Abstract
It has long been argued that face processing requires disproportionate reliance on holistic or configural processing, relative to that required for non-face object recognition, and that a disruption of such holistic processing may be causally implicated in prosopagnosia. Previously, we demonstrated that individuals with congenital prosopagnosia (CP) did not show the normal face inversion effect (better performance for upright compared to inverted faces) and evinced a local (rather than the normal global) bias in a compound letter global/local (GL) task, supporting the claim of disrupted holistic processing in prosopagnosia. Here, we investigate further the nature of holistic processing impairments in CP, first by confirming, in a large sample of CP individuals, the absence of the normal face inversion effect and the presence of the local bias on the GL task, and, second, by employing the composite face paradigm, often regarded as the gold standard for measuring holistic face processing. In this last task, we show that, in contrast with normal individuals, the CP group perform equivalently with aligned and misaligned faces and was impervious to (the normal) interference from the task-irrelevant bottom part of faces. Interestingly, the extent of the local bias evident in the composite task is correlated with the abnormality of performance on diagnostic face processing tasks. Furthermore, there is a significant correlation between the magnitude of the local bias in the GL and performance on the composite task. These results provide further evidence for impaired holistic processing in CP and, moreover, corroborate the critical role of this type of processing for intact face recognition.
Keywords: configural, faces, face perception, global processing, acquired prosopagnosia
1. Introduction
Face recognition presents one of the most demanding perceptual challenges to the visual system. Not only is a multiplicity of dimensions such as emotional expression and gaze direction conveyed via the face, but the individual identity of each face must be rapidly and accurately established. Despite this apparent complexity, humans are expert at face recognition and the robustness of this ability is further attested to by the fact that recognition remains remarkably accurate even under relatively poor lighting conditions, under changes in view of the face, and with changes in the age and appearance of the face (for example, with changes of facial hair). Surprisingly, however, there are a number of conditions under which normal face recognition is adversely impacted. Common to many, if not all, of these conditions is that there is a disruption of the configural or holistic processing of the face. Consequently, the observer resorts to relying on the featural information rather than on the configural information, in which the relations among the features of the face rather than just the features themselves, are represented (Maurer, Le Grand, & Mondloch, 2002; Tanaka & Farah, 1993).
Configural processing in face recognition
Given that all faces share the same local internal components (eyes, nose and mouth), the claim is that deriving a rapid and accurate representation of the face requires disproportionate reliance on the configuration of the features relative to that required for non-face object recognition (Maurer et al., 2002). Any manipulation that disrupts the configuration of the face, then, would be predicted to affect face processing disproportionately. Evidence to support this prediction comes from a number of experimental paradigms. For example, it is now well-known that face processing is adversely affected by changes in orientation: when the face is inverted, recognition is adversely affected to a greater degree, relative to upright, than is true for other classes of objects (Farah, Tanaka, & Drain, 1995; Freire, Lee, & Symons, 2000; Leder & Bruce, 1998; Malcolm, Leung, & Barton, 2004; Searcy & Bartlett, 1996). It is also the case that face perception benefits from the presence of the entire face, compared with the presence of just some components of the face and this whole versus part advantage holds to a greater degree for faces than for other objects (Gauthier & Tarr, 2002; Tanaka & Farah, 1993).
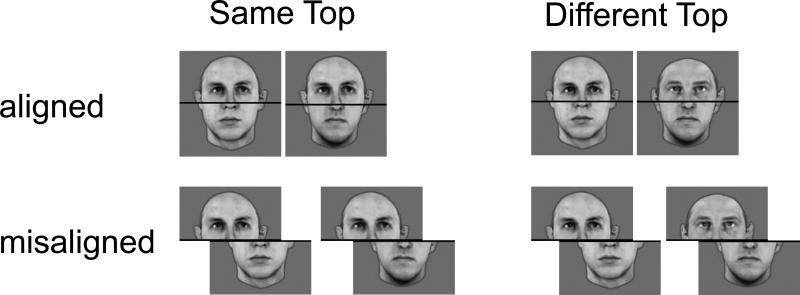
Interestingly, the derivation of the configuration of the face is apparently so automatic that even when instructed to attend selectively to only some parts of a face, normal observers cannot help but be sensitive to the entire face (Amishav & Kimchi, 2010). Data to support this claim comes from a well-established paradigm using composite faces (Boutet, Gentes-Hawn, & Chaudhuri, 2002; Farah, Wilson, Drain, & Tanaka, 1998; Gauthier, Curran, Curby, & Collins, 2003; Le Grand, Mondloch, Maurer, & Brent, 2004; Young, Hellawell, & Hay, 1987). In the version of this paradigm used here (Figure 1), individuals view two consecutively presented composite faces, and make same/different decisions based only on the top part of the face (Le Grand et al., 2004). The bottom part of the face is to be ignored. The two faces are created such that the two top parts could either be the same or different while the bottom part is always different. Additionally, the top and bottom parts of a single face can be either aligned or misaligned. Due to the holistic nature of face processing, even when instructed to judge only the top halves of aligned faces and to ignore the bottom parts, normal observers exhibit significant interference induced by the presence of the task-irrelevant bottom half of the composite face (which is always different). Thus, erroneously, they tend to judge two faces with identical tops as ‘different’ rather than ‘same’ (i.e. make false alarms). This interference from the task-irrelevant bottom of the face is substantially reduced when configural information is disrupted, as in the misaligned condition (Figure 1, bottom row) (Young et al., 1987) and also when the faces are inverted (Hole, 1994; Hole, George, & Dunsmore, 1999).
Figure 1.
Composite experiment: Examples of the stimuli used in the experiment showing the aligned (top row) and misaligned (bottom row) conditions.
Disrupted configural processing in prosopagnosia
If it is indeed the case that individuals with prosopagnosia are impaired at configural processing, one direct prediction is that their judgments about the top parts of faces will be impervious to the (different) bottom part of faces, even in the especially taxing aligned condition. That is, they will not process the task-irrelevant lower part of the face automatically.
Considerable empirical evidence supports the notion that a breakdown in configural processing is related to the impairment in face processing (for review see Barton, 2009; Rivest, Moscovitch, & Black, 2009). For example, individuals with prosopagnosia were substantially impaired, relative to matched controls, when deciding which of 3 faces was ‘odd’ when the interocular distance or the distance between the nose and mouth were altered (Barton, Press, Keenan, & O'Connor, 2002). Based on these findings, the authors argued that the need to represent the spatial relations between the features (and they note that the distance between the eyes is especially important) is integral to the ability to process faces. Moreover, PS, a well-characterized patient with acquired prosopagnosia but no deficits in other perceptual domains, exhibited abnormal holistic processing on several behavioral tests, including the composite face paradigm (Ramon, Busigny, & Rossion, 2010).
This disruption in configural processing skills appears to be true not only of individuals with acquired prosopagnosia (Barton, 2009) but also of individuals with congenital prosopagnosia (CP) (Lobmaier, Bolte, Mast, & Dobel, 2010). CP is an apparently lifelong deficit in face processing that occurs along with intact sensory visual abilities, normal intelligence and adequate opportunity to acquire face recognition skills (Behrmann & Avidan, 2005). Although there is some evidence that CP is related to a difficulty in deriving the configural or holistic relations between the features of a face, this claim is still controversial.
On the one hand, CP individuals, similar to individuals with AP (Busigny & Rossion, 2010a), are minimally (if at all) affected by face inversion and a few even show better performance for inverted than upright faces (the “inversion” superiority effect) but this latter effect is not very common in either forms of prosopagnosia (Behrmann, Avidan, Marotta, & Kimchi, 2005; Busigny & Rossion, 2010a; Farah et al., 1995) (and see also Table 2 in the present study). Additionally, these same individuals show a bias for local processing of elemental features, even for non-face stimuli. Thus, shown hierarchical, compound Navon stimuli, these individuals are faster at local than global letter identification and, when the letter identities are inconsistent at the two levels, show no interference from global to local letter identification, a pattern markedly discrepant from that of normal observers (Behrmann et al., 2005; Kimchi, 1992; Navon, 2003) (and see also Table 2, Figure 2 and Supplementary Figure 1 in the present study). Finally, along similar lines, Palermo et al., (2011) recently showed reduced holistic processing (i.e. reduced interference indicating atypical configural processing) in a group of 12 individuals with CP on the composite task.
Table 2.
Individual and group average of CP participants and large control group (accuracy, RT and z scores) on the upright/inverted face discrimination task and mean performance of the large control group (n=37).
