Abstract
Cerebral amyloid beta (Aβ) deposition occurs in a substantial fraction of cognitively normal (CN) older individuals. However, it has been difficult to reliably detect evidence of amyloid-related cognitive alterations in CN using standard neuropsychological measures. We sought to determine whether a highly demanding face-name associative memory exam (FNAME) could detect evidence of Aβ-related memory impairment in CN. We studied 45 CN subjects (mean age = 71.7 ± 8.8) with Clinical Dementia Rating (CDR) scores = 0 and MMSE ≥ 28, using Positron Emission Tomography with Pittsburgh Compound B (PiB PET). Memory factor scores were derived from a principal components analysis for FNAME name retrieval (FN-N), FNAME occupation retrieval (FN-O) and the 6-Trial Selective Reminding Test (SRT). Using multiple linear and logistic regression analyses, we related the memory factor scores to PiB distribution volume ratios (DVR, cerebellar reference) as either a continuous or a dichotomous variable in frontal cortex and a posterior cortical region representing the precuneus, posterior cingulate and lateral parietal cortices (PPCLP), co-varying for age and AMNART IQ (a proxy of cognitive reserve (CR)). A significant inverse relationship for FN-N was found with Aβ deposition in frontal (R2 = .29, β = −2.2, p = 0.02) and PPCLP cortices (R2 = .26, β = −2.4, p = 0.05). In contrast, neither FN-O nor the SRT were significantly related to Aβ deposition. Performance on a demanding test of face-name associative memory was related to Aβ burden in brain regions associated with memory systems. Associative memory for faces and names, a common complaint among older adults, may be a sensitive marker of early Aβ-related impairment.
Keywords: Preclinical Alzheimer’s Disease, Early Detection, Normal Aging, Amyloid Imaging
Introduction
Cognitively normal (CN) older individuals without evidence of cognitive or functional impairment are frequently found to harbor a substantial burden of fibrillar amyloid beta (Aβ) pathology when imaged with Positron Emission Tomography (PET) using Pittsburgh Compound B (PiB) (Fagan, et al., 2006; Johnson, 2006; Mintun, et al., 2006). This observation is consistent with postmortem data indicating that substantial numbers of Aβ plaques are found in some individuals who showed no evidence of memory impairment or dementia during their lifetime (Bennett, et al., 2006; Katzman, et al., 1989; J. L. Price & Morris, 1999). Such individuals may represent a preclinical stage of Alzheimer’s disease (AD) (Morris, et al., 2009; Sperling, et al, 2011), however, it has been difficult to reliably detect evidence of Aβ-related cognitive alternations in CN subjects using standard neuropsychological measures.
Several studies examining increased Aβ deposition with PiB PET imaging in CN subjects were unable to find a relationship between cognitive test performance and Aβ burden (Aizenstein, et al., 2008; Jack, et al., 2008; Mormino, et al., 2009; Villemagne, et al., 2011). One study (Pike, et al., 2007) of 32 healthy control subjects was able to find a modest relationship (r= −0.38) between Aβ burden and episodic memory (EM) in CN subjects but the general findings were limited by sample selection bias toward family history and the presence of an apolipoprotein (APOE) ε4 allele, primary risk factors for AD. Another study by Mormino et al. (Mormino, et al., 2009) found that PiB retention was related to EM and to hippocampal volume (HV) in a subset (N=20) of the healthy control subjects studied. However, when HV and PiB were included in a regression model predicting EM, the HV variable was significant and the PiB variable was not. Storandt and colleagues (Storandt, Mintun, Head, & Morris, 2009) reported an association of Aβ burden with longitudinal cognitive decline prior to diagnosis of AD but a single time point of cognitive performance was not predictive of Aβ-related cognitive change.
