Abstract
Background
Previous studies have found individuals with family histories of alcohol use disorders are more impulsive on some but not all laboratory behavioral measures, suggesting deficits on specific forms of impulse control. However, drawing conclusions is tenuous because these different measures have not been administered together in the same group of participants.
Methods
In the present study, we compared healthy 21–35 year old adults with family histories of alcohol related problems (FHAP+) or without such histories (FHAP−) on behavioral measures of response inhibition, response initiation, and consequence sensitivity impulsivity. FHAP+ (n=36) and FHAP− (n=36) participants were compared on performance on the Immediate Memory Task (IMT, response initiation), GoStop Impulsivity Paradigm (GoStop, response inhibition), Two Choice Impulsivity Paradigm (TCIP, consequence sensitivity) and Single Key Impulsivity Paradigm (SKIP, consequence sensitivity).
Results
FHAP+ individuals were more impulsive on the IMT and GoStop but not on the TCIP or SKIP.
Conclusions
These results suggest that response initiation and response inhibition impulsivity are increased in individuals with family histories of alcohol related problems despite not having alcohol or drug use disorders themselves. In contrast, increased consequence sensitivity impulsivity may be associated with additional risk factors such as more severe family histories of alcohol use disorders, or it may be increased as a consequence of heavy drug or alcohol use.
Keywords: impulsivity, alcohol use disorder, family history, risk, vulnerability
1. Introduction
Individuals with a family history of alcohol use disorders (FHA+) are at increased risk for developing alcohol and other substance use disorders compared with those lacking such a history (FHA; Finn et al., 1990; Lieb et al., 2002; Merikangas et al., 1998), however the underlying behavioral endophenotype contributing to this risk is not fully understood. The FHA+ associated risk appears to have a strong genetic component, as indicated by twin, adoption, cross-fostering, and pedigree analysis studies (Cloninger et al., 1981; Merikangas, 1990; Reich et al., 1998; Slutske et al., 2002). FHA+ is also linked with a pattern of “behavioral undercontrol” or “neurobehavioral disinhibition”, which consists of increased sensation seeking, risk-taking, aggressiveness, and antisocial behaviors (Sher et al., 2004; Sher and Trull, 1994; Tarter et al., 2003). FHA+ is also associated with subtle impairments on tests of executive functioning, attention (Corral et al., 2003; Deckel, 1999; Stevens et al., 2003), and altered activity in neural circuits regulating processes such as impulse control, decision making, and emotional reactivity (Acheson et al., 2009; Glahn et al., 2007; Schweinsburg et al., 2004).
It is plausible that impulsivity contributes to the increased risk for alcohol and other drug use disorders in FHA+ individuals as increased impulsivity has been observed in individuals with alcohol and other substance use disorders, and impulsivity is generally considered to be a risk factor for developing these disorders (Bornovalova et al., 2005; de Wit, 2009; Reynolds, 2006). Consequently, impaired impulse control may be a prominent component of the behavioral endophenotype of FHA+ individuals even in the absence of alcohol or drug use disorders. To date however, findings on the effects of FHA+ status on behavioral measures of impulsivity have been mixed. Impulsive performance on stop signal tasks can predict the development of problem drinking among FHA+ adolescents and the development of alcohol dependence among adult heavy drinkers (Nigg et al., 2006; Rubio et al., 2008). Additionally, FHA+ young adults with high behavioral undercontrol were modestly more impulsive on a go/no go task than FHA− young adults with low behavioral undercontrol (Saunders et al., 2008). However, other studies reported small or no effects of FHA status on impulsive responding on preferences for immediate and delayed hypothetical monetary rewards (Acheson et al., in press; Crean et al., 2002; Herting et al., 2010; Petry et al., 2002). These studies have varied in potentially important factors such as age of subjects and methods used to classify family histories, and it is possible that these differences account for the lack of consistent effects of FHA+ across studies using different behavioral impulsivity measures.