| participant | accuracy upright faces | accuracy inverted faces | inversion index accuracy | RT upright faces | RT inverted faces | inversion index RT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % corr | z-score | % corr | z-score | index | z-score | RT (msec) | z-score | RT (msec) | z-score | index | z-score | |
| MT* | 100 | 0.4 | 100 | 0.71 | 0 | 0.37 | 8386 | 7.7 | 8106 | 4.22 | -0.02 | -0.85 |
| BE* | 100 | 0.4 | 90 | -0.61 | -0.05 | -0.7 | 8307 | 7.61 | 7642 | 3.87 | -0.04 | -1.01 |
| KM* | 100 | 0.4 | 100 | 0.71 | 0 | 0.37 | 6365 | 5.33 | 7973 | 4.12 | 0.11 | -0.04 |
| IT** | 93.3 | -0.91 | 93.3 | -0.17 | 0 | 0.37 | 6500.3 | 5.49 | 5381.6 | 2.2 | -0.09 | -1.34 |
| WS** | 100 | 0.4 | 93 | -0.21 | -0.04 | -0.36 | 3532.1 | 2 | 1394.6 | -0.76 | -0.43 | -3.49 |
| KE** | 100 | 0.4 | 96.7 | 0.27 | -0.02 | 0.03 | 3879.4 | 2.41 | 4018.6 | 1.19 | 0.02 | -0.63 |
| IM* | 76.7 | -4.19 | 80 | -1.93 | 0.02 | 0.8 | 5016.9 | 3.74 | 6542.5 | 3.06 | 0.13 | 0.09 |
| TD* | 100 | 0.4 | 96.4 | 0.24 | -0.02 | 0.003 | 1511.6 | -0.37 | 1563 | -0.63 | 0.02 | -0.64 |
| BT | 86.7 | -2.22 | 65.5 | -3.85 | -0.14 | -2.45 | 1140 | -0.81 | 959.2 | -1.08 | -0.09 | -1.29 |
| SI | 100 | 0.4 | 100 | 0.71 | 0 | 0.37 | 2336.2 | 0.6 | 2026.8 | -0.29 | -0.07 | -1.19 |
| JT | 100 | 0.4 | 93.3 | -0.17 | -0.03 | -0.33 | 3374.6 | 1.82 | 4911.7 | 1.85 | 0.19 | 0.43 |
| ID | 96.7 | -0.25 | 100 | 0.71 | 0.02 | 0.72 | 1077.8 | -0.88 | 1196.8 | -0.9 | 0.05 | -0.41 |
| SW | 100 | 0.4 | 93.3 | -0.17 | -0.03 | -0.33 | 4560.3 | 3.21 | 6953.1 | 3.36 | 0.21 | 0.57 |
| TZ | 100 | 0.4 | 100 | 0.71 | 0 | 0.37 | 3800 | 2.32 | 3320.8 | 0.67 | -0.07 | -1.17 |
| CP mean±s.d. | 96.7 ± 6.9 | 93 ± 9.6 | -0.02 ± 0.04 | 4270.5 ± 2422.6 | 4427.8 ± 2709.8 | -0.01 ± 0.16 | ||||||
| Controls mean±s.d. | 98 ± 5.1 | 94.6 ± 7.6 | -1.8 ± 4.9 | 1827.9 ± 851.7 | 2415.6 ± 1349.8 | 0.1 ± 0.2 | ||||||
Color code as in Table 1.
Performance on upright and inverted face discrimination task of these CP participants was published previously in (Avidan & Behrmann, 2008; Behrmann et al., 2005; Nishimura et al., 2010).
Performance on upright face discrimination task of these CP participants was published previously in (Avidan & Behrmann, 2008)
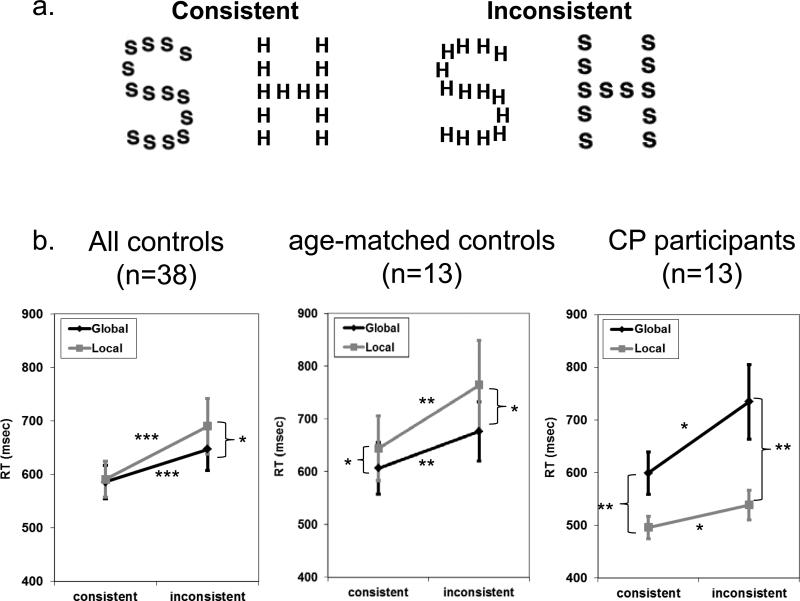
Figure 2.
Supporting evidence for impaired holistic/configural processing in CP. a. Stimuli used in the compound letter global/local task b. Results obtained for the entire group of control participants, age-matched controls and CP participants on the global/local task showing that CPs do not exhibit the expected global advantage and, instead, evince a local advantage and local-to-global interference. Asterisks denote significance level * p<0.05; **p<0.005 ***p<0.0005. Error bars indicate ± standard error of the mean across participants.
This apparent trend towards featural or elemental processing may not be ubiquitous, however. For example, Duchaine (2000) tested a congenital (or ‘developmental’) prosopagnosic on three tests from the Kit of Factor-Referenced Cognitive Tests and showed that this individual performed normally on these gestalt completion tasks. More pertinent perhaps and contrary to our findings, Duchaine, Yovel, & Nakayama (2007) tested a group of 14 developmental prosopagnosia participants on the global/local task and did not find a local processing bias in these individuals. Additionally, Le Grand et al., (2006) employed the composite face task and found abnormal performance in only one out of 8 CP participants. We return to the discrepancies among these studies, as well as others, and offer a possible, albeit tentative, resolution in the discussion section.
To explore further whether CP individuals do indeed evince an impairment in holistic processing, here, we conduct 3 experiments, all of which are designed to tap configural processing, in a relatively large group of 14 well-characterized CP individuals. We expect to replicate the lack of an inversion effect and the local bias in the global/local task, both of which we reported previously in smaller groups of participants, and, furthermore, predict that the very same individuals should be less affected by the incongruency effects afforded by the discrepant bottom parts of aligned faces in the composite task. In other words, and counterintuitively, in this last task, CP individuals should perform better than controls and produce fewer false alarms. Finally, a correlation between performance on these tasks would further support an account of abnormal holistic processing in CP.
Before reporting our empirical findings, we note that the terms ‘holistic’ and ‘configural’ are used interchangeably in the literature and, indeed, there continues to be heated debate on the differences, if any, that are implied by these two terms (Gauthier & Tarr, 2002). In the context of the present study, we use these terms operationally to refer to the obligatory integrated coding of all the face elements, as has been suggested by others (Farah et al., 1998; Ramon et al., 2010). Others use the term configural/spacing processing to refer to the coding of the spatial relations and distances between facial (or even non-facial) features, and to the perception of manipulations of second order relations such as the distance between local features, such as the eyes (Barton, 2009; Maurer et al., 2002; Yovel & Duchaine, 2006). Since the experimental paradigms used here are not diagnostic of the ability to process the spatial relations between facial elements, our results cannot speak directly to the debate regarding the role of holistic vs. spatial relation processing in face perception. Rather, our focus is on characterizing holistic processing in CP individuals and examining the robustness of this finding using three different but converging paradigms.
2. Methods
2.1. Participants
All participants provided informed consent to a protocol approved by the Institutional Review Boards of Carnegie Mellon University and of the University of Pittsburgh and by the Ethics committee of the Psychology Department at Ben-Gurion University. All participants have normal or corrected-to-normal visual acuity.
2.1.1 Congenital prosopagnosics
Fourteen individuals who were diagnosed with CP took part in the study (11 females), ages 31-79 years (mean± s.d. 52.6±16.4). Eight of these individuals have participated in previous studies and some of their data, obtained from a subset of the tasks, have been reported previously (Avidan & Behrmann, 2008, 2009; Behrmann et al., 2005; Nishimura, Doyle, Humphreys, & Behrmann, 2010). For more details regarding previous publications including data of these individuals, see Tables 1-3; figure legends of Figure 2 and Supplementary Figure 1. Of the remaining 6 CPs, 5 were tested in Israel and one was tested in the US and their data have not been included in any previous publications.
Table 1.
Demographic details of CP individuals and performance (raw values and z-scores) on famous faces questionnaire, CFMT and CFPT diagnostic tests
| Participant | Sex | Age | famous faces questionnaire | CFMT (total) | CFPT (upright) | |||
|---|---|---|---|---|---|---|---|---|
| 14 | % corr. | z-score | score | z-score** | score | z-score** | ||
| MT* | M | 41 | 62.5 | -1.64 | 36 | -3.11 | 40 | 0.26 |
| BE* | F | 33 | 37.5 | -3.53 | ----- | ----- | ----- | ----- |
| KM* | F | 60 | 42.9 | -3.12 | 41 | -2.45 | 34 | -0.18 |
| IT* | F | 72 | 33.9 | -3.8 | ----- | ----- | ----- | ----- |
| WS* | F | 64 | 37.5 | -3.53 | 27 | -4.29 | 68 | 2.29 |
| KE* | F | 67 | 42.9 | -3.12 | 40 | -2.58 | 48 | 0.84 |
| IM* | M | 79 | 21.4 | -4.75 | 37 | -2.97 | 90 | 3.88 |
| TD* | F | 38 | 46.4 | -2.85 | 41 | -2.45 | 36 | -0.03 |
| BT | M | 32 | 55.4 | -2.18 | 58 | -0.21 | 52 | 1.13 |
| SI | F | 66 | 37.5 | -3.53 | 45 | -1.92 | 42 | 0.4 |
| JT | F | 31 | 52.4 | -2.4 | 32 | -3.63 | ----- | ----- |
| ID | F | 41 | 37.5 | -3.53 | 37 | -2.97 | 74 | 2.72 |
| SW | F | 50 | 55.4 | -2.18 | 48 | -1.53 | 44 | 0.55 |
| TZ | F | 62 | 69.6 | -1.1 | 43 | -2.18 | 56 | 1.42 |
| CP mean±s.d. | 52.5±16.4 | 45.2±12.7 | 40.4±7.9 | 54±18.3 | ||||
| Controls mean±s.d. | Age range 20-70 (famous faces) 45±9.1 (CFMT) 36.3±13.6 (CFPT) | 84.1±13.2 | 59.6±7.6 | 36.4±13.8 | ||||
Color code: green = - 1SD below control performance; blue = - 1.6SD below control performance; red = - 2SD below control performance. Note that in the famous faces questionnaire and the CFMT, abnormal performance is indicated by a negative z-score while in the CFPT it is indicated by a positive z-score (higher scores on this test indicate low accuracy).
