Abstract
Although renin, the rate-limiting enzyme of the renin-angiotensin system (RAS), was first discovered by Robert Tigerstedt and Bergman more than a century ago, the research on the RAS still remains stronger than ever. The RAS, once considered to be an endocrine system, is now widely recognized as dual (circulating and local/tissue) or multiple hormonal systems (endocrine, paracrine and intracrine). In addition to the classical renin/angiotensin I-converting enzyme (ACE)/angiotensin II (Ang II)/Ang II receptor (AT1/AT2) axis, the prorenin/(Pro)renin receptor (PRR)/MAP kinase axis, the ACE2/Ang (1-7)/Mas receptor axis, and the Ang IV/AT4/insulin-regulated aminopeptidase (IRAP) axis have recently been discovered. Furthermore, the roles of the evolving RAS have been extended far beyond blood pressure control, aldosterone synthesis, and body fluid and electrolyte homeostasis. Indeed, novel actions and underlying signaling mechanisms for each member of the RAS in physiology and diseases are continuously uncovered. However, many challenges still remain in the RAS research field despite of more than one century's research effort. It is expected that the research on the expanded RAS will continue to play a prominent role in cardiovascular, renal and hypertension research. The purpose of this article is to review the progress recently being made in the RAS research, with special emphasis on the local RAS in the kidney and the newly discovered prorenin/PRR/MAP kinase axis, the ACE2/Ang (1-7)/Mas receptor axis, the Ang IV/AT4/IRAP axis, and intracrine/intracellular Ang II. The improved knowledge of the expanded RAS will help us better understand how the classical renin/ACE/Ang II/AT1 receptor axis, extracellular and/or intracellular origin, interacts with other novel RAS axes to regulate blood pressure and cardiovascular and kidney function in both physiological and diseased states.
Keywords: Angiotensin converting enzyme 2 (ACE2), angiotensin II (Ang II), AT1 receptor signaling, insulin-regulated aminopeptidase (IRAP), Mas receptor, (Pro)renin receptor (PRR)
1. Introduction
Since renin, the rate-limiting enzyme of the renin-angiotensin system (RAS), was first discovered by Robert Tigerstedt and Bergman more than a century ago [210], the RAS is now well recognized as a dual vasoactive system, acting as both a circulating endocrine system and a local tissue paracrine system [25,77,111]. A local RAS has been demonstrated in nearly every target tissues including the kidney, adrenal glands, heart, blood vessels, pancreas, liver, brain, and even adipose tissues. The localization and functional properties of a local RAS in various target tissues have been reviewed elsewhere [12,29,40,61,65,117,236]. The objective of this review therefore focuses solely on the new insights and perspectives on the endocrine, paracrine and intracrine RAS in the kidney. In the kidney, all major components of the RAS, including the precursor angiotensinogen, the rate-limiting enzyme renin and angiotensin I-converting enzyme (ACE), and the receptors for angiotensin II (Ang II), AT1 and AT2, Ang (1-7) and Ang (3-8) have been demonstrated (Fig. 1) [29,35,107,153,163,238]. Although there are continuous debates on whether renin and angiotensinogen are synthesized or taken up in tissues other than the juxtaglomerular apparatus (JGA) or hepatocytes, respectively, there is a consensus that the presence of renin, angiotensinogen and ACE in tissues is necessary for local formation of Ang II independent of circulating Ang II. In spite of recent discovery of several new components of the RAS, such as prorenin or (Pro)renin receptor, PRR [168,169], the second ACE (ACE2) [51,68], biologically active angiotensin fragments such as Ang (1-7) and Ang (3-8) (Ang IV) [35,82], and the novel receptors for Ang (1-7), Mas [187], and Ang IV, AT4 [3], Ang II is still and will remain to be the principle effector of the RAS in the kidney and other tissues. Likewise, although five major classes of Ang receptors, AT1a, AT1b and AT2 for Ang II, the Mas receptor for Ang (1-7), and AT4 for Ang IV have been cloned, the AT1 (AT1a) receptor remains to be the principal receptor that mediates the majority of the known actions of Ang II in the kidney [30,39,76,172,199,235,239]. Nevertheless, new progress has indeed been made in several areas during last several years. First, what we have known as the classical RAS for many decades has now expanded to include prorenin/PRR [168,169] and ACE2 [51,68] (Fig. 2). Second, there are renewed interests in the physiological and pathophysiological roles of several Ang II metabolites including Ang III, Ang (1-7) and Ang IV [33,82,173]. Third, the so-called intracrine/intracellular RAS or Ang II, which was previously considered not to be physiologically important, has been recently shown to have blood pressure-increasing effects in rodents [135,180]. The critical review or timely update of these new developments in the RAS research may provide new insights and perspectives into both classic and novel roles of the RAS in the physiological regulation of blood pressure, cardiovascular and kidney functions, and in the development of hypertension, cardiovascular and kidney diseases.
Figure 1.
In vitro autoradiographic mapping of: (A) active renin binding in justaglomerular apparatus in the dog kidney pretreated with sodium depletion using the radiolabeled renin inhibitor, 125I-H77 [202,237], (B) angiotensin I-converting enzyme binding in proximal tubules of the rat kidney using 125I-351A [98,238], (C) AT1 receptor in the presence of the AT2 receptor blocker PD123319 or (D) AT2 receptor binding in the presence of the AT receptor blocker losartan using 125I-[Sar1,Ile8]-Ang II [236,239,241], (E) Ang (1-7) receptor binding in the rat kidney using 125I-Ang (1-7) as the radioliagnd, and (F) Ang IV receptor binding in the rat kidney using 125I-Ang (3-8) [133,238]. The levels of binding are indicated by color calibration bars with red representing the highest, whereas blue showing the lowest levels of enzyme or receptor binding. G: glomerulus. IM: inner medulla. IS: inner stripe of the outer medulla. JGA: juxtaglomerular apparatus. P: proximal tubule.
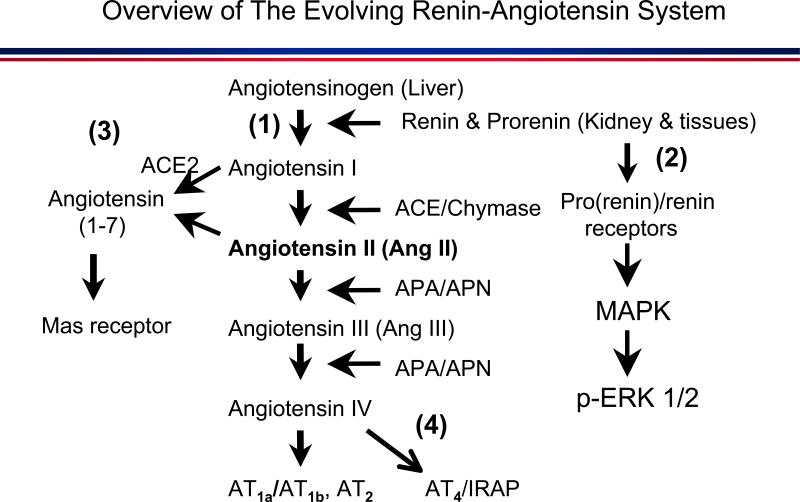
Figure 2.
Overview of the expanded and evolving renin-angiotensin system. (1): the classical angiotensinogen/renin/ACE/Ang II/AT1 receptor axis. (2): the prorenin/(Pro)renin receptor/MAP kinases ERK 1/2 axis. (3) the ACE2/Ang (1-7)/Mas receptor axis. (4) the Ang IV/AT4/IRAP axis. APA: aminopeptidase A. APN: aminopeptidase N. IRAP: insulin-regulated aminopeptidase.
2. Overview of the prorenin/PRR/MAP kinases ERK 1/2 axis in renal physiology and diseases
2.1. Localization and roles of prorenin in the kidney
The molecular biology, structure, biochemistry and the roles of prorenin and (Pro)renin receptors (PRR) in physiology and diseases have been critically reviewed elsewhere [26,58,166,198]. In the current article, only the progress on the kidney- and blood pressure-related prorenin and PRR research is briefly reviewed. The kidney was initially thought to be the only tissue to produce renin in JGA cells. Indeed, renin was found to decrease markedly after the kidneys were removed in humans and animals [27,28]. However, it is now recognized that prorenin is also produced in extrarenal tissues including adrenal glands, reproductive tissues such as ovary and testis, and the retina of eyes [26,59,226]. A physiological or pathophysiological role of prorenin is indicated by the observations that the levels of prorenin may be 100-time higher in ovarian follicular fluid and amniotic fluid in physiology, and in vitreous fluid of diabetic retinopathy [59,226]. In the kidney, renin and prorenin are primarily located in JGA cells, afferent arteries and interlobular arterioles, where prorenin may be activated by sodium depletion [60,202,205,237]. Despite of its presence in the kidney (or other tissues), prorenin was not previously considered to have a biological role due to its insignificant intrinsic enzymatic activity [26]. For example, when recombinant human prorenin was infused into rhesus monkeys or rats transgenic for human angiotensinogen, it failed to increase blood pressure [132,159]. Likewise, blood pressure was either unchanged or decreased in rats transgenic for rat or human prorenin with ~ 60 to 400-fold increases in plasma prorenin levels [154,220]. These studies strongly suggest that prorenin may not exert a physiological role in blood pressure, cardiovascular and renal regulation.
2.2. Localization and roles of (Pro)renin receptors (PRR) in the kidney
The discovery of PRR rapidly changes the dynamics of prorenin research in the kidney and other tissues [26,58,166,198]. The term of the (Pro)renin receptor may be confusing, because both renin and prorenin recognize and bind this same receptor, and activate the same intracellular signaling pathway, the MAP kinases ERK1/2. It is not quite sure whether it is renin or prorenin that physiologically activates this PRR. The PRR is a 350-amino acid protein with a single transmembrane domain and was first pharmacologically characterized in glomerular mesangial cells [168] and subsequently cloned by Nguyen et al. in 2002 [169]. PRR was later found structurally identical to CAPER protein (endoplasmic reticulum-localized type 1 transmembrane adaptor precursor) and vacuolar H+-ATPase (V-ATPase) [1,57,198]. The interactions between PRR and V-ATPase are particularly relevant in the renal physiology and diseases. V-ATPase is present on the membranes of intracellular endosomes, lysosomes and endoplasmic reticulum, where V-ATPase activity plays an important role in maintaining an acidification environment of endosomal and lysosomal compartments, and therefore in intracellular pH homeostasis and G protein-coupled endocytosis and recycling. In contrast to its initial localization and characterization in human mesangial cells [168], mRNAs and immunoreactive proteins of PRR were recently localized primarily in the distal nephron segments especially in collecting ducts [1,198]. However, PRR is in fact expressed widely in different tissues.
2.3. Signaling mechanisms underlying the activation of the prorenin/PRR/MAP kinases ERK 1/2 axis
Activation of PRR by prorenin or renin has been shown to induce diverse biological and pathophysiological responses ranging from activation of MAP kinases ERK1/2, p38 MAP kinase, TGF-β1, PAI-1, COX-2, PI3 kinase and fibronectin, to proteinuria and nephropathy [165,166,198]. Most if not all of studies on prorenin and PPR suggest that renin and prorenin bind PRR and activates MAP kinases ERK1/2 independent of Ang II production and/or activation of AT1 (AT1a) receptors [166,167]. It has been further suggested that this pathway may play an important role in cardiovascular and kidney diseases in which the classical renin and ACE inhibitors or AT1 receptor blockers (ARBs) are ineffective [164,165]. However, this conclusion remains to be further established due to several reasons [26,57,80,198]. First, there is evidence that proreinin binding to PRR induces non-proteolytic activation of prorenin to elicit full renin enzymatic activity [13,161,169]. Activation of prorenin on the cell surface almost certainly leads to Ang II formation on the cell membrane, which in turn activates downstream MAP kinases ERK1/2 signaling via an AT1 receptor-dependent mechanism. The renin- and/or Ang II-dependent activation of MAP kinases ERK1/2 appears to have been well established. Ang II also appears to stimulate V-ATPase activity in rat proximal tubule cells via mechanisms involving tyrosine kinase, p38 MAPK, and PI3K [31]. Since PRR is structurally identical to CAPER protein (endoplasmic reticulum-localized type 1 transmembrane adaptor precursor) and vacuolar H+-ATPase (V-ATPase), intracellular PRR may also mediate the intracellular actions of prorenin or renin [1,57,198]. Second, in most published studies nanomolar concentrations of prorenin have been used to induce PRR activation in vitro, whereas only fentomolar to picomolar concentrations of prorenin are reported in the circulation and tissues [26,57]. Thus physiological roles of PRR remain uncertain. Third, renin and ACE inhibitors and ARBs were not simultaneously used to block the entire RAS cascade before in vitro or in vivo studies involving prorenin or PRR overexpression were performed, and Ang II production was rarely determined in those studies. And finally, a specific PRR inhibitor or antagonist remains to be developed, which will play an important role in defining the physiological and pathophysiological roles of prorenin and PRR. The results of the so-called Handle Region Peptide (HRP) to block PRR at best have been mixed and often remain controversial. Both positive and negative effects of this decoy peptide in blocking PRR have been reported [104,80]. Even if HRP is indeed effective in blocking prorenin and PRR interactions, its clinical relevance remains unknown due to its peptide properties. The renin-specific inhibitors have been developed to treat hypertension and cardiovascular and kidney diseases. Whether the renin inhibitors are therapeutically superior to classical ACE inhibitors or ARBs remains an issue of continuous debates. If prorenin and PRR indeed play important physiological and pathophysiological roles in blood pressure regulation and pathologies of cardiovascular, renal and diabetic diseases, the development of orally active PRR-specific inhibitors to block prorenin-induced activation of PRR will be highly necessary.
