Abstract
Purpose
Epidermal keratinocytes, which can be severely damaged after ionizing radiation (IR), are rapid turnover cells that function as a barrier protecting the host from pathogenic invasion and fluid loss. We tested fibroblast growth factor-peptide (FGF-P), a small peptide derived from the receptor-binding domain of FGF-2, as a potential mitigator of radiation effects via proliferation and the barrier function of keratinocytes.
Methods and Materials
Keratinocytes isolated from neonatal foreskin were grown on transwells. After 0, 5, or 10-Gy, the cells were treated with a vehicle or FGF-P. The permeability of IR cells was assessed through transepithelial electrical resistance (TEER) and a paracellular tracer flux of fluorescein isothiocyanate-conjugated bovine serum albumin (FITC-BSA) with Ussing chambers. The cell proliferation was measured with yellow tetrazolium salt (MTT) and tritiated thymidine (3H-TdR) assays. The phosphorylation of extracellular signal-regulated kinases (ERK) was measured in an enzyme-linked immunosorbent (ELISA)-like assay, and the proteins related to tight junctions (TJ) and adherens junctions (AJ) were examined with Western blotting. We used a mouse model to assess the ability of FGF-P to promote the healing of skin β-burns created with a strontium applicator.
Results
1) FGF-P reduced the permeability of irradiated keratinocytes, as evidenced by increased TEER and decreased diffusion of FITC-BSA both associated with the regulation of different proteins and levels of TJ and AJ; 2) FGF-P enhanced the proliferation of irradiated keratinocytes, as evidenced by increased MTT activity and 3H-TdR incorporation, which was associated with activation of the ERK pathway; and 3) FGF-P promoted the healing of skin β-burns.
Conclusions
FGF-P enhances the barrier function, including up-regulation of TJ proteins, increases proliferation of human keratinocytes, and accelerates the healing of skin β-burns. FGF-P is a promising mitigator that improves the proliferation and barrier function of keratinocytes after IR.
Keywords: FGF-P, radiation, keratinocyte, barrier function, proliferation
INTRODUCTION
Human skin, the largest organ in the body, functions as a barrier between the external and internal environments. It prevents body fluid loss and the invasion of pathogens and reduces the impact of trauma (e.g., mechanical trauma and UV radiation) (1–3). To fulfill these functions, the epidermal keratinocytes are constantly replaced by cells originating from proliferating keratinocyte stem cells (KSC) in the stratum basale (4,5). Turnover of skin can occur in 4–5 days. In addition, the conjunction between keratinocytes must be very tight in order to form a strong barrier (2,3).
Exposure to ionizing radiation (IR), from radiotherapy, space exploration, or nuclear events, can result in dermatitis, erythema, disruption of epidermal barrier function and other radiation-induced complications (3,6–8). A method to protect keratinocyte function and cutaneous health would be valuable in case of incidental or malevolent IR exposure.
Normally, growth factors are critical for the proliferation of keratinocytes. For example, the various members of the fibroblast growth factor (FGF) family have been shown to protect against normal tissue damage, including radiation (9–13). Lin et al (14) show that the small peptide generated from the binding domain of FGF-2 possesses the biological function of an intact protein (14) and has the following advantages over the full-length peptide: 1) it is stable under severe conditions (e.g.,boiling); 2) it is enzyme-resistant as a dry powder and has a long shelf-life compared to most protein drugs; 3) it can be self-administered via intramuscular (IM) or subcutaneous (SC) injection by a victim who has been exposed to radiation; and 4) it can be synthesized in large quantities with high purity at a lower cost than the production of an intact protein.
To explore the utility of FGF-P, a dimer form of FGF-2’s binding domain, in promoting the barrier function of irradiated skin, we studied permeability and proliferation in irradiated normal human epidermal keratinocytes (NHEK) in vitro and a β-burn skin model in vivo.
METHODS AND MATERIALS
Cell culture
Following standard protocol (15), NHEK were isolated from discarded neonatal foreskin. Briefly, the epidermis was separated from the dermis by incubation in dispase/Hank’s buffered salt solution (HBSS) (Invitrogen by Life Technologies, Carlsbad, CA) overnight at 4°C, followed by incubation in 0.05% trypsin-ethylenediaminetetraacetic acid (EDTA) for 5 minutes at 37°C and blocking with Dulbecco’s modified Eagle's medium (DMEM). The material was then passed through a 40 mm cell strainer (BD Falcon, Franklin Lakes, NJ) and centrifuged at 1,500 RPM for 5 minutes at room temperature. NHEK were cultured in keratinocyte serum-free medium (SFM), and cells were grown at 37°C in a humidified 5% CO2 incubator.
Measurements of transepithelial electrical resistance
The 2nd passage of NHEK was seeded (105/well) on top of 12 transwell-permeable supports (0.4 zm-pore size, polyester membrane, surface area = 1.13 cm2, Costar #3401) in keratinocyte growth-medium. After 3 days of culture, the cells were irradiated with a 137Cs γ - ray source at 1.84 Gy/minute dose rate. Some wells were treated with FGF-P (200 ng/ml) 24 hours after IR. We performed experiments after continuing to culture the cells for 10–14 days until they were confluent. Transepithelial electrical resistance (TEER) was measured with modified Ussing chambers (VCC MC8, Physiologic Instruments, San Diego, CA) (16,17).
