Abstract
A common clinical presentation of Plasmodium falciparum is parasitemia complicated by an encephalopathy for which other explanations cannot be found, termed cerebral malaria—an important cause of death in young children in endemic areas. Our objective was to study hepatic histopathology in Malawian children with fatal encephalopathy, with and without P falciparum parasitaemia, in order to assess the contributions of severe malaria. We report autopsy results from a series of 87 Malawian children who died between 1996 and 2008. Among 75 cases with P falciparum parasitaemia, 51 had intracerebral sequestered parasites, while 24 without sequestered parasites had other causes of death revealed by autopsy including 4 patients with clinicopathological findings which may represent Reye’s Syndrome. Hepatic histology in parasitaemic cases revealed very limited sequestration of parasites in hepatic sinusoids, even in cases with extensive sequestration elsewhere, but increased numbers of hemozoin-laden Kupffer cells were invariably present with a strong association with histological evidence of cerebral malaria by quantitative analysis. Of 12 patients who were consistently aparasitaemic during their fatal illness, 5 had clinicopathological findings which may represent Reye’s Syndrome. Hepatic sequestration of parasitized erythrocytes is not a feature of fatal malaria in Malawian children, and there is no structural damage in the liver. Reye’s syndrome may be an important cause of fatal encephalopathy in children in Malawi with and without peripheral parasitemia and warrants close scrutiny of aspirin use in malaria endemic areas.
Introduction
Malaria is one of the world’s major infectious causes of morbidity and mortality. It can cause severe disease often manifested by coma and seizures, designated cerebral malaria (CM). The liver histopathology in malaria has not been systematically studied in African children. Previous investigations have been in adults from the western world or India [1–4]. Jaundice associated with malaria infections, a relatively common clinical finding in adults in India has been attributed to ‘malarial hepatitis’[1–5]. Spitz reported parasitized red blood cells (PRBCs) in liver sinusoids and infrequent hepatocyte necrosis in fatal malaria in adult American soldiers in World War II [6].
We studied the histological features of the liver in Malawian children dying of encephalopathic syndromes. We included a quantitative assessment both of sequestered parasites and of the size and distribution of granules of hemozoin pigment, a by-product of the consumption of hemoglobin by malaria parasites. Our aim was to assess hepatic changes associated with severe malaria and describe pathological changes in pediatric comatose patients in this context. We report the histopathology of the liver in Malawian children who died of an encephalopathic illness between 1996 and 2008 in Blantyre, Malawi.
Materials and Methods
This study was reviewed and approved by The University of Malawi College of Medicine Research and Ethics Committee, and by the ethics committees of the University of Liverpool and Michigan State University. The patients were all admitted to the Blantyre Malaria Project as part of an ongoing study of the clinicopathological features of cerebral malaria. All patients were evaluated with comprehensive historical, physical and laboratory examinations. Patients with documented parasitemia were treated with intravenous quinine. When clinically indicated, patients were given antipyretics, glucose, anticonvulsive drugs, crystalloid infusions, antibiotics, blood transfusion and other supportive therapies. No salicylate containing compounds were used in the hospital. A history of drug use including salicylates was sought. A full post-mortem examination was performed by a pathologist and all tissue samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 3 – 6 microns and stained with hematoxylin and eosin. Histology samples for the present study (see details below) were evaluated by two pathologists (RW and DM). Liver slides were also evaluated by a hepatopathology specialist (MMY).
Hepatic histology was available and examined in all patients who had an autopsy performed and was interpreted in the context of the full histological analysis of cases including brain, heart, lung, genitourinary system, spleen/lymph nodes, gastrointestinal tract, endocrine organs, and soft tissue. Cases were classified as cerebral malaria (CM) if the patient met the clinical case definition of cerebral malaria (Blantyre Coma Score ≤ 2, parasitemia, and no other cause of coma including meningitis, hypoglycemia, and post-ictal state) and were found to have parasite sequestration in the brain at autopsy without other evidence of anatomic cause of death. Cases were classified as ‘Other’ if they met the clinical case definition of cerebral malaria, but had no evidence of parasite sequestration in the brain at autopsy, and were found to have another anatomic cause of death. Cases were classified as ‘Aparasitemic’ if they presented in a coma without parasitemia (i.e., did not meet the full clinical case definition of cerebral malaria). Cases were considered to have clinicopathological findings which may represent Reye’s Syndrome if the liver histology showed microvesicular fatty liver (with at least 50% of fatty hepatocytes showing very fine or small fat droplets rather than a single cytoplasm-filling or ‘macrovesicular’ fat droplet), with little or no hepatic inflammation or necrosis.
Liver histopathological changes were graded as absent (0) or, if present, on a scale of 1 to 4 and included the presence of parasites (i.e., intraerythrocytic circulating parasites and sequestered parasites), inflammation, necrosis, steatosis, degenerative changes, and Kupfer cell patterns.
A subset of patients (n = 47) were analyzed quantitatively for the size and area occupied by pigment within the liver; these included 20 CM, 21 Other, and 6 Aparasitemic patients. Histological slides of liver (hematoxylin and eosin stained 6 um sections) were polarized using a two filter system (U-ANT and U-POT T2 Filters) on a standard light microscope (BX41TF Microscope, Olympus Corporation, Tokyo, Japan). For the liver parenchyma with central vein (centered), three random 200x fields were photographed under ultra bright conditions (Light intensity = 6; Exposure = 106 ms) using a digital microscope camera (Q Color 3, Olympus America, Inc.). For the liver portal areas with a portal triad (centered), three random 400x fields were photographed under ultra bright conditions (Light intensity = 6; Exposure = 233 ms) with the same camera. Within ImageJ, each image was split into three channels and the red channel was analyzed because the pigment under the above conditions appears primarily bright red to white. An automated macro within ImageJ was written which performed the following functions for each image: a) set a standard threshold for each image (central vein = 46,255, portal triad = 57, 255), b) partitioned an area of the field, c) analyzed particles for size and area, d) repeated b & c 9 times for a total of 10 different partitions (with overlap). The thirty measurements (i.e., 3 images × 10 partitions/image) for size of particles and area occupied by particles of each case were averaged and a standard deviation was calculated. All measurements and calculations were carried out blinded to the final diagnosis. A logistic regression model with diagnostic category (CM vs. non-CM) as the outcome was constructed to determine the association of the average area of polarized pigment in the parenchyma around the central vein including platelet count (quartiles), the duration of fever before admission, and the hematocrit as confounders.