Alternatively, it is possible that FHA+ status may be associated with impairments on some behavioral measures of impulsivity but not others. Behavioral measures of impulsivity are not interchangeable but rather appear to index distinct neuropsychological processes (de Wit and Richards, 2004; Dougherty et al., 2009; Evenden, 1999; Moeller et al., 2001; Winstanley et al., 2006). There are at least three processes that are measured by commonly used behavioral impulsivity tasks: (1) rapid responding that occurs prior to complete processing and evaluation of a stimulus (i.e., response initiation, as measured by go/no go tasks); (2) failure to inhibit an already initiated response (i.e., response inhibition, as measured by stop signal tasks); and (3) reward-directed responding that persists despite less than optimal outcomes (i.e., consequence sensitivity, as measured by delay discounting and related delayed reward choice measures) (Dougherty et al., 2005a). These three processes measured by behavioral impulsivity tasks appear to be independent, as indicated by large sample behavioral studies in humans (Dougherty et al., 2009; Reynolds et al., 2006) and neurobiological studies in humans and animals identifying distinctions in neural circuits required for performing these measures (Eagle et al., 2008; McClure et al., 2004; Robbins, 2007). Consequently, FHA+ individuals may have phenotypical impairments on specific impulse control processes (response initiation and response inhibition) while leaving others (consequence sensitivity) relatively unaffected (Crean et al., 2002; Nigg et al., 2006; Petry et al., 2002; Rubio et al., 2008; Saunders et al., 2008).
In the present study, we examined healthy adults who reported one or both biological parents had one or more serious alcohol-related problems (i.e., divorce, job loss or arrests due to alcohol use) and compared them with controls who reported no alcohol-related problems in any 1st or 2nd degree biological relative (FHAP−). This objective criteria was based on specific problem behaviors and negative life events in parents that constitute significant, real world alcohol-related impairments. Participants were compared on four behavioral impulsivity tasks collectively indexing the three impulsive processes identified above: response initiation, response inhibition, and consequence sensitivity. Based on previous studies, we expected FHAP+ individuals to be more impulsive on the response initiation and response inhibition measures but not the consequence sensitivity measures.
2. Method
2.1 Participants
Thirty-six FHAP+ participants (20 men, 16 women) and 36 FHAP− participants (21 men, 15 women) were compared on laboratory measures of behavioral impulsivity. Participants were recruited from the community through radio, newspaper, and television advertisements. Respondents to advertising completed an initial telephone interview to assess suitability for study participation, and potential participants were invited to the laboratory for a more comprehensive screening assessment of physical and psychiatric health, drug/alcohol use history, and intelligence. Generally about 20 to 30% of potential participants fail our screening procedures. Typical reasons for screen failures include low IQ, psychiatric conditions, or other health issues. Psychiatric health was assessed using the Structured Clinical Interview for DSM-IV psychiatric disorders (SCID; First et al., 2001) administered by trained research assistants and reviewed by a staff psychiatrist board-certified in adult psychiatry. Intelligence was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI; Psychological Corporation, 1999). Exclusionary criteria included physical conditions that would interfere with task performance, DSM-IV Axis I psychiatric disorder (including lifetime alcohol or drug abuse/dependence), positive alcohol or drug screen, or IQ < 80. All participants were between 21 and 35 years old. This age range was selected so all participants had at least some opportunity to legally drink alcohol and because in our experience exclusions due to serious health problems occur more frequently in older participants, and often more commonly in high risk populations. We have also found that individuals in this age range are unlikely to show age-related differences on our laboratory behavioral impulsivity measures. Two hundred seventy adults who were of good medical health with no past or current alcohol or drug use disorders were screened for our FHAP criteria (see below). Of these individuals, 36 met our FHAP+ criteria, all of whom were included in the study. These 36 FHAP+ participants were age, gender and ethnicity matched to 36 individuals from the same sample who meet our FHAP− criteria, all of whom were also included in the study. Written informed consent was obtained prior to study participation. The experimental protocol was approved by Institutional Review Boards of Wake Forest University Health Sciences and The University of Texas Health Science Center at San Antonio.