Measurements of paracellular tracer flux
We then measured the paracellular tracer flux through the keratinocyte sheet by adding fluorescein isothiocyanate-conjugated bovine serum albumin (FITC-BSA). FITC-BSA was added to the apical side of the transwell and then collected at 30-minute intervals from both the apical and basal compartments of the keratinocyte sheet grown on the transwell filters (18). A SpectraMax M2 reader (Molecular Devices, Inc., Silicon Valley, CA) was used to measure the amount of FITC-BSA that diffused from the apical to the basal side of the cellular sheet. The FITC permeability was expressed as the ratio based on the percentage of basal and apical FITC-BSA density.
MTT assay for cell proliferation
Keratinocytes were seeded into 96-well tissue-culture plates and irradiated, and a yellow tetrazolium salt (MTT) assay was performed (19). Briefly, the cells were incubated until ready for experimental use. After addition of the MTT solution, the cultures were incubated at 37°C for 4 hours. Then after centrifuging, the supernatant was discarded and dimethyl sulfoxide (DMSO) was added to each well. The absorbance was measured using a SpectraMax M2 reader with a test wavelength of 560 nm.
Metabolic incorporation 3H-TdR proliferation assay
Keratinocytes were seeded into 96-well tissue-culture plates and grown to confluence. After the cells were irradiated and incubated for 3 days, followed by the addition of tritiated thymidine (3H-TdR), we used standard protocols (20,21) to measure 3H-TdR uptake by keratinocytes.
Cell-based ELISA for Phospho-ERK1/2
Phospho–extracellular signal-regulated kinases (ERK) 1/2 (p-ERK) is involved in the regulation of proliferation. To further study the proliferative effects of FGF-P on keratinocytes, an enzyme-linked immunosorbent assay (ELISA) was performed to observe the level of p-ERK. Keratinocytes were seeded onto a 96-well tissue-culture plate, which was then irradiated. p-ERK was examined by a cell-based ELISA 24 hours after IR, and the absorbance was measured on a SpectraMax M2 reader (22).
Protein extraction and immunoblot analysis
Protein samples were prepared from cultured keratinocytes and analyzed for claudin-1, -7, JAM-A, occludin, E-cadherin, and β-catenin (Invitrogen by Life Technologies, Carlsbad, CA) by Western blotting (23). The protein concentration was determined in samples using the bicinchoninic acid (BCA) assay. Equivalent loads of protein from irradiated and non-irradiated samples were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred onto polyvinylidene fluoride (PVDF) membranes, and the primary antibodies were detected using affinity-purified polyclonal antibodies.
Effect of FGF-P on healing of irradiated mouse skin
Eight-week-old male BALB/C mice from the National Cancer Institute (NCI) were used in the experiment. The right hind-legs of BALB/C mice were irradiated (50 Gy) with a strontium applicator (25.21 Gy/min) to create an IR β-burn on a 7 mm in diameter section of skin. Then, mice were randomly divided into 2 groups (4 mice/group) and treated topically with a vehicle control or FGF-P (200 ng/ml in cream form) and either with a vehicle control (0.2 ml saline/mouse) or FGF-P (0.4 mg/0.2 ml/day) through IM injection. After 16 days, pictures were taken to document the extent of healing in the IR skin area. All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the University of Rochester (Rochester, NY).
Statistical analysis
Data are expressed as mean ± standard deviation. The difference between treatment and control was evaluated with a student’s t-test; p<0.05 was considered to be significant.
RESULTS
FGF-P increases TEER after radiation
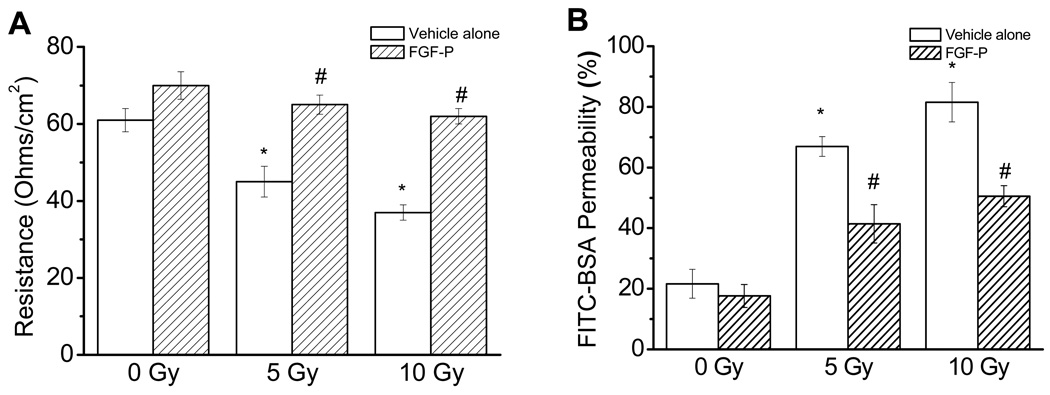
The permeability barrier function of NHEK cells was examined with the TEER assay, which is a reliable gauge of the permeability of water-soluble ions. The values were obtained by multiplying the recorded electrical resistance by the area of effective membrane on the filter inserts at the condition of voltage clamp. Results were expressed in standard units—Ohms/cm2 (Ω/cm2). As shown in Figure 1A, there was a significant decrease of TEER in 5 and 10 Gy compared to 0 Gy (45.30 and 37.27 Ω/cm2 vs61.21 Ω/cm2, p<0.05). After the addition of FGF-P, TEER was restored to a baseline level, which suggests an improved permeability barrier function.