Plasma salicylate assays were performed by spectrophotometry on Beckman Synchron LX or CX chemistry analyzers using the timed endpoint method where salicylate hydrozylase catalyzes the conversion of salicylate and NADH to catechol and NAD in the presence of oxygen.
Results
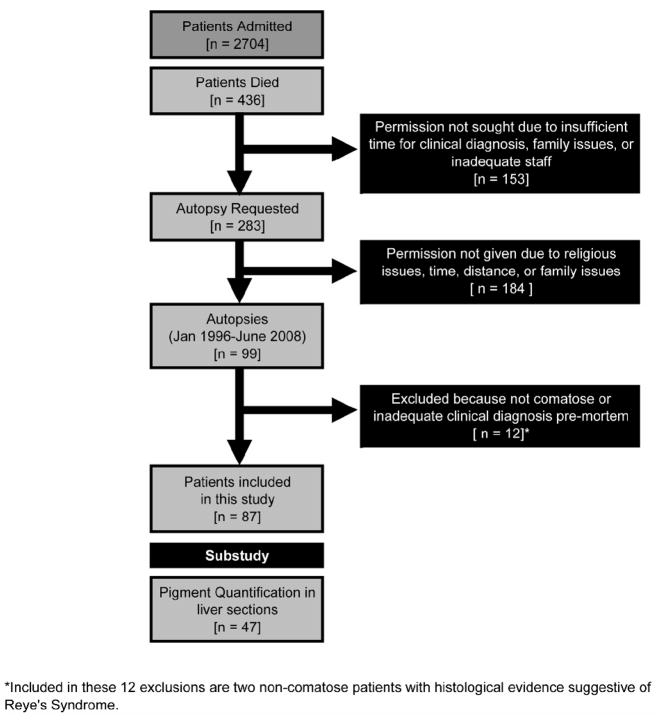
We conducted autopsies on 99 children between 6 and 156 months of age (Figure 1). Autopsies were performed from 1.5 to 17.5 hours after death. We excluded 12 cases from further analysis because the patients either had an inadequate clinical diagnosis or were never comatose, and we here report on the remaining 87 cases. Of these, 75 had P falciparum parasitaemia on admission to hospital and fulfilled the clinical criteria defining cerebral malaria, and the other 12 had been comatose but consistently aparasitaemic during the illness. At autopsy histopathological examination revealed sequestered parasites within cerebral microvasculature in 51 cases, all of whom were parasitaemic during life. In these cases no alternative cause of death was revealed by autopsy, and we considered the final diagnosis to be fatal CM. In a further 24 cases with parasitaemia, there were no sequestered parasites in the brain and a cause of death other than malaria was found (classified as ‘Other’) with the exception of 4 patients which had only clinicopathological findings which may represent Reye’s Syndrome. Among the 12 without parasitaemia (‘Aparasitemic’ group), none had intracerebral sequestration of P falciparum, and in all of these another anatomic cause of death was found with the exception of 5 which had only clinicopathological findings which may represent Reye’s Syndrome. A summary of the clinical and laboratory features of the three diagnostic groups is presented in Table 1.
Figure 1.
Study Design. Between 1996 and 2008 we admitted 2704 children to the Malaria Research Ward of Queen Elizabeth Central Hospital in Blantyre, Malawi. Deaths occurred in 436 patients (16.1%) and in 283 cases we requested permission for autopsy, employing detailed discussion directed by Malawian nurses and physicians. The decision to request autopsy was dependent upon a) the presence of appropriate parents or guardians, b) the family structure and religious affiliation, c) and the availability of the pathologist and adequate support team. Approval for autopsy was given in 99 cases (35.0% consent rate). Of these, 12 cases were excluded from this analysis owing to an indeterminate clinical diagnosis or lack of coma at admission.
Table 1.
