Abstract
Patients with peripheral arterial disease (PAD) have lower functional capacity and worse clinical outcomes than age and gender matched patients. Few data exist on the relationship of PAD with functional and clinical outcomes in heart failure (HF) patients. We sought to compare HF patients with and without PAD for baseline functional capacity, response to exercise training, and clinical outcomes. HF-ACTION was a randomized controlled trial comparing usual care to structured exercise training plus usual care in HF patients with an ejection fraction ≤ 35% and NYHA class II – IV heart failure symptoms. Cardiopulmonary exercise (CPX) testing occurred at enrollment, 3 months, and 1 year. Clinical follow-up occurred up to 4 years. Of the 2331 HF-ACTION patients, 157 (6.8%) had PAD. At baseline, HF patients with PAD had a lower exercise duration (8.0 vs. 9.8 minutes, p<0.001), lower peak oxygen consumption (VO2) (12.5 vs. 14.6 mL/kg/min, p<0.001), and shorter six minute walking distance (306 vs. 371 meters, p<0.001) compared to HF patients without PAD. At three months, HF patients with PAD had less improvement on CPX testing [exercise duration (0.5 vs. 1.1 minutes; p=0.002) and peak VO2 (mean change; 0.1 vs. 0.6 mL/kg/min; p=0.04)] compared to HF patients without PAD. PAD was an independent predictor of all-cause death or hospitalization [hazard ratio (95% CI); 1.31 (1.06 – 1.62), p=0.011]. PAD patients with HF have depressed baseline exercise capacity and decreased response to exercise training. In conclusion, PAD is an independent predictor of all-cause death or hospitalization in HF patients.
Keywords: Peripheral Arterial Disease, Heart Failure, Exercise Training
Evaluation of the influence of PAD on HF patients is warranted to better understand the response to exercise training and clinical outcomes in patients with these disease states. We hypothesized that the presence of PAD in HF patients would be associated with worse baseline exercise capacity, a limited response to exercise training in an exercise training study, more hospitalizations, and a higher morbidity and mortality than HF patients without PAD.
Methods
The design of the HF-ACTION clinical trial has been described previously.1 Briefly, HF-ACTION was a multinational, randomized controlled trial investigating outpatients with a left ventricular (LV) ejection fraction (EF) ≤ 35% and New York Heart Association (NYHA) class II to IV heart failure symptoms. These patients were randomized to a usual care plus structured exercise training group or a usual care group. Enrollment occurred from 2003 to 2007 in the United States, Canada, and France. Patients were excluded if they had major co-morbidities or limitations that would interfere with exercise training, recent (≤ 6 weeks) or planned major cardiovascular events or procedures, performed regular exercise training, or used devices that limited the ability to achieve target heart rates. The study protocol was reviewed and approved by the institutional review board or ethics committee for each enrolling center and by the coordinating center's institutional review board. All patients provided written informed consent prior to randomization.
All patients were treated with evidence-based medical therapies for heart failure at enrollment. Enrolled patients were to undergo a baseline cardiopulmonary exercise (CPX) test and eligible patients were then randomized in a 1:1 fashion using permuted block randomization which was stratified by clinical site and heart failure etiology (ischemic vs. non-ischemic). Patients with a history of PAD as documented on the case report form were included in the PAD cohort. If patients did not have a diagnosis of PAD, they were included in the `no PAD' cohort.
Patients randomized to the usual care plus exercise training group participated in a supervised exercise program with a goal of 36 sessions over 3 months. Full details of the exercise training protocol have been reported.2 Patients were to begin home-based exercise after completing 18 supervised sessions and were to fully transition to home exercise after 36 supervised sessions. Patients in the usual care group were not provided with formal exercise instructions. All patients received a detailed HF educational booklet at the time of enrollment, including information on medications, fluid management, symptom exacerbation, sodium intake, and activity level of 30 minutes of moderate-intensity activity on most days of the week, consistent with the guidelines from the American College of Cardiology and the American Heart Association.3
All patients were asked to return for clinic visits every 3 months for the first 2 years and yearly thereafter for up to 4 years. CPX testing and a 6-minute walk test were to be performed at the 3-, 12-, and 24-month follow-up visits, while the 6-minute walk test was also to be performed at the 3-year and final visits. Patients made their final visit at the end of the study follow-up period or at 4 years. For patients lost to follow-up, searches of the Social Security Death Index and the National Death Index were performed to assess whether any of these patients had died during the follow-up period.