Figure 1. Effects of FGF-P on permeability of irradiated NHEK cells.
NHEK cells were cultured in triplicate on top of a transwell to 90% confluence, irradiated with 0, 5, or 10 Gy, and treated with the vehicle alone or FGF-P (200 ng/ml). After 10–14 days, the permeability of irradiated NHEK cells was measured as described in the “Methods and Materials” section. A) TEER was measured with an Ussing chamber under voltage-clamp conditions; B) FITC-BSA diffusion from the top well to the bottom well was measured every 30 minutes. The * represents that compared to the 0-Gy control group, p<0.05. The # represents that compared to the 5-Gy control group, the FGF-P treatment achieved p<0.05.
FGF-P decreases paracellular tracer flux after radiation
As shown in Figure 1B, the FITC-BSA permeability of NHEK increased significantly in both the 5 and 10-Gy groups compared to the 0Gy group (66.91% and 81.53% vs 21.61%, p<0.05). The addition of FGF-P significantly decreased permeability of irradiated NHEK at 5 Gy and 10 Gy (41.4 % and 50.52%, p<0.05).
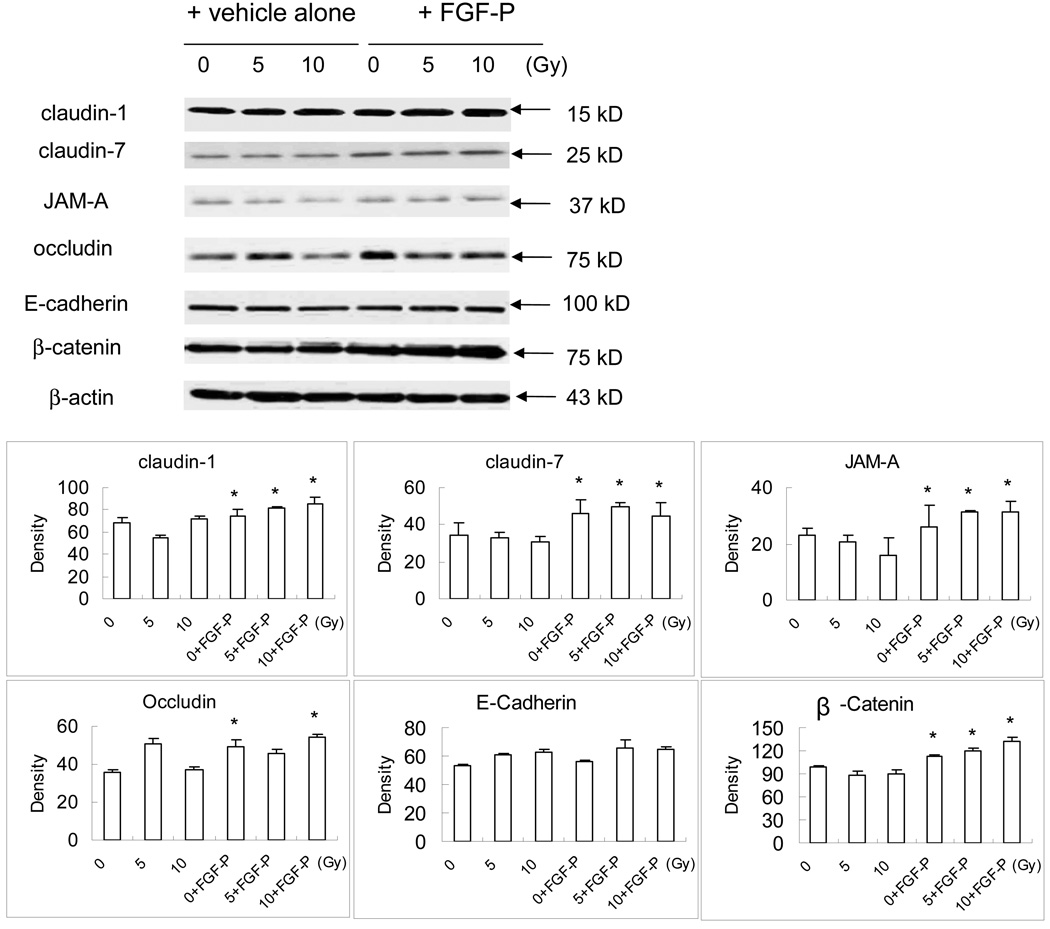
FGF-P improves some of the cell tight junction proteins after radiation
We investigated whether the permeability increased by FGF-P is related to the regulation of TJ proteins, such as claudin-1, -7, JAM-A, occludin, and the AJ members E-cadherin and β-catenin. The result of Western blotting and the Quantity 1 software analysis showed a small but significant increase in claudin-1 and occludin (except at 5 Gy) expression (p<0.05) and a larger increase in claudin-7, JAM-A, and β-catenin after FGF-P treatment (p<0.05) (Fig. 2). There was no change in E-cadherin expression when the vehicle alone or FGF-P was tested on the irradiated keratinocytes.
