Abstract
Background
Vascular and immune alterations in the prefrontal cortex may contribute to major depression in elderly subjects. Intercellular adhesion molecule-1 (ICAM-1), major inflammatory mediator in vessels and astrocytes, could be altered in geriatric depression, but little is known about its age-dependent expression in subjects with depression and its relationship to astrocytes identified by the marker glial fibrillary acidic protein (GFAP), found to be reduced in depression.
Methods
We measured the percentage of gray matter area fraction covered by ICAM-1 immunoreactivity in blood vessels and in extravascular accumulations of ICAM-1 immunoreactivity in 19 non-psychiatric comparison subjects and 18 subjects with major depression, all characterized by postmortem psychological diagnosis. Association of extravascular ICAM-1 to GFAP-positive astrocytes was investigated by double-labeling immunofluorescence.
Results
Vascular and extravascular fractions of ICAM-1 immunoreactivity were lower in subjects with MDD than in non-psychiatric comparison subjects. Non-psychiatric comparison subjects older than 60 experienced dramatic increase in extravascular ICAM-1 immunoreactivity, but this increase was attenuated in elderly subjects with MDD, particularly in those dying by suicide. Most extracellular ICAM-1 immunoreactivity was coextensive with GFAP-immunoreactive astrocytes in both groups.
Limitations
Heterogeneity in type and dosage of antidepressant medication. Difficulty in determining the exact onset of depression in subjects older than 60 at the time of death. Routine cerebrovascular pathological screening may miss subtle subcellular and molecular changes.
Conclusions
There is significant attenuation of extravascular and vascular ICAM-1 immunoreactivity in elderly subjects with major depression suggesting an astrocyte-associated alteration in immune function in the aging orbitofrontal cortex of subjects with MDD.
Keywords: Depression, prefrontal cortex, astrocytes, GFAP, ICAM-1, postmortem
INTRODUCTION
Major depressive disorder (MDD) has been associated with reductions or increases in specific proteins mediating immune responses in the vascular system, suggesting possible linkage between depression, inflammation and cardiovascular events (Dinan, 2009). Within the prefrontal cortex vascular pathology may contribute to the development of MDD in elderly patients (Alexopoulos et al., 1997a; Alexopoulos et al., 1997c; Krishnan et al., 1997). Increased levels of cytokines and other proteins related to lymphocyte motility occur in the brains of subjects with depression (5-8). However, the mechanisms of cerebrovascular involvement and the extent of the contribution of immune disturbances, such as altered lymphocyte endothelial transmigration, to the development of depression are unknown. In contrast to inflammatory activation, other research points to impairment of some aspects of immune reactivity in subjects with depression (Irwin et al., 1990; Schleifer, 2009).
Astrocytes, microglia and endothelial cells, produce mediators of immune and vascular effects (Arvin et al., 1996; Benveniste, 1992). One of these mediators, intercellular adhesion molecule-1 (ICAM-1), is constitutively expressed in endothelium and can be induced in astrocytes (Bell and Perry, 1995). ICAM-1 is a transmembrane protein that facilitates lymphocyte migration into tissues during inflammation or ischemia (Lee and Benveniste, 1999). We recently observed that aging in non-psychiatric subjects results in dramatic increases of astrocyte-associated ICAM-1 immunoreactivity in the gray matter of the orbitofrontal cortex (ORB), and also in a lesser but significant increase in blood vessel-associated ICAM-1 immunoreactivity (Miguel-Hidalgo et al., 2007). The ORB displays major morphological and functional changes in depression (Cotter et al., 2005; Drevets, 2000; Lai et al., 2000; Rajkowska et al., 1999).
Because age-related ischemic lesions in the prefrontal cortex (PFC) may result in depression (Alexopoulos, 2006; Alexopoulos et al., 1997b; Camus et al., 2004), some studies have focused on determining whether ICAM-1 expression in PFC is altered in elderly subjects with vascular depression (Thomas et al., 2004; Thomas et al., 2002; Thomas et al., 2000). In a study targeting ICAM-1 immunoreactive vessels in dorsolateral PFC of elderly subjects, Thomas et al. (2002) described increased area of ICAM-1-vessels in gray matter in MDD patients, suggesting that depression is associated with increased ICAM-1 expression or increased size of ICAM-1 positive vessels. Furthermore, in patients with symptoms of depression after coronary syndromes (Lesperance et al., 2004), elevated levels of soluble ICAM-1 have been detected, suggesting a possible involvement of soluble ICAM-1 in vascular depression. A relationship of soluble ICAM-1 levels to depression might be mediated by factors not directly related to cardiovascular disease. For example, soluble ICAM-1 is increased in patients treated with interferon-alpha, which occasionally elicits depressive symptoms (Schaefer et al., 2004). However, it is unknown whether alterations in ICAM-1 immunoreactive vessels or astrocytes in the ORB also occur in younger subjects with MDD and whether putative late-life changes in astrocyte- and vessel-associated ICAM-1 immunoreactivity in comparison subjects (Miguel-Hidalgo et al., 2007) will also be found in elderly subjects with MDD without a history of stroke or ischemia. Thus, our investigation sought to determine whether morphometrically-quantified extravascular and vascular ICAM-1 immunoreactivity differs in the ORB between subjects with MDD and comparison subjects relative to their age at the time of death and the age of disease onset.
METHODS
Subjects
The research protocol was approved by the University IRB. Human postmortem brain tissue originated from autopsies at the Cuyahoga County Coroner's Office, Cleveland, OH. Informed written consent was obtained from the next-of-kin for all subjects. Tissue blocks of frontal lobe were frozen in isopentane, immersed in dry ice and stored at –80°C until sectioning. Informant-based psychiatric assessments were performed for MDD and comparison subjects, as described (Stockmeier et al., 2004) (see Table 1 for demographic information). A trained interviewer administered the Schedule for Affective Disorders and Schizophrenia: Lifetime Version (SADS-L) (Spitzer and Endicott, 1978) to next-of-kin of 9 non-psychiatric comparison subjects and 14 subjects with depression. The Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID) was administered to next-of-kin of the remaining subjects. Blood and urine samples were examined for psychotropic medications and substances of abuse. Consensus diagnosis on Axis I psychopathology was reached by a psychiatrist and a clinical psychologist. SADS-L responses for the subjects with MDD were recorded in the SCID. All subjects with depression met criteria for MDD based on the DSM-IV (American Psychiatric Association, 1994). None of the subjects with MDD were considered in remission at the time of death or met criteria for other Axis I psychiatric disorders. The non-psychiatric comparison subjects did not currently or previously meet criteria for DSM-IV disorders. Hypertension was reported for 6 subjects in the non-psychiatric comparison group and 4 subjects in the MDD group and they appear equally distributed among younger and older subjects in comparison and subjects with MDD. None of the subjects in either group (MDD or comparison subjects) was reported to have experienced a stroke. Two subjects in the comparison group and one in the MDD group had diabetes.