The primary end point for this study was a composite of all-cause death or all-cause hospitalization. All-cause death, the composite of cardiovascular death or cardiovascular hospitalization, and the composite of cardiovascular death or heart failure hospitalization were secondary endpoints. In addition, data were collected to measure the change from baseline in peak oxygen consumption (peak VO2) on a progressive exercise test to exhaustion at 3 months and 1 year and change in distance from baseline in the 6-minute walk test at 3 months and 1 year. A clinical end point committee adjudicated deaths and many cardiovascular hospitalizations for each patient.
Statistical analyses were performed by the coordinating center (Duke Clinical Research Institute, Durham, North Carolina) using SAS software version 8.2 (SAS Institute Inc, Cary, North Carolina). Baseline patient characteristics were summarized using medians for continuous variables and percentages for categorical variables. All statistical tests were 2-tailed. Cumulative event rates were calculated using the Kaplan-Meier method and a plot of unadjusted Kaplan-Meier rates for the primary endpoint by PAD status was produced.
Cox proportional hazards modeling was used to statistically compare the two study groups with respect to the time until the first occurrence of either component of the primary composite end point and the secondary time-to-event outcomes, adjusting for covariates identified in models developed by the process described below. Relative risks were expressed as hazard ratios (HR) with 95% confidence intervals (C.I.) and were calculated using the Cox proportional hazards model.
The baseline predictors of the primary and secondary outcomes were objectively selected using a stepwise variable selection based on a bootstrap-backward selection process (see Supplementary Appendix). Multiple imputation was used to replace missing data for covariates. A mixed model analysis was used to estimate changes from baseline to specified repeated measures time points in physiologic variables (6-minute walk (meters), CPX exercise time (minutes), and peak VO2 (mL/kg/min)) at 3 months and 12 months among PAD and non-PAD patients. Comparisons of change at these time points between the two groups were made as contrasts within the model.
Results
Of the 2331 HF-ACTION patients, 2320 (99.5%) patients had baseline data on PAD and 157 (6.8%) patients had a diagnosis of PAD. Table 1 demonstrates the baseline demographic characteristics, exercise capacity, and randomized treatment assignment of the PAD and non-PAD patients. The mean modeled change from baseline in peak VO2, exercise duration and the 6-minute walk distance at 3 months are presented in Table 2.
Table 1.
Baseline Demographic and Clinical Characteristics, Exercise Testing Results, and Randomized Treatment Assignments
| Patient Characteristics | All HF Patients (N=2320) | HF with PAD (N=157) | HF no PAD (N=2163) |
|---|---|---|---|
| Age (years, median) | 59 | 67 | 59 |
| Female Sex | 28% | 10% | 30% |
| United States | 89% | 89% | 89% |
| Canada | 8% | 10% | 8% |
| France | 3% | 1% | 3% |
| Body Mass Index (kg/m2, median) | 30 | 28 | 30 |
| Ischemic Etiology of HF | 51% | 83% | 49% |