Characteristics of comatose patients included in this study (n = 87)
| Characteristic | Cerebral Malaria (n=51) | Comatose, Parasitemic, non-CM (n = 24) | Comatose, Aparasitemic, non-CM (n = 12] | p-value* | Suggested Reye’s Syndrome | p-value*** | ||
|---|---|---|---|---|---|---|---|---|
| Age (months, med, IQR) | 31 (22–77) | 30.6 (19.5 – 52.5) | 43.5 (11 – 79.5) | 0.7867 | 25 (19 – 51) | 0.5620 | ||
| Gender (% Male) | 47.1 | 62.5 | 50.0 | 0.4450 | 44.4 | 0.5880 | ||
| Duration of Coma (hours, med, IQR) | 32 (24–48) | 20.5 (13.5 – 37) | 72 (14 – 92) | 0.0821 | 28.5 (16 – 65) | 0.8765 | ||
| History of Fever (% Yes) | 96.1 | ≫ | 78.3 | ~ | 58.3 | 0.0010 | 77.8 | 0.1030 |
| Fever Duration (hours, mean, sd) | 57 (± 38) | ~ | 32 (± 32) | ≪ | 199 (± 213) | <0.0001 | 119 (± 68) | 0.0159 |
| History of Seizures (% Yes) | 68.6 | 75.0 | 45.5 | 0.2570 | 33.3 | 0.0520 | ||
| Seisure Duration (hours, med, IQR) | 6.5 (5 – 13) | 7 (5 – 13) | 9.5 (5 – 23) | 0.8073 | 7 (6 – 23) | 0.4588 | ||
| History of Aspirin (% Yes) | 25.5 | 20.8 | 25.0 | 0.9380 | 33.3 | 0.4490 | ||
| Temperature (°C, mean, sd) | 38.5 (± 1.3) | ≫ | 37.6 (± 2.1) | ~ | 37.7 (± 1.8) | 0.0587 | 36.8 (± 0.5) | 0.0005 |
| Pulse (bpm, mean, sd) | 151 (± 31) | 133 (± 38) | 139 (± 29 | 0.0849 | 148 (± 7) | 0.3959 | ||
| Blood Pressure (sys, med, IQR) | 103 (90 – 110) | 110 (90 – 120) | 94 (74 – 110) | 0.3497 | 90 [60 – 110) | 0.2026 | ||
| Respiration (bpm, med, IQR) | 48 (40–58) | 42 (36 – 53) | 44 (36 – 50) | 0.2011 | 42 (36 – 52) | 0.2164 | ||
| BMI (kg/m2, mean, sd) | 14.4 (± 2.3) | 15.2 (± 2.2) | 13.9 (± 2.2) | 0.1693 | 14.2 (± 0.9) | 0.4143 | ||
| Glucose (g/dL, mean, sd) | 5.2 (± 3.0) | ~ | 4.7 (± 4.7) | ≪ | 8.7 (± 4.4) | 0.0097 | 6.5 (± 2.0) | 0.1705 |
| Liver > 1cm below diaphragm? (% Yes) | 54.0 | 65.2 | 66.7 | 0.6020 | 44.4 | 0.4330 | ||
| Parasitemia (p/uL, med, IQR) | 81840 (11399–442705) | < | 4438 (436.5 – 93740) | 0** | 0.0018 | 0 (0-4716) | 0.0015 | |
| Hematocrit (%, mean, sd) | 20.9 (± 7.1) | ≪ | 29.8 (± 9.6) | ~ | 27.2 (± 11.9) | 0.0002 | 26.0 (± 3.7) | 0.0365 |
| White Blood Cell Count (K/uL, med, IQR) | 12.7 (9.7–17.1) | ~ | 14.1 (10.5 – 23.0) | ≪ | 19.3 (16.1 – 39.8) | 0.0323 | 18.0 (11.5 – 30.4) | 0.0797 |
| Platelet Count (K/uL, med, IQR) | 57 (35.5–89) | ≪ | 207 (45 – 276) | ~ | 350 (277 – 556) | 0.0001 | 350 (222 – 370) | 0.0009 |
| HIV status (% Positive) | 28.0 | 4.5 | 16.7 | 0.0600 | 22.2 | 0.5370 |
P-values represent Kruskal-Wallis tests (for medians), ANOVA (for means), and Fisher’s Exact (for proportions). For significant p-values in multiple comparisons, the results of Bonferroni (ANOVA) or Test for Trend (Kruskal-Wallis) are shown between columns to demonstrate equivalency (“~”) or significant differences (“≪” or “&Gt”)
The comatose, aparasitemic group, by definition, has a zero value for parasitemia at admission; therefore, the P-value shown in this table is for a Wilcoxan rank sum test between the cerebral malaria versus non-CM, parasitemic patients.
P-values are Reye’s vs. Cerebral Malaria and represent Wilcoxon Rank-Sum tests (for medians), T-test of means (for means), and Fisher’s Exact (for proportions).
Liver histopathology in parasitemic cases
All but one (50/51) of the cases in the ‘CM’ group showed variable, but usually extensive, Kupffer cell hyperplasia and accumulation of malaria pigment in enlarged Kupffer cells in the hepatic sinusoids (Figure 2A). Similar pigment-laden Kupffer cells were found in only 3 of 24 cases in the ‘Other’ group. The pigment was coarse, birefringent on polarizing microscopy, and consisted of generally larger granules than noted within Plasmodium trophozoites (Figure 2B). The histological features of all patients are summarized in Table 2.
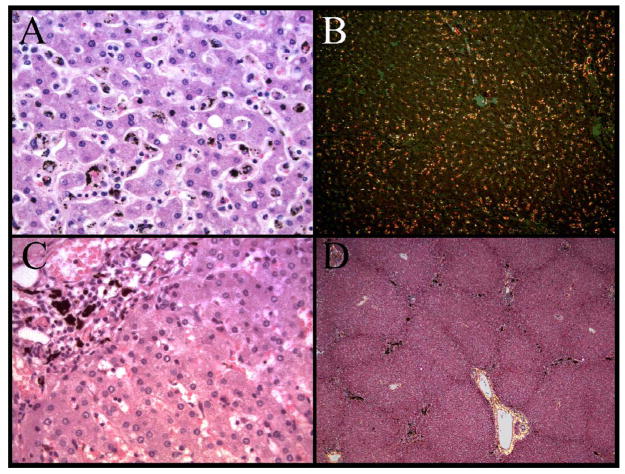
Figure 2.
The distribution of pigment within the liver of the autopsy cohort showed two distinct patterns. A diffuse, speckled pattern predominantly in the lobular parenchyma was present and associated with increased histiocytes within sinusoids (A). At low power, polarized microscopy reveals the extent of the histiocyte hyperplasia (B). Within portal triads, large globules of pigment were present in many cases (C). At low power, the lobular architecture and the increased pigment (present as black areas) in the portal triads is evident (D).
Table 2.