Figure 2. Effect of FGF-P on proteins related to TJ and AJ.
NHEK cells were cultured in triplicate on top of a transwell to 90% confluence, irradiated with 0, 5, or 10 Gy and treated with vehicle alone or FGF-P (200 ng/ml). After 10–14 days, the cell sheets were harvested and lysed with lysis buffer cocktail containing protenase inhibitors. Thirty µg/lane of lysate from each treatment group was loaded on 10% SDS-PAGE gel, electrophoresized, transferred to PVDF membrane, and the different antibodies against the proteins related with TJ and AJ were used in the Western blot staining. The quantization analysis was done by scanning the density of each band and relative ratio to β-actin accessed with Quantity 1 software (Bio-Rad). The * represents p<0.05 as a comparison of the density of the FGF-P treated group to the vehicle-alone group.
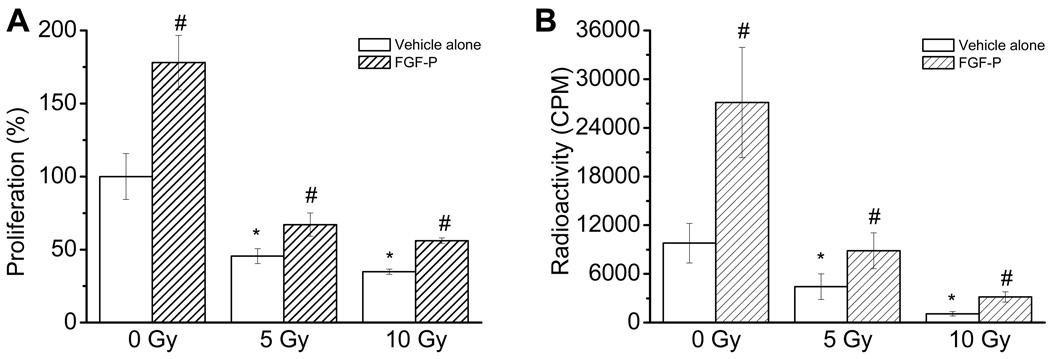
FGF-P improves cell proliferation after radiation
Proliferation is critical in maintaining the healthy barrier layer of keratinocytes. To study the maintenance of proliferation, we performed MTT and 3H-TdR assays. As shown in Figure 3A, radiation reduced the MTT activity of keratinocytes in a dose-dependent manner, which was reversed by treatment with FGF-P (p<0.05). Similarly, the results from 3H-TdR incorporation were consistent with the MTT assay, suggesting that FGF-P indeed promotes the proliferation of irradiated keratinocytes (Fig. 3B).
Figure 3. Effect of FGF-P on proliferation of irradiated keratinocytes.
NHEK cells were cultured in triplicate in 96 well plates to 80% confluence, irradiated with 0, 5, or 10 Gy, and treated with vehicle alone or FGF-P (200 ng/ml). Three days later, the proliferation was determined by 2 methods: A) MTT assay; and B) 3H-TdR incorporation assay. The * represents that compared to the 0-Gy control group, p<0.05. The # represents that compared to the 5-Gy control group, the FGF-P treatment achieved p<0.05.
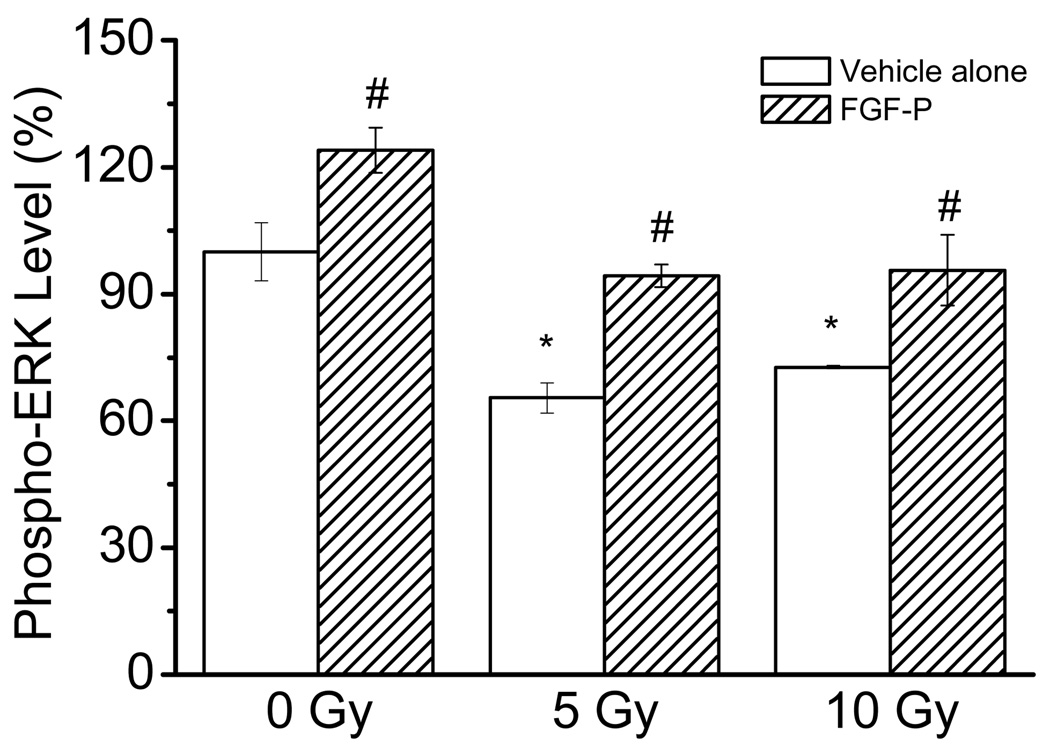
FGF-P increases phospho-ERK levels after radiation
Since the phosphorylation of ERK is a pathway for FGF-2 to trigger cell proliferation, we performed an in-plate ELISA-like assay for FGF-P treated keratinocytes. As shown in Figure 4, the p-ERK level was significantly decreased following IR (p<0.05); however, after FGF-P treatment, the phosphorylation of ERK markedly increased (p<0.05).