2.1.1 Group Classification
Family history of alcohol related problems were determined using the Family History Questionnaire (Schuckit, 1985). FHAP+ individuals endorsed one or more of the following six items for at least one biological parent: (1) marital separation or divorce because of their drinking; (2) laid off from work or fired because of their drinking; (3) two or more drunk driving arrests; (4) two or more arrests for public intoxication or drunk and disorderly conduct; (5) a physician said alcohol itself had harmed their health; and (6) repeatedly unable to care for the house or family because of alcohol use. FHAP− individuals endorsed no alcohol or drug FHQ items for any 1st or 2nd degree relatives. Potential FHAP− participants who lacked information on 1st or 2nd degree relatives were not included in the study. Potential FHAP+ who lacked information on their parents were not included in the study. This classification relied on readily observable parental behaviors and life outcomes. A positive response on any one of these items would indicate a serious alcohol-related problem that likely had signficant negative consequences and a real-world impairment.
2.2 Experimental procedure
Participants completed two days of testing. Each day, participants arrived at the laboratory at 0800 h and provided expired-air samples to screen for recent alcohol use (AlcoTest® 7110 MKIII C, Draeger Safety Inc., Durango, CO) and urine samples to screen for recent drug use (THC, cocaine, benzodiazepines, opiates, and amphetamines; Panel/Dip Drugs of Abuse Testing Device, Redwood Biotech, Santa Rosa, CA). A total of 7 participants were excluded due to positive alcohol or drug screen on either test session. Participants completed questionnaires to assess current and lifetime alcohol and drug use, socioeconomic status (Four Factor Index of Socioeconomic Status, FFISS; Hollingshead, 1975), self-reported impulsivity (Barratt Impulsiveness Scale, BIS-11; Patton et al., 1995), and performed four laboratory measures of impulsivity (described below) between the hours of 0830 and 1600, over a two day period. The administration of the laboratory behavioral measures of impulsivity was counterbalanced, with standardized instructions given prior to each of the tasks. Study procedures were completed in a sound-attenuated chamber equipped with a computer monitor and mouse. Participants earned points during each task and were told they would earn more money by earning more points, however all participants were actually paid a flat $5 task bonus in addition to their daily payment to keep compensation consistent across participants.
2.3 Laboratory-Behavioral Measures of Impulsivity
The behavioral impulsivity measures described below have been developed within our laboratory and have been demonstrated to be appropriate for assessing impulsivity across the life-span (Dougherty et al., 2009; Dougherty et al., 2003b) as well as sensitive to both population differences and pharmacological manipulations (Dougherty et al., 1999; Dougherty et al., 2008; Dougherty et al., 2007; Dougherty et al., 2004). We have also demonstrated through factor analyses conducted in two large independent samples that the response initiation, response inhibition, and consequence sensitivity measures described below correspond to three distinct components with factor solutions accounting for 81% and 79% of the variance respectively (Dougherty et al., 2009). In both samples factor loadings within each component were nearly identical, with no significant loadings across the different task types. These findings indicate that the behavioral impulsivity measures outlined below sensitive assays of distinct underlying processes.
2.3.1 Immediate Memory Task (IMT)
The IMT (Dougherty et al., 2003a; Dougherty et al., 2002) is a go/no go task used to measure response initiation impulsivity. In this task, a series of 5-digit numbers (e.g., 38391) were displayed on a computer monitor. The sequence of numbers was randomly generated and each number appeared for 500 ms at a rate of one per second. Participants were instructed to click a mouse button when the 5-digit number they saw was identical to the one that preceded it. The three main types of numeric stimuli were target, catch, and filler stimuli. A target stimulus was a 5-digit number identical to the preceding number. Participants were instructed to respond only to these numbers and these responses were recorded as correct detections. A catch stimulus was a number that differed from the preceding number by only one digit (its position and value determined randomly). Responses to catch stimuli were recorded as commission errors. A filler stimulus was a random 5-digit number. Responses to filler stimuli were designated filler errors. The probabilities of either target or catch stimulus presentations were 33% each and the probability of a filler stimulus presentation was 34%. Participants earned points based on performance accuracy, and points earned were displayed at the end of the session. There were a total of 546 trials divided across two 5-min testing blocks separated by a 30-sec rest period, thus the task lasted 10.5 minutes. The primary dependent measure for this task was the IMT Ratio, (i.e., the proportion of commission errors to correct detections).