Summary of the histological findings of 87 patients.
| Histological Characteristic of Patients by Diagnosis | CM | Other* | Aparasitemic** | p-value*** | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | ||
| Parasitized Red Blood Cells | 21 | 19 | 7 | 3 | 21 | 3 | 0 | 0 | 12 | 0 | 0 | 0 | <0.0001 | |||
| Sequestration | 45 | 4 | 1 | 24 | 0 | 0 | 12 | 0 | 0 | 0.5570 | ||||||
| Lobular inflammation | 14 | 22 | 12 | 2 | 6 | 14 | 0 | 4 | 5 | 5 | 2 | 0 | 0.0420 | |||
| Portal Inflammation | 17 | 17 | 12 | 3 | 1 | 10 | 7 | 3 | 3 | 1 | 8 | 2 | 1 | 1 | 0 | 0.5270 |
| Necrosis | 45 | 4 | 1 | 19 | 4 | 1 | 9 | 2 | 1 | 0.3250 | ||||||
| Microvesicular Fat | 42 | 7 | 1 | 0 | 0 | 16 | 4 | 2 | 1 | 1 | 6 | 1 | 1 | 2 | 2 | 0.0080 |
| Macrovesicular Fat | 47 | 2 | 1 | 24 | 0 | 0 | 12 | 0 | 0 | 1.0000 | ||||||
| Mixed Fat | 48 | 2 | 0 | 0 | 0 | 20 | 0 | 0 | 2 | 2 | 10 | 1 | 0 | 0 | 1 | 0.0340 |
| Ballooning of Hepatocytes | 49 | 1 | 20 | 4 | 9 | 3 | 0.0090 | |||||||||
| Kupfer Cells Prominent | 0 | 0 | 3 | 21 | 26 | 3 | 7 | 9 | 4 | 1 | 4 | 4 | 3 | 1 | 0 | <0.0001 |
Includes 4 patients with Reye’s histology
Includes 5 patients with Reye’s histology
Fisher’s Exact P-value from a full contingency table (for first three categories) and for Reye’s vs. all other patients.
Hemozoin pigment was diffusely dispersed throughout the lobule in the parasitaemic cases, but was concentrated in the portal areas in those without parasitemia, suggesting movement or concentration of pigment to the portal areas when a malaria infection has resolved (figure 2C & 2D). Polarizing light microscopy revealed distinct patterns of pigment distribution including no pigment (Figure 3A & 3B), diffuse speckling (Figure 3C & 3D), or a mixture of speckling with increasing amounts of large globules within the portal triads (Figure 3E – 3H). The quantitative assessment of the polarized pigment within the liver demonstrated that the size of pigment granules and the area occupied by pigment in the parenchyma around the central veins were both larger in cerebral malaria patients than in non-CM. There were no differences in the size or area of pigment in the portal triads by diagnosis (Table 3). Each 0.1% increase in the area occupied by pigment in the lobules was significantly associated with the diagnosis of cerebral malaria (Odds Ratio = 1.57 95% CI [1.04 – 2.38], p-value = 0.033) when adjusted for haematocrit, fever duration, and platelet count.
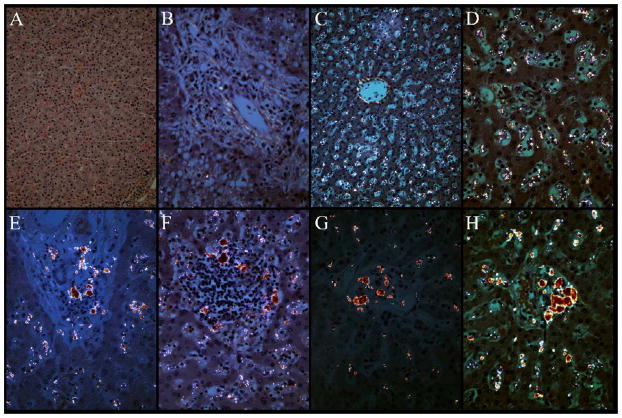
Figure 3.
The polarized images used for quantification of malaria pigment demonstrate several distinct reproducible patterns which include the following: no pigment evident in the lobular parenchyma (A) or portal triads (B); diffuse speckled pigment in the lobular parenchyma (C) and portal triads (D); large globules of pigment of increasing size and concentration in the portal triads (E – H). Globules of pigment were never seen in the lobular parenchyma (Panels A and C at 200x magnification; panels B, D, E–f at 400x magnification).
Table 3.
Quantification of polarized pigment compared between diagnostic groups
| Diagnosis in Parasitemic Patients | p-value* | |||
|---|---|---|---|---|
| Cerebral Malaria | Other | Aparasitemic | ||
|
Lobule Pigment | ||||
| Size of polarized pigment | 45 ± 16 | 30 ± 12 | 31 ± 23 | 0.0078 |
| Area of polarized pigment | 1.47 ± 0.61 | 0.46 ± 0.48 | 0.35 ± 0.46 | <0.0001 |
|
Portal Pigment | ||||
| Size of polarized pigment | 101 ± 35 | 113 ± 82 | 114 ± 68 | 0.8001 |
| Area of polarized pigment | 1.51 ± 1.07 | 1.47 ± 1.36 | 1.4 ± 1.2 | 0.9796 |
P-value for diagnostic groups is ANOVA
Greater numbers of PRBCs were present in the liver than in the general circulation in only 5 of 87 cases, and in no case was there margination of PRBCs, or filling of the sinusoids with PRBCs (Figure 4A & 4B). Rare PRBCs were noted in livers of 29 of 51 cases with CM, 3 of 24 in the ‘Other’ group and 0 of 12 in the Aparasitemic group; however, such PRBCs were not judged to represent sequestration, as no more PRBCs were present than were circulating in the peripheral blood. The absence of sequestered PRBCs in the liver was an obvious feature, including in CM cases which had intense sequestration of PRBCs in cerebral and other microvascular beds. Fatty change within the liver in ‘CM’ cases was largely microvesicular and only rarely purely macrovesicular (Table 2). However, most of the fatty change was minimal. There were usually only mild inflammatory changes present, equal in all groups, consisting of portal and lobular lymphocytic infiltrates. Necrosis was minimal, composed of single apoptotic hepatocytes, lobular in location, and seen in all patient groups (13 total cases). Patients in the aparasitemic group were less likely to have lobular inflammation than those in the ‘CM’ and ‘Other’ groups (p-value = 0.0420). No significant fibrosis was noted, except for two cases of incidental schistosomiasis with portal fibrous expansion surrounding degenerated eggs, without bridging or cirrhosis (Figure 4C & 4D). Hepatocyte ballooning and pleomorphism were rare. There was no evidence of microthrombi, bile duct injury or cholestasis. Merozoite filled hepatocytes were not identified.