Performance on famous faces questionnaire of these CP participants was published previously in (Avidan & Behrmann, 2008; Behrmann, Avidan, Marotta, & Kimchi, 2005; Nishimura, Doyle, Humphreys, & Behrmann, 2010)
Calculation of z-score is based on control data provided in (Duchaine, Germine, & Nakayama, 2007; Duchaine, Yovel, & Nakayama, 2007)
Table 3.
Individual and group average of CP participants and of the large control groups on the experimental tasks (accuracy, RT and z scores). Global bias index obtained from the global/local task; raw accuracy and RT and interference index (accuracy and RT) obtained from the composite face task.
| Participant | Global bias index | composite task interference index - accuracy | composite task interference index- RT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Index (RT) | z-score | missalignend | aligned | Index | z-score | missalignend | aligned | Index | z-score | |
| MT* | 22 | -0.2 | 86.15 | 74.24 | 7.43 | 0.17 | 933.61 | 881.8 | 2.85 | 0.86 |
| BE* | -59 | -1.19 | 98.48 | 93.94 | 2.36 | -0.56 | 860.12 | 708.63 | 9.66 | 2.01 |
| KM* | -46 | -1.03 | 100 | 95.45 | 2.33 | -0.57 | 847.03 | 813.63 | 2.01 | 0.71 |
| IT | ----- | ----- | 86.36 | 84.85 | 0.88 | -0.78 | 933.53 | 1046.54 | -5.71 | -0.59 |
| WS | -102 | -1.72 | 92.31 | 87.88 | 2.46 | -0.55 | 1048.4 | 860.12 | 9.87 | 2.04 |
| KE | -112 | -1.83 | 81.82 | 86.36 | -2.7 | -1.3 | 949.83 | 882.51 | 3.67 | 0.99 |
| IM | -46 | -1.03 | 84.85 | 83.33 | 0.9 | -0.78 | 1086.93 | 902.22 | 9.29 | 1.94 |
| TD | 27 | -0.14 | 90.91 | 89.39 | 0.84 | -0.79 | 835.3 | 787.02 | 2.98 | 0.88 |
| BT | -9 | -0.58 | 92.19 | 98.36 | -3.24 | -1.38 | 547.61 | 685.23 | -11.6 | -1.51 |
| SI | -182 | -2.7 | 95.45 | 93.94 | 0.8 | -0.79 | 774 | 777.68 | -0.24 | 0.33 |
| JT | -18 | -0.69 | 90.77 | 87.88 | 1.62 | -0.67 | 794.66 | 836.22 | -2.55 | -0.06 |
| ID | -392 | -5.27 | 90.63 | 96.97 | -3.38 | -1.4 | 604.34 | 455.16 | 14.08 | 2.75 |
| SW | -57 | -1.16 | 95.45 | 89.39 | 3.28 | -0.43 | 809.77 | 952.55 | -8.1 | -0.99 |
| TZ | -227 | -3.25 | 93.65 | 95.16 | -0.8 | -1.02 | 853 | 991.48 | -7.51 | -0.89 |
| CP mean±s.d. | -92.4 ± 116.5 | 91.4 ± 5.2 | 89.8 ± 6.5 | 0.9 ± 2.9 | 848.4 ± 147.3 | 827.2 ± 146.9 | 3 ± 7.2 | |||
| Controls mean±s.d. | 38.1 ± 81.5 | 88.1 ± 13.1 | 78.4 ± 15 | 6.3 ± 6.9 | 775.2 ± 162 | 811.6 ± 169.9 | -2.2 ± 5.9 | |||
Performance on the global/local task of these CP participants was published previously in (Behrmann et al., 2005)
2.1.1 Control participants
Control participants, none of whom had a history of neurological or psychiatric illness, were healthy community volunteers and undergraduate students who participated in the study in return for a course credit. For each experiment, we used 2 control groups: one relatively large (n>30) heterogeneous control group was used to establish the extent of the predicted normal behavioral effect and to permit calculation of normalized z-scores in CPs. In addition, as is common in the neuropsychological literature (e.g. (Le Grand et al., 2004)), when conducting direct statistical comparisons between CP and controls using ANOVA, we also used a group of age-matched controls for the CP participants, sub-selected from the large control group (in cases were more than one participant could serve as an age-matched control, one of these subjects was randomly selected). This second group not only serves as a direct matched control group but also, because the group sizes are equated between CP and controls, this avoid any biases that may result from the differences in group size. Below, we report data from two sets of studies: the first are a series of diagnostic tasks in which we establish who is CP and included in this group and the second are a series of experimental tasks which are designed to explore the possible configural impairment in the CP versus control individuals.
2.2 Stimuli and experimental design
All CP participants were tested individually. With the exception of the famous face questionnaire and the Cambridge Face Memory Test (CFMT) and Cambridge Face Perception Test (CFPT), the experiments were run using E-Prime (Psychology software Tools Inc.) either on a laptop or a desktop PC. The famous faces questionnaire was conducted as a pencil and paper survey and the CFMT and CFPT were run using a Java script. For the computer tasks, participants were seated at a viewing distance of approximately 60 cm from the computer screen.
2.3 Diagnostic tasks for CP
All CP participants reported experiencing substantial life-long difficulties with face processing. In order to establish objective diagnostic criteria for CP individuals, we used 4 different experiments previously used in the literature. These included a famous face questionnaire, the CFMT, the CFPT and a task measuring discrimination of novel upright and inverted faces. To determine whether each CP participant is impaired, for each of these tests, we calculated z-scores for each participant based on data obtained from large control groups (details below). As is common in the neuropsychology literature, we establish a cut-off of 2 SD away from the control mean (red in the tables indicate values beyond 2SDs from the controls). Moreover, as is the standard in other studies describing individuals with CP (e.g. (Bowles et al., 2009; Rivolta, Palermo, Schmalzl, & Coltheart, 2011)), we also label those cases with borderline z-scores that are between 1 to 2 SD away from the control mean and consider them as possibly having some, albeit milder, difficulties with face processing (in all tables, green values indicate z-scores 1-1.6 SD from control mean and blue values indicate z-scores 1.6-2 SD from control mean). However, our strict diagnostic criteria is to include only participants with clear impairments (more than 2SDs) on at least two of our four diagnostic measures (see more about this in the specific description of each experiment).
2.3.1 Famous face questionnaire
All participants were tested on a famous faces questionnaire. This task has been described in detail previously (Avidan & Behrmann, 2008). Briefly, the questionnaire included photographs of faces of 56 celebrities, randomly intermixed with 56 photos of faces of unknown individuals (celebrities who are famous in other countries). Face images were inserted into a table printed on paper, and participants had to indicate the name of the individual, provide some contextual information (e.g. occupation) or respond ‘do not know’. Participants were allowed as much time as they needed to fill out this questionnaire. We created two versions of this questionnaire, one suitable for US participants and the other suitable for Israeli participants. A total of 58 control participants (age range 20-70) completed the questionnaire, some of whose data were included in previous studies (Avidan & Behrmann, 2008; Behrmann et al., 2005). Performance on these two versions of the questionnaire did not differ (mean ± SEM: 83.4%±2.7; 84.9%±2.3, for 30 US and 28 Israeli participants respectively, p=0.67).
2.3.2 CFMT and CFPT
These tests were provided by Dr. Bradley Duchaine and detailed description of their procedures can be found in (Duchaine, Germine, & Nakayama, 2007; Duchaine, Yovel et al., 2007). The CFMT is designed to examine memory of unfamiliar faces and the CFPT is designed to examine visual perception of unfamiliar faces. Both tests have been widely used in recent years in the congenital/developmental prosopagnosia literature and hence we use them here to permit direct comparison of our results to other studies. For these tests, we used 2 sets of control data. The first set was provided to us by Dr. Bradley Duchaine and included 20 controls for CFMT (aged 45.1±9.1) and 37 controls for the CFPT (aged 36.3±13.6). These data have been used in previous studies for diagnostic purposes for individuals with developmental prosopagnosia (Duchaine, Germine et al., 2007; Duchaine, Yovel et al., 2007). The second set was taken from data published in (Bowles et al., 2009); we used specific age-matched norms for each CP individual for both the CFMT and CFPT tests (for the comparison across z-scores of CP individuals obtained using these different data set and see Supplementary Table 1).
2.3.2 Unfamiliar face discrimination
All CP participants were tested on a simultaneous face discrimination task used previously in our studies (see (Behrmann et al., 2005) for details). The control group was comprised of 37 participants (age range 20-76) with a subgroup of 14 age-matched controls (mean age 44.1±15.5). On each trial, three unfamiliar faces, shown from a frontal view, appeared on the screen in a pyramid format (one face at the top and the two choice faces presented below it to the left and right) for unlimited exposure duration. Participants had to decide whether the ‘target’ face presented at the top was the exact same face as the face on the bottom left or the bottom right. In one block, faces appeared in the upright orientation while, in the second, they were inverted. Note that results from both the upright and inverted trials are informative and that, in addition, the difference between these two conditions may provide supporting evidence for impaired holistic processing in CP.
In this task, as well as in the next two tasks (compound letter global/local task and composite task) where both RT and accuracy are recorded, mean RT is calculated only from correct trials, and trials which are 2SD above or below the mean are removed prior to RT analyses.
2.4. Experimental tasks
2.4.1 Compound letter global/local (GL) task
The stimuli were four hierarchical letters of two types (Figure 2a): consistent letters, in which the global and the local letters shared identity (a large H made of smaller Hs and a large S made of small Ss), and inconsistent letters in which the letters at the two levels had different identities (a large H made of small Ss and a large S made of small Hs). The global letter subtended 3.2° in height and 2.3° in width, and the local letter subtended 0.44° in height and 0.53° in width. Participants identified the letter at either the global or local level in separate blocks of trials in which consistent and inconsistent letters were randomized. Each block (n=96 trials) was preceded by instructions to identify at the local or global level. A trial was initiated with a central fixation cross of 500 msec duration, which was immediately replaced by one of the four possible stimuli. Participants pressed one of two keys on the keyboard to indicate a response of ‘S’ or ‘H’. The stimuli remained visible until subject made a response. The order of the blocks and the response key (S/H) was counterbalanced.