TABLE 1.
Demographic features of the subject groups included in the present study
| AGE | Gender | Race | PMD | pH | Storage (months) | CAUSE OF DEATH | |||
|---|---|---|---|---|---|---|---|---|---|
| COMP | |||||||||
| 1 | 27 | F | C | 15 | 7.01 | 98 | Hypertensive cardiovascular disease | ||
| 2 | 30 | M | AA | 19 | 6.98 | 89 | Hypertrophic cardiomyopathy | ||
| 3 | 35 | M | C | 25 | 6.74 | 79 | Heart disease | ||
| 4 | 37 | F | C | 13 | 5.93 | 53 | Viral myocarditis | ||
| 5 | 43 | M | C | 23 | 6.49 | 71 | Pulmonary thromboembolism | ||
| 6 | 46 | F | C | 24 | 6.32 | 91 | Homicide | ||
| 7 | 46 | M | AA | 11 | 6.95 | 72 | Heart disease | ||
| 8 | 50 | F | C | 27 | 6.74 | 78 | Heart complications | ||
| 9 | 54 | M | C | 29 | 6.79 | 19 | Myocardial infarct | ||
| 10 | 60 | F | AA | 19 | 6.735 | 98 | Aortic aneurism | ||
| 11 | 67 | M | C | 24 | 6.96 | 79 | Atherosclerotic cardiovascular disease | ||
| 12 | 69 | M | C | 18 | 6.7 | 114 | Heart-dissecting aortic aneurism | ||
| 13 | 70 | M | C | 20 | 6.81 | 84 | Heart problems | ||
| 14 | 71 | M | C | 24 | 6.82 | 115 | Myocardial infarct, cardiac rupture | ||
| 15 | 73 | M | AA | 24 | 6.665 | 113 | Heart disease | ||
| 16 | 75 | F | C | 32 | 6.425 | 27 | Myocardial infarct | ||
| 17 | 77 | M | C | 24 | 6.56 | 95 | Heart disease | ||
| 18 | 80 | F | C | 21 | 6.78 | 65 | coronary sclerotic heart disease | ||
| 19 | 86 | F | C | 18 | 6.805 | 51 | Coronary sclerotic heart disease | ||
| Avg | 57.7 | 8F, 11M | 4AA, 15C | 21.6 | 6.7 | 78.5 | |||
| MDD | Onset (years old) | Duration (years) | |||||||
| 1 | 30 | M | AA | 18 | 6.91 | 120 | Suicide-SIGSW to chest | 27 | 3.0 |
| 2 | 34 | F | C | 24 | 6.27 | 93 | Suicide-CO | 14 | 20.0 |
| 3 | 35 | M | C | 11 | 6.965 | 59 | Undetermined | 33 | 1.5 |
| 4 | 40 | F | C | 25 | 6.32 | 81 | Heart disease | 35 | 5.0 |
| 5 | 42 | F | C | 24 | 6.62 | 101 | Suicide-overdose | 15 | 27.0 |
| 6 | 42 | M | C | 20 | 6.64 | 98 | Suicide-drowning | 40 | 2.0 |
| 7 | 46 | M | AA | 17 | 6.26 | 104 | Homicide | 45 | 1.0 |
| 8 | 50 | F | C | 23 | 6.83 | 95 | Suicide-hanging | 45 | 5.0 |
| 9 | 54 | M | C | 23 | 6.24 | 102 | Suicide-SIGSW to head | 51 | 3.0 |
| 10 | 63 | F | C | 24 | 6.32 | 104 | Pulmonary thromboembolism | 33 | 30.0 |
| 11 | 65 | M | C | 30 | 6.245 | 42 | Suicide-SIGSW to chest | 65 | 0.1 |
| 12 | 73 | M | C | 10 | 6.57 | 108 | Suicide-hanging | 68 | 5.0 |
| 13 | 73 | F | C | 17 | 6.57 | 93 | Aortic aneurism | 22 | 51.0 |
| 14 | 74 | M | C | 25 | 6.67 | 93 | Suicide-SIGSW to head | 50 | 24.0 |
| 15 | 78 | F | C | 25 | 6.94 | 95 | Suicide-jumping | 73 | 5.0 |
| 16 | 78 | M | C | 21 | 6.675 | 62 | Coronary sclerotic heart disease | 56 | 22.0 |
| 17 | 78 | M | A | 21 | 6.74 | 71 | Suicide hanging | 78 | 0.5 |
| 18 | 87 | F | C | 21 | 6.56 | 101 | Aortic aneurism/heart disease | 69 | 11.0 |
| Avg | 57.9 | 8F, 10M | 1A, 2AA, 15C | 21.1 | 6.6 | 90.0 | 45.5 | 12.0 |
Abbreviations: A=East Asian, AA= African American; Avg= Averages; C= Caucasian; COMP= non-psychiatric comparison subjects; F= Female; M= Male; MDD= subjects with major depressive disorder; PMD= Postmortem delay (time between death and freezing of brain samples); SIGSW= self-inflicted gunshot wound.
Potential study subjects were excluded with any evidence of head trauma, neurological or neurodegenerative disease, including Alzheimer's disease, or an active psychotropic use disorder. Sections from formalin-fixed samples of PFC, hippocampus and anterior temporal cortex were examined by a neuropathologist. Brains with a postmortem interval shorter than 32 hours were included. Further details on diagnostic procedures and methods for collecting information on subjects are provided elsewhere (Rajkowska et al., 1999; Stockmeier et al., 2002).