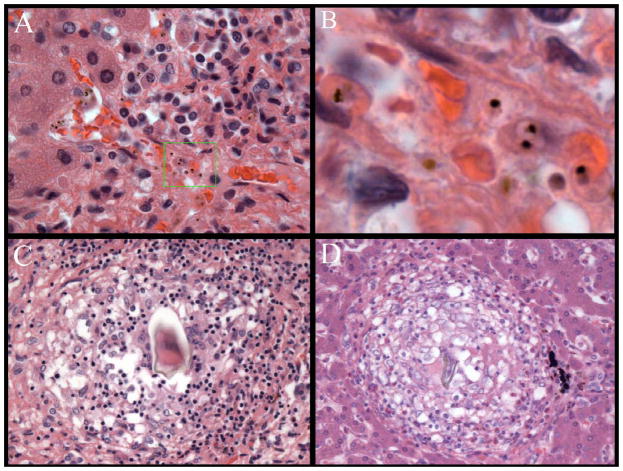
Figure 4.
Rare features of liver pathology within this autopsy cohort included sequestered parasites (A) which on high magnification show pigmented trophozoite stages (B) as well as two patients with granulomata around eggs of Schistosoma species parasites (C – D).
In the 24 parasitaemic patients without cerebral sequestration of P falciparum (‘Other’), autopsy diagnoses included pneumonia (5 cases), bacterial sepsis, bacterial meningoencephalitis, organophosphate poisoning, salicylate toxicity, massive hepatic necrosis, traumatic skull fracture, subdural haematoma, ruptured arteriovenous malformation, giant cell myocarditis, rapidly progressive glomerulonephritis, small bowel obstruction, anemia, left ventricular failure, indeterminate (2 cases), and 4 cases with clinicopathological findings which may represent Reye’s Syndrome.
Evidence of Reye’s syndrome?
In 9 of the 36 cases in the ‘Other’ and ‘Aparasitemic’ groups, hepatic histological examination showed extensive microvesicular fatty change in the majority of hepatocytes, as may be seen in Reye’s syndrome (Figure 5A–D). Among these cases, a pattern of microvesicular fatty change was predominant (always more than 50% microvesicular fat). There was no inflammation or necrosis. Seven of the 9 cases had mild to moderate Kupffer cell hyperplasia and pigment, but none had any PRBCs in hepatic sinusoids or vessels, consistent with current or recent malaria infection. None of the possible cases showed fatty change in myocardium, and only one case showed vacuolization of the cytoplasm adjacent to the basement membrane in proximal convoluted tubular epithelium of the kidney.
Figure 5.
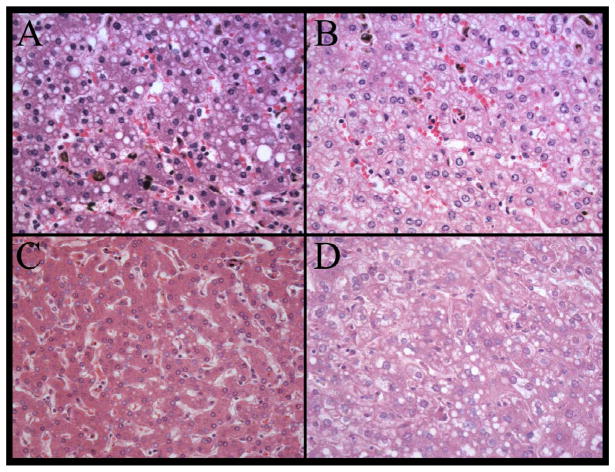
Four examples of patients with histology suggestive of Reye’s syndrome are shown as follows: A) 3 year old boy; B) 4 year old girl with history of salicylate use, aparasitemic; C) A 10 year old boy, aparasitemic; D) An 8 year old girl, aparasitemic. Liver, hematoxylin and eosin, original magnification 400x. Microvesicular fatty change is present with no other pathologic features.
Plasma salicylate levels could be measured in 6 of the 9 cases and salicylate was detectable in 2 (4.6 and 17.5 mg/dL). The intervals from hospital admission to death in the salicylate-positive cases were 9 and 14 hours, and in the 4 negative cases were 9, 22, 44 and 48 hours.
In the 9 patients who had clinicopathological findings which may represent Reye’s Syndrome, 6 reported known prior use of salicylates, 6 had received antibiotics (antibacterials, either penicillin or chloramphenicol or both), and 2 had been treated with phenobarbitone. Of the 7 aparasitaemic cases with relatively normal liver histology, autopsy revealed causes of death to be sepsis (2 cases), meningitis or meningoencephalitis (2 cases), head trauma with pneumonia, diffuse anoxic brain injury, and one unclassified diagnosis.
We performed an exploratory analysis on the presenting clinical and laboratory features between children with an eventual clinical and pathological diagnosis of cerebral malaria with those with clinicopathological findings which may represent Reye’s Syndrome (Table 1). There were differences (lack of fever, longer duration of fever if present, absent or low parasitemia, normal hematocrit, and normal platelet count). In a multivariate analysis, platelet count remained significant and parasitemia was included in the model with AUC = 0.8965; this finding is consistent with comparisons of cerebral malaria to other fatal non-CM patients (Table 4).
Table 4.