3. Overview of the ACE2/Ang (1-7)/Mas receptor axis in renal physiology and diseases
3.1. Roles of ACE2 in Ang (1-7) formation in the kidney and other tissues
Recently, there has been an explosion of renewed interest in studying the biological, physiological and pathophysiological role of the angiotensin metabolite, Ang (1-7), following the discoveries of ACE2 and the Mas receptor specific for Ang (1-7) [35,82,106,186,195]. Ang (1-7) is a heptapeptide of the RAS, which may be generated in two different ways. First, a number of enzymes in tissues including neprilysin [EC 3.4.24.11], prolyl oligopeptidase [EC 3.4.24.26], and thimet oligopeptidase [EC 3.4.24.15] may cleave three end amino acids, Phe-His-Leu, from the decapeptide Ang I [Ang (1-10)] to form Ang (1-7) [35,82]. In the kidney, neprilysin appears to be the major enzyme to process Ang I to Ang (1-7) [35,82]. Second, it is now recognized that Ang (1-7) can also be generated by an alternative enzyme named ACE2 [69,212]. ACE2 was first cloned by Donoghue et al. from 5’ sequencing of a human heart failure ventricle cDNA library in 2000 [69]. It shares 42% of the genomic structure of ACE and is expressed not as widely as ACE in that ACE2 is localized mainly in the heart, kidney and testis in humans [69,130,201,223]. Both ACE and ACE2 are membrane-bound metallopeptidases and require both Zn2+ and Cl- for optimal activation [35,82]. However, the biochemical activities of ACE2 are different from those of ACE. For example, the main functions of ACE are to convert the inactive Ang I to form the active octapeptide Ang II [Ang (1-8)] and to degrade bradykinin. Additionally, ACE also appears to be a major route for Ang (1-7) metabolism in the plasma and proximal tubules of the kidney [35,82]. By contrast, ACE2 acts as a monocarboxypeptidase to cleave only a single residue or amino acid from the carboxyl terminus of Ang I to form Ang (1-9) [69], or degrade Ang II into Ang (1-7) [35,82]. ACE2 is not inhibited by classical ACE inhibitors, suggesting that ACE2 represents a novel and independent member of the RAS [35,82]. Interestingly, Ang (1-9) may further be cleaved by other endopeptidases to generate Ang (1-7) or other fragments, of which Ang (1-7) by far generates most of exciting investigations among all angiotensin metabolites.
3.2. Localization of Ang (1-7) receptors or the Mas receptors in the kidney
The specific binding sites or receptors for the heptapeptide Ang (1-7) had been elusive until Santos et al. identified the G protein-coupled receptor encoded by the Mas protooncogene as the Ang (1-7) receptor [187]. The association between the Mas protooncogene and Ang II receptors or Ang II-induced signalling is not entire new, since it has been suggested that the Mas gene may encode an Ang II receptor [109] and modulate AT1 receptor-mediated intracellular Ca2+ signalling [5]. However, Santos et al. showed that genetic deletion of the Mas receptor abolished Ang (1-7) binding in the mouse kidney and prevented in vitro and in vivo vascular and renal responses to Ang (1-7) stimulation [187]. There are questions remained as to whether the Mas receptor is specific only to Ang (1-7) or involves multiple receptor binding sites. For example, specific 125I-Ang (1-7) binding in kidney sections as demonstrated by Santos et al. was not much different between wild-type and Mas-KO mice [187]. Furthermore, even at pharmacological concentrations (10 µM) unlabeled Ang (1-7) or Ang (1-7) antagonist A-779 could still only displace approximately 40% of 125I-Ang (1-7) binding [187]. This raises the possibility that the Mas receptor is only partially specific to Ang (1-7) and additional receptor(s) may be involved. Nevertheless, there is evidence that the Mas receptor appears to mediate the known biological and physiological effects of Ang (1-7) in different tissues [35,82,187].
In the kidney, the Mas receptor is widely expressed in intrarenal blood vessels such as afferent arterioles and tubular epithelium especially in proximal tubules, where the known biological and physiological effects of Ang (1-7) have been extensively investigated [35,82] (for a more extensive review). Using 125I-Ang (1-7) as the ligand for its receptors, we found that specific 125I-Ang (1-7) receptor binding is localized primarily in the inner cortex and the outer stripe of the outer medulla in the rat kidney, which may be completely displaced by unlabeled Ang (1-7) (Fig. 1). This anatomical localization suggests that Ang (1-7) receptors are likely expressed predominantly in proximal tubules.
3.3. Roles of Ang (1-7) receptors or the Mas receptors in the kidney
In general, the activation of ACE2/Ang (1-7)/the Mas receptor axis largely opposes the known actions of the renin//ACE/Ang II/AT1 receptor axis through inhibition of MAP kinase- or cyclooxygenase-2 (COX-2)-dependent pathways, or stimulation of nitric oxide (NO)/cGMP-dependent pathway [35,82,106,186] (Fig. 3). While the renin/ACE/Ang II/AT1 receptor axis acts to raise blood pressure [53,108,137], cause renal vasoconstriction, decrease renal blood flow (RBF) and glomerular filtration rate (GFR) [32,98,117], stimulation of proximal tubule sodium transport [43,162,240] and increase urine concentration [138,170] etc., the ACE2/Ang (1-7)/the Mas receptor instead acts to reduce blood pressure, dilate intrarenal blood vessels, increase RBF and GFR, inhibit proximal tubule transport, and induce diuresis [35,82] (for a comprehensive review). Similarly, the renin/ACE/Ang II/AT1 receptor axis is widely implicated in the pathogenesis and progression of acute and chronic kidney diseases, including hypertension and diabetic nephropathy [52,77,142,158]. By contrast, overexpression of ACE2 to increase Ang (1-7) production or stimulation of the Mas receptor by Ang (1-7) in target tissues may be used to counter the pathological role of the renin/ACE/Ang II/AT1 receptor axis. Indeed, impairment of the ACE2/Ang (1-7)/the Mas receptor axis may be associated with increases in blood pressure [23,88] or acute renal ischemic and reperfusion injury [55].
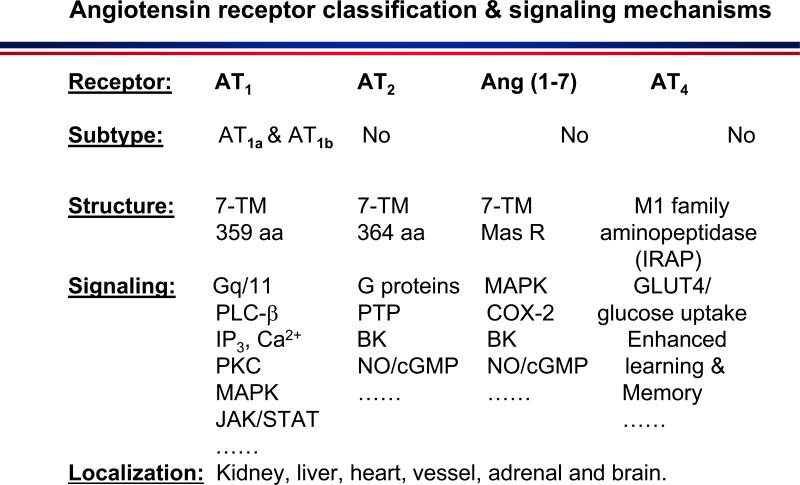
Figure 3.
Angiotensin receptor classification into four different classes and their signalling mechanisms. AT1: type 1 receptor specific for Ang II including type 1a and type 1b. AT2: type 2 receptor specific for Ang II. Mas receptor: specific for Ang (1-7). AT4: type 4 specific for Ang IV. 7-TM: seven transmembrane domains. BK: bradykinin. cGMP: cyclic guanosine monophosphate. COX-2: cyclooxygenase-2. Gq/11: a G protein subunit. IP3: inositol trisphosphate. JAK/STAT: janus kinase/signal transducer and activator of transcription. MAPK: mitogen-activated protein (MAP) kinases. NO: nitric oxide. PKC: protein kinase C. PLC-β: phospholipase C-β. PTP: protein tyrosine phosphatase.
It should be emphasized, however, that the activation of the ACE2/Ang (1-7)/the Mas receptor axis may not be involved in established hypertension in SHRSP or TGR (mRen2)27 models [114], but may be associated with stimulation of growth-stimulatory pathways in human mesangial cells [248], increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy [219], accelerated STZ-induced diabetic nephropathy [197], or Ang II-induced epithelial-to-mesenchymal transformation [24]. Ang-(1-7) also reportedly stimulated water transport in rat inner medullary collecting duct via interactions with vasopressin V2 receptors [150]. These effects of Ang (1-7) are quite similar to those induced by the activation of the renin/ACE/Ang II/AT1 receptor axis. It is tempting to speculate that these diverse effects of Ang (1-7) may be mediated by, or interacted with, different receptors that recognize the heptapeptide and may be expressed differentially in different cell types in the kidney. Further studies are required to establish the physiological and therapeutical roles of this novel ACE2/Ang (1-7)/the Mas receptor axis in hypertension, cardiovascular and renal diseases.
4. Overview of the Ang IV/AT4/IRAP axis in renal physiology and diseases
4.1. Roles of aminopeptidases A and N in Ang IV formation in the kidney and other tissues
Ang (3-8), also termed Ang IV, is another biologically active Ang II fragment that has been a subject of extensive studies during recent years. Ang IV is primarily derived from Ang II or Ang III through the enzymatic actions of aminopeptidases A and N to cleave one or two N terminal amino acids [8,9]. The Ang IV/AT4 receptors and their biological and pharmacological properties and tissue distribution have been comprehensively reviewed [61]. In the current article, we will focus on the Ang IV/AT4 receptor axis in the kidney, because Aminopeptidases A and N are particularly abundant in this organ, especially in apical membranes of proximal nephron where these enzymes degrade Ang II to form Ang IV [174]. Ang IV can also be generated in the glomeruli of the kidney [9].
4.2. Hemodynamic and tubular effects of Ang IV in the kidney
In the kidney, Ang IV has been shown to induce diverse biological and physiological effects, which are often inconsistent or controversial. For example, Ang IV was initially shown to induce renal cortical vasodilatation probably through nitric oxide independent of AT1/AT2 receptors [44,203]. However, subsequent studies showed that Ang IV remains a potent renal vasoconstrictor in the kidney via activation of AT1 receptor [83,133,218,229,230]. One of the most significant studies is that genetic over-expression of Ang IV in the brain of a transgenic mouse model leads to hypertension that is reversed by an AT1 receptor antagonist [148]. Some of Ang IV-induced intracellular signaling pathways also appear to resemble those elicited by Ang II.via AT1 receptor activation. For instance, Ang IV was reported to increase intracellular calcium in mesangial cells [34,133], human proximal tubules cells [94], opossum kidney cells [74], or Mardin-Darby bovine kidney epithelial cells [95]. Conversely, Ang IV either increased [54] or had no effect on cAMP production or intracellular calcium in kidney cells [37].
We have recently studied whether Ang IV exerts systemic and renal cortical effects on blood pressure (MAP), renal microvascular smooth muscle cells (VSMCs), or glomerular mesangial cells (MC) and, if so, whether AT1 receptor signaling is involved [133]. We demonstrated that in anesthetized rats, systemic infusion of Ang II, Ang III, or Ang IV caused dose-dependent increases in MAP and decreases in renal cortical blood flow. Ang II also induced dose-dependent reductions in renal medullary blood flow, whereas Ang IV did not. Ang IV-induced MAP response and renal cortical vasoconstriction were completely abolished by the AT1 receptor blocker losartan [133]. When Ang IV (1 nmol/kg/min) was infused directly in the renal artery, renal cortical blood flow was reduced by >30%, and the response was also blocked by losartan. Furthermore, unlabeled Ang IV displaced 125I-labeled [Sar1,Ile8]Ang II binding, whereas unlabeled Ang II (10 μM) also inhibited 125I-labeled Nle1-Ang IV (AT4) binding in a concentration-dependent manner (Fig. 1). In freshly isolated renal VSMCs, Ang IV (100 nM) increased intracellular Ca2+ concentration, and the effect was blocked by losartan and U-73122, a selective inhibitor of phospholipase C/inositol trisphosphate/ Ca2+ signaling (1 μM) [133]. In cultured rat MCs, Ang IV (10 nM) induced MAP kinases ERK 1/2 phosphorylation also via AT1 receptor- and phospholipase C-activated signaling. Our results suggest that, at nanomolar concentrations, Ang IV can increase MAP and induce renal cortical effects by interacting with AT1 receptor-activated signalling pathways [133].
4.3. Insulin-regulated aminopeptidase as the receptor for Ang IV (AT4)
The identification of the AT4 receptor to be insulin-regulated aminopeptidase (IRAP) may open new opportunities to further study the roles of Ang IV via this unique receptor [3]. IRAP is a type II integral membrane spanning protein associated with the M1 family of aminopeptidases and GLUT4 vesicles in insulin-responsive cells [3,33]. Albiston et al. have recently mapped the distribution of both GLUT4 and IRAP in the kidney [4]. IRAP and GLUT4 immunostaining was localized, but not overlapped, in proximal and distal tubules and thick ascending limbs in the cortex. GLUT4 staining appears to be associated with intracellular vesicles, whereas IRAP staining was predominantly associated with the apical membrane [4]. IRAP immunoreactivity was also localized in the principal cells of the inner medulla collecting ducts (IMCD), with limited overlap with the vasopressin responsive water channel aquaporin-2 (AQP-2) [4]. The role of IRAP in the regulation of renal hemodynamics or renal tubular transport has not been investigated. The localization of IRAP and specific 125I-Ang IV binding sites in the renal cortex suggests that the Ang IV/AT4/IRAP axis may play an important role in the regulation of glucose uptake or transport in proximal tubules of the kidney [4,96,133]. Mice deficient in IRAP (IRAP-/-) have been generated [116] and may be a useful model for further studies of the physiological and pathophysiological roles of the Ang IV/AT4/IRAP axis in the kidney.
5. New insights into the classical renin/ACE/Ang II/AT1 receptor axis in renal physiology and diseases: focus on intracrine/intracellular Ang II in proximal tubules
5.1. Overview of the knowledge gap on the roles of intracrine/intracellular Ang II
Angiotensin II is undoubtedly the major effector of the renin/ACE/Ang II/AT1 receptor axis in long-term cardiovascular, renal and blood pressure regulation (Fig. 1 & Fig. 2). There is also no doubt that extracellular (circulating and paracrine) Ang II plays the classical roles of Ang II through activation of cell surface GPCRs (Fig. 3) [25,29,61,111,152,211,213]. Thus, no wonder many have assumed that: 1) Ang II only needs to activate cell surface receptors to induce all of its responses, 2) all renin and ACE inhibitors and ARBs would block all responses to extracellular Ang II to produce the same beneficial effects, and 3) an intracrine/intracellular Ang II system may not be required or involved in the regulation of cardiovascular and renal physiology or diseases.