Figure 4. Effect of FGF-P on phosphorylation of ERK.
NHEK cells were cultured in triplicate in 96 well plates to 80% confluence, irradiated with 0, 5, or 10 Gy, and treated with vehicle alone or FGF-P (200 ng/ml). Three days later, the cells were fixed with 4% formalin for 3 minutes and subjected to an in-plate ELISA-like assay with rabbit anti-phosphorylated ERK followed by HRP-anti-rabbit and TMB colorization. The * represents that compared to the 0-Gy control group, p<0.05. The # represents that compared to the 5-Gy control group, the FGF-P treatment achieved p<0.05.
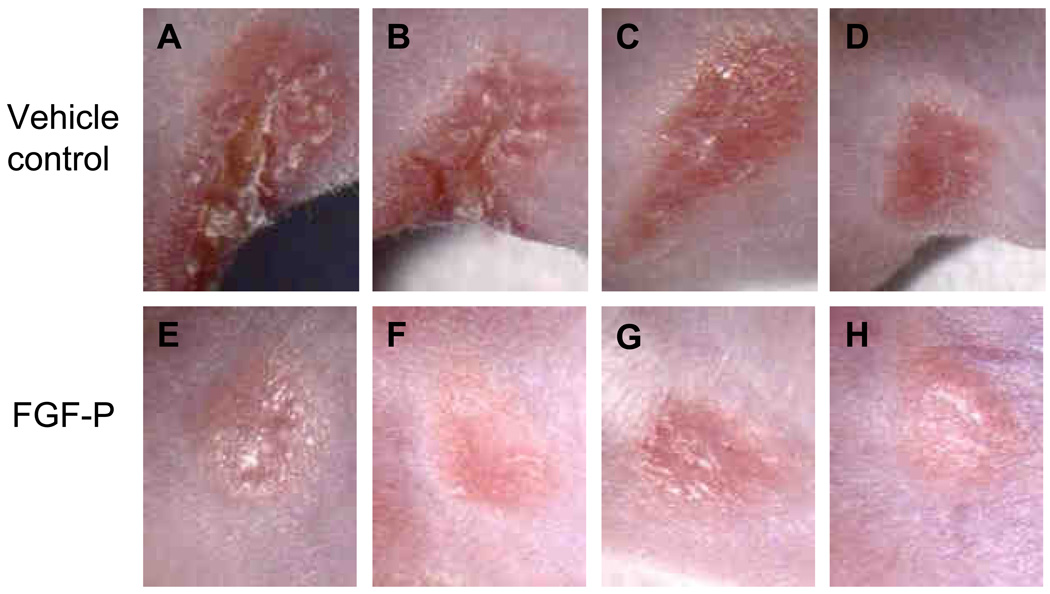
FGF-P improves healing of irradiated mouse skin
To further elucidate whether FGF-P is a potent healing mitigator after radiation in vivo, we studied IR-induced skin injury in a mouse β-burn model. In contrast to the vehicle-treated mice, scabs and red inflammation in the irradiated spot were much less pervasive in the FGF-P treated mice (Fig. 5A–H).
Figure 5. Effect of FGF-P on healing of irradiated skin.
The right hind-leg of BALB/C mice were irradiated 50 Gy with a strontium applicator (25.21 Gy/min) to create a β-burn 7 mm in diameter on the skin. They were then treated topically with vehicle alone or FGF-P (200 ng/ml in cream form, daily) and an IM of either saline as vehicle control (0.2 ml/mouse) or FGF-P (0.4 mg/0.2 ml/day). After 16 days, pictures were taken to document the extent of healing in the IR-skin area. A–D: the 4 mice in the vehicle group; E–H: the 4 mice in the FGF-P treated group.