| Current Angina Pectoris Class | |||
| None | 84% | 79% | 84% |
| I | 9% | 9% | 9% |
| II–IV | 8% | 12% | 7% |
| Moderate to Severe or Severe Mitral Regurgitation | 11% | 12% | 11% |
| Rest Electrocardiographic Ventricular Conduction | |||
| Normal | 43% | 35% | 44% |
| Left Bundle Branch Block | 17% | 17% | 17% |
| Right Bundle Branch Block | 4% | 6% | 4% |
| Intraventricular Conduction Delay | 13% | 13% | 13% |
| Paced | 23% | 29% | 23% |
| Creatinine in mg/dL (median) | 1.2 | 1.4 | 1.2 |
| Baseline Beta Blocker Dose Using Carvedilol Equivalent (mg/day, median) | 27 | 31 | 27 |
| Baseline Loop Diuretic Dose Using Furosemide Equivalent (mg/day, median) | 40 | 40 | 40 |
| KCCQ Overall Summary Score at Baseline (median) | 68 | 65 | 68 |
| Smoking Status | |||
| Never | 37% | 19% | 39% |
| Current | 17% | 28% | 16% |
| Past | 46% | 53% | 46% |
| Left Ventricular Ejection Fraction (%, median) | 25% | 25% | 25% |
| History of Hypertension | 60% | 77% | 59% |
| History of Diabetes Mellitus | 32% | 45% | 31% |
| Prior Myocardial Infarction | 42% | 68% | 40% |
| Prior Chronic Obstructive Pulmonary Disease | 11% | 30% | 9% |
| Prior Stroke | 10% | 17% | 10% |
| Baseline Exercise Testing Medians (Q1, Q3) | |||
| Exercise Duration on Cardiopulmonary Exercise Test (minutes) | 9.6 (6.9, 12.0) | 8.0 (5.7, 10.0) | 9.8 (7.0, 12.1) |
| Peak Oxygen Consumption (mL/kg/min) | 14.4 (11.4, 17.7) | 12.5 (10.0, 15.0) | 14.6 (11.6, 17.8) |
| 6 Minute Walk Distance (meters) | 370 (299, 435) | 306 (235, 393) | 375 (302, 438) |
| Randomized Treatment Assignment | |||
| Usual Care | 50% | 54% | 50% |
| Exercise Training + Usual Care | 50% | 46% | 50% |
Table 2.
Change in Exercise Testing Results from Baseline to 3 Months
| Mean Modeled Change in Exercise Testing from Baseline to 3 Months Means (95% CI) | HF with PAD (N=157) | HF no PAD (N=2163) | p value |
|---|---|---|---|
| Exercise Duration on Cardiopulmonary Exercise Test (minutes) | 0.5 (0.1, 0.9) | 1.1 (1.0, 1.2) | 0.002 |
| Peak Oxygen Consumption (mL/kg/min) | 0.1 (−0.4, 0.5) | 0.6 (0.4, 0.7) | 0.04 |
| 6 Minute Walk Distance (meters) | 10.1 (−3.0, 23.1) | 14.4 (11.1, 17.7) | 0.53 |
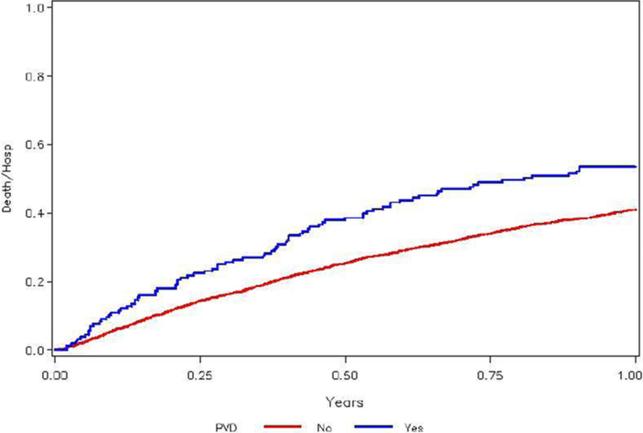
Data for the primary and secondary endpoints are presented in Table 3. The one-year Kaplan-Meier rate of the primary endpoint (all-cause death or all-cause hospitalization) was 53.5% vs. 41.0% of HF patients with and without PAD, respectively. PAD remained an independent predictor of the primary endpoint after adjustment (HR (95% C.I.): 1.31 (1.06 –1.62); p=0.011; Figure 1). The one-year Kaplan-Meier rate of the secondary endpoint of all-cause death was 11% vs. 4.5% of HF patients with and without PAD, respectively. However, PAD only trended towards significance for this endpoint after adjustment (HR (95% C.I.) = 1.36 (0.98 – 1.91); p=0.07). Incidence of the composite secondary endpoints cardiovascular (CV) death / CV hospitalization and CV death / HF hospitalization was also increased among PAD patients, with PAD making a significant contribution to previously defined adjusted models for CV death / CV hospitalization (HR (95% C.I.) = 1.41 (1.12 – 1.76); p=0.003) and CV death / HF hospitalization (HR (95% C.I.) = 1.72 (1.31 – 2.26); p< 0.001).
Table 3.