2.3.2 GoStop Impulsivity Paradigm (GoStop)
The GoStop (Dougherty et al., 2005b) is a stop signal task used to measure response inhibition impulsivity. Similar to the IMT (above), the GoStop involved rapid presentation of a series of 5-digit numbers during a 12-min session; i.e., each stimulus was displayed for 500 ms with a 1500 ms inter-stimulus interval. Unlike the IMT, half of the 5-digit numbers were target trials (matching stimuli) and half are filler trials (non-matching stimuli). The primary feature of this response inhibition task was that half of all target trials were stop trials, where participants were required to withhold responding. On target trials, participants were instructed to respond while a number was still on the monitor, but to withhold responding if that number turned from black (go signal) to red (stop signal). The failure to withhold a response on stop trials was a response inhibition failure. On stop trials, the stop delays (interval between the onset of the go signal and onset of the stop signal) were 50, 150, 250, or 350 ms, with more response inhibition failures expected at longer stop delays. To encourage rapid initiation of responses on target trials, participants were also instructed that late responses (i.e., after the stimulus disappears from the screen) would not be counted as points earned for that response even though the response may have been correct. A session consisted of 40 go trials, 40 stop trials (10 at each delay), and 80 filler trials for a total of 160 trials. Each session was divided into two blocks of 80 trials separated by a 30 s rest break. Participants received points for correct responses to go trials and for withheld responses to stop trials. Participants received performance feedback as points earned and points lost, shown on the monitor during the rest break and at the end of the session. The primary dependent measure is the number of response inhibition failures (i.e., responses on stop trials).
2.3.3 Two Choice Impulsivity Paradigm (TCIP)
The TCIP (Dougherty et al., 2005b) is a discrete-choice procedure designed to assess consequence sensitivity aspects of impulsivity. Participants made 50 reward choices by clicking on a circle or a square to add points to a counter. The left/right orientation of the two shapes was randomly determined. Participant chose between clicking on the circle to earn 5 points after waiting 5 s (short delay) or clicking on the square to earn 15 points after waiting 15 s (long delay). After selecting a shape, the other shape disappeared and the selected shape faded to gray. After the scheduled delay had elapsed, the shape changed back to black and flashed for 500 ms once per second. At this point participants clicked on the shape to add the reward to the counter. Prior to the actual session participants completed a practice session of five forced choices on each option, allowing an association to be made between the two delay-reward contingencies without explicit information being provided during instructions. The primary dependent measure for the TCIP was the proportion of smaller-sooner reward choices.
2.3.4 Single Key Impulsivity Paradigm (SKIP)
The SKIP (Dougherty et al., 2005b) is used to test consequence sensitivity aspects of impulsivity by measuring free operant choices for rewards. In a 20-min session, participants were free to respond as often as desired by clicking a computer mouse to accumulate points. Participants were instructed: “Nothing in this task will tell you when to press the button. You can press the button whenever you want to, but keep in mind, the longer you wait before pressing the button, the more points that press will be worth.” Each response added points that increased exponentially as the length of the delay between responses increase, and the delay/reward contingency was calculated as [seconds elapsed + (3 × [seconds elapsed]2)]/1000. For example, a response emitted 5 s after the previous response earned 8 points, a response after 10 s earned 31 points, and a response after 15 s earned 69 points. Two point counters were displayed on a computer monitor. A counter at the bottom of the screen displayed earnings for each response, giving feedback about the delay contingency, and a counter at the top of the screen displayed the total accumulated earnings. The primary dependent measures was the longest delay interval between responses.
2.4 Data Analyses
The primary dependent measures from the GoStop (inhibition failures at all 4 stop delays) were analyzed together in a repeated-measures ANOVA with GoStop delay as a within-subject factor and group as a between subjects factor. Effect sizes for significant main effects and interactions were evaluated with Cohen’s f test and were followed by post hoc independent samples t-tests with Bonferroni correction. The primary dependent measures from the IMT, TCIP and SKIP were also analyzed using independent samples t-tests, and effect sizes for significant results were evaluated with Cohen’s d tests. Demographic and drug use measures were analyzed with independent samples t-tests or chi-square tests as appropriate. All analyses were performed using SPSS® version 17.0 (SPSS Inc; Chicago, IL) with alpha criteria of 0.05.