Thirty-eight healthy individuals (18 females), ages 18-82 (mean years 42.16±18.35) comprise the large control group for the global/local task and a subgroup of 13 age-matched participants (mean years 52.15±15.88) was used for direct statistical comparisons with CP subjects (note that one CP subject did not perform this experiment and hence the control group here was comprised of 13 participants).
2.4.2 Composite face experiment
Stimuli included twelve grayscale images of male faces (provided by Isabel Gauthier). A horizontal black line was added on top of each face to conceal the area where the face is cut. Faces were cropped horizontally at the center of the black line into two halves containing the top and bottom part of the face (Figure 1). The size of each face was ~4.3° horizontally and ~4.6° vertically. The experiment was composed of two separate blocks, one in which faces were presented such that the two halves composing the face were aligned and one in which the two halves were misaligned. The order of the blocks was counterbalanced across participants, and both were run consecutively in the same testing session with a short break between them (previous data suggest that order of blocks has no effect on the outcome; (Le Grand et al., 2004)). In the misaligned block, the two parts of the face were shifted from the center of the screen, such that the top half of the face was displaced 18 pixels rightward and the bottom part of the face was displaced 18 pixels leftward (for an example, see Figure 1). Each block contained 132 trials. On each trial, two consecutive faces were presented in the center of the screen. Each face was presented for 200 msec, with ISI of 300 msec and ITI of 2300 msec. On half of the trials, the tops of the two faces were the same and, on the other half, they were different. The bottom parts of the two faces within a trial were always different. The top and bottom parts of each face always originated from a different face such that the original faces were never presented.
Participants were instructed to make a same/different response by pressing designated keys with their right (dominant) hand on the computer keyboard. Critically, participants were instructed to maintain their gaze around the black horizontal line and to make their judgment based only on the top part of the face. Test trials were preceded by ten practice trials of both the aligned and misaligned condition. These trials were discarded from any further analysis. Both accuracy and reaction time were recorded. Note that this form of the task is referred to as the partial composite task (it is not the full orthogonal crossing of top/bottom × same/different × aligned/misaligned). While the partial design is thought to give rise to response biases (Richler, Cheung, Wong, & Gauthier, 2009), making its interpretation difficult, here we compare responses across two groups both of whom were subject to the same task design. Our focus then is on the group differences obtained under identical conditions of testing, rather than on any possible biases or confounds that emerge in a particular design of the task.
Fifty healthy individuals (39 females), ages 19-87 (mean years 46.78±24.31) comprise the control group for the composite face task with a subgroup of 14 age-matched participants (mean years 54.93±20.06) serving as a matched control group for CP.
3. Results
3.1 Diagnostic tests
Table 1 and 2 display the raw performance and standardized z-scores, as calculated based on performance of control groups, for the diagnostic tasks for all CP participants.
3.1.1 Famous faces
Twelve CP individuals were clearly impaired on the famous faces questionnaire. The remaining 2 participants (MT, TZ) still exhibited some mild difficulty with face recognition as evident by their relatively low performance and borderline z-scores.
3.1.2 CFMT, CFPT
Of the 12 CP participants who completed the CFMT task, 9 were clearly impaired, two (SI, SW) exhibited performance and only one participant (BT) was clearly in the normal range. As for the CFPT, of the 11 participants who completed this task, three were clearly impaired, two exhibited relatively low performance and the rest of the participants were in the normal range (see also supplementary Table 1 for results on the inverted CFPT).
3.1.3 Upright/inverted face discrimination
We have previously shown that CP individuals are slower than controls on such a novel face discrimination task and that they do not show the expected decrement in performance for inverted faces (the reader is referred to (Behrmann et al., 2005) for detailed analysis and discussion of these original findings). Critically, the results reported in the present study replicate and extend these findings with 6 additional CP participants whose performance on this task has not been reported previously (see Table 2 for details).
We start by discussing the results in terms of accuracy and then move to the RT analyses. As is evident in Table 2, accuracy on this task is generally high, likely as a result of the unlimited exposure. Accuracy for upright and inverted faces of all CP participants except for two (IM, BT) was in the normal range. Unsurprisingly, an ANOVA of these data, comparing the performance of the CP group and matched subgroup as the between-subjects factor and orientation (upright, inverted) as the within-subjects factor, revealed a significant main effect of orientation (F(1, 26)=5.35, p<0.03) but no effect of group (F<1) or group × orientation interaction (F<1). When directly comparing the accuracy for upright and inverted faces in each group, we find a trend for an inversion effect (reduced performance for inverted compared to upright faces) in the matched controls but not in the CP group (planned comparisons for upright vs. inverted faces, controls p=0.07, CP p=0.18). Finally, to quantify the extent of the well-documented inversion effect, we calculated an inversion index (separately for accuracy and RT) as follows:
When examining this index for accuracy, one CP (BT) is outside the normal range due to a dramatic decrease in accuracy for inverted faces (greater reduction than controls). Thus, to summarize, while controls here show a trend towards an inversion effect in terms of accuracy, CPs do not show a similar trend, that is they show equal performance for both upright and inverted faces. As suggested above, this pattern of general high accuracy for both CP and controls for both orientations likely emerges from the unlimited exposure duration and under such conditions, RT is considered the more telling dependent variable.
We first describe the RT results on the upright face discrimination condition alone. Ten of the 14 CPs fell outside the normal range with significantly longer RT for upright faces. One participant exhibited relatively slower performance compared to controls (JT) and one participant, BT, exhibited normal performance in terms of RT but was substantially impaired in accuracy, thus exhibiting a clear speed-accuracy trade-off (notably no other participant showed such a trade-off). Three additional participants (TD, BT, SI) exhibited normal RT for upright faces, but as will be evident below, these participants showed a trend towards faster RT for inverted compared to upright faces and hence exhibited overall abnormal performance in this task. Thus, most, if not all, CP individuals exhibit difficulty not only with recognition of famous faces but also with perceptual tasks involving novel faces both here and on the CFMT (for related results see (Behrmann et al., 2005; Bowles et al., 2009; Dobel, Bolte, Aicher, & Schweinberger, 2007)). Importantly, when directly comparing CP to their matched control group (between subjects) with orientation as the within-subjects factor, CP participants showed no RT difference between inverted and upright faces, while controls exhibited significantly faster RT for upright over inverted faces (significant group × orientation interaction F(1,26)=5.59, p<0.026, planned comparison for upright vs. inverted faces, controls: p<0.007; CP: p=0.63). Interestingly, a t-test comparing the CP and matched control data on inverted faces reveals no group difference (p=0.4) but controls were significantly faster than CP on upright faces. Thus, at the group level, CPs do not show the typical advantage for upright compared to inverted faces in terms of RT.
To explore the inversion effect (or lack thereof) at an individual level, we calculated an index for each participant, as we did for accuracy above. Interestingly, 6 CPs exhibited decreased RT for inverted compared to upright faces, as evident by their inversion index (5 who are more than 1 SD away from controls and 1 who is more than 3 SD away from controls). Thus, some CP individuals show evidence for inversion superiority in terms of RT. The lack of an inversion effect at the group level and the tendency towards inversion superiority exhibited by some CP individuals, provide further evidence for the disrupted holistic perception in CP. These results in themselves replicate our previous report of this finding and set the stage for the detailed discussion regarding holistic processing as directly tested by the two experimental tasks below.
3.2 Experimental tasks
3.2.1 Global/local task
Figure 2b shows the data from the hierarchical global/local letter experiment for the large control group (n=38), for the subgroup of age-matched controls (n=13) and for the 13 CP participants (one CP, IT, did not complete this task). We first describe the performance of our control groups (whole group and age-matched subgroup), to confirm that we have replicated the standard findings i.e. global advantage and global-to-local interference, and then we compare the CP group with the matched controls.
3.2.1.1 Control groups
The results for the large, as well as matched control groups are presented in Figure 2. A two-way ANOVA of consistency (consistent, inconsistent) × task (global, local) for the large control group (N=38) revealed a significant consistency × task interaction (F(1,37=8.3), p<0.007), main effect of consistency (F(1,37)=28.6, p<0.000005) and a trend toward main effect of task (F(1,37)=3.3, p=0.08) with RTs for local slower than for global trials. Similarly, for the matched controls (n=13), there is a two-way interaction (F(1,12=7.9), p<0.02), as well as main effects of task ((F(1,12=8), p<0.015) and consistency (F(1,12=18.8), p<0.001). Thus, both control groups show the same pattern with performance faster on the global than local trials and faster on the consistent than the inconsistent condition. There is also, however, disproportionate slowing on local inconsistent trials, relative to global inconsistent trials, reflecting the global-to-local interference.
3.2.1.2 CP
We first perform a similar analysis on the data from the CP group alone, with task and consistency as within-subject variables (Figure 2). Note that the performance of the CP group is qualitatively different from that of controls in that they are slower in the global than local task. This is confirmed by a main effect of task (F(1,12=15.13), p<0.002). Similarly to controls, CP also exhibited a main effect of consistency (F(1,12=8.9), p<0.01) (for more details see Figure 2b and see supplementary Figure 1 for raw data of individual CPs on this task). There is also an interaction between task and consistency, (F(1,12=8.2), p<0.015), that arises because the global inconsistent condition is even slower than global consistent, relative to the local conditions.