Unadjusted Kaplan-Meier Event Rates, Unadjusted and Adjusted Effect of Peripheral Arterial Disease for Primary and Secondary Endpoints.
| Kaplan-Meier 1 year Rate Estimates | PAD Effect in Unadjusted Cox Proportional Hazards Model | PAD Effect in Adjusted Cox Proportional Hazards Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | No PAD | PAD | Wald Chi-Square | P-Value | HR (95% CI) | Wald Chi-Square | P-Value | HR (95% CI) |
| All-Cause Death or All-Cause Hospitalization | 0.410 | 0.535 | 17.9 | <0.001 | 1.49 (1.24–1.79) | 6.4 | 0.011 | 1.31 (1.06–1.62) |
| All-Cause Death | 0.045 | 0.110 | 21.9 | <0.001 | 2.07 (1.53–2.81) | 3.3 | 0.070 | 1.36 (0.98–1.91) |
| Cardiovascular Death or Cardiovascular Hospitalization | 0.318 | 0.414 | 14.4 | <0.001 | 1.47 (1.20–1.79) | 8.8 | 0.003 | 1.41 (1.12–1.76) |
| Cardiovascular Death or Heart Failure Hospitalization | 0.149 | 0.210 | 21.5 | <0.001 | 1.76 (1.39–2.24) | 15.2 | <0.001 | 1.72 (1.31–2.26) |
Figure 1. Unadjusted Kaplan-Meier Curve Demonstrating Time to All-Cause Death or All-Cause Hospitalization by Presence or Absence of PAD.
Unadjusted Kaplan-Meier curve that demonstrates the time to all-cause death or all-cause hospitalization based on presence or absence of PAD.
Discussion
This analysis of PAD patients in the HF ACTION study identifies several important findings. The first is that patients with HF and PAD have decreased baseline functional capacity when compared to HF patients without PAD. The second is that patients with HF and PAD may not obtain the same benefits from structured exercise training afforded routine HF patients without PAD. Finally, patients with HF and PAD have substantially worse clinical outcomes when compared to patients without PAD. We believe these findings could have important clinical and health policy implications.
The decreased baseline functional capacity in HF patients with PAD is not surprising as these patients were more likely to be older, current smokers, to have an ischemic etiology of HF, and more likely to have a history of diabetes, hypertension, MI, and stroke. Multivariable adjustment was performed to control for these differences in baseline characteristics, and PAD remained an independent predictor of all-cause mortality or hospitalization in this study population. This is similar to a recent sub-study from the Beta-Blocker Evaluation of Survival Trial (BEST) dataset that showed that PAD was associated with increased mortality and hospitalization in a propensity-matched cohort of chronic HF patients.4
Importantly, we evaluated the response to exercise training in HF patients with PAD. Currently, supervised exercised training is a class I recommendation in PAD patients, based on moderate sized randomized controlled trials to increase walking distance.5–7 In HF publications, prior studies have observed that a lack of improvement in exercise capacity after exercise training has strong prognostic significance for clinical events.8, 9 To date no study has described the functional limitations in HF patients with PAD and the lack of functional improvement after exercise training in this population. The current study shows that a diagnosis of PAD is associated not only with an elevated risk of mortality and hospitalization in HF, but also with limited functional benefit after exercise training in this population.
The mechanism leading to the association of PAD with poorer outcomes and lack of improvement with exercise training in a large HF population is unknown. The lack of improvement with exercise training has been separately described in the PAD and HF literature, and it may relate to skeletal muscle abnormalities or a mixture of central and peripheral hemodynamic abnormalities.10–16 There are unfortunately no data on skeletal muscle changes or hemodynamic changes with exercise in this study. The association of PAD with poor outcomes may be related to more extensive atherosclerosis or a higher frequency of myocardial infarction or sudden cardiac death in this subpopulation of the HF-ACTION cohort. Additionally, patients with PAD are known to undergo more endovascular revascularization procedures than similar patients without PAD, and the associated hospitalizations and complications could contribute to the findings in our study. Unfortunately, our study was not designed to interrogate these factors.
The standard of care for management of HF patients consists of lifestyle modification, utilization of evidence-based medications, and a walking program at home.3 The current study finds that, despite standard HF care, patients who carry a diagnosis of PAD do poorly. Approximately 50% of patients with a confirmed diagnosis of PAD are asymptomatic, thus the prevalence of PAD in this HF population is likely underestimated.