Tissue sampling
Tissue samples were obtained from the left ORB (Brodmann's area 47) of 19 non-psychiatric comparison subjects and 18 subjects with MDD diagnosis (Table 1). We used established cytoarchitectonic criteria for area 47 (Hof et al., 1995; Rajkowska et al., 1999) to select the cortical region of interest (ROI). Tissue samples from comparison subjects and subjects with MDD were coded for image processing and morphometric analysis so that laboratory personnel were blinded to diagnoses. For each diagnostic group, subjects were divided into two age subgroups. One younger subgroup was less than 60 years (age range 27 to 54 years, 5 male, 4 female for non-psychiatric comparison subjects; age range 30 to 54 years, 5 male, 4 female for subjects with MDD) and the other subgroup 60 years or older at the time of death (range 60 to 86, 6 male, 4 female for non-psychiatric comparison subjects; range 63 to 87, 5 male, 4 female for subjects with MDD). Non-psychiatric comparison subjects in the present study were included in our previous study of ICAM-1 in normal aging (Miguel-Hidalgo et al., 2007), although measurements of immunoreactivity were done anew on brain sections simultaneously processed with MDD subject samples. There was no significant difference between all comparison and all subjects with MDD or the four subgroups in the following measures: freezer storage (ANOVA F(3,33)= 1.35, p = 0.276), postmortem interval (ANOVA F(3,33)= 0.255, p = 0.857), or brain tissue pH (ANOVA F(3,33)= 0.761, p = 0.524).
Frozen slabs of 1 cm in thickness containing the ORB were cut into 20 μm-thick sections, collected onto gelatin-coated slides, and stored at –80 °C. Neighbor sections were Nissl-stained to ensure inclusion of area 47. Three sections evenly spaced (400 μm), adjacent to Nissl-stained sections, were chosen per subject for ICAM-1 immunostaining and for measuring the area fraction of ICAM-1 immunoreactivity. Other sections were immunostained with fluorescent probes to investigate co-localization of GFAP and ICAM-1 immunoreactivity.
Immunohistochemistry
Sections on slides were washed in PBS and fixed in 4% paraformaldehyde for 15 minutes. Subsequently, some sections were subjected to ICAM-1 immunostaining using a primary sheep polyclonal antibody (R&D Systems, Cat#AF720; dilution 1:750) or a primary mouse monoclonal antibody (Zymed Laboratories, Cat#07-5403; dilution1:500) .Sections were then washed in 0.1 M Tris-HCl buffer (pH 7.6), and incubated with biotinylated anti-sheep or anti-mouse antibody, washed again, and antibody binding was visualized with the ABC kit (Vector Laboratories, Burlingham) and 3-3’-diaminobenzidine (DAB) enhanced with nickel ammonium sulfate as chromogen.
Immunofluorescence
In four randomly chosen subjects per group, neighbor sections were subjected to a double fluorescent immunolabeling procedure detect both ICAM-1 and the astrocytic marker GFAP in individual sections. Each section was incubated overnight with the ICAM-1 polyclonal antibody and a mouse monoclonal antibody (1:1000) (Millipore) to GFAP. After three washes sections were incubated with Cy2-conjugated anti-sheep antibody (1:500 dilution) (Jackson Immunoresearch) and a Cy5-conjugated donkey anti-mouse antibody (1:500 dilution) (Jackson Immunoresearch) and washed again before coverslipping.
Omission of primary or secondary antibodies resulted in absence of immunostaining. Specific immunostaining was also suppressed by pre-incubating primary antibodies with recombinant human ICAM-1 (R&D Systems, Cat#ADP4). Each experiment involved the same number of sections from all groups of subjects, to minimize interassay variability. Furthermore, there were no less than 4 subjects per group in each experiment. Non-specific background immunostaining was assessed by measuring the optical density in portions of sections devoid of specific immunolabeling with the microscope light at constant intensity. There were no significant differences in non-specific staining between experiments.
Extent of ICAM-1 Immunoreactivity
To morphometrically determine the extent of ICAM-1 immunoreactivity in blood vessels (vascular immunoreactivity) and in extravascular structures in ORB sections, published methods were used (Miguel-Hidalgo et al., 2007). Briefly, ROIs were centered on area 47 of the ORB. These ROIs spanned all cortical layers with a width of 1530 μm. The area fraction of immunoreactivity in vessels and extravascular structures were measured separately (details in reference (Miguel-Hidalgo et al., 2007)). Unlike a previous method for measuring ICAM-1 immunoreactive vessels (Thomas et al., 2002; Thomas et al., 2000), our approach did not use cresyl violet counterstaining. Only immunostaining was measured, but without manual drawing of ICAM-1 vessels, so parts of vessels not labeled by anti-ICAM-1 antibodies were not measured.
Co-labeling of GFAP and ICAM-1
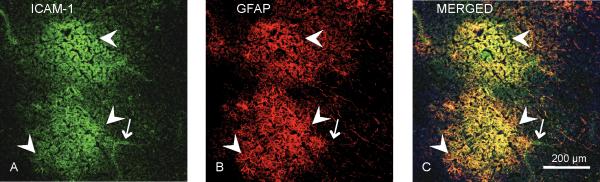
Spatial register of extravascular ICAM-1 immunoreactivity and astrocytes was confirmed by incubating sections simultaneously with a mouse antibody to GFAP and a sheep antibody to ICAM-1. Secondary antibodies conjugated either to Cy2 or Cy5 were used to detect ICAM-1 or GFAP, respectively. Since fluorophores Cy2 and Cy5 absorb and emit at non-overlapping wavelengths, ICAM-1 and GFAP immunoreactivities were unequivocally identified using a laser scanning confocal microscope (NIKON NC1) (Fig. 5) (Miguel-Hidalgo et al., 2007).
Figure 5.
Micrographs of a typical arrangement (top and bottom) of ICAM-1 immunoreactivity (green in A) in relation to GFAP-IR (red in B) astrocytes obtained simultaneously using antibodies to ICAM-1 and the astrocytic marker GFAP in a 70 year-old subject with MDD. Note in C (yellow areas, arrowheads) that the patches of diffuse ICAM-1 immunoreactivity (green in A) are largely confined within the area affected by GFAP-IR processes (red in B). ICAM-1 immunoreactivity (green in A and C, arrow) is also observable in structures with the morphology of blood vessels.
Statistics
Means of the area fraction of ICAM-1 immunoreactivity were compared using 2-way Analysis of Covariance (ANCOVA) with age group (younger vs. older) and diagnosis (non-psychiatric vs. MDD) as fixed factors and freezer storage time, postmortem interval and brain tissue pH as covariates. Potential correlations between ICAM-1 immunoreactivity and age or duration were determined with a Pearson correlation matrix. Summary data are reported as mean ± standard deviation. These data are shown in graphs without covariate adjustment.