However, recent research suggests that these views may be revised for a number of reasons [49,65,119,121,179,246]. First, extracellular Ang II is continuously internalized with the receptors immediately after it activates cell surface receptors. There is evidence that activated agonist/receptor complex internalized into endosomes may continue to transmit Ras/MAPK signaling from endosomes [18,50,149,160,176]. Second, Ang II exerts powerful and long-lasting genomic or gene transcriptional effects, which may be independent from the well-recognized systemic effects mediated by cell surface receptors [19,110,121,193]. In vitro and in vivo, Ang II induces the expression/transcription of growth factors and proliferative cytokines including nuclear factor-κB (NF-κB) [21,141,142,185], monocyte chemoattractant protein-1 (MCP-1) [141,204,242], TNF-α [204], and TGF-β1 [113,225,227]. Genomic effects induced by Ang II may be in part mediated by interactions with intracellular and nuclear mineralocorticoid receptors (MR) [19,110,131,156]. Although systemic hemodynamic responses to Ang II often occur in seconds or minutes, the genomic responses to Ang II including cellular growth, mitogenic and proliferative effects occur ranging from hours, weeks to months [124,156,224]. Thus the genomic effects of Ang II are probably mediated by intracellular Ang II system. Third, not all ARBs, ACE or renin inhibitors are created equal to block both extracellular and intracellular Ang II systems. Due to their structures, lipophilic properties, and affinity with AT1 receptors, ARBs may differ in their ability to enter the cells to block intracellular AT1 receptors [123]. Different effects of ARBs on uric acid metabolism, cell proliferation, oxidative stress, nitric oxide production and PPAR-γ activity have been reported [120,123]. For example, losartan has been shown to internalize with AT1a and AT1b receptors, albeit at a slower rate than Ang II [17,45], and to block intracellular and nuclear effects of Ang II [48,49,90,141,247]. Furthermore, telmisartan (less so for losartan or Irbesartan) is a partial activator of liver-specific peroxisome proliferator-activated receptor γ (PPAR-γ) in addition to blocking AT1 receptors [78,192]. Finally, although beneficial effects of ACE and renin inhibitors and ARBs are well documented, some patients continue to progress to hypertension and develop cardiovascular and renal complications despite being treated with one or two of these inhibitors [122,151]. This raises the possibility that the actions of intracellular Ang II may not be adequately blocked or other mechanisms may be involved [11]. Thus it is highly important to study the roles and underlying mechanisms of actions of intracellular Ang II and develop next generation of multifunctional ARBs that not only block cell surface but also cytoplasmic and nuclear AT1 receptors, and/or non-AT1 receptor-dependent mechanisms.
5.2. AT1 (AT1a) receptor-mediated uptake of Ang II by proximal tubules as a source of intracellular Ang II
GPCR-mediated endocytosis of extracellular Ang II plays an important role in the regulation of cellular responses to circulating and paracrine Ang II stimulation in target cells. The current dogma holds that the primary purpose of AT1 receptor-mediated endocytosis of Ang II is to desensitize cellular responses to continuous stimulation of cell surface AT1 receptor by extracellular Ang II by moving the Ang II/AT1 complex into the cells [81,85,103,233]. After endocytosis, Ang II is expected to be delivered first to endosomes and then to lysosomes for degradation, whereas the receptor recycles back to the cell surface [81,100,103]. Thus it has been long held that internalized Ang II plays little, if any, role in the regulation of cellular responses to Ang II. However, recent evidence suggests that the endosomal system may serve as a legitimate platform for the uptake of agonists via receptor-mediated endocytosis and continuous signaling from activated agonist/receptor complex in many cells [160]. Ras and mitogen-activated protein kinase (MAPK) signalling for several GPCRs including AT1a, vasopressin V2, and β2 adrenergic receptors (β2AR) have been reported in endosomal membranes [18,50,160], the endoplasmic reticulum and the Golgi independent of plasma membrane-initiated signalling [2,41,160]. We and others have recently shown that circulating and paracrine Ang II was taken up by the kidney via AT1 (AT1a) receptor-mediated endocytosis, and that high levels of internalized Ang II and AT1a receptors were found in the endosomal compartments [105,196,217,221,245,246,249]. We have further demonstrated for the first time that deletion of AT1a receptors markedly blocked intracellular uptake of [125I]Val5-Ang II [140] or unlabeled Val5-Ang II in the kidney of AT1a-KO mice [137]. However, these studies focused only on the entire kidney and the nephron segmental localization of Ang II uptake has not been determined.
Whether circulating and paracrine Ang II is taken up by proximal tubules from the luminal or the interstitial fluid compartments via apical (AP) or basolateral (BL) membrane AT1 (AT1a) receptor-mediated endocytosis has not been well studied. High density and high affinity Ang II receptors are localized in both AP and BL membranes of proximal tubule cells [22,70,76]. Ang II is known to stimulate both AP and BL membrane Ang II receptors to induce well-documented biphasic responses in proximal tubule sodium transport [43,97,101,222,240]. However, there is little information on AT1a or AT1b receptor-mediated uptake of extracellular Ang II from AP or BL membrane compartments. Becker et al. expressed a rabbit AT1 receptor in LLC-PKC14 cells, a porcine proximal tubule cell line, and showed that AT1 receptor-mediated endocytosis of [125I]-Ang II was strikingly different in AP and BL membranes [14]. [125I]-Ang II was internalized more rapidly and dominantly in AP membranes than in BL membranes [14]. By contrast, Thekkumkara et al. found that AT1a receptors were internalized 4-times faster in BL membranes than in AP membranes of opossum kidney (OK) cells [207]. We also demonstrated that extracellular Ang II was taken up via AT1 receptor-mediated endocytosis by rabbit proximal tubule cells [134,139,244,247], but the roles of AP vs. BL membrane AT1a receptors in mediating the uptake of extracellular Ang II by proximal tubules in the kidney have not been determined. Multi-photon intravital microscopic functional imaging may be an ideal tool to study AT1a receptor-mediated Ang II uptake by proximal tubules of the kidney in vivo.
In addition to AT1 (AT1a) receptor-mediated mechanisms, other factors may also regulate the uptake of extracellular Ang II by proximal tubules. Rodent kidneys express AT1a and AT1b receptors and both receptors undergo ligand-induced endocytosis in non-renal cells transiently expressing these mutant receptors [45,147,188]. Yet it remains unknown whether in the absence of dominant AT1a receptors, AT1b receptors play any role in mediating Ang II uptake in proximal tubules. We recently reported that losartan had a small but significant effect on intrarenal uptake of [125I]Val5-Ang II in AT1a-KO mice, suggesting a small role for AT1b receptors [140]. Furthermore, AP membranes of proximal tubules express abundant endocytic receptor megalin, which plays a crucial role in mediating low molecular weight (LMW) protein uptake [42,118,231]. Deletion of megalin in mice led to the development of LMW proteinuria [118,129]. Interestingly, megalin also binds and internalizes Ang II in immotalized yolk sac cells (BN-16 cells) [86]. Whether megalin plays any role in mediating the uptake of Ang II by proximal tubules is virtually unknown.
5.3. Canonical vs. noncanonical endocytic pathways in mediating Ang II uptake by proximal tubules
Although we demonstrated recently that AT1 receptors play a major role in mediating the uptake of extracellular Ang II by proximal tubule cells in vitro and circulating Ang II in mouse kidneys in vivo [134,137,139,140,244,247], the underlying cellular mechanisms and endocytic pathways involved have not been determined. In VSMCs, cardiomyocytes and COS-7 cells, GPCRs including β2AR, AT1a, EGFR, and insulin receptors are internalized via the canonical clathrin-coated pits-dependent pathway [6,81,177,209,232]. Clathrin-coated pits play an important role in invaginating and pinching off the plasma membranes to form coated vesicles and this process is powered by GTPase/dynamin with the vesicles being targeted to endosomes [178]. GPCR kinases (GRKs), small GTP-binding proteins, such as Rab5, and β-arrestins are reportedly involved in clathrin-dependent AT1a endocytosis [6,56,81,175,194,234]. However, dominant-negatives, siRNAs or knockout targeting dynamin, GRKs or β-arrestins have little effects on AT1a receptor endocytosis in some studies, suggesting that alternative (non-canonical) pathways may also be involved in AT1a receptor endocytosis [103,177]. AT1a receptors have been shown to internalize into lipid rafts/CAV-1 or non clathrin-coated vesicles in non-renal cells [215,250].
It is not known whether the canonical clathrin-coated pits/dynamin/β-arrestins or other non-canonical mechanisms mediate AT1a receptor endocytosis or uptake of extracellular Ang II by proximal tubule cells. In LLC-PKC14 cells expressing the rabbit AT1 receptor, Becker et al. showed that Ang II-induced AT1 receptor endocytosis in AP and BL membranes was significantly inhibited by phenylarsine oxide (PAO), an inhibitor of tyrosine phosphatases [7,87], but not by pertussis toxin [14,15]. Shelling and Linas showed that colchicine, an inhibitor of cytoskeleton microtubules [79], inhibited the effects of Ang II on rat proximal tubule cells by blocking Ang II receptor-mediated endocytosis [189,190]. Recently, we demonstrated that depletion of clathrin-coated pits with 400 mM sucrose or siRNA knockdown of clathrin light or high chain subunits had no effects on AT1 receptor-mediated uptake of Val5-Ang II, but colchicine or siRNA knockdown of microtubule-associated proteins MAP-1A or MAP-1B markedly inhibited AT1 receptor-mediated uptake of Val5- or FITC-labeled Ang II by proximal tubule cells [134,136,244,246]. These studies suggest that AT1 receptor-mediated uptake of Ang II by proximal tubule cells may involve the noncanonical microtubule-dependent endocytic pathway, rather than the canonical clathrin-dependent mechanisms [134,244,246]. However, the cellular mechanisms underlying the microtubule-dependent uptake of Ang II by proximal tubule cells remain to be elucidated.
Two important motifs in the 3rd cytoplasmic loop of the AT1a receptor have been identified to be critical for AT1a receptor internalization [102,208]. Triple alanine mutation or deletion of the motif Ser335-Thr336-Leu337 in the cytoplasmic tail of the receptor impaired ~95% of AT1 receptor internalization [102]. Furthermore, a distinct region of the cytoplasmic tail may also be involved in AT1a receptor endocytosis [208]. Mutation or truncation of the motif Leu316 to Tyr319 led to 2.5-fold decreases in rate and >70% reduction in maximal internalization [208,209]. Although these motifs are well conserved across various species, whether they are involved in AT1a receptor-mediated Ang II uptake by proximal tubule cells have not been investigated.
How Ang II and AT1 receptors are internalized into the endosomal compartments and transported to other organelles or the nucleus in proximal tubule cells also remains to be determined [38,46,141,157]. Radiolabeled Ang II has been found in peri-nuclear regions of rat VSMCs and cardiac myocytes [182] or the Golgi of adrenal cells after systemic administration [16]. Cook et al. demonstrated that Ang II induced AT1a receptor translocation to the nuclei of hepatocytes and VSMCs [46,47]. In HEK 293 cells stably expressing the AT1a receptor, internalized AT1a receptors were observed in peri-nuclear regions and the nuclei after stimulated by Ang II [38,157]. Recently, we also observed high levels of internalized FITC-labeled Ang II in perinuclear regions and the nucleus, which was completely inhibited by colchicine and siRNA knockdown of MAP-1A [134,136,139,141]. This suggests that the microtubule-dependent pathway may play an important role in nuclear trafficking of internalized Ang II and AT1 receptors in proximal tubule cells. Furthermore, a nuclear localization sequence (NLS, KKFKKY, aa307-312) within the AT1a receptor was recently identified to be important for nuclear trafficking and activation of AT1a receptors by Ang II [157].
5.4. In vitro effects of intracellular vs. extracellular Ang II in proximal tubule cells and signalling mechanisms
In cultured proximal tubule cells or isolated proximal tubule perfusion, exogenous Ang II stimulates the expression of NHE-3 [139,141], AP insertion of NHE-3 [72,139], Na+/H+ exchanger activity [20,84,112,143,181], or NHE-3-induced 22Na+ uptake [72,93,101,214]. The signaling mechanisms by which exogenous Ang II increases the expression and activity of NHE-3 in proximal tubule cells are well documented [73,115,191,222]. Ang II activation of cell surface GPCRs increases IP3 turnover and [Ca2+]i, generation of DG, and subsequent activation of PKC, leading to either inhibition or stimulation of proximal tubule sodium transport by extracellular Ang II [36,101,145,146,191]. The known downstream signaling pathways for extracellular Ang II that are either activated or inhibited by the PLC/[Ca2+]i/PKC signaling include calcium-dependent calcineurin activation [128], cAMP-dependent PKA [101,144,206], [Ca2+]i-independent PLA2 [14,15,75], phosphatidylinositol-3 kinase (PI 3-kinase) [72], c-Src/MAP kinases ERK 1/2 [75] and activation of NF-κB [21,142,184,244]. Thus extracellular Ang II is not the focus of the current review.
Neither the roles of intracellular Ang II nor the signaling mechanisms involved in the regulation of the NHE-3 expression, AP membrane insertion, and the Na+/H+ exchanger activity by intracellular Ang II has been well studied in proximal tubule cells. It is widely held that in order to induce intracellular actions, extracellular Ang II must bind to cell surface receptors and activate intracellular signaling mechanisms [62,71,213,241]. However, there is accumulating evidence that internalized Ang II may act as an intracellular peptide to regulate proximal tubule function. For instance, blockade of AT1 receptor endocytosis inhibits PKC activation, formation of IP3, and AP membrane Na+ flux [189,190]. Moreover, AP membrane AT1 receptor endocytosis is associated with activation of phospholipase A2 (PLA2) [14,15]. In OK cells expressing AT1a receptors, AT1 receptor endocytosis is associated with inhibition of BL membrane adenylyl cyclase and increases in AP membrane Na+ uptake [206,207]. Recently, we showed that AT1-mediated uptake of extracellular Val5-Ang II was also associated with inhibition of basal and forskolin-stimulated cAMP accumulation [134], Ang II-stimulated NHE-3 expression and AP membrane insertion [139], and Ang II-induced activation of NF-κB in proximal tubule cells [142,244]. Although these studies suggest that AT1 receptor-mediated uptake of extracellular Ang II may play an important role in the regulation of proximal tubule functions, they by no means confirmed whether these effects were due to activation of intracellular receptors.