3. Results
3.1 Participants
The demographic characteristics, intelligence scores, self-reported levels of impulsivity, and current and lifetime recreational drug use histories reported by participants are summarized in Table 1. The groups did not differ in age, ethnicity, gender, intelligence (WASI), socioeconomic status (FFISS) or self-reported impulsivity (BIS-11). Both groups were similar with respect to current alcohol use. The groups also did not differ in use of other drugs though observed means were higher for FHAP+ participants. Table 2 shows the types of alcohol related problems experienced by the parents of the FHAP+ participants. The FHAP+ participants reported FHQ alcohol items in their fathers more frequently than their mothers.
Table 1.
Participant Characteristics
| FHAP− | FHAP+ | |
|---|---|---|
| n = 36 | n = 36 | |
| Characteristics | Mean ± SD | Mean ± SD |
| Age | 27.3 ± 4.8 | 26.9 ± 4.7 |
| WASI Total IQ score | 98.4 ± 12.6 | 100.8 ± 12.6 |
| FFISS | 34.3 ± 11.1 | 35.6 ± 10.1 |
| BIS-11 attentional | 14.2 ± 3.4 | 14.6 ± 2.9 |
| BIS-11 motor | 23.3 ± 3.8 | 22.8 ± 3.1 |
| BIS-11 nonplanning | 21.5 ± 4.7 | 23.9 ± 6.2 |
|
Gender
| ||
| Male | 21 (68%) | 20 (66%) |
| Female | 15 (42%) | 16 (44%) |
|
Ethnicity
| ||
| African-American | 7 (19%) | 10 (28%) |
| Caucasian | 12 (33%) | 11 (31%) |
| Hispanic | 14 (39%) | 13 (36%) |
| Other | 3 (8%) | 2 (6%) |
WASI = Wechsler Abbreviated Scale of Intelligence; FFIS = Four Factor Index of Socioeconomic Status; BIS-11 = Barratt Impulsiveness Scale version 11
Table 2.
Family Healthy History Questionnaire (FHQ) Alcohol items endorsed for parents in FHAP+ participants
| Father | Mother | |
|---|---|---|
| FHQ Item | ||
| 1. Marital separation or divorce because of their drinking. | 17(47%) | 3(8%) |
| 2. Laid off from work or fired because of their drinking. | 11(31%) | 3(8%) |
| 3. Two or more drunk driving arrests. | 15(42%) | 2(6%) |
| 4. Two or more arrests for public intoxication and drunk and disorderly conduct. | 1(3%) | |
| 5. A physician said alcohol itself had harmed their health. | 8(22%) | 1(3%) |
| 6. Repeatedly unable to care for the house or family because of alcohol use. | 11(31%) | 3(8%) |
|
| ||
| Number of FHQ Items | ||
| Parent with 0 items | 6(17%) | 25(69%) |
| Parent with 1 item | 13(36%) | 8(22%) |
| Parent with 2 items | 5(14%) | 2(6%) |
| Parent with 3 or more items | 13(36%) | 1(3%) |
3.2 Impulsivity Tasks
3.2.1 IMT
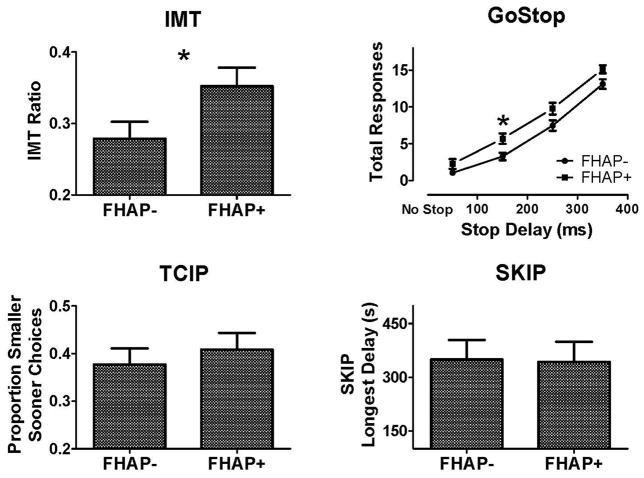
FHAP+ individuals had significantly higher IMT Ratios (greater proportions of commission errors to correct detections) [t (70) = 2.096, p = 0.04, d = 0.494] (Figure 1 upper left panel), indicating greater response initiation impulsivity in FHAP+ participants.