RESULTS
ICAM-1-LIKE IMMUNOREACTIVITY IN MDD AND NON-PSYCHIATRIC COMPARISON SUBJECTS
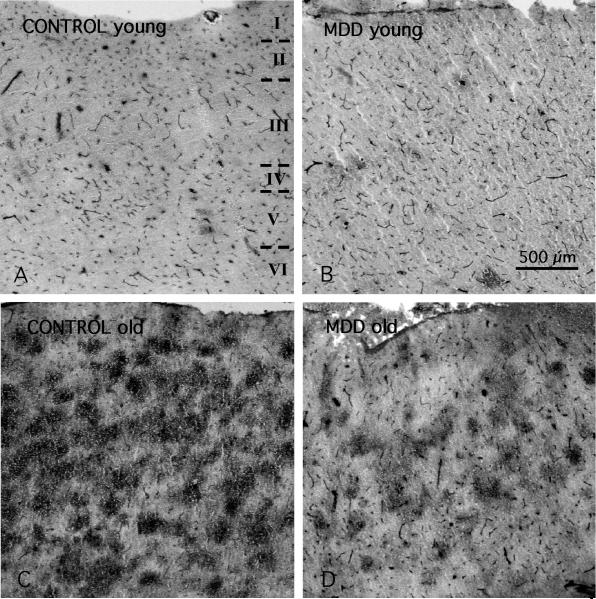
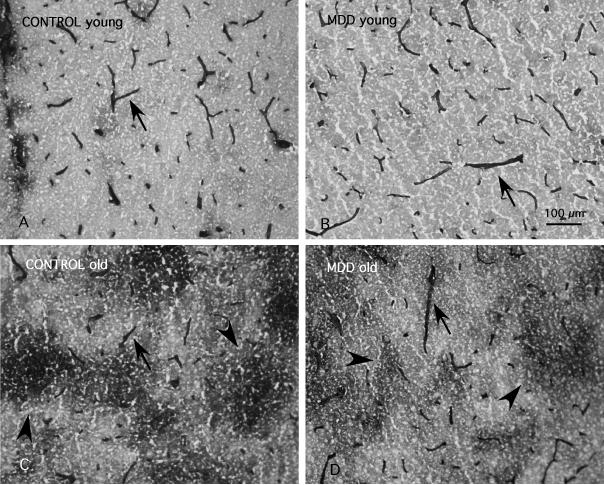
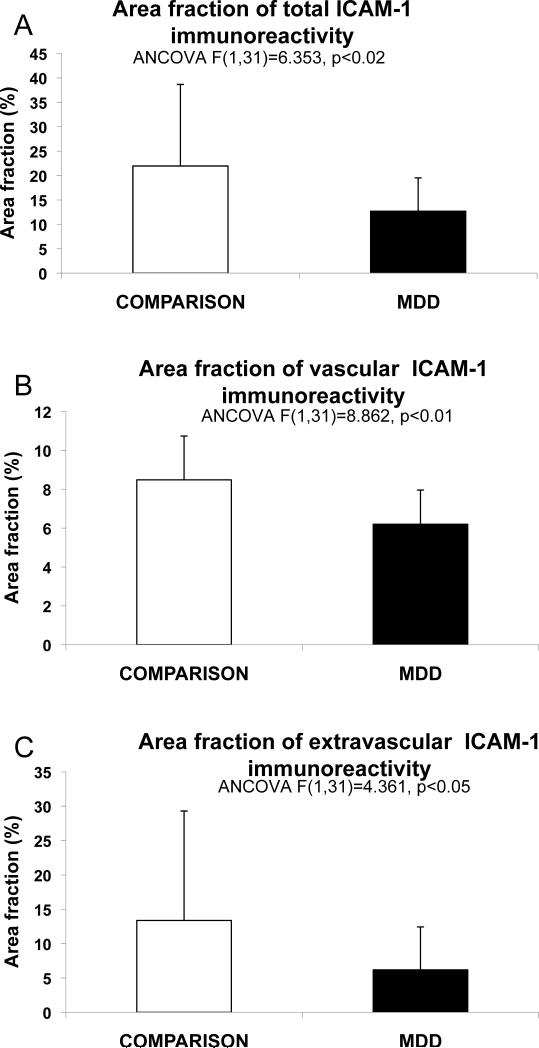
In all subjects blood vessels were easily identified by their strong ICAM-1 immunoreactivity (Figs. 1 and 2). Immunoreactive structures without blood vessel morphology were sparse in subjects younger than 60, but were common in all cortical layers in subjects over 60 (Fig.1 A, B). This extravascular ICAM-1 immunoreactivity, described previously (Miguel-Hidalgo et al., 2007), takes the appearance of round immunoreactive regions between 100 and 300 μm in diameter, at times fused into larger conglomerates (Fig. 2C,D). The average total area fraction of ICAM-1 immunoreactivity, including vascular and extravascular immunoreactivity, was lower in subjects with MDD than in non-psychiatric comparison subjects (F(1,31)=6.35, p<0.02) (Fig. 3A); ANCOVA using age, pH, postmortem interval, and storage time as covariates). The area fraction of vascular and extravascular ICAM-1 immunoreactivity was also significantly lower in subjects with MDD as compared to non-psychiatric comparison subjects (vascular F(1,31)=8.86 p<0.01; extravascular F(1,31)=4.85, p<0.04) (Fig. 3B,C).
Figure 1.
Low power micrographs of ICAM-1 immunoreactivity (I to VI between dashed lines indicate the extent of the six cortical layers in all the pictures) in area 47 of 4 human subjects of different age. A) 30 years old non-psychiatric comparison subject (CONTROL); B) 34 years old MDD subject; C) 75 years old non-psychiatric comparison subject (CONTROL); D) 87 years old MDD subject. Note that in all micrographs small and medium blood vessels are ICAM-1-IR (arrowheads). In addition, in the oldest subjects (C and D) there are many large, round patches of diffuse ICAM-1-IR (arrows) that are much less abundant in the younger subjects (A and B). Calibration bar: 500 μm.
Figure 2.
High power magnification micrographs of ICAM-1 immunoreactivity in layer III of cortical area 47. The top panels correspond to younger non-psychiatric comparison subject (CONTROL)(A) and subjects with MDD (B), ages 30 and 34 at the time of death, respectively. The bottom panels correspond to older non-psychiatric comparison subject (CONTROL) (C) and MDD (D) subjects, ages 75 and 87, respectively. Arrowheads point to the diffuse patches composed of extravascular ICAM-1 immunoreactivity. Arrows denote immunoreactive vessels. Calibration bar: 100 μm
Figure 3.
Bar graphs representing the average total area fraction of all ICAM-1-IR structures combined (A), the area fraction of immunoreactive structures with vascular morphology (B), and the area fraction of extravascular patches of ICAM-1 immunoreactivity (C) in non-psychiatric comparison subjects (CONTROL) and subjects with MDD. Whiskers on top of the columns are error bars representing the standard deviation.