The challenge in proving a role for intracrine/intracellular Ang II is primarily due to the lack of approaches that can distinguish between the responses induced by intracellular Ang II acting on cytoplasmic or nuclear receptors and those evoked by extracellular Ang II via activation of cell surface receptors. To overcome this difficulty, Haller et al. microinjected Ang II directly into rat VSMCs to induce intracellular and nuclear calcium responses to Ang II [91,92]. De Mello et al. dialyzed Ang II or renin directly into hamster cardiomyocytes and demonstrated that Ang II enhanced the L-type calcium currents [63,64]. Single cell microinjection or microdialysis of Ang II directly into the cells may provide an innovative approach for the proof of concept studies. We have confirmed that intracellular microinjection of Ang II directly into single rabbit proximal tubule cells also induced intracellular [Ca2+]i responses [243,246,247]. We found that co-microinjection of the AT1 blocker losartan abolished the [Ca2+]i response induced by microinjected Ang II but not completely by extracellular Ang II [247]. We further showed that Ang II directly stimulated nuclear AT1a receptors to increase in vitro transcription of mRNAs for TGF-β1, MCP-1 and NHE-3 in freshly isolated rat renal cortical nuclei [141]. Likewise, Ang II was found to increase nitric oxide or super oxide production in isolated renal cortical nuclei via AT1 or AT2 receptors [89,90]. These studies strongly suggest that intracellular Ang II may activate cytoplasmic and nuclear AT1 receptor to produce important intracellular or genomic effects in proximal tubule cells.
The other novel strategy to determine the effects of intracellular Ang II may be to express intracellular Ang II proteins in proximal tubule cells with or without expression of the wild-type or mutant Ang II receptors. Cook et al. recently transfected rat VSMCs or hepatocytes with a cyan fluorescent intracellular Ang II protein (ECFP/Ang II) and/or a rat yellow fluorescent AT1a receptor (AT1R/EYFP), and demonstrated that intracellular Ang II stimulated VSMC proliferation via activation of cAMP response element-binding protein (CREB), p38 MAP kinase and MAP kinases ERK 1/2 phosphorylation [46,48]. The construct encodes the Ang II protein without possessing secretory signal sequences and thus its expression will not lead to extracellular secretion [46,48]. Baker and Kumar have also expressed an intracellular Ang II (pcDNA/TO-iAng II) in CHO cells and interestingly they found that the intracellular Ang II induced cell proliferation independent of AT1 receptors [11,119]. How intracellular Ang II induces CHO cell proliferation without activation of AT1 receptors is not fully understood. Future studies should be designed to elucidate the signaling mechanisms by which intracellular Ang II induces short-term biological and long-term genomic effects in cardiovascular and renal systems.
5.5. Physiological effects of intracellular vs. extracellular Ang II on proximal tubule transport and blood pressure
Whether intracellular and/or internalized Ang II may physiologically regulate proximal tubular sodium transport and therefore blood pressure has also not been well studied so far. The progress has been stymied in this field due to the lack of suitable animal models that express an intracellular Ang II protein and the technical challenges in distinguishing between the effects induced by intracellular vs. extracellular Ang II. Reudelhuber's group first generated transgenic mice expressing an Ang II-producing fusion protein in cardiomyocytes to distinguish the effects between circulating and paracrine Ang II [216,228]. The expression of the Ang II-releasing fusion protein in cardiomyocytes was controlled by the α myosin heavy chain promoter. Cardiac Ang II levels are 10-fold higher than wild-type mice without elevating circulating Ang II [216,228]. Because this cardiac-specific Ang II construct contains a signal peptide sequence from human prorenin and a furin cleavage site, Ang II fusion protein will be cleaved by furin, released into the secretory pathway, and secreted into the cardiac interstitium [216,228]. Thus, Ang II secreted from cardiomyocytes activates cell surface rather than intracellular receptors to induce paracrine effects. Baker et al. used an adenoviral vector encoding an intracellular Ang II peptide to study the hypertrophic effect of cardiac-specific Ang II [10]. Intra-cardiac injection of this vector in mice surprisingly resulted in cardiac hypertrophy 48 h later, without affecting blood pressure and circulating Ang II levels [10]. How this intracellular Ang II rapidly induces cardiac hypertrophy and the effect is not blocked by the AT1 receptor blocker losartan is not known, but it may suggest an AT1 receptor-independent effect [10,11].
No attempt had been made to express an intracellular Ang II protein in any cell type of the kidney to study the physiological roles of intracellular Ang II until recently. It is not clear whether the in vitro effects of internalized or intracellular Ang II can be reproduced in proximal tubules of the kidney to regulate proximal tubule sodium and fluid reabsorption, promote salt retention and alter blood pressure in animals. Expression of an intracellular Ang II protein selectively in proximal tubules of the kidney under the control of a proximal tubule cell-specific promoter would be an ideal approach to test this hypothesis. Sigmund's group first used the kidney androgen-regulated protein gene (KAP) to drive tissue-specific expression of human angiotensinogen and renin in the kidney [66,67,125]. The KAP gene is widely expressed in the kidney, with its expression reportedly confined in proximal tubules and being regulated by androgen and estrogen [66,155,200]. Alternatively, Coffman's group generated transgenic mice in which AT1a receptors was expressed under the control of the gamma-glutamyl transpeptidase (γGT) promoter, which is specific for proximal tubule cells [126]. These approaches may be useful to investigate sexual dimorphic regulation of angiotensinogen expression or the roles of AT1a receptors in proximal tubules. However, they may not differentiate the roles of intracellular vs. extracellular Ang II in an animal model [66,125,126], because both intracellular and extracellular pathways may be involved. Potential ectopic expression of these transgenes in tissues other than proximal tubules of the kidney under the control of these promoters may also pose problems for the selective expression of target genes in proximal tubules. Most recently, transgenic mice expressing an intracellular fluorescent fusion of Ang II, ECFP/AII, have been generated using the mouse metallothionein promoter [180]. Global overexpression of this transgene in the brain, heart, kidney, liver, lung and testes led to increases in blood pressure and induced microangiopathy in the kidney [180]. This is the first transgenic mouse model demonstrating blood pressure in animals.
In collaboration with Dr. Julia Cook of Ochsner Clinic Foundation and Dr. Isabelle Rubera of University of Nice-Sophia, France, we have developed an adenoviral construct (Ad-sglt2-ECFP/AII), which encodes the cyan fluorescent fusion of Ang II protein, ECFP/AII [46,48]. We used the sodium and glucose co-transporter 2 promoter, sglt2, to drive the expression of ECFP/AII selectively in proximal tubules of the rat and mouse kidneys [183]. The sglt2 is expressed almost exclusively in early segment(s) of proximal tubules in the kidney [99]. We demonstrated that blood pressure was significantly increased by proximal tubule-specific transfer of ECFP/AII in a time-related manner [135]. The net increases in systolic blood pressure 1, 2 or 4 weeks after ECFP/Ang II transfer averaged between 15 mmHg to 30 mmHg. These levels of increased blood pressure are similar to those reported in transgenic mice globally expressing ECFP/Ang II [180] or in AT1a-KO mice with the knockin of AT1a receptors selectively in proximal tubules [127], but much lower than those induced by chronic infusion of exogenous Ang II in rats or mice [137,171,245]. Since we did not observe ectopic expression in extrarenal tissues and the expressed ECFP/AII was not released into the tubular lumen or interstitial compartment, this effect is proximal tubule- and intracellular specific. Furthermore, because the blood pressure-elevating effect of proximal tubule-selective transfer of ECFP/AII was blocked by losartan and prevented in AT1a-KO mice, our study suggests that AT1 (AT1a) receptors likely mediate this blood pressure-increasing effect [135].
6. Future Perspectives of the evolving endocrine, paracrine and intracrine RAS in cardiovascular, renal and hypertension research
In summary, the great progress has been made in the RAS research field since renin was first discovered by Tigerstedt and Bergman more than a century ago. The RAS, once considered as an endocrine system, is now recognized as dual (circulating and local/tissue) or multiple systems (endocrine, paracrine and intracrine). In addition to the classical renin/ACE/Ang II/Ang II receptor axis, we have now added several new axes to the RAS family, namely the prorenin/PRR/MAPK axis, the ACE2/Ang (1-7)/Mas receptor axis, and the Ang IV/AT4/IRAP axis. With the addition of these new members, the roles of the evolving RAS have been extended far beyond the regulation of blood pressure, aldosterone synthesis, and body fluid and electrolyte homeostasis. Indeed, novel actions for each member of the RAS are continuously discovered in physiology and diseases. However, many challenges still remain in the RAS research despite of more than one century's research. It is expected that the research on several areas of the expanded RAS will continue to play a prominent role in cardiovascular, renal and hypertension research. For example, it still remains poorly understood how the prorenin/PRR/MAPK axis, which appears to play a very limited role in physiology, is activated in many diseases such as diabetes. It is also not quite certain whether pharmacological blockade of PRR can provide additional benefits beyond the well-established renin and ACE inhibitors and ARBs in preventing and treating cardiovascular, renal and diabetic complications. Although the ACE/Ang (1-7)/Mas receptor axis appears to play a counter-regulatory role to buffer the effects of the renin/ACE/Ang II/AT1 receptor axis, the development and clinical relevance of the orally active agonists or compounds that aim to metabolize Ang II to increase Ang (1-7) production or to activate the Mas receptor still await clinical trials. In term of the Ang IV/AT4/IRAP axis, its cardiovascular and renal effects appear to be similar to the activation of the renin/ACE/Ang II/AT1 receptor axis, albeit at much higher concentrations. The focus on the Ang IV/AT4/IRAP axis should therefore be directed to its role and signalling mechanisms by which it regulate glucose uptake in health and diabetes via interactions with GLUT4. Finally, there is a renewed interest in the biology, biochemistry and physiological roles of the intracrine/intracellular RAS. It remains poorly understood how extracellular Ang II is internalized and after internalization, escapes the lysosome degradation pathways and trafficks to other intracellular organelles and the nucleus, whether and how internalized Ang II can transmitting signaling from endosomes independent of cell surface GPCR-mediated signaling, and whether and how internalized/intracellular Ang II interacts with the nuclear receptors to regulate growth and transcriptional responses. The additional challenges ahead are to develop novel strategies or compounds to block intracellular and nuclear actions of intracrine/intracellular Ang II to treat hypertension, cardiovascular and kidney diseases.
Research Highlights.
The renin-angiotensin system is now evolving as an endocrine, paracrine and intracrine system.
The renin/ACE/Ang II/AT1 receptor axis is the most recognized cascade of the entire system.
The prorenin/(Pro)renin receptor/MAP kinases ERK 1/2 axis is activated in diseased states.
The ACE2/Ang (1-7) Mas receptor axis opposes the effects induced by Ang II via AT1 receptors.
Intracrine/intracellular Ang II plays important roles in cardiovascular and renal regulation.
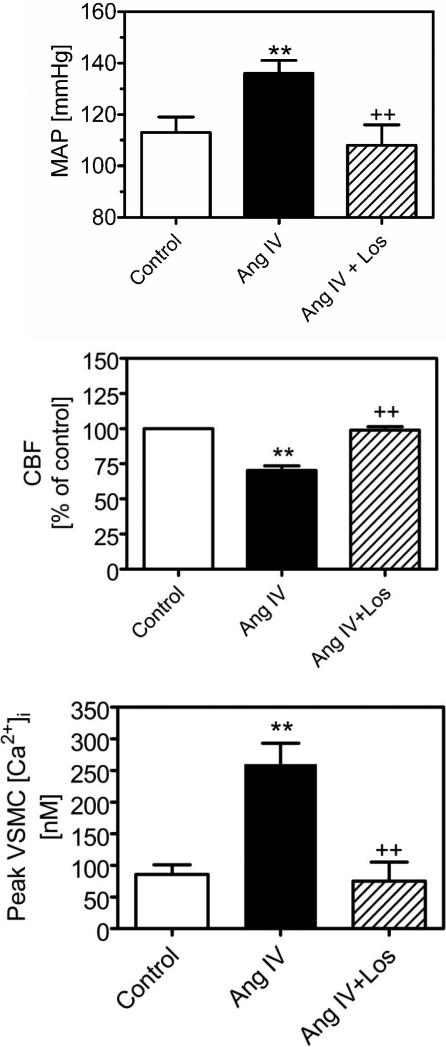
Figure 4.
AT1 receptor-dependent effects of Ang IV in the rat kidney. Top: mean arterial blood pressure (MAP) response to Ang IV in anesthetized rats. Middle: renal cortical blood flow response (CBF) to Ang IV, as determined by laser-doppler flowmeter in anesthetized rats. Bottom: intracellular calcium response to Ang IV in freshly isolated rat preglomerular vascular smooth muscle cells (VSMCs). All three responses to Ang IV were blocked by losartan, suggesting AT1 receptor-mediated effects for Ang IV in the kidney. Data are derived from [133]
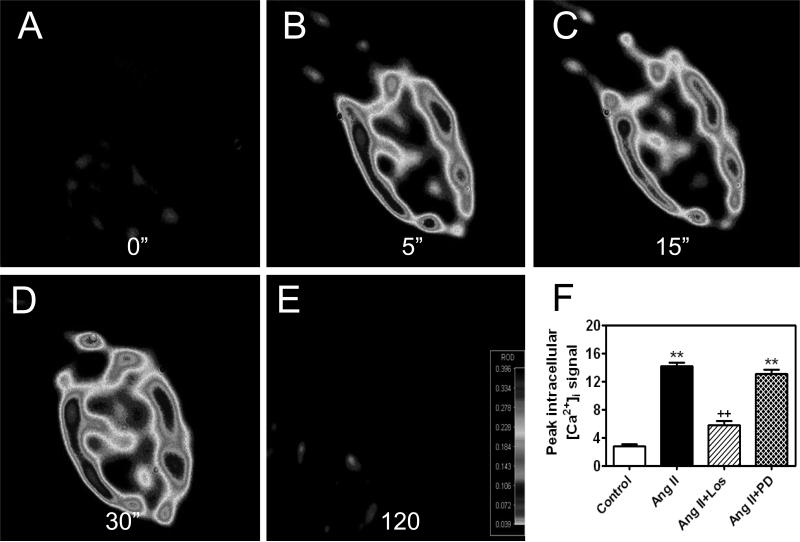
Figure 5.