As a potential confounding factor in the relationship between Aβ burden and cognitive performance, several studies, including our own, found that cognitive reserve (CR) may influence this association (Kemppainen, et al., 2008; Rentz, et al., 2010; Roe, et al., 2008; Roe, et al., 2010; Yaffe, et al., 2011). CR is a construct that indicates a reduced susceptibility to the clinical expression of a dementia, despite advanced neuropathology(Stern, 2009). This reduced susceptibility could be due to individual characteristics such as increased synaptic or neuronal capacity, greater efficiency engaging brain networks, or the use of alternative strategies to solve task demands. In a previous study with 66 CN subjects, the Aβ relation to cognitive performance was strongly attenuated in subjects with higher CR (Rentz, et al., 2010) suggesting that high CR subjects were performing normally on standardized cognitive tests despite increased Aβ burden. When a more challenging verbal associative memory task (i.e., Memory Capacity Test) was administered, we were able to find a significant relationship between memory performance and Aβ deposition but performance on the MCT was also sensitive to the modifying effects of CR.
As the field moves toward detecting and treating asymptomatic individuals during the very earliest stages of preclinical AD, it will be increasingly important to develop cognitive tests that are both sensitive to early pathological change and useful in subjects with all levels of CR. Since previous work with face-name associative memory tasks has demonstrated sensitivity to memory impairments related to preclinical AD (Clare, Wilson, Carter, Roth, & Hodges, 2002; Parra, et al., 2010; Werheid & Clare, 2007) and to impaired neural activity during face-name memory formation on fMRI tasks in subjects with amyloid deposition, (Sperling, et al., 2009; Vannini, et al., 2011) we speculated that this type of associative memory task may help to clinically differentiate older individuals with early amyloid deposition, irrespective of CR. Here, we tested the hypothesis that performance on a highly demanding test of face-name associative memory (FNAME), is related to Aβ burden in CN older adults and might be useful in overcoming the modifying effects of CR. In particular, we hypothesized that forming and retrieving novel cross-modal face-name associations (FN-N) would be particularly challenging, compared to face-occupation associations (FN-O), and might be a sensitive marker of early amyloid-associated memory impairment, even among the range of performance in CN older adults.
Materials and Methods
Subjects
Forty-five CN subjects enrolled in the Harvard Aging Brain Study at the Center for Alzheimer Research and Treatment at the Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH) Alzheimer’s Disease Research Center were studied using protocols and informed consent procedures approved by the Partners Human Research Committee.
The CN subjects were defined as having a Clinical Dementia Rating (CDR) (Morris, 1993) score of 0, a Mini Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975) score of greater than or equal to 28 and a Geriatric Depression Scale (GDS) score of less than 11 (Yesavage, et al., 1983) (See Table 1). A detailed review of medical history and functional performance as well as physical and neurological examinations confirmed their status as clinically normal (CN). Medical history profiles were typical of an aging sample with 13% having controlled hypertension, 3% with controlled hypercholesterolemia, 6% with remote history of resolved breast cancer, 4% with asthma and 2% with gastroesophageal reflux disease, atrial fibrillation, remote history of resolved prostate cancer and resolved depression. None of the participants had a history of alcoholism, drug abuse, head trauma or current serious medical or psychiatric illness.
Table 1.
Sample Characteristics (N=45)
| Sex, M/F, (Percent) | 19 (42%) | 26 (58%) | ||
|---|---|---|---|---|
| Mean | SD | Range | ||
| Age | 71.72 | 8.81 | 46.2 | 88.4 |
| Education | 16.73 | 2.64 | 12.0 | 20.0 |
| AMNART IQ | 123.47 | 7.05 | 102.0 | 132.0 |
| MMSE | 29.27 | 0.75 | 28.0 | 30.0 |
| GDS | 3.07 | 3.27 | 0.0 | 10.0 |
| FNAME Name Composite | 0.02 | 0.96 | −1.65 | 2.16 |
| FNAME Occupation Composite | −0.01 | 0.94 | −2.16 | 1.50 |
| SRT Composite | −0.02 | 0.78 | −1.74 | 1.95 |
| Frontal DVR Median | 1.11 | 0.15 | 0.95 | 1.70 |
| PPCLP DVR Median | 1.13 | 0.11 | 0.96 | 1.66 |
AMNART IQ= American National Adult Reading Test Intelligence Quotient; MMSE= Mini Mental State Exam, GDS= Geriatric Depression Scale, FNAME- Face Name Associative Memory Exam, SRT= Selective Reminding Test, DVR= Distribution Volume Ratio, PPCLP= Precuneus, Posterior Cingulate and Lateral Parietal
Neuropsychological (NP) evaluation
Subjects were administered an extensive battery of NP tests that covered the cognitive realms of attention, executive functions, memory, language and visuospatial processing. For this study, we focused only on episodic memory (EM) tests because declines in EM are reportedly the earliest signs of preclinical AD; (Albert, Moss, Tanzi, & Jones, 2001; Johnson, et al., 2007a) and performance on tests of EM tend to decline 7 years before conversion to AD (Grober, et al., 2008). EM tests administered to our subjects included the 6-Trial Selective Reminding Test (SRT), (Masur, et al., 1989) the Free and Cued Selective Reminding Test (FCSRT) (Grober, Merling, Heimlich, & Lipton, 1997) and a challenging cross-modal associative memory test we developed based on fMRI experiments, called the Face-Name Associative Memory Exam (FNAME) (Sperling, et al., 2003a; Sperling, et al., 2001).