Clinical Indicators of Reye’s Syndrome vs. Cerebral Malaria
| Univariate Associations | OR | 95% CI | P-value |
|---|---|---|---|
| Fever Duration | 1.00 | 1.00 – 1.02 | 0.1130 |
| Haematocrit | 1.09 | 0.99 – 1.19 | 0.0820 |
| Platelets (quartiles) | 6.94 | 1.99 – 24.30 | 0.0020 |
| Parasitemia (quartiles) | 0.28 | 0.11 – 0.69 | 0.0060 |
| Multivariate Adjusted Model* | OR | 95% CI | P-value |
| Platelets (Quartiles) | 5.49 | 1.51 – 19.95 | 0.0100 |
| Parasitemic (Quartiles) | 0.43 | 0.17 – 1.07 | 0.0700 |
| AUC | 0.8965 |
Fever duration and haematocrit were removed from the model because the p-value was not significant and neither changed the odds ratios of platelets or parasitemic by more than 10%
Discussion
Our study included 51 children who died with P falciparum infection and cerebral sequestration of parasites with no other identifiable cause of death, suggesting that they died of malaria (CM). The liver in these cases showed little histopathological evidence of injury. Spitz [6] reported the largest malaria autopsy series prior to the current study, but the 50 cases described were all adult soldiers serving in World War II. Her findings of heavily pigmented and hyperplastic Kupffer cells, and no fatty change, are similar to ours, but she also reported PRBCs in the sinusoids, which we did not find in significant numbers in Malawian children. Four of her cases showed moderate centrilobular necrosis which we did not encounter in our study and which may have indicated an additional element of outflow obstruction, drug effect, or right heart failure in her adult population. Spitz also reported abundant acidophilic bodies in 11 cases which we interpret as apoptotic hepatocytes or Councilman bodies. There is evidence from mouse models to suggest malaria infection induces apoptosis particularly in the liver through oxidative stress [7–11]. We did not see apoptotic hepatocytes in any of our cases. Mice infected with P berghei develop a hepatic lobular T lymphocyte infiltrate [12]; we found only occasional and minimal lobular lymphocytic inflammation in our subjects. These histopathological differences at the hepatic level seem to reflect the overall observed difference in adult vs. pediatric vs. mouse malaria models.
The malaria pigment distribution pattern in the liver appears to reflect the malaria pathophysiology of the patient overall. In patients with severe malaria (i.e., cerebral malaria in this cohort), the major pattern is the presence of large amounts of pigment within macrophages spread throughout the sinusoids; this pigment is larger, on average, and occupies more space than in non-cerebral malaria patients. This is consistent with the concept of a high total body parasite mass being a feature of severe disease and reflects the increased processing of parasite waste products. The finding of no difference between the patient groups in the size or area of pigment granules in the portal triads suggests that the triads are a final collecting area for parasite pigment and reflect previous malaria infections rather than acute disease.
Brito et al and Rosen et al reported abundant PRBC and non-parasitized RBCs clumped in the hepatic sinusoids identified by electron microscopy in a small number of adults with falciparum and vivax malaria [13, 14]. We did not see PRBC accumulation by light microscopy, nor did we see it in our cases studied by electron microscopy.
We found no histological basis for the term ‘malarial hepatitis’ sometimes reported or assumed in descriptions of malaria in adults [1–5]. Only a few of these previous studies report histopathological findings including inflammation, hepatocyte necrosis and cholestatic changes, which were absent in our patients.
Reye first described the syndrome of hepatic encephalopathy and fatty liver in 1963 in Australian children [15]. . It is usually preceded by a viral illness, often influenza B, measles or varicella, includes elevated serum transaminase and plasma ammonia levels, and is not associated with jaundice [16, 17]. Aspirin and other salicylates are believed to be cofactors in this disease [18]. Some authors argue that the clinical label of Reye’s syndrome applied to the combination of hepatic dysfunction in the setting neurological compromise may represent a heterogeneous range of disorders which can include infectious, metabolic, and toxic etiologies and not solely be due to a post-viral/salicylate syndrome[19].
Reye’s syndrome may present with similar clinical features as cerebral malaria [20–22]. Malarial infections are often treated with salicylate-containing drugs which are widely available in Malawi and other regions where malaria is endemic. Reye’s syndrome is said to have nearly disappeared, at least in the western world, since warnings about an association with the use of aspirin were published in 1980 [20]. Despite evidence that some cases of Reye’s-like illness are in fact inborn errors of metabolism such as urea cycle disorders or fructosemia [23], most researchers conclude that Reye’s syndrome is a distinct syndrome associated with, if not caused by, salicylates [17, 18]. The histopathology is specific with microvesicular fatty change of hepatocytes, absent or minimal spotty necrosis, absent or minimal inflammatory cell infiltrates, and absent or minimal fibrosis and cholestasis [24]. Ultrastructurally there may be enlarged misshapen mitochondria with reduced cristae [25], or no specific findings [26]. We found mitochondrial pleomorphism to some degree in one case of possible Reye’s syndrome, but the poor preservation of our samples for electron microscopy purposes precluded accurate ultrastructural assessment. Brown and Madge reported fatty change in the cytoplasm of the cardiac myocytes and renal cells of type not specified, in cases of Reye’s syndrome [27]. We did not see these fatty changes outside of the liver, but postmortem artifact may obscure them in the kidney where renal tubular epithelium is particularly susceptible to post-mortem autolysis. Post-mortem intervals were not mentioned in Brown and Madge’s study. Few other disorders cause exclusive or dominant microvesicular fatty change, other than rare inborn errors of metabolism, and most of the other conditions were absent among our patients (e.g. acute fatty liver of pregnancy, heatstroke, Jamaican vomiting sickness, and certain drugs and toxins such as tetracycline, valproic acid and nucleoside analogues) [28]. None of the drugs that were used on the research ward, or that were elicited in the clinical histories, could be implicated in causing fatty liver change [24].
Our observations suggest that the initial clinical pictures are similar in patients eventually diagnosed as cerebral malaria and those with possible Reye’s syndrome. As asymptomatic/incidental parasitaemia is not uncommon in African pediatric populations, it is possible that some patients with Reye’s syndrome will be parasitaemic at presentation, causing further difficulty in making the diagnosis. This could be the case with our group of possible Reye’s syndrome cases. A raised plasma ammonia concentration may strengthen a suspicion of Reye’s syndrome, but the value of this is uncertain and the facility rarely available [29]. Conversely the presence of heavy parasitemia, together with thrombocytopenia and malarial retinopathy suggest that the illness is due to malaria [30–34]. None of our possible Reye’s syndrome patients had evidence of malarial retinopathy which is an established clinical marker for cerebral malaria [30–34].