Intracellular microinjection of Ang II directly into single rabbit proximal tubule cells induces time-dependent increases in intracelluar calcium levels (0 to 120 seconds), which is blocked by co-microinjection of losartan (Los), an AT1 receptor blocker, but not by PD123319 (PD), an AT2 receptor blocker. **p<0.01 vs. Control. ++p<0.01 vs. Ang II. Data are derived from [246,247].
Figure 6.
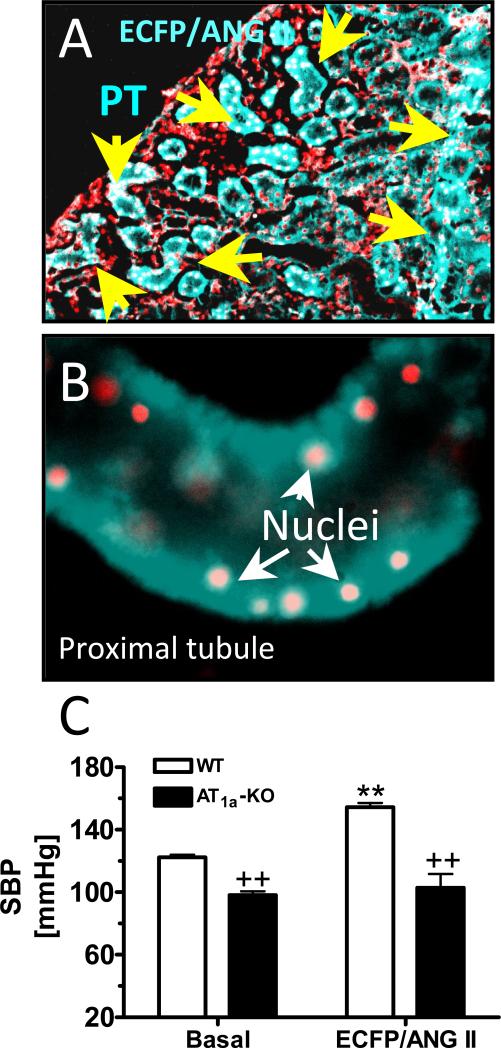
Intrarenal adenoviral transfer of an intracellular cyan fluorescent fusion of Ang II protein, ECFP/Ang II, selectively in proximal tubules of the rat or mouse kidney increases systolic blood pressure in rat and wild-type mice. A: a proximal tubule-specific promoter for the sodium and glucose co-transporter 2 was used to drive ECFP/Ang II expression selectively in proximal tubules (PT) [135]. B: ECFP/Ang II expression in a representative, freshly isolated proximal tubule. C: proximal tubule-specific transfer of ECFP/Ang II in wild-type (WT) mouse kidneys increased blood pressure, which was blocked in mice deficient of AT1a receptors (AT1a-KO). **p<0.01 vs. Control in WT mice. ++p<0.01 vs. WT mice at baselines and after ECFP/Ang II transfer. The data are derived from [135].
Acknowledgements
The authors’ work was supported in part by National Institute of Diabetes, Digestive and Kidney Diseases grants (5RO1DK067299, 2R56DK067299, and 2RO1DK067299), American Society of Nephrology M. James Scherbenske grant, and institutional supports from Henry Ford Health System, Detroit, Michigan and the University of Mississippi Medical Center, Jackson, Mississippi to Dr. Jia L. Zhuo. We sincerely thank all of our past and current collaborators for their expertise and our laboratory assistants/associates for their technical help. We apologize to other outstanding investigators whose work has not been included in this review due to the topics selected and space restriction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This is part of a series of reviews on local renin-angiotensin systems edited by Walmor C. De Mello.
References
- 1.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009 Aug;54(2):261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 2.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279(34):35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 3.Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn FA, Simpson RJ, Connolly LM, Chai SY. Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin- regulated aminopeptidase. J Biol Chem. 2001;276(52):48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- 4.Albiston AL, Yeatman HR, Pham V, Fuller SJ, Diwakarla S, Fernando RN, Chai SY. Distinct distribution of GLUT4 and insulin regulated aminopeptidase in the mouse kidney. Regul Pept. 2011;166(1-3):83–89. doi: 10.1016/j.regpep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Ambroz C, Clark AJ, Catt KJ. The mas oncogene enhances angiotensin-induced [Ca2+]i responses in cells with pre-existing angiotensin II receptors. Biochim Biophys Acta. 1991;1133(1):107–111. doi: 10.1016/0167-4889(91)90248-v. [DOI] [PubMed] [Google Scholar]
- 6.Anborgh PH, Seachrist JL, Dale LB, Ferguson SS. Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol Endocrinol. 2000;14(12):2040–2053. doi: 10.1210/mend.14.12.0565. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KM, Peach MJ. Receptor binding and internalization of a unique biologically active angiotensin II-colloidal gold conjugate: morphological analysis of angiotensin II processing in isolated vascular strips. J Vasc Res. 1994;31(1):10–17. doi: 10.1159/000159026. [DOI] [PubMed] [Google Scholar]
- 8.Ardaillou R. Active fragments of angiotensin II: enzymatic pathways of synthesis and biological effects. Curr Opin Nephrol Hypertens. 1997;6(1):28–34. doi: 10.1097/00041552-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ardaillou R, Chansel D. Synthesis and effects of active fragments of angiotensin II. Kidney Int. 1997;52(6):1458–1468. doi: 10.1038/ki.1997.476. [DOI] [PubMed] [Google Scholar]
- 10.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept. 2004;120(1-3):5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Baker KM, Kumar R. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol. 2006;291:C995–1001. doi: 10.1152/ajpcell.00238.2006. [DOI] [PubMed] [Google Scholar]
- 12.Baltatu OC, Campos LA, Bader M. Local renin-angiotensin system and the brain-A continuous quest for knowledge. Peptides. 2011 Feb. doi: 10.1016/j.peptides.2011.02.008. in press. [DOI] [PubMed] [Google Scholar]
- 13.Batenburg WW, Krop M, Garrelds IM, de VR, de Bruin RJ, Burckle CA, Muller DN, Bader M, Nguyen G, Danser AH. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens. 2007;25(12):2441–2453. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 14.Becker BN, Cheng HF, Burns KD, Harris RC. Polarized rabbit type 1 angiotensin II receptors manifest differential rates of endocytosis and recycling. Am J Physiol. 1995;269(4 Pt 1):C1048–C1056. doi: 10.1152/ajpcell.1995.269.4.C1048. [DOI] [PubMed] [Google Scholar]
- 15.Becker BN, Cheng HF, Harris RC. Apical ANG II-stimulated PLA2 activity and Na+ flux: a potential role for Ca2+-independent PLA. Am J Physiol. 1997;273(4 Pt 2):F554–62. doi: 10.1152/ajprenal.1997.273.4.F554. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi C, Gutkowska J, De Lean A, Ballak M, Anand-Srivastava MB, Genest J, Cantin M. Fate of [125I]angiotensin II in adrenal zona glomerulosa cells. Endocrinology. 1986;118:2605–2607. doi: 10.1210/endo-118-6-2605. [DOI] [PubMed] [Google Scholar]
- 17.Bihoreau C, Monnot C, Davies E, Teutsch B, Bernstein KE, Corvol P, Clauser E. Mutation of Asp74 of the rat angiotensin II receptor confers changes in antagonist affinities and abolishes G-protein coupling. Proc Natl Acad Sci U S A. 1993;90(11):5133–5137. doi: 10.1073/pnas.90.11.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bivona TG, Philips MR. Ras pathway signaling on endomembranes. Curr Opin Cell Biol. 2003;15(2):136–142. doi: 10.1016/s0955-0674(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 19.Blaxall BC, Miano JM, Berk BC. Angiotensin II: a devious activator of mineralocorticoid receptor-dependent gene expression. Circ Res. 2005;96(6):610–611. doi: 10.1161/01.RES.0000162163.75564.8b. [DOI] [PubMed] [Google Scholar]
- 20.Bloch RD, Zikos D, Fisher KA, Schleicher L, Oyama M, Cheng JC, Skopicki HA, Sukowski EJ, Cragoe EJ, Jr., Peterson DR. Activation of proximal tubular Na(+)-H+ exchange by angiotensin II. Am J Physiol. 1992;263(1 Pt 2):F135–F143. doi: 10.1152/ajprenal.1992.263.1.F135. [DOI] [PubMed] [Google Scholar]
- 21.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem. 2000;212:155–169. [PubMed] [Google Scholar]
- 22.Brown GP, Douglas JG. Angiotensin II binding sites on isolated rat renal brush border membranes. Endocrinology. 1982;111(6):1830–1836. doi: 10.1210/endo-111-6-1830. [DOI] [PubMed] [Google Scholar]
- 23.Burgelova M, Vanourkova Z, Thumova M, Dvorak P, Opocensky M, Kramer HJ, Zelizko M, Maly J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1-7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens. 2009;27(10):1988–2000. doi: 10.1097/HJH.0b013e32832f0d06. [DOI] [PubMed] [Google Scholar]
- 24.Burns WC, Velkoska E, Dean R, Burrell LM, Thomas MC. Angiotensin II mediates epithelial-to-mesenchymal transformation in tubular cells by ANG 1-7/MAS-1-dependent pathways. Am J Physiol Renal Physiol. 2010;299(3):F585–F593. doi: 10.1152/ajprenal.00538.2009. [DOI] [PubMed] [Google Scholar]
- 25.Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79(1):1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell DJ. Critical review of prorenin and (pro)renin receptor research. Hypertension. 2008;51(5):1259–1264. doi: 10.1161/HYPERTENSIONAHA.108.110924. [DOI] [PubMed] [Google Scholar]
- 27.Campbell DJ, Kladis A, Duncan AM. Nephrectomy, converting enzyme inhibition, and angiotensin peptides. Hypertension. 1993;22(4):513–522. doi: 10.1161/01.hyp.22.4.513. [DOI] [PubMed] [Google Scholar]
- 28.Campbell DJ, Kladis A, Skinner SL, Whitworth JA. Characterization of angiotensin peptides in plasma of anephric man. J Hypertens. 1991;9(3):265–274. doi: 10.1097/00004872-199103000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 30.Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35(1 Pt 2):155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Carraro-Lacroix LR, Girardi AC, Malnic G. Long-term regulation of vacuolar H(+)-ATPase by angiotensin II in proximal tubule cells. Pflugers Arch. 2009;458(5):969–979. doi: 10.1007/s00424-009-0668-9. [DOI] [PubMed] [Google Scholar]
- 32.Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40(5):735–741. doi: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- 33.Chai SY, Fernando R, Peck G, Ye SY, Mendelsohn FA, Jenkins TA, Albiston AL. The angiotensin IV/AT4 receptor. Cell Mol Life Sci. 2004;61(21):2728–2737. doi: 10.1007/s00018-004-4246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chansel D, Vandermeersch S, Oko A, Curat C, Ardaillou R. Effects of angiotensin IV and angiotensin-(1-7) on basal and angiotensin II-stimulated cytosolic Ca2+ in mesangial cells. Eur J Pharmacol. 2001;414(2-3):165–175. doi: 10.1016/s0014-2999(01)00791-9. [DOI] [PubMed] [Google Scholar]
- 35.Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: more than regulation of blood pressure? Hypertension. 2007;50(4):596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 36.Chatsudthipong V, Chan YL. Inhibitory effect of angiotensin II on renal tubular transport. Am J Physiol. 1991;260(3 Pt 2):F340–F346. doi: 10.1152/ajprenal.1991.260.3.F340. [DOI] [PubMed] [Google Scholar]
- 37.Chen JK, Zimpelmann J, Harris RC, Burns KD. Angiotensin IV induces tyrosine phosphorylation of focal adhesion kinase and paxillin in proximal tubule cells. Am J Physiol Renal Physiol. 2001;280(6):F980–F988. doi: 10.1152/ajprenal.2001.280.6.F980. [DOI] [PubMed] [Google Scholar]
- 38.Chen R, Mukhin YV, Garnovskaya MN, Thielen TE, Iijima Y, Huang C, Raymond JR, Ullian ME, Paul RV. A functional angiotensin II receptor-GFP fusion protein: evidence for agonist-dependent nuclear translocation. Am J Physiol Renal Physiol. 2000;279(3):F440–F448. doi: 10.1152/ajprenal.2000.279.3.F440. [DOI] [PubMed] [Google Scholar]
- 39.Cheng HF, Becker BN, Burns KD, Harris RC. Angiotensin II upregulates type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest. 1995;95(5):2012–2019. doi: 10.1172/JCI117886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Q, Leung PS. An update on the islet renin-angiotensin system. Peptides. 2011 Mar. doi: 10.1016/j.peptides.2011.03.003. in press. [DOI] [PubMed] [Google Scholar]
- 41.Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL, Cox AD, Philips MR. Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol. 2002;4(5):343–350. doi: 10.1038/ncb783. [DOI] [PubMed] [Google Scholar]
- 42.Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol. 2001;280(4):F562–F573. doi: 10.1152/ajprenal.2001.280.4.F562. [DOI] [PubMed] [Google Scholar]
- 43.Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension. 1990;15(5):451–458. doi: 10.1161/01.hyp.15.5.451. [DOI] [PubMed] [Google Scholar]
- 44.Coleman JK, Krebs LT, Hamilton TA, Ong B, Lawrence KA, Sardinia MF, Harding JW, Wright JW. Autoradiographic identification of kidney angiotensin IV binding sites and angiotensin IV-induced renal cortical blood flow changes in rats. Peptides. 1998;19(2):269–277. doi: 10.1016/s0196-9781(97)00291-x. [DOI] [PubMed] [Google Scholar]
- 45.Conchon S, Monnot C, Teutsch B, Corvol P, Clauser E. Internalization of the rat AT1a and AT1b receptors: pharmacological and functional requirements. FEBS Lett. 1994;349(3):365–370. doi: 10.1016/0014-5793(94)00703-9. [DOI] [PubMed] [Google Scholar]
- 46.Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol. 2006;40(5):696–707. doi: 10.1016/j.yjmcc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Cook JL, Mills SJ, Naquin RT, Alam J, Re RN. Cleavage of the angiotensin II type 1 receptor and nuclear accumulation of the cytoplasmic carboxy-terminal fragment. Am J Physiol Cell Physiol. 2007;292(4):C1313–C1322. doi: 10.1152/ajpcell.00454.2006. [DOI] [PubMed] [Google Scholar]
- 48.Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol. 2004;36(1):75–90. doi: 10.1016/j.yjmcc.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Cook JL, Zhang Z, Re RN. In vitro evidence for an intracellular site of angiotensin action. Circ Res. 2001;89(12):1138–46. doi: 10.1161/hh2401.101270. [DOI] [PubMed] [Google Scholar]
- 50.Cottrell GS, Padilla BE, Amadesi S, Poole DP, Murphy JE, Hardt M, Roosterman D, Steinhoff M, Bunnett NW. Endosomal endothelin-converting enzyme-1: a regulator of beta-arrestin-dependent ERK signaling. J Biol Chem. 2009;284(33):22411–22425. doi: 10.1074/jbc.M109.026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveirados-Santos AJ, da CJ, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 52.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115(4):1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Czekalski S, Chansel D, Vandermeersch S, Ronco P, Ardaillou R. Evidence for angiotensin IV receptors in human collecting duct cells. Kidney Int. 1996;50(4):1125–1131. doi: 10.1038/ki.1996.419. [DOI] [PubMed] [Google Scholar]
- 55.da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, de Sousa LP, Teixeira MM, Santos RA, Simoes e Silva AC, Ribeiro Vieira MA. ACE2-angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond) 2010;119(9):385–394. doi: 10.1042/CS20090554. [DOI] [PubMed] [Google Scholar]