ICAM-1 IMMUNOREACTIVITY IN AGE SUBGROUPS
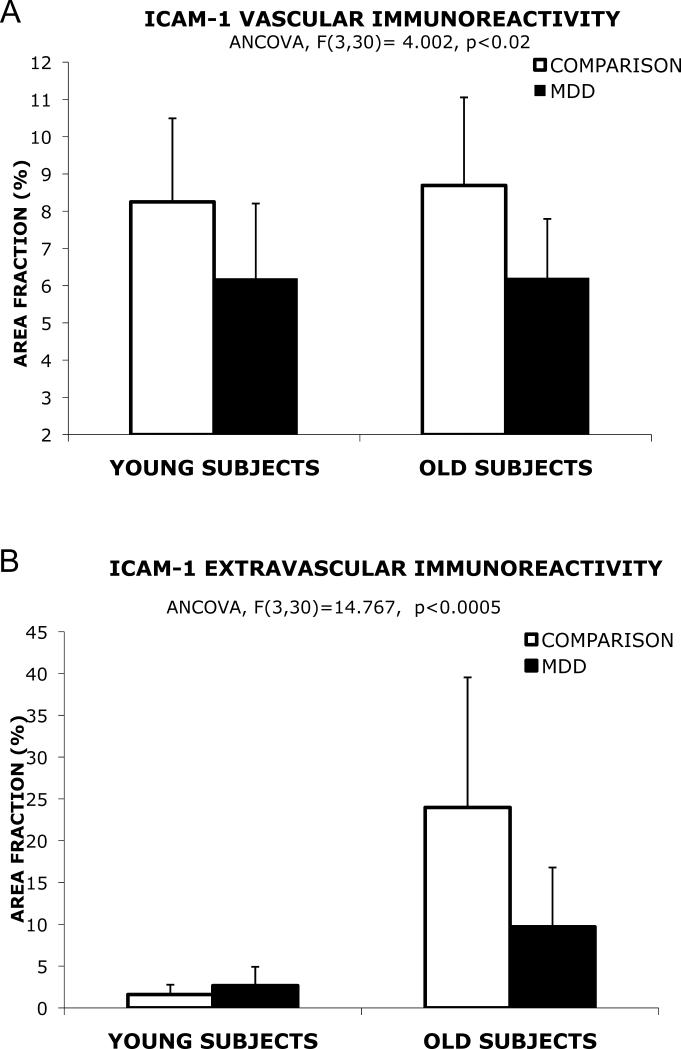
To asses whether differences between the comparison group and subjects with MDD are age-dependent, each diagnostic group was divided into one subgroup of subjects younger than 60 and another subgroup of subjects older than 60, following a previously reported criterion (Miguel-Hidalgo et al., 2007). The cut-off at 60 was chosen because in another study on ICAM-1 in the PFC subjects were considered elderly above 60 years old (25) an over that age there are significant increases in GFAP. Furthermore several neuroimaging studies also consider 60 years old as a cutoff for studying elderly patients (Lai et al., 2000; Steffens et al., 2003; Taylor et al., 2004). Two-way ANCOVA using age group (older vs. younger) and disease group (subjects with MDD vs. comparison subjects) as fixed factors revealed a significant difference in the total area fraction (F(1,30)=5.75, p=0.023), and in the area fraction of the vascular (F(1,30)=7.67, p<0.01) and extravascular compartments (F(1,30)=3.51, p=0.071) of ICAM-1 immunoreactivity (with brain pH, storage time, and postmortem delay used as covariates) between subjects with MDD and subjects in the comparison group. There was a strong significant interaction between age group and disease group for total ICAM-1 (F(1,30)=11.835, p<0.005) and for extravascular ICAM-1 (F(1,30)=10.35, p<0.005) indicating a much higher level of ICAM-1 in the older subjects of the comparison group. ANCOVAs for the four resulting groups revealed a significant difference in the total area fraction of ICAM-1 (F(3,30)=16.17, p<0.0005) and the area fraction of the vascular (F(3,30)=4.00, p<0.02) and extravascular compartments of ICAM-1 (F(3,30)=14.77, p<0.001)(with brain pH, storage time, and postmortem interval used as covariates)
Pairwise univariate contrasts showed that the area fraction of vascular ICAM-1 immunoreactivity was significantly higher in older comparison subjects than in older subjects with MDD by 40% (F(1,30)=10.59, p< 0.005) (Fig. 4A). The area fraction of vascular ICAM-1 in the comparison group was not significantly higher in older subjects as compared to younger subjects. There was also no significant difference in older subjects with MDD as compared to younger subjects with MDD. There was a tendency for a lower area fraction in young subjects with MDD as compared to young subjects of the comparison non-psychiatric group, but in the posthoc univariate analysis the difference was not statistically significant (F(1,30)=1.124, p=0.298).
Figure 4.
Bar graphs representing the area fraction of vascular (A) and extravascular ICAM-1 (B) immunoreactivity according to age (young are subjects younger than 60, old are subjects 60 and older). There is very little or no change in vascular area fraction in old subjects as compared to young in each diagnostic group (A). However, the dramatic increase in area fraction observed in old comparison subjects (as compared to young non-psychiatric comparison subjects) is greatly mitigated in old subjects with MDD compared to young subjects with MDD (B). Whiskers on top of the columns are error bars representing the standard deviation.
In the non-psychiatric comparison groups the area fraction of extravascular ICAM-1 in older subjects was dramatically higher than in younger subjects (F(1,30)=37.59, p< 0.0005) by 1496%. This area fraction in older subjects with MDD was also significantly lower as compared to older comparison subjects (F(1,30)=13.86, p<0.001) by 247% (Fig. 4B). The area fraction in older subjects with MDD, although with a trend for higher values, was not significantly different from that in younger subjects with MDD (F(1,30)=3.099, p<0.089). The area fraction of extravascular ICAM-1 immunoreactivity in younger subjects with MDD was not significantly different from younger comparison subjects (F(1,30)=0.698, p< 0.410).
ASSOCIATION WITH SUICIDE
In the MDD group 11 subjects had died by suicide, so the possibility existed that ICAM-1 immunoreactivity might differ in subjects with MDD dying by suicide as compared to those dying by other causes. For the extravascular compartment of ICAM-1 immunoreactivity, 2-way ANCOVA (with age group and suicide as factors, and pH, PMI and storage time as covariates) revealed a significant difference between suicides and non-suicides (F(1, 11) = 21.85 , p<0.005), and a significant interaction between suicide and age (F(1, 11) = 19.36 , p<0.005), with those subjects dying by suicide after 60 having significantly lower values of ICAM-1 immunoreactivity than subjects with MDD not dying by suicide. For the vascular ICAM-1 compartment there was no difference between suicide and non-suicide subjects with MDD (F(1, 11) = 1.66 , p=0.224), and no significant interaction between age group and suicide (F(1, 11) = 2.02 , p=0.183).