Taken together, these facts imply that simple diagnostic testing such as ankle-brachial index (ABI) testing may identify HF patients at higher risk for poorer outcomes. When PAD is identified, the medical treatment of PAD in HF patients is limited due to the current FDA black box warning against the use of cilostazol, a phosphodiesterase inhibitor proven to mildly improve walking distance in PAD patients. Further investigation into the influence of other co-morbid conditions, use of disease-specific medications, and evaluation of invasive treatment options for PAD should be considered in this population.
Several limitations of this study must be recognized. This is a post-hoc analysis of a randomized controlled trial, and the representation of patients may not be generalizable to a “real world” HF population. We relied on an existing diagnosis of PAD rather than on a screening diagnostic test, such as ankle-brachial index testing, for inclusion in the PAD cohort. Since PAD is often asymptomatic and under-diagnosed, the prevalence of PAD may be underestimated in this study population, possibly leading to overestimated hazard ratios for PAD. Furthermore, the extent of PAD was not defined in our cohort. Missing data may have biased the follow-up exercise testing results. Unmeasured confounding factors may have affected the results of the Cox modeling as well as the results on the three-month change in exercise testing.
Supplementary Material
Acknowledgments
NIH/NHLBI Information: This study was funded by grants from the National Heart, Lung, and Blood Institute: 5U01HL063747 to Dr O'Connor at Duke University and Duke Clinical Research Institute (coordinating center).
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT00047437
References
- 1.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA, Pina IL. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): Design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MI, Aronow WS, Criqui MH, Aban I, Love TE, Eichhorn EJ, Ahmed A. Effects of peripheral arterial disease on outcomes in advanced chronic systolic heart failure: A propensity-matched study. Circ Heart Fail. 2009;3:118–124. doi: 10.1161/CIRCHEARTFAILURE.109.866558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 6.Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–609. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 7.Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000:CD000990. doi: 10.1002/14651858.CD000990. [DOI] [PubMed] [Google Scholar]
- 8.Tabet JY, Meurin P, Beauvais F, Weber H, Renaud N, Thabut G, Cohen-Solal A, Logeart D, Ben Driss A. Absence of exercise capacity improvement after exercise training program: A strong prognostic factor in patients with chronic heart failure. Circ Heart Fail. 2008;1:220–226. doi: 10.1161/CIRCHEARTFAILURE.108.775460. [DOI] [PubMed] [Google Scholar]
- 9.Katzel LI, Sorkin J, Bradham D, Gardner AW. Comorbidities and the entry of patients with peripheral arterial disease into an exercise rehabilitation program. J Cardiopulm Rehabil. 2000;20:165–171. doi: 10.1097/00008483-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson KR, Duscha BD, Hranitzky PM, Kraus WE. Chronic heart failure and exercise intolerance: The hemodynamic paradox. Curr Cardiol Rev. 2008;4:92–100. doi: 10.2174/157340308784245757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges LD, Sandercock GR, Das SK, Brodie DA. Randomized controlled trial of supervised exercise to evaluate changes in cardiac function in patients with peripheral atherosclerotic disease. Clin Physiol Funct Imaging. 2008;28:32–37. doi: 10.1111/j.1475-097X.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 12.Duscha BD, Schulze PC, Robbins JL, Forman DE. Implications of chronic heart failure on peripheral vasculature and skeletal muscle before and after exercise training. Heart Fail Rev. 2008;13:21–37. doi: 10.1007/s10741-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 13.Arad M, Adler Y, Koren-Morag N, Natanzon S, Sela BA, Ben Dov I, Shechter M, Schwammenthal E, Freimark D. Exercise training in advanced heart failure patients: Discordance between improved exercise tolerance and unchanged NT-proBNP levels. Int J Cardiol. 2008;126:114–119. doi: 10.1016/j.ijcard.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Hannukainen JC, Janatuinen T, Toikka JO, Jarvisalo MJ, Heinonen OJ, Kapanen J, Nagren K, Nuutila P, Kujala UM, Kaprio J, Knuuti J, Kalliokoski KK. Myocardial and peripheral vascular functional adaptation to exercise training. Scand J Med Sci Sports. 2007;17:139–147. doi: 10.1111/j.1600-0838.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- 15.Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39:1170–1174. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- 16.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.