- 56.Dale LB, Seachrist JL, Babwah AV, Ferguson SS. Regulation of angiotensin II type 1A receptor intracellular retention, degradation, and recycling by Rab5, Rab7, and Rab11 GTPases. J Biol Chem. 2004;279:13110–13118. doi: 10.1074/jbc.M313333200. [DOI] [PubMed] [Google Scholar]
- 57.Danser AH. (Pro)renin receptor and vacuolar H+-ATPase. Hypertension. 2009;54(2):219–221. doi: 10.1161/HYPERTENSIONAHA.109.135236. [DOI] [PubMed] [Google Scholar]
- 58.Danser AH. (Pro)renin receptors: are they biologically relevant? Curr Opin Nephrol Hypertens. 2009;18(1):74–78. doi: 10.1097/MNH.0b013e3283196aaf. [DOI] [PubMed] [Google Scholar]
- 59.Danser AH, van den Dorpel MA, Deinum J, Derkx FH, Franken AA, Peperkamp E, de Jong PT, Schalekamp MA. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab. 1989;68(1):160–167. doi: 10.1210/jcem-68-1-160. [DOI] [PubMed] [Google Scholar]
- 60.Darby IA, Aldred P, Crawford RJ, Fernley RT, Niall HD, Penschow JD, Ryan GB, Coghlan JP. Renin gene expression in vessels of the ovine renal cortex. J Hypertens. 1985;3(1):9–11. doi: 10.1097/00004872-198502000-00002. [DOI] [PubMed] [Google Scholar]
- 61.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–472. [PubMed] [Google Scholar]
- 62.de Gasparo M. AT1 and AT2 angiotensin II receptors: key features. Drugs. 2002;62(1):1–10. [PubMed] [Google Scholar]
- 63.De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension. 1998;32(6):976–982. doi: 10.1161/01.hyp.32.6.976. [DOI] [PubMed] [Google Scholar]
- 64.De Mello WC. Renin increments the inward calcium current in the failing heart. J.Hypertens. 2006;24(6):1181–6. doi: 10.1097/01.hjh.0000226209.88312.db. [DOI] [PubMed] [Google Scholar]
- 65.De Mello WC, Danser AH. Angiotensin II and the heart: on the intracrine renin-angiotensin system. Hypertension. 2000;35(6):1183–1188. doi: 10.1161/01.hyp.35.6.1183. [DOI] [PubMed] [Google Scholar]
- 66.Ding Y, Davisson RL, Hardy DO, Zhu LJ, Merrill DC, Catterall JF, Sigmund CD. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1997;272(44):28142–28148. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 67.Ding Y, Sigmund CD. Androgen-dependent regulation of human angiotensinogen expression in KAP-hAGT transgenic mice. Am J Physiol Renal Physiol. 2001;280(1):F54–F60. doi: 10.1152/ajprenal.2001.280.1.F54. [DOI] [PubMed] [Google Scholar]
- 68.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 69.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 70.Douglas JG. Angiotensin receptor subtypes of the kidney cortex. Am J Physiol. 1987;253(1 Pt 2):F1–F7. doi: 10.1152/ajprenal.1987.253.1.F1. [DOI] [PubMed] [Google Scholar]
- 71.Douglas JG, Hopfer U. Novel aspect of angiotensin receptors and signal transduction in the kidney. Annu Rev Physiol. 1994;56:649–669. doi: 10.1146/annurev.ph.56.030194.003245. [DOI] [PubMed] [Google Scholar]
- 72.du CD, Chalumeau C, Defontaine N, Klein C, Kellermann O, Paillard M, Poggioli J. Angiotensin II stimulates NHE3 activity by exocytic insertion of the transporter: role of PI 3-kinase. Kidney Int. 2003;64(3):939–949. doi: 10.1046/j.1523-1755.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 73.Du Z, Ferguson W, Wang T. Role of PKC and calcium in modulation of effects of angiotensin II on sodium transport in proximal tubule. Am J Physiol Renal Physiol. 2003;284(4):F688–F692. doi: 10.1152/ajprenal.00261.2002. [DOI] [PubMed] [Google Scholar]
- 74.Dulin N, Madhun ZT, Chang CH, Berti-Mattera L, Dickens D, Douglas JG. Angiotensin IV receptors and signaling in opossum kidney cells. Am J Physiol. 1995;269(5 Pt 2):F644–F652. doi: 10.1152/ajprenal.1995.269.5.F644. [DOI] [PubMed] [Google Scholar]
- 75.Dulin NO, Alexander LD, Harwalkar S, Falck JR, Douglas JG. Phospholipase A2-mediated activation of mitogen-activated protein kinase by angiotensin II. Proc Natl Acad Sci U S A. 1998;95(14):8098–8102. doi: 10.1073/pnas.95.14.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dulin NO, Ernsberger P, Suciu DJ, Douglas JG. Rabbit renal epithelial angiotensin II receptors. Am J Physiol. 1994;267(5 Pt 2):F776–F782. doi: 10.1152/ajprenal.1994.267.5.F776. [DOI] [PubMed] [Google Scholar]
- 77.Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37(4):1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 78.Edgley AJ, Nichols NR, Anderson WP. Acute intrarenal infusion of ANG II does not stimulate immediate early gene expression in the kidney. Am J Physiol Regul Integr Comp Physiol. 2002;282(4):R1133–R1139. doi: 10.1152/ajpregu.00187.2001. [DOI] [PubMed] [Google Scholar]
- 79.Elkjaer ML, Birn H, Agre P, Christensen EI, Nielsen S. Effects of microtubule disruption on endocytosis, membrane recycling and polarized distribution of Aquaporin-1 and gp330 in proximal tubule cells. Eur J Cell Biol. 1995;67(1):57–72. [PubMed] [Google Scholar]
- 80.Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A, Jan Danser AH, Bader M, Nguyen G, Luft FC, Muller DN. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension. 2008;51(3):682–688. doi: 10.1161/HYPERTENSIONAHA.107.101444. [DOI] [PubMed] [Google Scholar]
- 81.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53(1):1–24. [PubMed] [Google Scholar]
- 82.Ferrario CM, Varagic J. The ANG-(1-7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Renal Physiol. 2010;298(6):F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardiner SM, Kemp PA, March JE, Bennett T. Regional haemodynamic effects of angiotensin II (3-8) in conscious rats. Br J Pharmacol. 1993;110(1):159–162. doi: 10.1111/j.1476-5381.1993.tb13786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3- cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A. 1990;87(20):7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonzalez-Gaitan M. Signal dispersal and transduction through the endocytic pathway. Nat Rev Mol Cell Biol. 2003;4(3):213–224. doi: 10.1038/nrm1053. [DOI] [PubMed] [Google Scholar]
- 86.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol. 2005;288:F420–F427. doi: 10.1152/ajprenal.00243.2004. [DOI] [PubMed] [Google Scholar]
- 87.Griendling KK, Delafontaine P, Rittenhouse SE, Gimbrone MA, Jr., Alexander RW. Correlation of receptor sequestration with sustained diacylglycerol accumulation in angiotensin II-stimulated cultured vascular smooth muscle cells. J Biol Chem. 1987;262(30):14555–14562. [PubMed] [Google Scholar]
- 88.Gurley SB, Allred A, Le TH, Griffiths R, Mao L, Philip N, Haystead TA, Donoghue M, Breitbart RE, Acton SL, Rockman HA, Coffman TM. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest. 2006;116(8):2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gwathmey T, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II - type 2 (AT2) receptors are functionally linked to nitric oxide production. Am.J Physiol Renal Physiol. 2009;296(6):F1484–93. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1-7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55(1):166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res. 1996;79(4):765–772. doi: 10.1161/01.res.79.4.765. [DOI] [PubMed] [Google Scholar]
- 92.Haller H, Lindschau C, Quass P, Luft FC. Intracellular actions of angiotensin II in vascular smooth muscle cells. J Am Soc Nephrol. 1999;10(Suppl 11):S75–S83. [PubMed] [Google Scholar]
- 93.Han HJ, Park SH, Koh HJ, Taub M. Mechanism of regulation of Na+ transport by angiotensin II in primary renal cells. Kidney Int. 2000 Jun;57(6):2457–2467. doi: 10.1046/j.1523-1755.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 94.Handa RK. Characterization and signaling of the AT4 receptor in human proximal tubule epithelial (HK-2) cells. J Am Soc Nephrol. 2001;12(3):440–449. doi: 10.1681/ASN.V123440. [DOI] [PubMed] [Google Scholar]
- 95.Handa RK, Harding JW, Simasko SM. Characterization and function of the bovine kidney epithelial angiotensin receptor subtype 4 using angiotensin IV and divalinal angiotensin IV as receptor ligands. J Pharmacol Exp Ther. 1999;291(3):1242–1249. [PubMed] [Google Scholar]
- 96.Handa RK, Krebs LT, Harding JW, Handa SE. Angiotensin IV AT4-receptor system in the rat kidney. Am J Physiol. 1998;274(2 Pt 2):F290–F299. doi: 10.1152/ajprenal.1998.274.2.F290. [DOI] [PubMed] [Google Scholar]
- 97.Harris PJ, Navar LG. Tubular transport responses to angiotensin II. Am J Physiol Renal Physiol. 1985;248:F621–F630. doi: 10.1152/ajprenal.1985.248.5.F621. [DOI] [PubMed] [Google Scholar]
- 98.Harrison-Bernard LM, Cook AK, Oliverio MI, Coffman TM. Renal segmental microvascular responses to ANG II in AT1A receptor null mice. Am J Physiol Renal Physiol. 2003;284(3):F538–F545. doi: 10.1152/ajprenal.00340.2002. [DOI] [PubMed] [Google Scholar]
- 99.Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74(4):993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- 100.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11(9):1266–1277. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- 101.Houillier P, Chambrey R, Achard JM, Froissart M, Poggioli J, Paillard M. Signaling pathways in the biphasic effect of angiotensin II on apical Na+/H+ antiport activity in proximal tubule. Kidney Int. 1996;50(5):1496–1505. doi: 10.1038/ki.1996.464. [DOI] [PubMed] [Google Scholar]
- 102.Hunyady L, Bor M, Balla T, Catt KJ. Identification of a cytoplasmic Ser-Thr-Leu motif that determines agonist-induced internalization of the AT1 angiotensin receptor. J Biol Chem. 1994;269(50):31378–31382. [PubMed] [Google Scholar]
- 103.Hunyady L, Catt KJ, Clark AJ, Gaborik Z. Mechanisms and functions of AT1 angiotensin receptor internalization. Regul Pept. 2000;91(1-3):29–44. doi: 10.1016/s0167-0115(00)00137-3. [DOI] [PubMed] [Google Scholar]
- 104.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114(8):1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Imig JD, Navar GL, Zou LX, O'Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol. 1999;277(2 Pt 2):F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 106.Ingelfinger JR. Angiotensin-converting enzyme 2: implications for blood pressure and kidney disease. Curr Opin Nephrol Hypertens. 2009;18(1):79–84. doi: 10.1097/MNH.0b013e32831b70ad. [DOI] [PubMed] [Google Scholar]
- 107.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol. 1999;276(2 Pt 2):F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 108.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A. 1995;92(8):3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jackson TR, Blair LA, Marshall J, Goedert M, Hanley MR. The mas oncogene encodes an angiotensin receptor. Nature. 1988;335(6189):437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- 110.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96(6):643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 111.Johnston CI. Franz Volhard Lecture. Renin-angiotensin system: a dual tissue and hormonal system for cardiovascular control. J Hypertens Suppl. 1992;10(7):S13–S26. [PubMed] [Google Scholar]
- 112.Jourdain M, Amiel C, Friedlander G. Modulation of Na+-H+ exchange activity by angiotensin II in opossum kidney cells. Am J Physiol. 1992;263(6 Pt 1):C1141–C1146. doi: 10.1152/ajpcell.1992.263.6.C1141. [DOI] [PubMed] [Google Scholar]
- 113.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kamilic J, Hamming I, Kreutz R, Bolbrinker J, Siems WE, Nassar I, Sluimer JC, Walther T, Navis GJ, van GH. Renal ACE2 expression and activity is unaltered during established hypertension in adult SHRSP and TGR(mREN2)27. Hypertens Res. 2010;33(2):123–128. doi: 10.1038/hr.2009.191. [DOI] [PubMed] [Google Scholar]
- 115.Karim Z, Defontaine N, Paillard M, Poggioli J. Protein kinase C isoforms in rat kidney proximal tubule: acute effect of angiotensin II. Am J Physiol. 1995;269(1 Pt 1):C134–C140. doi: 10.1152/ajpcell.1995.269.1.C134. [DOI] [PubMed] [Google Scholar]
- 116.Keller SR, Davis AC, Clairmont KB. Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. J Biol Chem. 2002;277(20):17677–17686. doi: 10.1074/jbc.M202037200. [DOI] [PubMed] [Google Scholar]
- 117.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 118.Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, utry-Varsat A, Gburek J, Willnow TE, Christensen EI, Moestrup SK. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci U S A. 2001;98(22):12491–12496. doi: 10.1073/pnas.211291398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: implications in cardiovascular remodeling. Curr Opin Nephrol Hypertens. 2008;17(2):168–173. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- 120.Kurtz TW. Beyond the classic angiotensin-receptor-blocker profile. Nat Clin Pract Cardiovasc Med. 2008;5(Suppl 1):S19–26. doi: 10.1038/ncpcardio0805. S19-S26. [DOI] [PubMed] [Google Scholar]
- 121.Kurtz TW, Gardner DG. Transcription-modulating drugs: a new frontier in the treatment of essential hypertension. Hypertension. 1998;32:380–386. doi: 10.1161/01.hyp.32.3.380. [DOI] [PubMed] [Google Scholar]
- 122.Kurtz TW, Klein U. Next generation multifunctional angiotensin receptor blockers. Hypertens Res. 2009;32(10):826–34. doi: 10.1038/hr.2009.135. [DOI] [PubMed] [Google Scholar]
- 123.Kurtz TW, Pravenec M. Molecule-specific effects of angiotensin II-receptor blockers independent of the renin-angiotensin system. Am J Hypertens. 2008;21(8):852–859. doi: 10.1038/ajh.2008.202. [DOI] [PubMed] [Google Scholar]
- 124.Larkin JE, Frank BC, Gaspard RM, Duka I, Gavras H, Quackenbush J. Cardiac transcriptional response to acute and chronic angiotensin II treatments. Physiol Genomics. 2004;18(2):152–166. doi: 10.1152/physiolgenomics.00057.2004. [DOI] [PubMed] [Google Scholar]