DISCUSSION
Since FGF-2, a potent mitogen that is crucial for stem cell renewal, is involved in multiple functions, including cell proliferation, differentiation, motility, wound healing and survival following multiple traumas (24–26), we designed FGF-P, a structure-modified analog of FGF-2, and found that it increases survival of mice that have been exposed to lethal doses of total body IR (data not shown). In the present study, FGF-P demonstrates its ability to improve barrier function and cell proliferation of irradiated human keratinocytes as evidenced by: 1) increased TEER; 2) decreased paracellular permeability; 3) up-regulation of some TJ and AJ proteins; 4) increased proliferation of keratinocytes; 5) increased phosphorylation of ERK; and 6) accelerated IR-induced wound healing. Indeed, these effects are connected; accordingly, the mitogenic effect of FGF-P is beneficial to irradiated cells at different levels: the cellular level (Fig. 2, 3A and 3B, and 4), the cell-layer level (Fig. 1A and B), and the skin tissue/organ level (Fig. 5A–H).
The permeability (one of the barrier functions) of the cell layer growing on the transwells is related to 2 factors: 1) the number of cells; and 2) the tightness of cell-cell conjunction. After radiation, the number of cells and their capacity to produce tight junction proteins are reduced. Both the radiation-induced reduction of cell numbers and the radiation-altered tight junction proteins affect the permeability of the cell layer, as evidenced by the low TEER (Fig. 1A) and high flux of FITC-BSA (Fig. 1B). To further dissect these 2 contributing factors, we performed several assays. When the amount of cell lysate protein loaded was equal, the cell proliferation assay (Fig. 3A and B) and Western blotting for tight junction proteins indicated that the increased permeability in FGF-P-treated cells was related to enhanced cell proliferation and increased levels of claudin-1, -7, JAM-A, occludin, and β-catenin (Fig 2).
TEER, an electrophysiological method to study permeability, reflects the transepithelial and paracellular permeability of the keratinocyte layer on transwells with polycarbonate filters (27). A high TEER resistance indicates a low water or ionic loss and the barrier function of tight junctions (26,27). In our study, after irradiation, both contact among the keratinocytes and their ability to express proteins related to TJ and AJ were reduced or altered (Fig. 2); therefore, they lost their normally high resistance tested by the TEER assay in an IR dose-dependent manner. FGF-P reversed this loss (Fig. 1A). A FITC-BSA assay was another means through which we measured the permeability of cell layers (28). Consistent with the TEER results, IR caused an increased leakage of the monolayer of keratinocytes, leading to a 3–4-fold increase in the amount of FITC-BSA crossing from the top well to the bottom well as compared with un-irradiated cells (Fig. 1B). This phenomenon was also normalized by FGF-P. Thus, 2 sets of assays suggest that FGF-P promotes the repair of IR-induced cell damage by stimulating the recovery of cells and their ability to produce proteins related to TJ and AJ.
To confirm the ability of FGF-P to regulate the production of proteins related to TJ and AJ, we used Western blotting and Quantity 1 software analysis. As shown by Martin-Padura et al, TJ plays an important role in the maintenance and regulation of barrier functions (29). Thus, we studied various TJ and AJ that were selected based on their importance in maintaining the barrier function of keratinocytes. Knowing that radiation could reduce cell numbers, we normalized the alteration of TJ/AJ proteins by loading the same amount of protein into each lane of Western blotting. The decrease in TJ/AJ may be a direct effect of FGF-P or an indirect effect due to reduced cell contact. The results (Fig. 2) revealed that IR decreased some of the TJ proteins, such as JAM-A. FGF-P slightly increased expression of claudin-1 and occludin (except after 5 Gy + FGF-P) and greatly increased claudin-7, JAM-A, and β-cateni. There was no effect on E-cadherins. Claudins constitute the major diffusion barrier of TJ and, together with occludin, zonula occludens (ZO) family members, and other cytoplasmic proteins, generate apical and basolateral domains in the plasma membrane to maintain cell polarity (30–33). Cadherins constitute the major adhesive component of AJ and recruit p210ctn, β-catenin, and the cytoskeleton to the plasma membrane while forming homophilic and heterophilic interactions between adjacent cells to generate a structural framework for the entire cell sheet (33–35). However, AJ is porous in mammals and plays no role as a diffusion barrier. Claudins are directly involved not only in the formation of TJ strands but also in their barrier function in simple epithelia. Continuous claudin-based TJ occur in the epidermis, and these are crucial for the barrier function of mammalian skin (36,37). Therefore, claudins express and play an important role in skin barrier function. Furuse et al have found in functional analyses that claudin-1 is essential for TJ function (30); similarly, Alexandre et al show that claudin-7 is involved in modulating paracellular Cl− permeability (38). JAM-A is a tight junction-associated adhesion protein implicated in TJ assembly, the regulation of barrier function, and paracellular permeability and has been reported to influence several cellular processes (39,40). The decrease of JAM-A expression after IR increased the permeability; meanwhile, increased JAM-A was detected after addition of FGF-P, which corresponds to a decrease in the permeability of keratinocytes. On the other hand, both E-cadherin and β-catenin are components of AJ; our data show that FGF-P increases β-catenin but not E-cadherin thus improving the adhesive function in keratinocytes. Altogether, FGF-P increases selected TJ and AJ proteins expressed in keratinocytes after IR.