EFFECTS OF ONSET AND DURATION OF DEPRESSION
To determine whether the estimated onset of depression correlated with area fraction of vascular and extravascular ICAM-1, partial correlations were determined between age of onset and area fraction of ICAM-1 immunoreactivity, controlling for age at time of death, postmortem interval, brain pH and storage time. We observed a strongly significant negative correlation between area fraction of extravascular ICAM-1 and age at onset (r = -0.712, p = 0.004, df = 12). Controlling for the same variables, a significant positive correlation was found between extravascular ICAM-1 and the estimated duration of depression. However, the area fraction of vascular ICAM-1 was not significantly correlated with the age of onset (r= -0.186, p = 0.525, df = 12) or the duration of depression (r = 0.179, p = 0.541, df = 12).
EFFECT OF ANTIDEPRESSANT TREATMENT
Thirteen out of 18 subjects with MDD received antidepressant (AD) treatment prior to death. Nine of the 18 subjects had not taken AD medication during the last month of life. To determine whether there was a difference in ICAM-1-I area fraction between AD-medicated and non-AD-medicated subjects with MDD, we first compared the 13 subjects with AD treatment (MDD+AD) to the 5 subjects with MDD without AD treatment; we then compared the 9 subjects with AD treatment during the last month of life (MDD+AD[final month]) to those without treatment, using ANCOVA for both comparisons (covariates: age, brain pH, postmortem interval and storage time). There was no significant effect of AD treatment on the area fraction of either vascular or extravascular ICAM-1 immunoreactivity (for no AD treatment vs. AD treatment F(1,12)=0.117, p=0.738 vascular and F(1,12)=1.785, p=0.206 extravascular; for no treatment during last month vs. treatment within last month, F(1,12)=0.362, p=0.559 vascular and F(1,12)=1.202, p=0.294 extravascular).
COLOCALIZATION OF ICAM-1 IMMUNOREACTIVITY WITH GFAP-POSITIVE ASTROCYTES
We previously found that extravascular ICAM-1 structures were spatially associated almost exclusively with GFAP immunoreactive processes in non-psychiatric comparison subjects (10). We therefore examined whether extravascular ICAM-1 immunoreactivity in subjects with MDD was associated with GFAP-immunofluorescent processes in sections near those used for non-fluorescent labeling of ICAM-1 (three sections per brain). Then, we determined the area percentage of ICAM-1-I extravascular structures in spatial register with GFAP immunoreactive structures (Fig. 5) In all subjects we found no less than 96% of ICAM-1 extravascular immunoreactivity in register with GFAP immunoreactive structures.
DISCUSSION
The present results support an age-dependent increase in the coverage of extravascular ICAM-1 immunoreactivity in the gray matter of the ORB in comparison subjects and possibly in subjects with MDD. In addition, the dramatically increased extravascular ICAM-1 immunoreactivity observed in comparison subjects older than 60 is significantly attenuated in subjects with MDD of comparable age. This attenuation is especially conspicuous in later onset depression for extravascular ICAM-1, but not for vascular ICAM-1 immunoreactivity. Longer durations of depression are positively correlated with the area fraction of extravascular ICAM-1 but not of vascular ICAM-1 immunoreactivity.
ASSOCIATION WITH SUICIDE
The present study was not specifically directed to determine the influence of suicide on ICAM-1 immunoreactivity. However, suicide is an important cause of death among subjects with MDD, and 11 out of 18 subjects in the MDD group had died by suicide. Thus, the possibility remained that differences between groups might be associated with this mode of death frequent in MDD subjects. In fact, the analysis showed a significant association of low values of extravascular ICAM-1 immunoreactivity with death by suicide particularly in older subjects, but no association of suicide to the extent of vascular immunoreactivity. Establishing a functional connection between ICAM-1 changes and changes in neurotransmission relevant to depression or suicide is not straightforward. However, a link between inflammatory alterations and death by suicide is supported by recent research showing that both significant increases and decreases of specific blood inflammatory cytokines are detected in subjects dying by suicide (Janelidze et al., 2011) as compared to non-suicides. Furthermore, significant changes in microglial cells have been found in subjects dying by suicide (Steiner et al., 2008). Since ICAM-1 can directly interact with integrin receptors expressed in microglial cells (Akiyama et al., 1993; Akiyama and McGeer, 1990) lower levels of extravascular ICAM-1 in elderly MDD subjects dying by suicide might be related to microglial changes in the brain parenchima. In the present research the age-related changes observed were mainly in extravascular ICAM-1, which is almost entirely in register with processes of GFAP-immunoreactive astrocytes in the elderly. If astrocyte-associated ICAM-1 mediates interactions between astrocytes and microglia, these interactions, when altered, could be involved in plastic changes in neuronal circuits during aging and be related to an increased probability of death by suicide. This view is highly speculative, and, clearly, further research is needed on the possibility that astrocyte-related extravascular ICAM-1 and a possible link to microglia are factors in normal brain function during aging that might be altered in MDD subjects with suicidal behavior.