Figure 1.
Comparison of individuals with family histories of alcohol related problems (FHAP+) or without such histories (FHAP−) on the Immediate Memory Task (IMT, upper left panel), Go Stop Impulsivity Paradigm (GoStop, upper right panel), Two Choice Impulsivity Paradigm (TCIP, lower left panel), and the Single Key Impulsivity Paradigm (SKIP, lower right panel). FHAP+ participants were more impulsive on both the IMT and GoStop tasks but there were no group differences on either the TCIP or SKIP tasks.
3.2.2 GoStop
FHAP+ participants had relatively more inhibition failures across stop delays [main effect of group; F (1,70) = 7.741 p = 0.007, f = 0.333] (Figure 1, upper right panel). There were no differences in total responses on go trials (Mean ± SD: FHAP− = 75.2 ± 6.7; FHAP+ = 75.2 ± 5.9), indicating the performance differences on stop delays reflect differences in response inhibition rather than general attention or performance impairments. Post hoc t-tests revealed FHAP+ participants had significantly more inhibition failures at the 150 ms stop delays and a trend towards more inhibition failures at the 250 and 350 ms stop delays (Figure 1, upper right panel). All participants had more inhibition failures with increasing stop delays [main effect of delay; F (3, 210) = 299.16, p < 0.001], indicating greater difficult withholding responding with longer intervals between the onsets of the go and stop signals.
3.2.3 TCIP and SKIP
There were no significant effects of FHAP status on proportions of smaller-sooner reward choices on the TCIP or longest delay intervals on the SKIP (Figure 1, lower panels), indicating consequence sensitivity impulsivity was not affected by FHAP status.
4. Discussion
In the present study, we observed that individuals with self-reported family histories of alcohol related problems were more impulsive on the IMT and GoStop, measures of response initiation and response inhibition impulsivity, but there were no significant effects of FHAP status on TCIP or SKIP performance, measures of consequence sensitivity impulsivity. We also observed no group differences in self-reported impulsivity on the BIS-11. These results suggest that response initiation and response inhibition impulsivity may be generally increased across individuals with a broad range of family histories of alcohol use disorders. In contrast increased consequence sensitivity impulsivity may be more apparent in individuals who are at greater risk for developing alcohol and drug use disorders, or they may be increased as a consequence of heavy alcohol or drug use.
This study is the first to examine four distinct measures of impulsivity within the same sample of individuals who were at risk for developing alcohol use disorders based on family history. Our results are consistent with previous studies that have examined the effects of FHA status on similar measures separately despite those studies using samples of different ages and varied methods for classifying FHA status (Crean et al., 2002; Nigg et al., 2006; Petry et al., 2002; Saunders et al., 2008). The present study found FHAP+ individuals were more impulsive on both the IMT and GoStop tasks, which together with previous studies suggests that increased response initiation and response inhibition impulsivity may be a relatively robust characteristics in individuals at risk based on family history (Nigg et al., 2006; Saunders et al., 2008). Additionally, the lack of significant differences in impulsive choice on the TCIP and SKIP are consistent with previous studies finding small or no FHA associated differences in relative preferences for immediate and delayed rewards (Acheson et al., in press; Crean et al., 2002; Herting et al., 2010; Petry et al., 2002) and suggest that consequence sensitivity impulsivity is not prominently affected by FHA status.