The healthy skin function also relies on continuous renewal of keratinocytes; therefore, we studied the effect of FGF-P on the proliferation of keratinocytes. IR damages the DNA, inhibits proliferation, and mediates apoptosis in the epidermis (41). Both the MTT and 3H-TdR assays showed that IR reduced DNA synthesis and cell proliferation; however, proliferation was salvaged by FGF-P treatment (Fig. 3A and B and 4). Since the phosphorylation of ERK is a major pathway triggered by FGF-2 (42), we examined the effect of FGF-P on this pathway. The results (Fig. 4) demonstrated that IR-reduced phosphorylation of ERK could be reversed by FGF-P. Since the assay for phosphorylation of ERK is performed in whole wells, the increase represents either increased cell numbers or increased ERK phosphorylation in each cell. Either provides further evidence that FGF-P is a bioactive peptide that acts against radiation-induced suppression of cell growth.
To determine if the in vitro effect of FGF-P on keratinocytes can be translated in vivo, an IR-induced skin wound was administered in a mouse β-burn model with a strontium applicator. On day 16 after the 50-Gy, scabs and red inflammation were observed at the IR-wound sites. The severity of the IR-wounds was reduced by FGF-P delivered topically and systemically (Fig. 5), which suggests that FGF-P has the potential to be used to promote the healing of IR-damaged skin lesions.
CONCLUSION
In conclusion, the treatment of irradiated human keratinocytes with FGF-P enhances the barrier function and tight-junction protein production and improves cell proliferation in vitro. It also accelerates IR-induced-wound healing in vivo. FGF-P is a promising radiation mitigator for skin. Further experiments are necessary to explore the mechanisms of FGF-P on keratinocyte proliferation and to optimize the treatment dose/course for other forms of IR damage (γ- or neutron-irradiation) to the skin.
Acknowledgements
This research was partially supported by 1RC1AI078519-01 (to LZ) and the Centers for Medical Countermeasures against Radiation program and U19-AI067733 (to PO) and the National Institute of Allergy and Infectious Diseases (NIAID). The authors wish to thank Dr. Andrei Ivanov and Nayden Naydenov for their kind help with the Western blot, Rob Howell for the thoughtful editing and discussion, and the Research Office at the University of Florida for final edits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification
Paul Okunieff and Lurong Zhang, as former employees of the University of Rochester, were founders of the intellectual property related to FGF-P now held by the University of Rochester. Therefore, they may benefit financially as a result of the outcome of the research or work reported in this publication.
Reference List
- 1.Huang CM, Xu H, Wang CC, et al. Proteomic characterization of skin and epidermis in response to environmental agents. Expert Rev Proteomics. 2005;2:809–820. doi: 10.1586/14789450.2.5.809. [DOI] [PubMed] [Google Scholar]
- 2.Cork MJ, Robinson DA, Vasilopoulos Y, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 3.Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrath JA. Keratinocyte heal thyself: a new form of "natural gene therapy". J Invest Dermatol. 2004;122:x–xi. doi: 10.1046/j.0022-202X.2003.22141.x. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich J, Hartmann JT, Dorr W, et al. Skin toxicity of anti-cancer therapy. J Dtsch Dermatol Ges. 2008;6:959–977. doi: 10.1111/j.1610-0387.2008.06831.x. [DOI] [PubMed] [Google Scholar]
- 7.Leong QM, Lai HK, Lo RG, et al. Radiation dermatitis following radioembolization for hepatocellular carcinoma: a case for prophylactic embolization of a patent falciform artery. J Vasc Interv Radiol. 2009;20:833–836. doi: 10.1016/j.jvir.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Healy ZR, Dinkova-Kostova AT, Wehage SL, et al. Precise determination of the erythema response of human skin to ultraviolet radiation and quantification of effects of protectors. Photodermatol Photoimmunol Photomed. 2009;25:45–50. doi: 10.1111/j.1600-0781.2009.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nylander O, Sjoblom M. Modulation of mucosal permeability by vasoactive intestinal peptide or lidocaine affects the adjustment of luminal hypotonicity in rat duodenum. Acta Physiol (Oxf) 2007;189:325–335. doi: 10.1111/j.1748-1716.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin MA, McKay DM, Yang PC, et al. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–119. doi: 10.1136/gut.47.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen ZY, Feng GG, Nishiwaki K, et al. Possible roles of neuropeptide Y Y3-receptor subtype in rat aortic endothelial cell proliferation under hypoxia, and its specific signal transduction. Am J Physiol Heart Circ Physiol. 2007;293:H959–H967. doi: 10.1152/ajpheart.00886.2006. [DOI] [PubMed] [Google Scholar]
- 12.Rossini M, Cheunsuchon B, Donnert E, et al. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int. 2005;68:2621–2628. doi: 10.1111/j.1523-1755.2005.00734.x. [DOI] [PubMed] [Google Scholar]