COMPARISON TO PREVIOUS STUDIES OF VASCULAR ICAM-1 IN DEPRESSION
In a previous study of ICAM-1 immunoreactive vessels in the dorsolateral PFC, using the same antibody as in this study, Thomas et al. (2002)(Thomas et al., 2002) reported an increase in the area fraction of gray matter ICAM-1 positive vessels in late-onset MDD elderly subjects as compared to non-psychiatric comparison subjects (Thomas et al., 2002). The discrepancy between ICAM-1 area fraction increase in Thomas et al. (2002) and the unchanged area fraction of vascular ICAM-1 in our study may have several explanations, which are not necessarily mutually exclusive. Our study was performed in the ORB, which is cytoarchitectonically and functionally clearly differentiated from the dlPFC. Thus, changes in immunity-related molecules in ORB may differ from those in the dlPFC in MDD. It is also possible that our method of measuring ICAM-1 immunostaining contributed significantly to the divergent results. In our study, sections were not paraffinated, but sectioned from fresh-frozen blocks and fixed in paraformaldehyde only for 30 minutes before immunohistochemical processing. However, in Thomas et al. (2002), sections were from blocks with much longer and variable lengths of fixation in formalin, and paraffin embedded. Furthermore, immunohistochemical processing in our investigation was for ICAM-1 alone, without counterstaining. Hence, the observable staining represents only antibody-binding . We only measured immunostained structures and our thresholding method (invariant for all sections) avoided manual drawing and excluded non-immunoreactive parts of vessel segments. As expected, the lumen of many vessels remained excluded from our immunoreactive area measurements. This difference may partly explain our results differing from those in Thomas et al. (2002) if, for instance, ICAM-1-positive vessels were in overall larger in MDD, but their immunoreactive portions were actually smaller. In addition, we were interested in extravascular ICAM-1 immunoreactivity and its possible association to astrocytes since age-dependent changes of GFAP astrocytes in PFC occur in depressed subjects (Miguel-Hidalgo et al., 2000; Si et al., 2004). Hence, unlike Thomas et al. (2002), we studied both vascular and extravascular compartments of ICAM-1 immunoreactivity. Another difference between the present study and that of Thomas et al. (2002) is that our sample included 11 Subjects with MDD (out of 18) that died by suicide and Thomas et al. (2002) only two subjects dying by suicide. Since we found that the ICAM-1 values (at least for extravascular ICAM-1) were lowest in the subjects with MDD dying by suicide, and did not change in the vascular compartment of subjects with suicide, this may partly explain higher ICAM-1 immunoreactivity values in subjects with MDD in Thomas et al. (2002). Thus, a combination of regional specificity, differences in cellular structures targeted, methodological divergences and a possible inverse correlation between ICAM-1 immunoreactivity and suicidality may explain apparent discrepancies between the present study and those of Thomas et al. (2000, 2002).
INFLUENCE OF AGE AND VASCULAR DISEASE
To clarify the role of age in ICAM-1 expression in psychiatric disorders, the present study examined a wide age range (young adults in their 20s and 30s to elderly subjects in their 70s and 80s), age at onset, and duration of depression. Inclusion of these covariates, while permitting determination of their influence on increases in ICAM-1 immunoreactive structures in PFC, might theoretically reduce power in detecting differences between MDD and non-psychiatric comparison subjects. Rather, the opposite occurred: depression did not result in age-related increases in area fraction of vascular ICAM-1 in the ORB and late-life depression was related to lower area fraction of extravascular ICAM-1.
Ischemic injury and white matter hyperintensities detected with magnetic resonance imaging have been implicated in late-life depression (Alexopoulos et al., 1997a; Alexopoulos et al., 1997c; Krishnan et al., 1997). However, in the present study there was no pre-mortem assessment for hyperintensities. Thus, it was impossible to determine if in vivo hyperintensities or vascular accidents were associated with ICAM-1 immunoreactivity changes. Subjects with a history of major neurological deficits or brain injury were excluded from the study. Accordingly, observed differences in ICAM-1 and GFAP immunoreactivity might not be necessarily a consequence of ischemic or vascular pathology. In fact, a certified pathologist examined systemic and brain samples from non-psychiatric comparison subjects and subjects with MDD and found no difference in macroscopic (and histologically determined) cerebrovascular pathology. However, since routine cerebrovascular pathological screening may miss subtle subcellular and molecular changes, the exact molecular nature of vascular and glial mechanisms underlying altered ICAM-1 and GFAP in depression remains to be determined. A limitation to understanding the effect of aging particularly in the group of subjects older than 60 is the difficulty in determining the exact onset of their depression, and further studies concentrating only on elderly subjects with well-characterized onsets of depression will be needed.
RELATIONSHIP OF ICAM-1 IMMUNOREACTIVITY TO GFAP IMMUNOREACTIVE ASTROCYTES
Virtually all the extravascular ICAM-1 immunoreactivity was co-extensive with GFAP-immunoreactive astrocytes. In subjects with MDD, the same high proportion of co-localization was present, although greater numbers of GFAP immunoreactive astrocytes were not associated with ICAM-1 immunoreactivity in elderly MDD than in non-psychiatric comparison subjects. Previously reported lower glial cell numbers and reduced GFAP in the ORB gray matter of younger subjects with MDD could be argued to account for the lower extravascular ICAM-1 area fraction in subjects with MDD. However, this relationship is not necessarily linear because the density of glial cells and GFAP levels, even if low in younger subjects with MDD, appear to increase towards the levels in the elderly non-psychiatric comparison subjects (Miguel-Hidalgo et al., 2000; Si et al., 2004). Furthermore the ICAM-1 area fraction in elderly MDD is only marginally higher than in younger subjects with MDD, and significantly lower than in older non-psychiatric comparison subjects. If we assume reduced astrocyte function in subjects with MDD, this may result in reduced ability to express ICAM-1. Alternatively, attenuated extravascular ICAM-1 in subjects with MDD might be due to activation of processes unrelated to the expression of GFAP in astrocytes.
ICAM-1 EXPRESSION AND MOLECULAR CHANGES IN DEPRESSION
Increased levels of ICAM-1 have been proposed after depression-inducing brain ischemic episodes (Rajagopalan et al., 2001; Thomas et al., 2002). However, ICAM-1 involvement in the pathophysiology of depression might be unrelated to ischemia, and depend on other depression-related molecular changes. For instance, NK-1-type substance P receptors are reduced in the ORB of subjects with MDD (Stockmeier et al., 2002), and the substance P antagonist spantide blocks TNF-α-induced ICAM-1 expression in brain endothelium (Annunziata et al., 2002). Thus, low NK-1 function may contribute to reduced ICAM-1 immunoreactivity. It remains to be determined whether extravascular ICAM-1 might be regulated by NK-1 receptors as well.
Elevated corticosteroid levels inhibit ICAM-1 expression (Sapolsky et al., 2000; van de Stolpe and van der Saag, 1996; van der Saag et al., 1996). Since elevated corticosteroids, or a failure in the dexamethasone suppression test, occur in many depressed subjects (Holsboer, 2001), reduced ICAM-1 immunoreactivity may reflect dysregulated corticosteroid signaling. It would be interesting to examine whether ICAM-1 immunoreactivity also normalizes during remission of depression, since the dexamethasone suppression test normalizes with remission.(Zobel et al., 2001).