To directly evaluate the pattern of CP performance relative to the age-matched controls, we performed an ANOVA that revealed a three-way interaction of group (CP, matched controls) × consistency (consistent, inconsistent) × task (global, local) F(1, 24)=14.97, p<0.0007). This analysis also revealed a robust task × group interaction (F(1, 24)=22.88, p<0.00007) but no consistency × group interaction (F<1). Interestingly, there was no main effect of group (F(1,24)=1.34, p=0.26), indicating that it is not the case that CP were simply slower compared to controls on this task. Rather, both the significant local advantage and the local-to-global interference in the CP group clearly diverge from the matched and large control groups, suggesting that the differences between the CP and control groups are robust and replicable.
3.2.1.3 Global/local index
To evaluate the extent of the local bias for the individual CP participants, we calculated a global bias index for each CP and each control [RT(local inconsistent – global inconsistent)-RT(local consistent-global consistent)] and this index along with its normalized z-score relative to the large control group is presented in Table 3. This index is a reflection of the graphic depiction of the results (Figure 2 and supplementary Figure 1), and shows the magnitude of the inconsistency interference and the global or local bias - a positive index indicates a global bias while a negative index indicates a local bias. The mean index for the controls (aggregated across all controls) is 38 msec, reflecting the global bias while the mean index for the CPs is -92.4 msec. Nine of the 13 CP participants exhibited a bias toward local advantage as evident by their global bias index (see Table 3). Thus, the local bias in this task is at both the group level and the individual subject level in many of the CPs.
3.2.2 Composite face experiment
Previous reports using the composite manipulation (Le Grand et al., 2004; Young et al., 1987) have demonstrated that the signature finding obtained with normal observers is an interference effect, in which there is a reduction in accuracy and/or increase in RT during the ‘same top’ trials of the aligned condition compared to the misaligned condition. For example, Le Grand et al., (2004) reported that control participants have a mean accuracy and RT of 63% and 780 msec for the same/aligned condition relative to the 91% and 616 msec in the same/misaligned condition. This reduced performance in the same/aligned condition compared with the same/misaligned is attributed to the automaticity of the holistic nature of face processing. The question here is whether CP participants also show a reduced interference effect compared to the controls.
We first describe the performance of our control groups (whole group and age-matched subgroup), showing that we have replicated the predicted composite effect, and then examine the performance of the CP group in relation to that of control. Following other studies which show that the alignment manipulation is only effective during the “same top” condition (Le Grand et al., 2004; Michel, Rossion, Han, Chung, & Caldara, 2006; Ramon et al., 2010), we focus only on this condition when examining the composite effect and use the performance on these trials as a measure of holistic perception (for raw data of these conditions for each CP see Table 3).
3.2.2.1 Control groups
We first establish that our control participants exhibit the well-documented composite effect (Figure 3). As described in the Methods section, we used a large control group (n=50) to ensure that we can replicate the standard results and we also sampled a subgroup of 14 age-matched controls to permit direct statistical comparison between CP and controls.
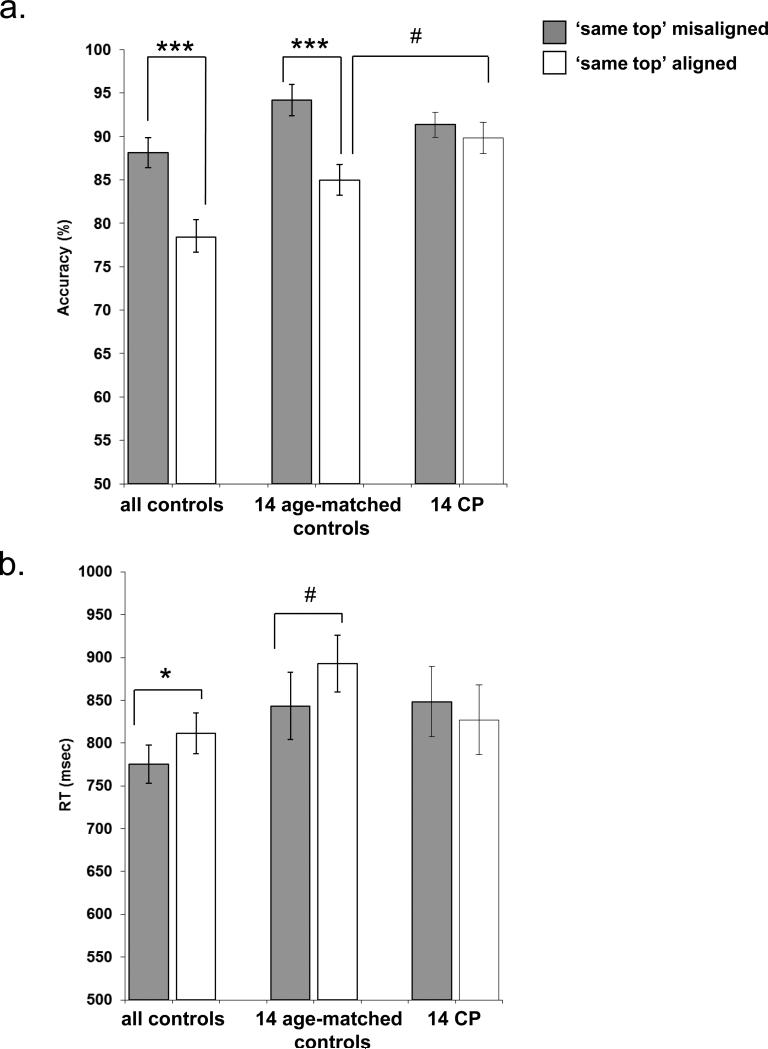
Figure 3.
Composite experiment: Mean accuracy (a) and reaction time (b) on the composite face experiment for the entire group of control participants, age-matched controls and CP participants on the ‘same top’ trials. Note the lack of interference effect in the CP group in accuracy and in RT, indicating that CPs are not affected by the experimental manipulation. Asterisks denote significance level * p<0.05; ***p<0.0005. # indicates p value that is marginally significant (p=0.06 in top graph and p=0.07 in the lower graph). Error bars indicate ± standard error of the mean across participants.
A one-way ANOVA comparing aligned and misaligned performance (only ‘same top’ trials) on the data from the large control group revealed, as expected, a significant influence of alignment on both accuracy, (F(1,49)=46.9, p<0.0001), and RT, (F(1,49)=8.9, p<0.005) (see Figure 3). Thus, overall, the large control group exhibits the expected interference effect during ‘same top’ trials. The same analyses conducted with the age-matched control group replicated these findings (alignment effect accuracy: F(1,13)=17.7, p<0.001); RT: F(1,13)=8.9, p<0.01) and assured us that even the smaller subset of matched controls evince the predicted effects (see Figure 3).
3.2.2.2 CP
A simple effect analysis of accuracy and RT revealed no effect of alignment [accuracy: F(1,13)=1.4, p=0.3; RT F<1], thus dramatically diverging from the results obtained with controls (Figure 3). To directly evaluate the pattern of CP performance relative the matched control group, we conducted an ANOVA with group (CP, matched controls) as the between-subject measure, and performance in terms of accuracy or RT on the experimental conditions (‘same top’: misaligned, aligned) as a within-subject repeated measure. The analysis for accuracy revealed a significant group × alignment interaction (F(1,26)=8.9, p<0.006), as well as a significant main effect of alignment (F(1,26)=17.6, p<0.0003) with no main effect of group (F<1). Importantly the group × alignment interaction stems from a difference in the ‘same top’ aligned condition (p=0.06) while no such difference was found in the “same top” misaligned condition (see Figure 3a). A similar analysis for RT comparing the CPs to the matched control group revealed a trend of alignment × group interaction (F(1,26)=3.8, p=0.06) with no main effects for alignment or group (F<1). Interestingly, as can be seen in Figure 3, while controls showed a trend towards RT increase for aligned compared to misaligned faces (p=0.07), no such effect was found for CP. The qualitative similarity of the findings when comparing the results of the CP to either of the control groups (Figure 3) is reassuring and attests to the robustness of the dramatic difference in performance obtained in the CPs relative to controls.
3.2.2.3 Interference index
To examine further the response pattern of individual CP participants, we calculated an interference index which directly compares the performance, in accuracy or RT (see Table 3), on the misaligned versus aligned conditions during ‘same top’ trials. The index was calculated separately for each CP and then was transformed to a z-score by normalizing the index by the mean index of the large group. We multiplied the results by 100 to obtain % index:
As can be seen in Table 3, at the individual subject level, half of the CPs exhibited an index that was one SD greater than that of controls, either for accuracy (three CPs), RT (three CPs) or both (one CP, BT – see below). It is important to note, however, that, for all CPs except for one (MT), the z-score based on accuracy was negative indicating a trend toward reduced interference compared to controls. Along similar lines, eight CPs had a positive z-score for RT, indicating faster RT for aligned compared to misaligned faces, a pattern that is reverse from controls. As noted above, CP participant (BT) exhibited a pattern that may reflect a speed-accuracy trade off – with greater accuracy for aligned compared to misaligned faces accompanied by an RT increase for aligned faces that is greater than controls. Thus, while it is not possible to show the reduced interference effect at the individual subject level for every participant, and, in this sense this task is not perfectly sensitive, the group effect described above is robust and informative regarding the nature of face processing in CP (for more discussion of this issue see the Discussion section).
3.2.3 Correlation between different experimental measures
Finally, in order to explore further the nature of the face processing deficit in the CP individuals and to explore possible relationships between the different experimental measures used here, we looked for correlations between performance in the different diagnostic and experimental tasks.
Interestingly, this analysis revealed a significant correlation between performance in the composite face task and two of the diagnostic tests. Specifically, we find a negative correlation between the famous faces questionnaire and the RT index of the composite task (r=-0.61, p<0.021) such that participants with low recognition scores exhibited a more positive index, indicating less interference (more abnormal performance) in the composite task. A significant negative correlation was also found between the same composite index and the CFMT z-scores, such that lower z-scores (more negative) were correlated with a more positive index (r=-0.72, p<0.009). Importantly, performance on the CFMT and famous face questionnaire was not correlated (p=0.32) and hence could not mediate the correlations described above. Such correlations between the performance on the composite task and the diagnostic tests are very important as they reveal that the local bias exhibited by CPs in the composite task is related to the way they process both familiar and unfamiliar faces. Moreover, these correlations suggest that even though the reduced composite effect cannot be shown significantly for each CP participant, this measure is still related to the general face processing skills of each CP.