- 13.Okunieff P, Li M, Liu W, et al. Keratinocyte growth factors radioprotect bowel and bone marrow but not KHT sarcoma. Am J Clin Oncol. 2001;24:491–495. doi: 10.1097/00000421-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Takahashi K, Campion SL, et al. Synthetic peptide F2A4-K-NS mimics fibroblast growth factor-2 in vitro and is angiogenic in vivo. Int J Mol Med. 2006;17:833–839. doi: 10.3892/ijmm.17.5.833. [DOI] [PubMed] [Google Scholar]
- 15.Poumay Y, Roland IH, Leclercq-Smekens M, et al. Basal detachment of the epidermis using dispase: tissue spatial organization and fate of integrin alpha 6 beta 4 and hemidesmosomes. J Invest Dermatol. 1994;102:111–117. doi: 10.1111/1523-1747.ep12371742. [DOI] [PubMed] [Google Scholar]
- 16.Yuki T, Haratake A, Koishikawa H, et al. Tight junction proteins in keratinocytes: localization and contribution to barrier function. Exp Dermatol. 2007;16:324–330. doi: 10.1111/j.1600-0625.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 17.Francis SA, Kelly JM, McCormack J, et al. Rapid reduction of MDCK cell cholesterol by methyl-beta-cyclodextrin alters steady state transepithelial electrical resistance. Eur J Cell Biol. 1999;78:473–484. doi: 10.1016/s0171-9335(99)80074-0. [DOI] [PubMed] [Google Scholar]
- 18.Mullin JM, Marano CW, Laughlin KV, et al. Different size limitations for increased transepithelial paracellular solute flux across phorbol ester and tumor necrosis factor-treated epithelial cell sheets. J Cell Physiol. 1997;171:226–233. doi: 10.1002/(SICI)1097-4652(199705)171:2<226::AID-JCP14>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 20.Delvenne P, al Saleh W, Gilles C, et al. Inhibition of growth of normal and human papillomavirus-transformed keratinocytes in monolayer and organotypic cultures by interferon-gamma and tumor necrosis factor-alpha. Am J Pathol. 1995;146:589–598. [PMC free article] [PubMed] [Google Scholar]
- 21.Malejczyk J, Malejczyk M, Kock A, et al. Autocrine growth limitation of human papillomavirus type 16-harboring keratinocytes by constitutively released tumor necrosis factor-alpha. J Immunol. 1992;149:2702–2708. [PubMed] [Google Scholar]
- 22.Protein Phosphorylation in Cell Growth Regulation. 1 ed. Amsterdam, Netherlands: Harwood Academic Publishers GmbH; 1996. [Google Scholar]
- 23.Zhang K, Xu J, Huang X, et al. Trichosanthin down-regulated p210Bcr-Abl and enhanced imatinib-induced growth arrest in chronic myelogenous leukemia cell line K562. Cancer Chemother Pharmacol. 2007;60:581–587. doi: 10.1007/s00280-007-0457-0. [DOI] [PubMed] [Google Scholar]
- 24.Yeoh JS, de Haan G. Fibroblast growth factors as regulators of stem cell self-renewal and aging. Mech Ageing Dev. 2007;128:17–24. doi: 10.1016/j.mad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaber M, DiSalvo J, Thomas KA. X-ray crystal structure of human acidic fibroblast growth factor. Biochemistry. 1996;35:2086–2094. doi: 10.1021/bi9521755. [DOI] [PubMed] [Google Scholar]
- 27.Lo CM, Keese CR, Giaever I. Cell-substrate contact: another factor may influence transepithelial electrical resistance of cell layers cultured on permeable filters. Exp Cell Res. 1999;250:576–580. doi: 10.1006/excr.1999.4538. [DOI] [PubMed] [Google Scholar]
- 28.Mishra KP. Cell membrane oxidative damage induced by gamma-radiation and apoptotic sensitivity. J Environ Pathol Toxicol Oncol. 2004;23:61–66. doi: 10.1615/jenvpathtoxoncol.v23.i1.60. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuse M, Sasaki H, Fujimoto K, et al. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gow A, Southwood CM, Li JS, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 32.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 33.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 34.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 35.Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001;13:600–603. doi: 10.1016/s0955-0674(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 36.Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunes FD, Lopez LN, Lin HW, et al. Distinct subdomain organization and molecular composition of a tight junction with adherens junction features. J Cell Sci. 2006;119:4819–4827. doi: 10.1242/jcs.03233. [DOI] [PubMed] [Google Scholar]
- 38.Alexandre MD, Jeansonne BG, Renegar RH, et al. The first extracellular domain of claudin-7 affects paracellular Cl- permeability. Biochem Biophys Res Commun. 2007;357:87–91. doi: 10.1016/j.bbrc.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 39.Laukoetter MG, Nava P, Lee WY, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandell KJ, Babbin BA, Nusrat A, et al. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–11674. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- 41.Trott KR, Shirazi A, Heasman F. Modulation of accelerated repopulation in mouse skin during daily irradiation. Radiother Oncol. 1999;50:261–266. doi: 10.1016/s0167-8140(98)00136-4. [DOI] [PubMed] [Google Scholar]
- 42.Chang YH, Lin HH, Wang YK, et al. Activation of caspase-8 and Erk-1/2 in domes regulates cell death induced by confluence in MDCK cells. J Cell Physiol. 2007;211:174–182. doi: 10.1002/jcp.20926. [DOI] [PubMed] [Google Scholar]