INFLUENCE OF ANTIDEPRESSANT MEDICATIONS
In vitro evidence shows that antidepressants can change responses from pro-inflammatory to anti-inflammatory (Leonard, 2001a, b). Therefore lower ICAM-1 immunoreactivity might result from antidepressant treatment of depressed subjects. However, the lack of differences in ICAM-1 area fraction between the 13 subjects with and the 5 subjects without antidepressant treatment, or between those that had no antidepressant treatment during the last month of life and the remaining subjects with MDD would suggest a non-significant effect (at the sample size used in the present study) of antidepressant treatment on ICAM-1 immunoreactivity. In addition, some studies reported no changes in cytokines after antidepressant treatment (Muller and Schwarz, 2007). Clearly, and despite the absence of detectable differences in our study, the full appreciation of a possible influence of antidepressant treatment on ICAM-1 immunoreactivity can be limited by the heterogeneity in the pharmacological nature and dosage of antidepressant medication in the various subjects, and the difficulty in precisely determining degree of compliance and the possible interactions between different types of medications.
In summary, this study suggests lower expression of extravascular ICAM-1 in the ORB of MDD than in non-psychiatric comparison subjects. In particular, extravascular ICAM-1 immunoreactivity is dramatically reduced in late-life in subjects with MDD and this is significantly correlated with death by suicide. This reduction in MDD appears to occur in spatial register with GFAP positive astrocytes. It remains to be determined which neurochemical or endocrine alterations mediate lower extravascular ICAM-1 immunoreactivity in subjects with MDD.
Role of the Funding Source
Funding for this study was provided by NIH Grants RR17701, MH60451 and MH67996. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
REFERENCES
- Akiyama H, Kawamata T, Yamada T, Tooyama I, Ishii T, McGeer PL. Expression of intercellular adhesion molecule (ICAM)-1 by a subset of astrocytes in Alzheimer disease and some other degenerative neurological disorders. Acta Neuropathol (Berl) 1993;85:628–634. doi: 10.1007/BF00334673. [DOI] [PubMed] [Google Scholar]
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Alexopoulos G, Meyers B, Young R, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997a;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997b;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [see comments] [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. Am J Psychiatry. 1997c;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- Annunziata P, Cioni C, Santonini R, Paccagnini E. Substance P antagonist blocks leakage and reduces activation of cytokine-stimulated rat brain endothelium. J Neuroimmunol. 2002;131:41–49. doi: 10.1016/s0165-5728(02)00262-x. [DOI] [PubMed] [Google Scholar]
- Arvin B, Neville LF, Barone FC, Feuerstein GZ. The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev. 1996;20:445–452. doi: 10.1016/0149-7634(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Bell MD, Perry VH. Adhesion molecule expression on murine cerebral endothelium following the injection of a proinflammagen or during acute neuronal degeneration. J Neurocytol. 1995;24:695–710. doi: 10.1007/BF01179819. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263:C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Camus V, Kraehenbuhl H, Preisig M, Bula CJ, Waeber G. Geriatric depression and vascular diseases: what are the links? J Affect Disord. 2004;81:1–16. doi: 10.1016/j.jad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005;7:358–369. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Inflammatory markers in depression. Curr Opin Psychiatry. 2009;22:32–36. doi: 10.1097/YCO.0b013e328315a561. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Hof PR, Mufson EJ, Morrison JH. Human orbitofrontal cortex: cytoarchitecture and quantitative immunohistochemical parcellation. J. Comp. Neurol. 1995;359:48–68. doi: 10.1002/cne.903590105. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Irwin M, Patterson T, Smith TL, Caldwell C, Brown SA, Gillin JC, Grant I. Reduction of immune function in life stress and depression. Biol Psychiatry. 1990;27:22–30. doi: 10.1016/0006-3223(90)90016-u. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin S, Traskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. 2011;25:335–339. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999;98:77–88. doi: 10.1016/s0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Changes in the immune system in depression and dementia: causal or co-incidental effects? Int J Dev Neurosci. 2001a;19:305–312. doi: 10.1016/s0736-5748(01)00014-4. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2001b;25:767–780. doi: 10.1016/s0278-5846(01)00155-5. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. GFAP-immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:860–872. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Nithuairisg S, Stockmeier C, Rajkowska G. Distribution of ICAM-1 immunoreactivity during aging in the human orbitofrontal cortex. Brain Behav Immun. 2007;21:100–111. doi: 10.1016/j.bbi.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196–198. A197. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Horn M, Schmidt F, Schmid-Wendtner MH, Volkenandt M, Ackenheil M, Mueller N, Schwarz MJ. Correlation between sICAM-1 and depressive symptoms during adjuvant treatment of melanoma with interferon-alpha. Brain Behav Immun. 2004;18:555–562. doi: 10.1016/j.bbi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ. Immunity and Depression: A Clinical Perspective. In: Siegel A, Zalcman SS, editors. The Neuroimmunological Basis of Behavior and Mental Disorders. Springer; New York: 2009. pp. 287–305. [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O'Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Endicott J. Schedule for affective disorders and schizophrenia (SADS) New York State Psychiatric Institute; New York: 1978. [Google Scholar]
- Steffens DC, McQuoid DR, Welsh-Bohmer KA, Krishnan KR. Left orbital frontal cortex volume and performance on the benton visual retention test in older depressives and controls. Neuropsychopharmacology. 2003;28:2179–2183. doi: 10.1038/sj.npp.1300285. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Shi X, Konick L, Overholser JC, Jurjus G, Meltzer HY, Friedman L, Blier P, Rajkowska G. Neurokinin-1 receptors are decreased in major depressive disorder. Neuroreport. 2002;13:1223–1227. doi: 10.1097/00001756-200207020-00031. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Davis S, Ferrier IN, Kalaria RN, O'Brien JT. Elevation of cell adhesion molecule immunoreactivity in the anterior cingulate cortex in bipolar disorder. Biol Psychiatry. 2004;55:652–655. doi: 10.1016/j.biopsych.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Ferrier IN, Kalaria RN, Davis S, O'Brien JT. Cell adhesion molecule expression in the dorsolateral prefrontal cortex and anterior cingulate cortex in major depression in the elderly. Br J Psychiatry. 2002;181:129–134. doi: 10.1017/s0007125000161847. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Ferrier IN, Kalaria RN, Woodward SA, Ballard C, Oakley A, Perry RH, O'Brien JT. Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex. Am J Psychiatry. 2000;157:1682–1684. doi: 10.1176/appi.ajp.157.10.1682. [DOI] [PubMed] [Google Scholar]
- van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- van der Saag PT, Caldenhoven E, van de Stolpe A. Molecular mechanisms of steroid action: a novel type of cross-talk between glucocorticoids and NF-kappa B transcription factors. Eur Respir J Suppl. 1996;22:146s–153s. [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression. a prospective study. J Psychiatr Res. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]