- 125.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol. 2004;286(5):F965–F971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 126.Le TH, Oliverio MI, Kim HS, Salzler H, Dash RC, Howell DN, Smithies O, Bronson S, Coffman TM. A gammaGT-AT1A receptor transgene protects renal cortical structure in AT1 receptor-deficient mice. Physiol Genomics. 2004;18(3):290–298. doi: 10.1152/physiolgenomics.00120.2003. [DOI] [PubMed] [Google Scholar]
- 127.Le TH, Kim HS, Allen AM, Spurney RF, Smithies O, Coffman TM. Physiological impact of increased expression of the AT1 angiotensin receptor. Hypertension. 2003;42(4):507–14. doi: 10.1161/01.HYP.0000092000.07559.57. [DOI] [PubMed] [Google Scholar]
- 128.Lea JP, Jin SG, Roberts BR, Shuler MS, Marrero MB, Tumlin JA. Angiotensin II stimulates calcineurin activity in proximal tubule epithelia through AT-1 receptor-mediated tyrosine phosphorylation of the PLC-gamma1 isoform. J Am Soc Nephrol. 2002;13(7):1750–1756. doi: 10.1097/01.asn.0000022029.50356.2c. [DOI] [PubMed] [Google Scholar]
- 129.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol. 1999;155(4):1361–1370. doi: 10.1016/S0002-9440(10)65238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lely AT, Hamming I, van GH, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204(5):587–593. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 131.Lemarie CA, Simeone SM, Nikonova A, Ebrahimian T, Deschenes ME, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-Induced Activation of Signaling Pathways Requires Activity of Angiotensin Type 1a Receptors. Circ Res. 2009;105(9):852–9. doi: 10.1161/CIRCRESAHA.109.196576. [DOI] [PubMed] [Google Scholar]
- 132.Lenz T, Sealey JE, Lappe RW, Carilli C, Oshiro GT, Baxter JD, Laragh JH. Infusion of recombinant human prorenin into rhesus monkeys. Effects on hemodynamics, renin-angiotensin-aldosterone axis and plasma testosterone. Am J Hypertens. 1990;3(4):257–261. doi: 10.1093/ajh/3.4.257. [DOI] [PubMed] [Google Scholar]
- 133.Li XC, Campbell DJ, Ohishi M, Yuan S, Zhuo JL. AT1 receptor-activated signaling mediates angiotensin IV-induced renal cortical vasoconstriction in rats. Am J Physiol Renal Physiol. 2006;290(5):F1024–F1033. doi: 10.1152/ajprenal.00221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li XC, Carretero OA, Navar LG, Zhuo JL. AT1 receptor-mediated accumulation of extracellular angiotensin II in proximal tubule cells: role of cytoskeleton microtubules and tyrosine phosphatases. Am J Physiol Renal Physiol. 2006;291:F375–F383. doi: 10.1152/ajprenal.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular cyan fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol. 2011 Feb. doi: 10.1152/ajprenal.00329.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li XC, Hopfer U, Zhuo JL. AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through the microtubule-dependent endocytic pathway. Am J Physiol Renal Physiol. 2009;297(5):F1342–52. doi: 10.1152/ajprenal.90734.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of angiotensin II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol. 2007;293:F586–F593. doi: 10.1152/ajprenal.00489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li XC, Shao Y, Zhuo JL. AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int. 2009;76(2):169–77. doi: 10.1038/ki.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li XC, Zhuo JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-Ang II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol. 2007;293:C367–C378. doi: 10.1152/ajpcell.00463.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li XC, Zhuo JL. In vivo regulation of AT1a receptor-mediated intracellular uptake of [125I]-Val5-angiotensin II in the kidneys and adrenal glands of AT1a receptor-deficient mice. Am J Physiol Renal Physiol. 2008;294:F293–F302. doi: 10.1152/ajprenal.00398.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-beta1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol. 2008;294(4):C1034–C1045. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li XC, Zhuo JL. Nuclear factor-kappaB as a hormonal intracellular signaling molecule: focus on angiotensin II-induced cardiovascular and renal injury. Curr Opin Nephrol Hypertens. 2008;17(1):37–43. doi: 10.1097/MNH.0b013e3282f2903c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu FY, Cogan MG. Angiotensin II stimulation of hydrogen ion secretion in the rat early proximal tubule. Modes of action, mechanism, and kinetics. J Clin Invest. 1988;82(2):601–607. doi: 10.1172/JCI113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu FY, Cogan MG. Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. J Clin Invest. 1989;84(1):83–91. doi: 10.1172/JCI114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu FY, Cogan MG. Effects of intracellular calcium on proximal bicarbonate absorption. Am J Physiol. 1990;259(3 Pt 2):F451–F457. doi: 10.1152/ajprenal.1990.259.3.F451. [DOI] [PubMed] [Google Scholar]
- 146.Liu FY, Cogan MG. Role of protein kinase C in proximal bicarbonate absorption and angiotensin signaling. Am J Physiol. 1990;258(4 Pt 2):F927–F933. doi: 10.1152/ajprenal.1990.258.4.F927. [DOI] [PubMed] [Google Scholar]
- 147.Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension. 1994;24:538–548. doi: 10.1161/01.hyp.24.5.538. [DOI] [PubMed] [Google Scholar]
- 148.Lochard N, Thibault G, Silversides DW, Touyz RM, Reudelhuber TL. Chronic production of angiotensin IV in the brain leads to hypertension that is reversible with an angiotensin II AT1 receptor antagonist. Circ Res. 2004;94(11):1451–1457. doi: 10.1161/01.RES.0000130654.56599.40. [DOI] [PubMed] [Google Scholar]
- 149.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62(2):305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Magaldi AJ, Cesar KR, de AM, Simoes e Silva AC, Santos RA. Angiotensin-(1-7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V2 receptors. Pflugers Arch. 2003;447(2):223–230. doi: 10.1007/s00424-003-1173-1. [DOI] [PubMed] [Google Scholar]
- 151.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 153.Mendelsohn FA. Angiotensin II: evidence for its role as an intrarenal hormone. Kidney Int Suppl. 1982;12:S78–S81. [PubMed] [Google Scholar]
- 154.Mercure C, Prescott G, Lacombe MJ, Silversides DW, Reudelhuber TL. Chronic increases in circulating prorenin are not associated with renal or cardiac pathologies. Hypertension. 2009;53(6):1062–1069. doi: 10.1161/HYPERTENSIONAHA.108.115444. [DOI] [PubMed] [Google Scholar]
- 155.Meseguer A, Catterall JF. Cell-specific expression of kidney androgen-regulated protein messenger RNA is under multihormonal control. Mol Endocrinol. 1990;4(8):1240–1248. doi: 10.1210/mend-4-8-1240. [DOI] [PubMed] [Google Scholar]
- 156.Min LJ, Mogi M, Li JM, Iwanami J, Iwai M, Horiuchi M. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ Res. 2005;97(5):434–442. doi: 10.1161/01.RES.0000180753.63183.95. [DOI] [PubMed] [Google Scholar]
- 157.Morinelli TA, Raymond JR, Baldys A, Yang Q, Lee MH, Luttrell L, Ullian ME. Identification Of A Putative Nuclear Localization Sequence Within The Angiotensin II AT1A Receptor Associated with Nuclear Activation. Am J Physiol Cell Physiol. 2007;292(4):C1398–408. doi: 10.1152/ajpcell.00337.2006. [DOI] [PubMed] [Google Scholar]
- 158.Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35(1 Pt 2):193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 159.Muller DN, Hilgers KF, Mathews S, Breu V, Fischli W, Uhlmann R, Luft FC. Effects of human prorenin in rats transgenic for human angiotensinogen. Hypertension. 1999;33(1 Pt 2):312–317. doi: 10.1161/01.hyp.33.1.312. [DOI] [PubMed] [Google Scholar]
- 160.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: A legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106(42):17615–22. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med. 2006;18(3):483–488. [PubMed] [Google Scholar]
- 162.Navar LG, Carmines PK, Huang WC, Mitchell KD. The tubular effects of angiotensin II. Kidney Int Suppl. 1987;20:S81–S88. [PubMed] [Google Scholar]
- 163.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17(5):412–422. [PubMed] [Google Scholar]
- 164.Nguyen G. The (pro)renin receptor: pathophysiological roles in cardiovascular and renal pathology. Curr Opin Nephrol Hypertens. 2007;16(2):129–133. doi: 10.1097/MNH.0b013e328040bfab. [DOI] [PubMed] [Google Scholar]
- 165.Nguyen G. The (pro)renin receptor in health and disease. Ann Med. 2010;42(1):13–18. [PubMed] [Google Scholar]
- 166.Nguyen G. Renin, (pro)renin and receptor: an update. Clin Sci (Lond) 2011;120(5):169–178. doi: 10.1042/CS20100432. [DOI] [PubMed] [Google Scholar]
- 167.Nguyen G, Contrepas A. Physiology and pharmacology of the (pro)renin receptor. Curr Opin Pharmacol. 2008;8(2):127–132. doi: 10.1016/j.coph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 168.Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int. 1996;50(6):1897–1903. doi: 10.1038/ki.1996.511. [DOI] [PubMed] [Google Scholar]
- 169.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J.Clin.Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Oliverio MI, Delnomdedieu M, Best CF, Li P, Morris M, Callahan MF, Johnson GA, Smithies O, Coffman TM. Abnormal water metabolism in mice lacking the type 1A receptor for ANG II. Am J Physiol Renal Physiol. 2000;278(1):F75–F82. doi: 10.1152/ajprenal.2000.278.1.F75. [DOI] [PubMed] [Google Scholar]
- 171.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292(1):F330–9. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30(5):1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 173.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension. 2009;53(2):338–343. doi: 10.1161/HYPERTENSIONAHA.108.124198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pullman TN, Oparil S, Carone FA. Fate of labeled angiotensin II microinfused into individual nephrons in the rat. Am J Physiol. 1975;228(3):747–751. doi: 10.1152/ajplegacy.1975.228.3.747. [DOI] [PubMed] [Google Scholar]
- 175.Qian H, Pipolo L, Thomas WG. Association of beta-Arrestin 1 with the type 1A angiotensin II receptor involves phosphorylation of the receptor carboxyl terminus and correlates with receptor internalization. Mol Endocrinol. 2001;15(10):1706–1719. doi: 10.1210/mend.15.10.0714. [DOI] [PubMed] [Google Scholar]
- 176.Rajagopal K, Lefkowitz RJ, Rockman HA. When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest. 2005;115(11):2971–2974. doi: 10.1172/JCI26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103(44):16284–9. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rappoport JZ, Kemal S, Benmerah A, Simon SM. Dynamics of clathrin and adaptor proteins during endocytosis. Am J Physiol Cell Physiol. 2006;291(5):C1072–81. doi: 10.1152/ajpcell.00160.2006. [DOI] [PubMed] [Google Scholar]
- 179.Re RN. On the biological actions of intracellular angiotensin. Hypertension. 2000;35(6):1189–1190. doi: 10.1161/01.hyp.35.6.1189. [DOI] [PubMed] [Google Scholar]
- 180.Redding KM, Chen BL, Singh A, Re RN, Navar LG, Seth DM, Sigmund CD, Tang WW, Cook JL. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am J Physiol Heart Circ Physiol. 2010;298(6):H1807–18. doi: 10.1152/ajpheart.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reilly AM, Harris PJ, Williams DA. Biphasic effect of angiotensin II on intracellular sodium concentration in rat proximal tubules. Am J Physiol. 1995;269(3 Pt 2):F374–F380. doi: 10.1152/ajprenal.1995.269.3.F374. [DOI] [PubMed] [Google Scholar]
- 182.Robertson A, Khairallah P. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science. 1971;172:1138–1139. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- 183.Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P, Tauc M. Specific Cre/Lox recombination in the mouse proximal tubule. J Am Soc Nephrol. 2004;15(8):2050–2056. doi: 10.1097/01.ASN.0000133023.89251.01. [DOI] [PubMed] [Google Scholar]
- 184.Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT1 and AT2 receptors. Am J Pathol. 2001;158(5):1743–1756. doi: 10.1016/s0002-9440(10)64130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ruiz-Ortega M, Lorenzo O, Ruperez M, Konig S, Wittig B, Egido J. Angiotensin II activates nuclear transcription factor kappaB through AT1 and AT2 in vascular smooth muscle cells: molecular mechanisms. Circ Res. 2000;86(12):1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 186.Santos RA, Ferreira AJ. Angiotensin-(1-7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16(2):122–128. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 187.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de B,I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100(14):8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sasamura H, Hein L, Saruta T, Pratt RE. Evidence for internalization of both type 1 angiotensin receptor subtypes (AT1a, AT1b) by a protein kinase C independent mechanism. Hypertens Res. 1997;20(4):295–300. doi: 10.1291/hypres.20.295. [DOI] [PubMed] [Google Scholar]
- 189.Schelling JR, Hanson AS, Marzec R, Linas SL. Cytoskeleton-dependent endocytosis is required for apical type 1 angiotensin II receptor-mediated phospholipase C activation in cultured rat proximal tubule cells. J Clin Invest. 1992;90(6):2472–2480. doi: 10.1172/JCI116139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schelling JR, Linas SL. Angiotensin II-dependent proximal tubule sodium transport requires receptor-mediated endocytosis. Am J Physiol. 1994;266(3 Pt 1):C669–C675. doi: 10.1152/ajpcell.1994.266.3.C669. [DOI] [PubMed] [Google Scholar]