Our finding of a number of deaths due to possible Reye’s syndrome contrasts with reports of the disappearance of this disease in non-tropical areas in parallel with the diminishing use of salicylates among children in such populations [20, 35, 36]. We found salicylate in the plasma of 41 of 198 (21%) other children admitted during the same period to the same research ward (data not shown), suggesting that there is still considerable use of salicylate-containing medications in Malawi, as there is in rural Kenya [22, 37]. The negative plasma salicylate results in 4 of 6 of our possible cases of Reye’s syndrome might be explained by the relative short half life of salicylates (~ two hours) and the prolonged hospital course in 3 of these cases. It is known that the plasma concentration of salicylate does not correlate with outcome in Reye’s syndrome [29].
A limitation of our study is that we cannot completely exclude inborn errors of metabolism that present in a clinically similar manner to Reye’s syndrome [19, 23, 36, 38–40], but these are rare disorders and the cytokine abnormalities induced by salicylates [21] may exacerbate these disorders as well, with malaria being an additional stressor. It would seem less likely to find 9 cases of inherited inborn errors of metabolism in our series of 87 cases than to find 9 cases of possible Reye’s syndrome in a cohort of known salicylate users. A related limitation of our study is our inability to perform blood ammonia and transaminase testing. Most of our aparasitaemic cases, including those with possible Reye’s syndrome, had Kupffer cell hyperplasia and malaria pigment accumulation in the liver indicating past, probably recent malaria infection. It is not possible to know whether this evidence of recent malaria infection has any relevance to the aetiology of Reye’s syndrome in this patient population, since malaria infections are widely prevalent in the community. The contribution of a malaria infection as a potential contributory trigger in the pathogenesis of Reye’s syndrome is not clear.
Our data demonstrate that in fatal P falciparum malaria there is little evidence of either intrahepatic sequestration or histologically evident damage to hepatic tissue. We also show that Reye’s syndrome may be an important cause of encephalopathy in children in the population studied. Salicylate use is the likely precipitating factor for Reye’s syndrome in this setting, and while efforts to control malaria are increasingly applied, we recommend that salicylates be used judiciously, if at all, among children in regions where malaria is endemic.
Acknowledgments
Funding Support: This work was supported by the U.S. National Institutes of Health (RO1 AO34969, K23 AI072033), and by The Wellcome Trust, U.K (042390/Z/94)
We would like to thank the parents and guardians of children studied in this ongoing project, for their helpfulness and their approval of the investigations.. Thanks to Cobie Whitten, PhD for editing and psychic and nutritional support, Kelly Kukas MT and Kathy D’acci MT for performing salicylate assays and to Beckman Instruments Inc. Fullerton, CA for kindly donating reagents for the salicylate assays. Further special thanks to the indispensable team in Malawi that made and continues to make the autopsy study possible: George Liomba, Charles Dzamalala, Fred MacInnes, Sam Wassmer, Jacqui Montgomery, Dumizulu Tembo, Jimmy Vareta, Karl Seydel, Fingani Mphande, Wales Namanya, Johanness Kaliwambe, Laston James Mbewe.
Footnotes
The authors have no financial or professional conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anand AC, Puri P. Jaundice in malaria. J Gastroenterol Hepatol. 2005;20(9):1322–32. doi: 10.1111/j.1440-1746.2005.03884.x. [DOI] [PubMed] [Google Scholar]
- 2.Devarbhavi H, Alvares JF, Kumar KS. Severe falciparum malaria simulating fulminant hepatic failure. Mayo Clin Proc. 2005;80(3):355–8. doi: 10.4065/80.3.355. [DOI] [PubMed] [Google Scholar]
- 3.Kochar DK, et al. Malarial hepatitis. J Assoc Physicians India. 2003;51:1069–72. [PubMed] [Google Scholar]
- 4.Murthy GL, et al. Hepatitis in falciparum malaria. Trop Gastroenterol. 1998;19(4):152–4. [PubMed] [Google Scholar]
- 5.Anand AC, et al. Malarial hepatitis: a heterogeneous syndrome? Natl Med J India. 1992;5(2):59–62. [PubMed] [Google Scholar]
- 6.Spitz S. The Pathology of Acute Falciparum Malaria. The Military Surgeon. 1946;99:555–572. [PubMed] [Google Scholar]
- 7.Dey S, et al. Malarial infection develops mitochondrial pathology and mitochondrial oxidative stress to promote hepatocyte apoptosis. Free Radic Biol Med. 2009;46(2):271–81. doi: 10.1016/j.freeradbiomed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Guha M, et al. Overexpression, purification and localization of apoptosis related protein from Plasmodium falciparum. Protein Expr Purif. 2007;52(2):363–72. doi: 10.1016/j.pep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Guha M, et al. Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. Faseb J. 2006;20(8):1224–6. doi: 10.1096/fj.05-5338fje. [DOI] [PubMed] [Google Scholar]