Finally, it is very interesting to note that the local bias index calculated from the global/local experiment and the composite interference index based on accuracy level, calculated across the CP participants, are positively correlated (r=0.52, p=0.06): a more negative index (increased local processing) in the global/local task is associated with a smaller interference index (less holistic or configural processing) in the composite task. This correlation provides support for the claim that, at least, in these individuals with face processing deficits, there may be an association between the impairments in holistic processing of faces (composite task) and non-face stimuli (global-local task). We note however, that this association may not be universally true; for example in the acquired prosopagnosic patient PS, there was a clear dissociation between impaired performance on the composite task and intact performance in the Navon task (Busigny & Rossion, 2010b), indicating that these two task do not necessarily tap onto the same perceptual skills. We consider this further in the Discussion below. None of the other correlation between the tasks and measures was significant (see Supplemenatary Table 2 for details).
Discussion
The goal of the present study was to explore the extent to which individuals who are impaired at face processing are also impaired at deriving global configurations from disparate elements present in the visual input. We showed that a relatively large group of CP individuals (n=14), although slower overall, performed equally well on inverted than upright faces, in contrast with the controls, and also showed a marked local bias, rather than the normal global superiority, in a global/local compound letter identification task. Additionally, using the composite face paradigm, often regarded as the gold standard for examining holistic face processing, we observed that the CP group was significantly less affected compared to controls, by the alignment manipulation when perceiving faces, performing equally well for aligned and misaligned faces and remaining impervious to the incongruent bottom-half of the composite display. Taken together, these findings provide converging evidence for impaired holistic processing in CP and suggest that such processing may play a critical role in intact face recognition.
Interestingly, as shown here, in tasks in which participants can rely on more local or elemental features, the CP individuals have a unique advantage over their control counterparts. This constitutes a rare and telling case of better performance by a neuropsychological than control population. The CP advantage is true both in the composite paradigm but also in the equal performance for inverted compared to upright faces relative to controls who are adversely affected by the misorientation of the faces. It is the case, however, that even though CPs perform equally well for upright and inverted faces, compared to controls, their performance (as reflected in RT) is still slower than the controls for both conditions (but see (Avidan & Behrmann, 2008) for overall faster RT in CP under time pressure). In contrast, in the composite paradigm, the CPs are actually more accurate than controls and their RT is similar to controls on the critical ‘same top’ aligned condition (see Figure 3). Hence, the lack of holistic processing in these individuals cannot be attributed to an overall lower level of accuracy or slower response (see also absence of main effect in global-local task).
Finally, we show that the impaired holistic processing, as revealed in the composite task, was correlated with the local bias, as assessed in the global/local hierarchical letter task. It is clear then that not only is this decrement in holistic processing robustly uncovered in the CP individuals but it is also the case that the impairment in global processing is not limited strictly to faces and may affect visual processing more generally at least in these individuals (but see (Busigny, Joubert, Felician, Ceccaldi, & Rossion, In press; Busigny & Rossion, 2010b) for different results in AP). Consistent with this, a recent study reports impairments in visual processing of biological motion in CP – biological motion perception requires integration of local elements and hence also engages global processing (Lange et al., 2009). However, the nature and extent to which the impairments in CP extend beyond face processing remain to be determined in future studies.
A key question concerns the relationship between face processing abilities and global visual processing in normal participants (and by extension, the perturbed relationship in CP). One recent study reported a significant correlation between face identification and the magnitude of the global processing bias, as assessed by the same Navon hierarchical letter task as we have used here: an increase in global precedence was positively correlated with better performance on a face identification task (Darling, Martin, Hellmann, & Memon, 2009). A second study provides additional consistent evidence, demonstrating that participants with greater global precedence (as assessed by the Navon task) were more affected by the face inversion manipulation; that is, compared with participants with a smaller global precedence, these individuals showed greater decrement in processing inverted versus upright faces (Martin & Macrae, 2009). Along similar lines, Weston & Perfect (2005) reported that participants who completed the composite task, after performing the Navon task using a local bias, were less affected by the composite manipulation (i.e. showed less interference), compared to participants who performed a different, control task prior to the composite task. Taken together, these findings, obtained from healthy individuals, provide strong support for the role of holistic processing, in general, in face perception and show that there is a close association between configural measures (global/local and upright/inverted) and face perception.
Interestingly, our findings also reveal a correlation between the severity of the face recognition impairment, as evidenced by performance on both the famous faces questionnaire and the CFMT with the index of local bias in the composite task (RT index). Such correlations are of great importance as they indicate that the local bias, as evident in this task, is associated with the processed adopted by our CP individuals in perceiving faces. We note, however, that there is some controversy in the literature regarding the extent to which these measures should correlate. For example, Konar, Bennett, & Sekuler (2010) report no obvious association between the magnitude of the composite face effect (using the same paradigm that we have used here) and face identification accuracy. In contrast, Richler, Cheung, & Gauthier (2010) do obtain such correlations. The explanation offered by Gauthier and colleagues is that the adoption of the partial composite design, as used by us and by Konar et al. (2010), may not be sufficiently sensitive to yield the underlying composite-face identification correlations and perhaps if we were to use the full design we would be able to show correlations even with the composite accuracy index and not only with the RT index. The full design of the composite task, which is uncontaminated and free from bias does tap into the configural skills mediating face perception (see Richler et al., 2010; Richler, Mack, Gauthier, & Palmeri, 2009). We note that because our focus was more in establishing the group differences between controls and CP, our use of the more streamlined partial design is not unreasonable. That we see correlations between performance on this partial design, the global/local task and some of the diagnostic tasks suggest that even this partial design remains sensitive to some aspects of holistic processing.
Impaired holistic processing in congenital prosopagnosia
Consistent with our findings of impaired holistic processing and a local bias in CP, many other studies have also reported undue focus on local elements in CP. There does, however, seem to be greater heterogeneity in CP than in AP (but, of course, there are more CP than AP individuals and so this may simply be a sampling restriction). For example, Bentin, Degutis, D'Esposito, & Robertson (2007) report a significant local bias, and an extreme deficit in global/configural processing in CP patient (KW). Surprisingly, normal global processing in this same hierarchical letter paradigm has been observed in a large group of DP individuals (Duchaine, Yovel et al., 2007). It is also the case that in the composite task, only 1 of 8 CP (called DP in that study) was immune to interference (Le Grand et al., 2006), while we observe this effect more generally in our group of 14 CPs. Consistent with our findings, Palermo et al., (2011) also found evidence for reduced holistic processing in the composite paradigm in a group of 12 individuals with CP. We also note that Duchaine (2000) reported normal configural processing in a DP patient on three tests, the Gestalt completion test, word fragment test and Snowy pictures test. All three of these tests explore aspects of pattern completion, which, arguably, might differ from the paradigms used to assess configural processing: in the former, the elements themselves are fragmented whereas in the latter, the elements are presented in their entirety but need to be integrated to give rise to a coherent unified perceptual entity.
The reasons for the apparent discrepancies across studies in the global/local and composite tasks are not readily apparent, and cannot be easily accounted for by differences in behavioral paradigms, as very similar paradigms were used across labs. It remains perplexing, then, that such discrepant findings are obtained by the different research groups. One potential difference concerns the manner in which participants are diagnosed as being prosopagnosic, with different studies possibly employing different diagnostic criteria although we note that the CFMT and CFPT are now widely used for diagnostic purposes by many labs. A more standard and widely agreed-upon set of criteria may remedy some of this observed variability and a clear future direction for the field would entail having a robust set of benchmark measures that allow for cross-investigator comparison.
It does remain a possibility, however, that the different researchers are testing slightly different populations, all of whom fall within the ambit of CP. As is evident in the results presented here, there is definitely some heterogeneity across participants. For example, some individuals fall outside of the z-score range (2SDs) on all four diagnostic tests (see Table 1 and 2), such as WS and IM, and several other individuals fall outside the range of three of the four or even two of the four measures, and we note that it is not perfectly consistent across the measures which are the most diagnostic.
Heterogeneity among CP individuals is not only evident in the behavioral measures but also in imaging studies in CP. Whereas some studies document normal face selective activation in occipito-temporal cortex in CP (Avidan & Behrmann, 2009; Avidan, Hasson, Malach, & Behrmann, 2005; Hasson, Avidan, Deouell, Bentin, & Malach, 2003), others report abnormal activation patterns (Bentin et al., 2007; Hadjikhani & De Gelder, 2002; Minnebusch, Suchan, Koster, & Daum, 2009; Furl, Garrido, Dolan, Driver, & Duchaine, 2011). This variability is also evident in the face selectivity of the M170 (Harris, Duchaine, & Nakayama, 2005) and N170 (Minnebusch, Suchan, Ramon, & Daum, 2007) components of DP individuals, even when tested at the same labs (and see also (Bentin, Deouell, & Soroker, 1999)). It remains possible, therefore, that the heterogeneous configural processing findings may reflect real variability among CP individuals or that there may be somewhat different subgroups with somewhat differing psychological and neural signatures. Of course, we also need to recognize that there is variability even in the normal population, with some normal observers not showing FFA activation (Kanwisher, McDermott, & Chun, 1997). Even in the data presented here, there is clearly variability in the controls (simply view the error bars in the Figures we include here – for example, the error bars for the age-matched controls are not that much smaller than those for the CP individuals), presumably reflecting some variability in this sample too (Stollhoff, Jost, Elze, & Kennerknecht, 2011). Future studies, perhaps employing more sensitive tasks, may be able to reveal distinct subtypes among CP participants that will perhaps enable us to better address the nature of variability in this population and its relation to the normal population, more generally.