- 191.Schelling JR, Singh H, Marzec R, Linas SL. Angiotensin II-dependent proximal tubule sodium transport is mediated by cAMP modulation of phospholipase C. Am J Physiol. 1994;267(5 Pt 1):C1239–C1245. doi: 10.1152/ajpcell.1994.267.5.C1239. [DOI] [PubMed] [Google Scholar]
- 192.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109(17):2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 193.Schupp N, Schmid U, Rutkowski P, Lakner U, Kanase N, Heidland A, Stopper H. Angiotensin II-induced genomic damage in renal cells can be prevented by angiotensin II type 1 receptor blockage or radical scavenging. Am J Physiol Renal Physiol. 2007;292(5):F1427–F1434. doi: 10.1152/ajprenal.00458.2006. [DOI] [PubMed] [Google Scholar]
- 194.Seachrist JL, Laporte SA, Dale LB, Babwah AV, Caron MG, Anborgh PH, Ferguson SS. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem. 2002;277(1):679–85. doi: 10.1074/jbc.M109022200. [DOI] [PubMed] [Google Scholar]
- 195.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292(1):F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 196.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-Ang II infused rats. Am J Physiol Renal Physiol. 2009;296(5):F1067–71. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shao Y, He M, Zhou L, Yao T, Huang Y, Lu LM. Chronic angiotensin (1-7) injection accelerates STZ-induced diabetic renal injury. Acta Pharmacol Sin. 2008;29(7):829–837. doi: 10.1111/j.1745-7254.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 198.Sihn G, Rousselle A, Vilianovitch L, Burckle C, Bader M. Physiology of the (pro)renin receptor: Wnt of change? Kidney Int. 2010;78(3):246–256. doi: 10.1038/ki.2010.151. [DOI] [PubMed] [Google Scholar]
- 199.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3', 5'-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97(8):1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Soler M, Tornavaca O, Sole E, Menoyo A, Hardy D, Catterall JF, Vandewalle A, Meseguer A. Hormone-specific regulation of the kidney androgen-regulated gene promoter in cultured mouse renal proximal-tubule cells. Biochem J. 2002;366(Pt 3):757–766. doi: 10.1042/BJ20011807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296(2):F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 202.Song K, Zhuo J, Chai SY, Mendelsohn FA. A new method to localize active renin in tissues by autoradiography: application to dog kidney. Kidney Int. 1992;42(3):639–646. doi: 10.1038/ki.1992.329. [DOI] [PubMed] [Google Scholar]
- 203.Swanson GN, Hanesworth JM, Sardinia MF, Coleman JK, Wright JW, Hall KL, Miller-Wing AV, Stobb JW, Cook VI, Harding EC. Discovery of a distinct binding site for angiotensin II (3-8), a putative angiotensin IV receptor. Regul Pept. 1992;40(3):409–419. doi: 10.1016/0167-0115(92)90527-2. [DOI] [PubMed] [Google Scholar]
- 204.Takahashi M, Suzuki E, Takeda R, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y. Angiotensin II and tumor necrosis factor-alpha synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-kappaB, p38, and reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;294(6):H2879–H2888. doi: 10.1152/ajpheart.91406.2007. [DOI] [PubMed] [Google Scholar]
- 205.Taugner R, Hackenthal E, Nobiling R, Harlacher M, Reb G. The distribution of renin in the different segments of the renal arterial tree: immunocytochemical investigation in the mouse kidney. Histochemistry. 1981;73(1):75–88. doi: 10.1007/BF00493135. [DOI] [PubMed] [Google Scholar]
- 206.Thekkumkara T, Linas SL. Role of internalization in AT(1A) receptor function in proximal tubule epithelium. Am J Physiol Renal Physiol. 2002;282(4):F623–F629. doi: 10.1152/ajprenal.00118.2001. [DOI] [PubMed] [Google Scholar]
- 207.Thekkumkara TJ, Cookson R, Linas SL. Angiotensin (AT1A) receptor-mediated increases in transcellular sodium transport in proximal tubule cells. Am J Physiol. 1998;274:F897–F905. doi: 10.1152/ajprenal.1998.274.5.F897. [DOI] [PubMed] [Google Scholar]
- 208.Thomas WG, Baker KM, Motel TJ, Thekkumkara TJ. Angiotensin II receptor endocytosis involves two distinct regions of the cytoplasmic tail. A role for residues on the hydrophobic face of a putative amphipathic helix. J Biol Chem. 1995;270(38):22153–22159. doi: 10.1074/jbc.270.38.22153. [DOI] [PubMed] [Google Scholar]
- 209.Thomas WG, Thekkumkara TJ, Baker KM. Molecular mechanisms of angiotensin II (AT1A) receptor endocytosis. Clin Exp Pharmacol Physiol Suppl. 1996;3:S74–S80. [PubMed] [Google Scholar]
- 210.Tigerstedt R, Bergman PG. Niere und Kreislauf. Scand.Arch.Physiol. 1898;8:223–271. [Google Scholar]
- 211.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45(2):205–251. [PubMed] [Google Scholar]
- 212.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 213.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 214.Tsuganezawa H, Preisig PA, Alpern RJ. Dominant negative c-Src inhibits angiotensin II induced activation of NHE3 in OKP cells. Kidney Int. 1998;54(2):394–398. doi: 10.1046/j.1523-1755.1998.00029.x. [DOI] [PubMed] [Google Scholar]
- 215.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res. 2005;97(8):829–36. doi: 10.1161/01.RES.0000185322.46009.F5. [DOI] [PubMed] [Google Scholar]
- 216.van Kats JP, Methot D, Paradis P, Silversides DW, Reudelhuber TL. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice. Direct and indirect effects of angiotensin II on the heart. J Biol Chem. 2001;276(47):44012–44017. doi: 10.1074/jbc.M106132200. [DOI] [PubMed] [Google Scholar]
- 217.van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001;60(6):2311–2317. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 218.van Rodijnen WF, van Lambalgen TA, van Wijhe MH, Tangelder GJ, Ter Wee PM. Renal microvascular actions of angiotensin II fragments. Am J Physiol Renal Physiol. 2002;283(1):F86–F92. doi: 10.1152/ajprenal.00121.2001. [DOI] [PubMed] [Google Scholar]
- 219.Velkoska E, Dean RG, Griggs K, Burchill L, Burrell LM. Angiotensin-(1-7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin Sci (Lond) 2011;120(8):335–345. doi: 10.1042/CS20100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Veniant M, Menard J, Bruneval P, Morley S, Gonzales MF, Mullins J. Vascular damage without hypertension in transgenic rats expressing prorenin exclusively in the liver. J Clin Invest. 1996;98(9):1966–1970. doi: 10.1172/JCI119000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.von Thun AM, Vari RC, El Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol. 1994;266(1 Pt 2):F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 222.Wang T, Chan YL. The role of phosphoinositide turnover in mediating the biphasic effect of angiotensin II on renal tubular transport. J Pharmacol Exp Ther. 1991;256(1):309–317. [PubMed] [Google Scholar]
- 223.Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem. 2005;280(47):39353–39362. doi: 10.1074/jbc.M508914200. [DOI] [PubMed] [Google Scholar]
- 224.Weber H, Taylor DS, Molloy CJ. Angiotensin II induces delayed mitogenesis and cellular proliferation in rat aortic smooth muscle cells. Correlation with the expression of specific endogenous growth factors and reversal by suramin. J Clin Invest. 1994;93(2):788–798. doi: 10.1172/JCI117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Weigert C, Brodbeck K, Klopfer K, Haring HU, Schleicher ED. Angiotensin II induces human TGF-beta 1 promoter activation: similarity to hyperglycaemia. Diabetologia. 2002;45(6):890–898. doi: 10.1007/s00125-002-0843-4. [DOI] [PubMed] [Google Scholar]
- 226.Wilkinson-Berka JL, Miller AG, Binger KJ. Prorenin and the (pro)renin receptor: recent advances and implications for retinal development and disease. Curr Opin Nephrol Hypertens. 2011;20(1):69–76. doi: 10.1097/MNH.0b013e328341328a. [DOI] [PubMed] [Google Scholar]
- 227.Wolf G, Ziyadeh FN, Stahl RA. Angiotensin II stimulates expression of transforming growth factor beta receptor type II in cultured mouse proximal tubular cells. J Mol Med. 1999;77(7):556–564. doi: 10.1007/s001099900028. [DOI] [PubMed] [Google Scholar]
- 228.Xu J, Carretero OA, Lin CX, Cavasin MA, Shesely EG, Yang JJ, Reudelhuber TL, Yang XP. Role of cardiac overexpression of ANG II in the regulation of cardiac function and remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293(3):H1900–H1907. doi: 10.1152/ajpheart.00379.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang R, Smolders I, De BD, Fouyn R, Halberg M, Demaegdt H, Vanderheyden P, Dupont AG. Brain and peripheral angiotensin II type 1 receptors mediate renal vasoconstrictor and blood pressure responses to angiotensin IV in the rat. J Hypertens. 2008;26(5):998–1007. doi: 10.1097/HJH.0b013e3282f5ed58. [DOI] [PubMed] [Google Scholar]
- 230.Yang R, Walther T, Gembardt F, Smolders I, Vanderheyden P, Albiston AL, Chai SY, Dupont AG. Renal vasoconstrictor and pressor responses to angiotensin IV in mice are AT1a-receptor mediated. J Hypertens. 2010;28(3):487–494. doi: 10.1097/HJH.0b013e3283343250. [DOI] [PubMed] [Google Scholar]
- 231.Zhai XY, Nielsen R, Birn H, Drumm K, Mildenberger S, Freudinger R, Moestrup SK, Verroust PJ, Christensen EI, Gekle M. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int. 2000;58(4):1523–1533. doi: 10.1046/j.1523-1755.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 232.Zhang J, Barak LS, Anborgh PH, Laporte SA, Caron MG, Ferguson SS. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J Biol Chem. 1999;274(16):10999–11006. doi: 10.1074/jbc.274.16.10999. [DOI] [PubMed] [Google Scholar]
- 233.Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels. 1997;5(3-4):193–199. [PubMed] [Google Scholar]
- 234.Zhang J, Ferguson SS, Barak LS, Menard L, Caron MG. Dynamin and beta-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. J Biol Chem. 1996;271(31):18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- 235.Zhuo JL, Alcorn D, Harris PJ, Mendelsohn FA. Localization and properties of angiotensin II receptors in rat kidney. Kidney Int Suppl. 1993;42:S40–S46. [PubMed] [Google Scholar]
- 236.Zhuo JL, Allen AM, Alcorn D, MacGregor D, Aldred GP, Mendelsohn FA. The distribution of angiotensin II receptors. In: Laragh JH, Brenner BM, editors. Hypertension: Pathology, Diagnosis & Management. 2nd Edition Raven Press; New York: 1995. pp. 1739–1762. [Google Scholar]
- 237.Zhuo JL, Anderson WP, Song K, Mendelsohn FA. Autoradiographic localization of active renin in the juxtaglomerular apparatus of the dog kidney: effects of sodium intake. Clin Exp Pharmacol Physiol. 1996;23(4):291–298. doi: 10.1111/j.1440-1681.1996.tb02826.x. [DOI] [PubMed] [Google Scholar]
- 238.Zhuo JL, Moeller I, Jenkins T, Chai SY, Allen AM, Ohishi M, Mendelsohn FA. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J Hypertens. 1998;16(12 Pt 2):2027–2037. doi: 10.1097/00004872-199816121-00026. [DOI] [PubMed] [Google Scholar]
- 239.Zhuo JL, Song K, Harris PJ, Mendelsohn FA. In vitro autoradiography reveals predominantly AT1 angiotensin II receptors in rat kidney. Ren Physiol Biochem. 1992;15(5):231–239. doi: 10.1159/000173458. [DOI] [PubMed] [Google Scholar]
- 240.Zhuo JL, Thomas D, Harris PJ, Skinner SL. The role of endogenous angiotensin II in the regulation of renal haemodynamics and proximal fluid reabsorption in the rat. J Physiol. 1992;453:1–13. doi: 10.1113/jphysiol.1992.sp019214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhuo JL, Mendelsohn FAO. Intrarenal angiotensin II receptors. In: Robertson JIS, Nicholls MG, editors. The Renin-Angiotensin System: Biochemistry, Pathophysiology and Therapeutics. Gower Medical Publishing; London & New York: pp. 251–25.14. [Google Scholar]
- 242.Zhuo JL. Monocyte chemoattractant protein-1: a key mediator of angiotensin II-induced target organ damage in hypertensive heart disease? J Hypertens. 2004;22(3):451–454. doi: 10.1097/01.hjh.0000098211.37783.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhuo JL. Intracrine renin and angiotensin II: a novel role in cardiovascular and renal cellular regulation. J Hypertens. 2006;24(6):1017–1020. doi: 10.1097/01.hjh.0000226188.90815.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhuo JL, Carretero OA, Li XC. Effects of AT1 receptor-mediated endocytosis of extracellular Ang II on activation of nuclear factor-kappa B in proximal tubule cells. Ann N Y Acad Sci. 2006;1091:336–45. doi: 10.1196/annals.1378.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension. 2002;39(1):116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 246.Zhuo JL, Li XC. Novel roles of intracrine angiotensin II and signalling mechanisms in kidney cells. J Renin Angiotensin Aldosterone Syst. 2007;8(1):23–33. doi: 10.3317/jraas.2007.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular angiotensin II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol. 2006;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zimpelmann J, Burns KD. Angiotensin-(1-7) activates growth-stimulatory pathways in human mesangial cells. Am J Physiol Renal Physiol. 2009;296(2):F337–F346. doi: 10.1152/ajprenal.90437.2008. [DOI] [PubMed] [Google Scholar]
- 249.Zou LX, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11(5):570–578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 250.Zuo L, Ushio-Fukai M, Hilenski LL, Alexander RW. Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts. Arterioscler.Thromb.Vasc.Biol. 2004;24(7):1223–1228. doi: 10.1161/01.ATV.0000132400.25045.2a. [DOI] [PubMed] [Google Scholar]