- 10.Guha M, et al. Melatonin inhibits free radical-mediated mitochondrial-dependent hepatocyte apoptosis and liver damage induced during malarial infection. J Pineal Res. 2007;43(4):372–81. doi: 10.1111/j.1600-079X.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, et al. Bilirubin inhibits Plasmodium falciparum growth through the generation of reactive oxygen species. Free Radic Biol Med. 2008;44(4):602–13. doi: 10.1016/j.freeradbiomed.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs T, et al. CTLA-4-dependent mechanisms prevent T cell induced-liver pathology during the erythrocyte stage of Plasmodium berghei malaria. Eur J Immunol. 2004;34(4):972–80. doi: 10.1002/eji.200324477. [DOI] [PubMed] [Google Scholar]
- 13.De Brito T, Barone AA, Faria RM. Human liver biopsy in P. falciparum and P. vivax malaria. A light and electron microscopy study. Virchows Arch A Pathol Pathol Anat. 1969;348(3):220–9. doi: 10.1007/BF00555648. [DOI] [PubMed] [Google Scholar]
- 14.Rosen S, et al. The Liver in Malaria. Arch Path. 1967;83:271–277. [PubMed] [Google Scholar]
- 15.Reye RD, Morgan G, Baral J. Encephalopathy and Fatty Degeneration of the Viscera. a Disease Entity in Childhood. Lancet. 1963;2(7311):749–52. doi: 10.1016/s0140-6736(63)90554-3. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh D, et al. Investigation of an epidemic of Reye’s syndrome in northern region of India. Indian Pediatr. 1999;36(11):1097–106. [PubMed] [Google Scholar]
- 17.Glasgow JF, Middleton B. Reye syndrome--insights on causation and prognosis. Arch Dis Child. 2001;85(5):351–3. doi: 10.1136/adc.85.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsyth BW, et al. New epidemiologic evidence confirming that bias does not explain the aspirin/Reye’s syndrome association. Jama. 1989;261(17):2517–24. [PubMed] [Google Scholar]
- 19.Casteels-Van Daele M, et al. Reye syndrome revisited: a descriptive term covering a group of heterogeneous disorders. Eur J Pediatr. 2000;159(9):641–8. doi: 10.1007/pl00008399. [DOI] [PubMed] [Google Scholar]
- 20.Belay ED, et al. Reye’s syndrome in the United States from 1981 through 1997. N Engl J Med. 1999;340(18):1377–82. doi: 10.1056/NEJM199905063401801. [DOI] [PubMed] [Google Scholar]
- 21.Clark I, et al. Salicylates, nitric oxide, malaria, and Reye’s syndrome. Lancet. 2001;357(9256):625–7. doi: 10.1016/S0140-6736(00)04061-7. [DOI] [PubMed] [Google Scholar]
- 22.English M, et al. Chronic salicylate poisoning and severe malaria. Lancet. 1996;347(9017):1736–7. doi: 10.1016/s0140-6736(96)90809-0. [DOI] [PubMed] [Google Scholar]
- 23.Chang PF, et al. Metabolic disorders mimicking Reye’s syndrome. J Formos Med Assoc. 2000;99(4):295–9. [PubMed] [Google Scholar]
- 24.MacSween RNM, et al. Pathology of the Liver. Livingstone. 2002:133–134. [Google Scholar]
- 25.Daugherty CC, et al. A morphometric study of Reye’s syndrome. Correlation of reduced mitochondrial numbers and increased mitochondrial size with clinical manifestations. Am J Pathol. 1987;129(2):313–26. [PMC free article] [PubMed] [Google Scholar]
- 26.Svoboda DJ, Reddy JK. Pathology of the liver in Reye’s syndrome. Lab Invest. 1975;32(5):571–9. [PubMed] [Google Scholar]
- 27.Brown RE, Madge GE. Hepatic degeneration and dysfunction in Reye’s syndrome. Am J Dig Dis. 1971;16(12):1116–22. doi: 10.1007/BF02235170. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig J, Batts K. Practical Liver Biopsy Interpretation. 2. ASCP Press; 1998. p. 68. [Google Scholar]
- 29.Partin JS, et al. Serum salicylate concentrations in Reye’s disease. A study of 130 biopsy-proven cases. Lancet. 1982;1(8265):191–4. doi: 10.1016/s0140-6736(82)90759-0. [DOI] [PubMed] [Google Scholar]
- 30.Beare NAV, et al. Prognostic significance and course of retinopathy in children with severe malaria. Arch Ophthalmol. 2004;122:1141–1147. doi: 10.1001/archopht.122.8.1141. [DOI] [PubMed] [Google Scholar]
- 31.Beare NAV, et al. Malaria Retinopathy: A Newly Established Diagnostic Sign in Severe Malaria. Am J Trop Med Hyg. 2006;75(5):790–797. [PMC free article] [PubMed] [Google Scholar]
- 32.Lewallen S, et al. A review of the spectrum of clinical ocular fundus findings in P. falciparum malaria in African children with a proposed classification and grading system. Trans R Soc Trop Med Hyg. 1999;93(6):619–22. doi: 10.1016/s0035-9203(99)90071-8. [DOI] [PubMed] [Google Scholar]
- 33.Lewallen S, et al. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology. 1993;100:857–861. doi: 10.1016/s0161-6420(93)31563-0. [DOI] [PubMed] [Google Scholar]
- 34.Lewallen S, et al. Clinical-histopathological correlation of the abnormal retinal vessels in cerebral malaria. Arch Ophthalmol. 2000;118(7):924–8. [PubMed] [Google Scholar]
- 35.Autret-Leca E, et al. Incidence of Reye’s syndrome in France: a hospital-based survey. J Clin Epidemiol. 2001;54(8):857–62. doi: 10.1016/s0895-4356(00)00366-8. [DOI] [PubMed] [Google Scholar]
- 36.Hardie RM, et al. The changing clinical pattern of Reye’s syndrome 1982–1990. Arch Dis Child. 1996;74(5):400–5. doi: 10.1136/adc.74.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Idro R, et al. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. Jama. 2007;297(20):2232–40. doi: 10.1001/jama.297.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bzduch V, et al. Metabolic cause of Reye-like syndrome. Bratisl Lek Listy. 2001;102(9):427–9. [PubMed] [Google Scholar]
- 39.Orlowski JP. Whatever happened to Reye’s syndrome? Did it ever really exist? Crit Care Med. 1999;27(8):1582–7. doi: 10.1097/00003246-199908000-00032. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan KM, et al. Epidemiology of Reye’s syndrome, United States, 1991–1994: comparison of CDC surveillance and hospital admission data. Neuroepidemiology. 2000;19(6):338–44. doi: 10.1159/000026274. [DOI] [PubMed] [Google Scholar]