Impaired holistic processing in acquired prosopagnosia
Our findings of impaired holistic processing in individuals with CP are consistent with results revealing similar impairments in individuals with acquired prosopagnosia (AP). Impaired holistic processing has long been considered a key characteristic of AP, as revealed by the face inversion effect but also by tasks involving non-face stimuli (e.g. (Levine & Calvanio, 1989) and see (Ramon et al., 2010) for a review of early studies showing holistic impairment in AP). Recent studies also document altered sensitivity to the role of spatial relations between features and the role of specific local elements, such as the eyes in face perception in AP (Ramon & Rossion, 2010). For example, Stephan and Caine (2009) reported abnormal scanning patterns of faces in an AP patient in which he focused on peripheral (hair, forehead) rather than internal (eyes, nose, mouth) regions of the face. A focus on local aspects of a face has also been reported in patient PS who does not use the optimal eye information to identify familiar faces, and, instead, focuses on the non-informative lower part of the face, including the mouth and the external contours (Caldara et al., 2005; Orban de Xivry, Ramon, Lefevre, & Rossion, 2008). Interestingly, such a pattern is typical in normal observers when processing unfamiliar faces. Also, when normal participants are engaged in a holistic face processing task, they tend to fixate the whole area of the eyes and nose, while, during an analytical mode of processing, their behavior is characterized by a more feature-specific gaze (Schwarzer, Huber, & Dummler, 2005). Finally, and most pertinent to the present study, Ramon et al., (2010) showed that patient PS did not exhibit the superiority of the whole over the parts in the well-known whole-part paradigm and, compatible with this, she demonstrated impaired holistic processing (no RT difference between misaligned and aligned faces). Ramon and Rossion (2010) suggest that the primary cause of AP lies in the inability to process faces configurally and that all AP are unable to “integrate the multiple features of an individual face simultaneously, into a unified perceptual representation” (Rossion, 2008; Tanaka & Farah, 1993). Consequently, on their account, this failure to represent information configurally results in an analytical feature-by-feature face processing style or in the ability to use only a small spatial window at a time (Bukach, Bub, Gauthier, & Tarr, 2006). They also suggest that the failure to focus on the eye region of the face (Bukach et al., 2006; Bukach, Le Grand, Kaiser, Bub, & Tanaka, 2008; Caldara et al., 2005; Rossion, Kaiser, Bub, & Tanaka, 2009) as well as the relative distances between features (Barton & Cherkasova, 2005; Barton et al., 2002) are direct consequences of defective holistic processing (Rivest et al., 2009). Taken together, these findings suggest that abnormal holistic perception with an exaggerated local bias and a tendency to focus on specific elements may be characteristic markers of AP. These reports are compatible with our findings in CP and point to potential commonalities between these disorders (Behrmann et al., 2005; Behrmann, Avidan, Thomas, & Humphreys, 2010).
To conclude, similar to previous reports in AP, we provide clear evidence for impaired holistic processing in a large group of CP individuals on three different tasks, involving face and non-face stimuli. These findings suggest that disruptions in holistic processing may be characteristic of CP and may underlie, at least, some of the face processing deficits in these individuals.
Supplementary Material
Research highlights manuscript NSY-D-10-00395.
Congenital prosopagnosic individuals outperform controls on face perception task.
This results from reliance on local rather than global face processing.
A local bias is also evident in tasks involving processing of non-faces stimuli.
Impaired face recognition is correlated with increased local bias.
An impairment in global processing may underlie congenital prosopagnosia.
Acknowledgements
This research was supported by a grant from the Israeli Science Foundation (ISF, 384/10) to GA, and from grants from the National Institutes of Mental Health (MH54246), and the National Science Foundation (NSF BCS0923763; NSF SBE-0542013 to the Temporal Dynamics of Learning Center, an NSF Science of Learning Center) to MB. We would like to thank Isabel Gauthier of Vanderbilt University for providing us with the stimuli used in the composite face experiments and also for valuable discussions about the data analysis. We also thank Daphne Maurer of McMaster University for her help with data interpretation and Bradley Duchaine of Dartmouth College for sharing his CFMT/CFPT tasks and control data with us. We thank Linda Moya for her help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amishav R, Kimchi R. Perceptual integrality of componential and configural information in faces. Psychon Bull Rev. 2010;17(5):743–748. doi: 10.3758/PBR.17.5.743. [DOI] [PubMed] [Google Scholar]
- Avidan G, Behrmann M. Implicit familiarity processing in congenital prosopagnosia. Journal of Neuropsychology. 2008;2:141–164. doi: 10.1348/174866407x260180. [DOI] [PubMed] [Google Scholar]
- Avidan G, Behrmann M. Functional MRI reveals compromised neural integrity of the face processing network in congenital prosopagnosia. Curr Biol. 2009;19(13):1146–1150. doi: 10.1016/j.cub.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan G, Hasson U, Malach R, Behrmann M. Detailed exploration of face-related processing in congenital prosopagnosia: 2. Functional neuroimaging findings. J Cogn Neurosci. 2005;17(7):1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- Barton JJ. What is meant by impaired configural processing in acquired prosopagnosia? Perception. 2009;38(2):242–260. doi: 10.1068/p6099. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Cherkasova MV. Impaired spatial coding within objects but not between objects in prosopagnosia. Neurology. 2005;65(2):270–274. doi: 10.1212/01.wnl.0000168901.98616.1c. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Press DZ, Keenan JP, O'Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002;58(1):71–78. doi: 10.1212/wnl.58.1.71. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G. Congenital prosopagnosia: face-blind from birth. Trends Cogn Sci. 2005;9(4):180–187. doi: 10.1016/j.tics.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Marotta JJ, Kimchi R. Detailed exploration of face-related processing in congenital prosopagnosia: 1. Behavioral findings. J Cogn Neurosci. 2005;17(7):1130–1149. doi: 10.1162/0898929054475154. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Thomas C, Humphreys K. Congenital and Acquired Prosopagnosia: Flip sides of the same coin? In: Bub D, Gauthier I, Tarr M, editors. Perceptual Expertise: Bridging Brain and Behavior. Oxford University Press; 2010. pp. 167–196. [Google Scholar]
- Bentin S, Degutis JM, D'Esposito M, Robertson LC. Too many trees to see the forest: performance, event-related potential, and functional magnetic resonance imaging manifestations of integrative congenital prosopagnosia. J Cogn Neurosci. 2007;19(1):132–146. doi: 10.1162/jocn.2007.19.1.132. [DOI] [PubMed] [Google Scholar]
- Bentin S, Deouell LY, Soroker N. Selective visual streaming in face recognition: evidence from developmental prosopagnosia. Neuroreport. 1999;10(4):823–827. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- Boutet I, Gentes-Hawn A, Chaudhuri A. The influence of attention on holistic face encoding. Cognition. 2002;84(3):321–341. doi: 10.1016/s0010-0277(02)00072-0. [DOI] [PubMed] [Google Scholar]
- Bowles DC, McKone E, Dawel A, Duchaine B, Palermo R, Schmalzl L, et al. Diagnosing prosopagnosia: effects of ageing, sex, and participant-stimulus ethnic match on the Cambridge Face Memory Test and Cambridge Face Perception Test. Cogn Neuropsychol. 2009;26(5):423–455. doi: 10.1080/02643290903343149. [DOI] [PubMed] [Google Scholar]
- Bukach CM, Bub DN, Gauthier I, Tarr MJ. Perceptual expertise effects are not all or none: spatially limited perceptual expertise for faces in a case of prosopagnosia. J Cogn Neurosci. 2006;18(1):48–63. doi: 10.1162/089892906775250094. [DOI] [PubMed] [Google Scholar]
- Bukach CM, Le Grand R, Kaiser MD, Bub DN, Tanaka JW. Preservation of mouth region processing in two cases of prosopagnosia. J Neuropsychol. 2008;2(Pt 1):227–244. doi: 10.1348/174866407x231010. [DOI] [PubMed] [Google Scholar]
- Busigny T, Joubert S, Felician O, Ceccaldi M, Rossion B. Holistic perception of the individual face is specific and necessary: evidence from an extensive case study of acquired prosopagnosia. Neuropsychologia. 48(14):4057–4092. doi: 10.1016/j.neuropsychologia.2010.09.017. In press. [DOI] [PubMed] [Google Scholar]
- Busigny T, Rossion B. Acquired prosopagnosia abolishes the face inversion effect. Cortex. 2010a;46(8):965–981. doi: 10.1016/j.cortex.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Busigny T, Rossion B. Holistic processing impairment can be restricted to faces in acquired prosopagnosia: Evidence from the global/local Navon effect. J Neuropsychol. 2010b doi: 10.1348/174866410X500116. [DOI] [PubMed] [Google Scholar]
- Caldara R, Schyns P, Mayer E, Smith ML, Gosselin F, Rossion B. Does prosopagnosia take the eyes out of face representations? Evidence for a defect in representing diagnostic facial information following brain damage. J Cogn Neurosci. 2005;17(10):1652–1666. doi: 10.1162/089892905774597254. [DOI] [PubMed] [Google Scholar]
- Darling S, Martin D, Hellmann JH, Memon A. Some witnesses are better than others. Personality and Individual Differences. 2009;47(4):369–373. [Google Scholar]
