a multisignaling process. Behav Brain Res. 1999;105:69–80. doi: 10.1016/s0166-4328(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Smith MT, Pencea V, Wang Z, Luskin MB, Insel TR. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm Behav. 2001;39:11–21. doi: 10.1006/hbeh.2000.1630. [DOI] [PubMed] [Google Scholar]
- So K, Moriya T, Nishitani S, Takahashi H, Shinohara K. The olfactory conditioning in the early postnatal period stimulated neural stem/progenitor cells in the subventricular zone and increased neurogenesis in the olfactory bulb of rats. Neuroscience. 2008;151:120–128. doi: 10.1016/j.neuroscience.2007.07.051. [DOI] [PubMed] [Google Scholar]
- Spehr M, Spehr J, Ukhanov K, Kelliher KR, Leinders-Zufall T, Zufall F. Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell Mol Life Sci. 2006;63:1476–1484. doi: 10.1007/s00018-006-6109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Suarez R, Mpodozis J. Heterogeneities of size and sexual dimorphism between the subdomains of the lateral-innervated accessory olfactory bulb (AOB) of Octodon degus (Rodentia: Hystricognathi) Behav Brain Res. 2009;198:306–312. doi: 10.1016/j.bbr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Sugai T, Yoshimura H, Kato N, Onoda N. Component-dependent urine responses in the rat accessory olfactory bulb. Neuroreport. 2006;17:1663–1667. doi: 10.1097/01.wnr.0000239950.14954.59. [DOI] [PubMed] [Google Scholar]
- Thompson RN, Napier A, Wekesa KS. Chemosensory cues from the lacrimal and preputial glands stimulate production of IP3 in the vomeronasal organ and aggression in male mice. Physiol Behav. 2007;90:797–802. doi: 10.1016/j.physbeh.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirindelli R, Dibattista M, Pifferi S, Menini A. From pheromones to behavior. Physiol Rev. 2009;89:921–956. doi: 10.1152/physrev.00037.2008. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Proceedings: Pheromonal stimulation of puberty in female mice. J Endocrinol. 1975;64:38P. [PubMed] [Google Scholar]
- Veyrac A, Sacquet J, Nguyen V, Marien M, Jourdan F, Didier A. Novelty determines the effects of olfactory enrichment on memory and neurogenesis through noradrenergic mechanisms. Neuropsychopharmacology. 2009;34:786–795. doi: 10.1038/npp.2008.191. [DOI] [PubMed] [Google Scholar]
- Wagner S, Gresser AL, Torello AT, Dulac C. A multireceptor genetic approach uncovers an ordered integration of VNO sensory inputs in the accessory olfactory bulb. Neuron. 2006;50:697–709. doi: 10.1016/j.neuron.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten WK. Occurrence of anoestrus in mice caged in groups. J Endocrinol. 1959;18:102–107. doi: 10.1677/joe.0.0180102. [DOI] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Inamura K, Kashiwayanagi M. Increases in Fos-immunoreactivity after exposure to a combination of two male urinary components in the accessory olfactory bulb of the female rat. Brain Res. 2000;876:211–214. doi: 10.1016/s0006-8993(00)02651-2. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Takase LF, Kochman LJ, Fornal CA, Miczek KA, Jacobs BL. Repeated brief social defeat episodes in mice: effects on cell proliferation in the dentate gyrus. Behav Brain Res. 2006;172:344–350. doi: 10.1016/j.bbr.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Yoshikage M, Toshiaki I, Seiichi K, Nobuo K, Nishimura M. Sex steroids modulate the signals from volatile female odors in the accessory olfactory bulb of male mice. Neurosci Lett. 2007;413:11–15. doi: 10.1016/j.neulet.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]