The present findings should be considered in the context of the participant classification and recruitment strategy. The positive family history group only had to have at least one major, alcohol-related problem in at least one biological parent, and this criteria likely resulted in relatively heterogeneous backgrounds of parental alcohol use disorders in this sample. Furthermore, participants in this study were adults with no histories of alcohol or other drug use disorders, and while some of the younger participants may still develop drug or alcohol use problems, this seems less likely for the older participants in the study. Consequently, it is possible our selection criteria excluded potential FHAP+ participants most strongly predisposed to alcohol and drug problems because they would already be afflicted. The fact that significant increases in response initiation and response were still observed suggest that increases in these forms of impulsivity are strongly associated with family history of alcohol use disorders and may be persistent phenotypical characteristics of this population. However the lack of significant differences on consequence sensitivity impulsivity in the present sample and previous studies do not rule out a prominent role for consequence sensitivity impulsivity in risk for alcohol use disorders. As alcohol dependence is associated with increased consequence sensitivity impulsivity (Mitchell et al., 2005; Petry, 2001), it is possible that increased consequence sensitivity impulsivity may be increased in individuals more strongly predisposed to alcohol and drug use disorders (individuals who may have been excluded from this study because of the age group recruited). For instance, consequence sensitivity may be increased in individuals with more serious and denser family histories of alcohol use disorders or additional risk factors such as high behavioral undercontrol (Acheson et al., in press; Sher et al., 2004; Sher and Trull, 1994). Alternatively, it is possible that increased consequence sensitivity impulsivity may develop as alcohol and other substance use disorders progress.
It is possible that increased response initiation and response inhibition impulsivity relates to risk for alcohol use disorders by an association with the trait of urgency, or the tendency to act rashly when experiencing extremely negative or positive moods (Billieux et al., 2010; Dick et al., 2010). Urgency was one of four distinct impulsivity-related traits identified from a factor analysis performed on multiple self-report measures of personality and impulsivity administered to a large sample of undergraduate students (Whiteside and Lynam, 2001), and subsequent research has found that high levels of self-reported urgency are associated alcohol and drug use disorders (Verdejo-Garcia et al., 2007; Whiteside and Lynam, 2003). It has been proposed that high response initiation and inhibition impulsivity may underlie urgency (Bechara and Van Der Linden, 2005), and there is emerging empirical support for this assertion (Billieux et al., 2010; Gay et al., 2008). Although we observed no group differences in self-reported impulsivity (BIS-11), it is possible we would have detected group differences in self-reported urgency had we administered the full UPPS Impulsive Behavior Scale (Whiteside and Lynam, 2001). Therefore, high response initiation and response inhibition impulsivity may increase risk for alcohol use disorders by making individuals more emotionally reactive and thereby making alcohol misuse more likely. Alternatively, high response initiation and response inhibition impulsivity may be less directly related to problem alcohol use and instead contribute to the general, relatively subtle cognitive impairments associated with FHA (Corral et al., 2003; Deckel, 1999; Stevens et al., 2003), and these cognitive impairments may be primarily responsible for biasing these individuals towards alcohol and drug use disorders as well other maladaptive behaviors. More research is needed, particularly in at risk and alcohol and drug abusing populations, to better understand relationships between response initiation and inhibition impulsivity, urgency, other behavioral and self-report measures of impulsivity, and alcohol and drug use disorders.
Table 3.
Alcohol and Drug Use Summary
| FHAP− | FHAP+ | |
|---|---|---|
| Current alcohol and drug use | ||
| Alcohol (Mean ± SD; drinks/week) | 11.9 ± 8.1 | 12.4 ± 9.2 |
| Cigarettes (n >2 cigarettes/day) | 8 | 10 |
| Marijuana (n >0.5/week) | 0 | 2 |
| Lifetime drug use (n ever used) | ||
| Marijuana (n ever used) | 20 | 27 |
| Stimulants | 3 | 8 |
| Opiates | 14 | 18 |
| Benzodiazepines | 4 | 5 |
Acknowledgments
Role of Funding Source
Funding for this study was provided by the National Institutes of Health, R01-AA12046, R01-AA014988, and T32-AA00765. These funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
This research was supported by grants from the NIH/NIAAA: R01-AA12046, R01-AA014988, and T32-AA00765. Samantha John, Jessica Harrison, Dina Chaviara, and Sharon Cates provided excellent technical assistance.