- Dobel C, Bolte J, Aicher M, Schweinberger SR. Prosopagnosia without apparent cause: overview and diagnosis of six cases. Cortex. 2007;43(6):718–733. doi: 10.1016/s0010-9452(08)70501-x. [DOI] [PubMed] [Google Scholar]
- Duchaine BC. Developmental prosopagnosia with normal configural processing. Neuroreport. 2000;11(1):79–83. doi: 10.1097/00001756-200001170-00016. [DOI] [PubMed] [Google Scholar]
- Duchaine BC, Germine L, Nakayama K. Family resemblance: Ten family members with prosopagnosia and within-class object agnosia. Cognitive Neuropsychology. 2007;24(4):419–430. doi: 10.1080/02643290701380491. [DOI] [PubMed] [Google Scholar]
- Duchaine BC, Yovel G, Nakayama K. No global processing deficit in the Navon task in 14 developmental prosopagnosics. Soc Cogn Affect Neurosci. 2007;2(2):104–113. doi: 10.1093/scan/nsm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? J Exp Psychol Hum Percept Perform. 1995;21(3):628–634. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychol Rev. 1998;105(3):482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K, Symons LA. The face-inversion effect as a deficit in the encoding of configural information: direct evidence. Perception. 2000;29(2):159–170. doi: 10.1068/p3012. [DOI] [PubMed] [Google Scholar]
- Furl N, Garrido L, Dolan RJ, Driver J, Duchaine B. Fusiform Gyrus Face Selectivity Relates to Individual Differences in Facial Recognition Ability. J Cogn Neurosci. 2011 doi: 10.1162/jocn.2010.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Curran T, Curby KM, Collins D. Perceptual interference supports a non-modular account of face processing. Nat Neurosci. 2003;6(4):428–432. doi: 10.1038/nn1029. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ. Unraveling mechanisms for expert object recognition: bridging brain activity and behavior. J Exp Psychol Hum Percept Perform. 2002;28(2):431–446. doi: 10.1037//0096-1523.28.2.431. [DOI] [PubMed] [Google Scholar]
- Harris AM, Duchaine BC, Nakayama K. Normal and abnormal face selectivity of the M170 response in developmental prosopagnosics. Neuropsychologia. 2005;43(14):2125–2136. doi: 10.1016/j.neuropsychologia.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Hasson U, Avidan G, Deouell LY, Bentin S, Malach R. Face-selective activation in a congenital prosopagnosic subject. J Cogn Neurosci. 2003;15(3):419–431. doi: 10.1162/089892903321593135. [DOI] [PubMed] [Google Scholar]
- Hole GJ. Configurational factors in the perception of unfamiliar faces. Perception. 1994;23(1):65–74. doi: 10.1068/p230065. [DOI] [PubMed] [Google Scholar]
- Hole GJ, George PA, Dunsmore V. Evidence for holistic processing of faces viewed as photographic negatives. Perception. 1999;28(3):341–359. doi: 10.1068/p2622. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi R. Primacy of wholistic processing and global/local paradigm: a critical review. Psychol Bull. 1992;112(1):24–38. doi: 10.1037/0033-2909.112.1.24. [DOI] [PubMed] [Google Scholar]
- Konar Y, Bennett PJ, Sekuler AB. Holistic processing is not correlated with face-identification accuracy. Psychol Sci. 2010;21(1):38–43. doi: 10.1177/0956797609356508. [DOI] [PubMed] [Google Scholar]
- Lange J, de Lussanet M, Kuhlmann S, Zimmermann A, Lappe M, Zwitserlood P, et al. Impairments of biological motion perception in congenital prosopagnosia. PLoS One. 2009;4(10):e7414. doi: 10.1371/journal.pone.0007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand R, Cooper PA, Mondloch CJ, Lewis TL, Sagiv N, de Gelder B, et al. What aspects of face processing are impaired in developmental prosopagnosia? Brain Cogn. 2006;61(2):139–158. doi: 10.1016/j.bandc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP. Impairment in holistic face processing following early visual deprivation. Psychol Sci. 2004;15(11):762–768. doi: 10.1111/j.0956-7976.2004.00753.x. [DOI] [PubMed] [Google Scholar]
- Leder H, Bruce V. Local and relational aspects of face distinctiveness. Q J Exp Psychol A. 1998;51(3):449–473. doi: 10.1080/713755777. [DOI] [PubMed] [Google Scholar]
- Levine DN, Calvanio R. Prosopagnosia: a defect in visual configural processing. Brain Cogn. 1989;10(2):149–170. doi: 10.1016/0278-2626(89)90051-1. [DOI] [PubMed] [Google Scholar]
- Lobmaier JS, Bolte J, Mast FW, Dobel C. Configural and featural processing in humans with congenital prosopagnosia. Adv Cogn Psychol. 2010;6:23–34. doi: 10.2478/v10053-008-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm GL, Leung C, Barton JJ. Regional variation in the inversion effect for faces: differential effects for feature shape, feature configuration, and external contour. Perception. 2004;33(10):1221–1231. doi: 10.1068/p5372. [DOI] [PubMed] [Google Scholar]
- Martin D, Macrae CN. Processing style and person recognition: Exploring the face inversion effect. Visual Cognition. 2009;18(2):161–170. [Google Scholar]
- Maurer D, Le Grand RL, Mondloch CJ. The many faces of configural processing. Trends Cogn Sci. 2002;6(6):255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Michel C, Rossion B, Han J, Chung CS, Caldara R. Holistic processing is finely tuned for faces of one's own race. Psychol Sci. 2006;17(7):608–615. doi: 10.1111/j.1467-9280.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- Minnebusch DA, Suchan B, Ramon M, Daum I. Event-related potentials reflect heterogeneity of developmental prosopagnosia. Eur J Neurosci. 2007;25(7):2234–2247. doi: 10.1111/j.1460-9568.2007.05451.x. [DOI] [PubMed] [Google Scholar]
- Navon D. What does a compound letter tell the psychologist's mind? Acta Psychol (Amst) 2003;114(3):273–309. doi: 10.1016/j.actpsy.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Doyle J, Humphreys K, Behrmann M. Probing the face-space of individuals with prosopagnosia. Neuropsychologia. 2010;48(6):1828–1841. doi: 10.1016/j.neuropsychologia.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Ramon M, Lefevre P, Rossion B. Reduced fixation on the upper area of personally familiar faces following acquired prosopagnosia. J Neuropsychol. 2008;2(Pt 1):245–268. doi: 10.1348/174866407x260199. [DOI] [PubMed] [Google Scholar]
- Palermo R, Willis ML, Rivolta D, McKone E, Wilson CE, Calder AJ. Impaired holistic coding of facial expression and facial identity in congenital prosopagnosia. Neuropsychologia. 2011;49(5):1226–1235. doi: 10.1016/j.neuropsychologia.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon M, Busigny T, Rossion B. Impaired holistic processing of unfamiliar individual faces in acquired prosopagnosia. Neuropsychologia. 2010;48(4):933–944. doi: 10.1016/j.neuropsychologia.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Ramon M, Rossion B. Impaired processing of relative distances between features and of the eye region in acquired prosopagnosia--two sides of the same holistic coin? Cortex. 2010;46(3):374–389. doi: 10.1016/j.cortex.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Richler JJ, Cheung OS, Gauthier I. When the measure is valid, holistic processing predicts face expertise. 2010. In preparation.
- Richler JJ, Cheung OS, Wong AC, Gauthier I. Does response interference contribute to face composite effects? Psychon Bull Rev. 2009;16(2):258–263. doi: 10.3758/PBR.16.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler JJ, Mack ML, Gauthier I, Palmeri TJ. Holistic processing of faces happens at a glance. Vision Res. 2009;49(23):2856–2861. doi: 10.1016/j.visres.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest J, Moscovitch M, Black S. A comparative case study of face recognition: the contribution of configural and part-based recognition systems, and their interaction. Neuropsychologia. 2009;47(13):2798–2811. doi: 10.1016/j.neuropsychologia.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Rivolta D, Palermo R, Schmalzl L, Coltheart M. Covert face recognition in congenital prosopagnosia: A group study. Cortex. 2011 doi: 10.1016/j.cortex.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Rossion B. Picture-plane inversion leads to qualitative changes of face perception. Acta Psychol (Amst) 2008;128(2):274–289. doi: 10.1016/j.actpsy.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Rossion B, Kaiser MD, Bub D, Tanaka JW. Is the loss of diagnosticity of the eye region of the face a common aspect of acquired prosopagnosia? J Neuropsychol. 2009;3(Pt 1):69–78. doi: 10.1348/174866408X289944. [DOI] [PubMed] [Google Scholar]
- Schwarzer G, Huber S, Dummler T. Gaze behavior in analytical and holistic face processing. Mem Cognit. 2005;33(2):344–354. doi: 10.3758/bf03195322. [DOI] [PubMed] [Google Scholar]
- Searcy JH, Bartlett JC. Inversion and processing of component and spatial-relational information in faces. J Exp Psychol Hum Percept Perform. 1996;22(4):904–915. doi: 10.1037//0096-1523.22.4.904. [DOI] [PubMed] [Google Scholar]
- Stephan BC, Caine D. Aberrant pattern of scanning in prosopagnosia reflects impaired face processing. Brain Cogn. 2009;69(2):262–268. doi: 10.1016/j.bandc.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Stollhoff R, Jost J, Elze T, Kennerknecht I. Deficits in long-term recognition memory reveal dissociated subtypes in congenital prosopagnosia. PLoS One. 2011;6(1):e15702. doi: 10.1371/journal.pone.0015702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. Q J Exp Psychol [A] 1993;46(2):225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Weston NJ, Perfect TJ. Effects of processing bias on the recognition of composite face halves. Psychon Bull Rev. 2005;12(6):1038–1042. doi: 10.3758/bf03206440. [DOI] [PubMed] [Google Scholar]
- Young AW, Hellawell D, Hay DC. Configurational information in face perception. Perception. 1987;16(6):747–759. doi: 10.1068/p160747. [DOI] [PubMed] [Google Scholar]
- Yovel G, Duchaine B. Specialized face perception mechanisms extract both part and spacing information: evidence from developmental prosopagnosia. J Cogn Neurosci. 2006;18(4):580–593. doi: 10.1162/jocn.2006.18.4.580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.