Footnotes
Contributors
Authors Dougherty, Richard and Mathias designed the study and wrote the protocol. Author Acheson undertook the statistical analysis and wrote the manuscript with input from the coauthors. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2011.01507.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Billieux J, Gay P, Rochat L, Van der Linden M. The role of urgency and its underlying psychological mechanisms in problematic behaviours. Behav Res Ther. 2010;48:1085–1096. doi: 10.1016/j.brat.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Lejuez CW, Daughters SB, Zachary Rosenthal M, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clin Psych Rev. 2005;25:790–812. doi: 10.1016/j.cpr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: cross fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Corporation P. Psychological Coorporation, Wechsler Abbreviated Scale of Intelligence (WASI) Manual. Harcourt Brace and Company; San Antonio, TX: 1999. [Google Scholar]
- Corral M, Holguin SR, Cadaveira F. Neuropsychological characteristics of young children from high-density alcoholism families: a three-year follow-up. J Stud Alcohol. 2003;64:195–199. doi: 10.15288/jsa.2003.64.195. [DOI] [PubMed] [Google Scholar]
- Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav Brain Res. 2002;136:349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- Deckel AW. Tests of executive functioning predict scores on the MacAndrew Alcoholism Scale. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:209–223. doi: 10.1016/s0278-5846(98)00108-0. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, Swann AC. Behavioral impulsivity paradigms: a comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psychol Psychiatry. 2003a;44:1145–1157. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Huckabee HC, Moeller FG, Swann AC. Laboratory measures of aggression and impulsivity in women with borderline personality disorder. Psychiatry Res. 1999;85:315–326. doi: 10.1016/s0165-1781(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96:111–120. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW. Immediate and delayed memory tasks: a computerized behavioral measure of memory, attention, and impulsivity. Behav Res Methods Instrum Comput. 2002;34:391–398. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW, Dawes MA, Bradley DM, Morgan CJ, Badawy AA. The effects of alcohol on laboratory-measured impulsivity after L: -Tryptophan depletion or loading. Psychopharmacology (Berl) 2007;193:137–150. doi: 10.1007/s00213-007-0763-6. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW, Swann AC. The conceptualization and role of impulsivity: bipolar disorder and substance abuse. Psychiatr Times. 2005a;XXII:32–35. [Google Scholar]
- Dougherty DM, Mathias CW, Marsh-Richard DM, Furr RM, Nouvion SO, Dawes MA. Disinctions in behavioral impulsivity: implications for substance abuse research. Addict Disord Their Treat. 2009;8:61–73. doi: 10.1097/ADT.0b013e318172e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM. Laboratory measures of impulsivity. In: Coccaro EF, editor. Aggression: Assessment and Treatment Medical Psychiatric Series No 22. Marcel Dekker Publishers; New York: 2003b. [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behav Res Methods. 2005b;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Moeller FG, Swann AC. Suicidal behaviors and drug abuse: impulsivity and its assessment. Drug Alcohol Depend. 2004;76(Suppl):S93–S105. doi: 10.1016/j.drugalcdep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kleinman I, Pihl RO. The lifetime prevalence of psychopathology in men with multigenerational family histories of alcoholism. J Nerv Ment Dis. 1990;178:500–504. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; N.Y: 2001. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version, Non-patient Edition (SCID-I/NP) [Google Scholar]
- Gay P, Rochat L, Billieux J, d’Acremont M, Van der Linden M. Heterogeneous inhibition processes involved in different facets of self-reported impulsivity: evidence from a community sample. Acta Psychol (Amst) 2008;129:332–339. doi: 10.1016/j.actpsy.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University; New Haven, CT: 1975. p. 22. [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol Med. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Merikangas KR. The genetic epidemiology of alcoholism. Psychol Med. 1990;20:11–22. doi: 10.1017/s0033291700013192. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Indiv Differ. 2006;40:305–315. [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Avila C, Ferre F, Jimenez-Arriero MA, Ponce G, Palomo T. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res. 2008;32:1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Jr, Sorocco KH, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcohol Clin Exp Res. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Genetics and the risk for alcoholism. JAMA. 1985;254:2614–2617. [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann NY Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Ann Rev Clin Psychol. 2004;22:1–22. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. J Abnorm Psychol. 2002;111:124–133. [PubMed] [Google Scholar]
- Stevens MC, Kaplan RF, Hesselbrock VM. Executive-cognitive functioning in the development of antisocial personality disorder. Addict Behav. 2003;28:285–300. doi: 10.1016/s0306-4603(01)00232-5. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 2007;91:213–219. doi: 10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Indiv Differ. 2001;30:669–689. [Google Scholar]
- Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS Impulsive Behavior Scale. Exp Clin Psychopharmacol. 2003;11:210–217. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]