FNAME Procedure
The FNAME requires the subject to remember 16 unfamiliar face-name pairs and 16 face-occupation pairs for a total of 32 cross-modal paired associates to be remembered. The test has an initial study phase as well as free recall and cued recall trials.
Initial Face Study Phase
The test begins with an exposure to all 16 faces. Subjects are shown 4 faces to a page, one face in each quadrant. They are asked to look at each face for a total of 2 seconds until they have seen all 16 faces.
Initial Study of Face-Name Pairs
Subjects are then presented the same faces with names underneath and asked to study the name that goes with the face. To ensure that the subject is learning the face-name pairs, the examiner points to the face and asks the subject to read the name associated with that face. After all 4 items are correctly identified; another 4 face-name pairs are presented until all 16 face-name pairs are studied. Subjects are given only one exposure to learn all 16 face-name pairs.
Initial Recall of Face-Name Pairs
The subjects are then shown the face and asked to recall the name that goes with the face. The correct number of face-name pairs is recorded as an initial learning score for names (ILN).
Initial Study of Face-Occupation Pairs
Subjects are then shown the same faces but this time with occupations underneath. The face-occupation pairs are presented in the same manner as the face-name pairs until all 16 face-occupation pairs are studied.
Initial Recall of Face-Occupation Pairs
Subjects are again shown the face and asked to recall the occupation that goes with the face. Correct recall of faces and occupations are tabulated as initial learning of occupations (ILO).
FNAME Recall Trials
The FNAME included both “free” and “cued” recall trials at immediate and delayed intervals.
Free Recall Trials
After the initial study phase, subjects are then asked to freely recall all the names (FRN) and occupations (FRO).
Cued Recall Trials
Following the Free Recall trial, subjects are shown the face and asked to recall the name (CRN) and occupation (CRO) that was associated with the face.
30-Minute Delayed Free Recall
Subjects are again asked to freely recall the names (FRN30) and occupations (FRO30) following a 30-minute delay.
30-Minute Delayed Cued Recall
Subjects are again shown the face and asked to recall the name (CRN30) and occupation (CRO30) associated with the face. A Reliability Analysis indicated good internal consistency among the 10 performance scores of the FNAME with a Cronbach alpha coefficient of .96. The mean inter-item correlation was 0.72 with values ranging from 0.48 to .93 suggesting a strong relationship among the items.
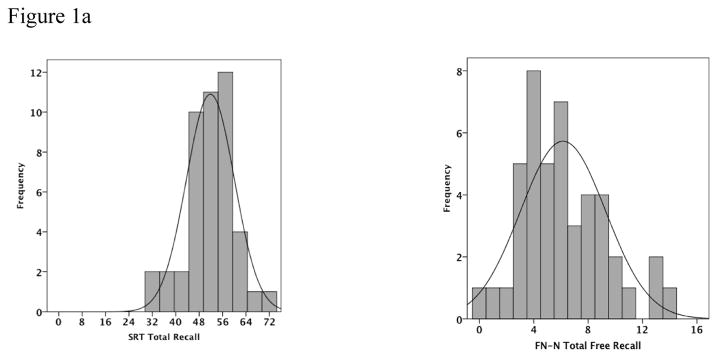
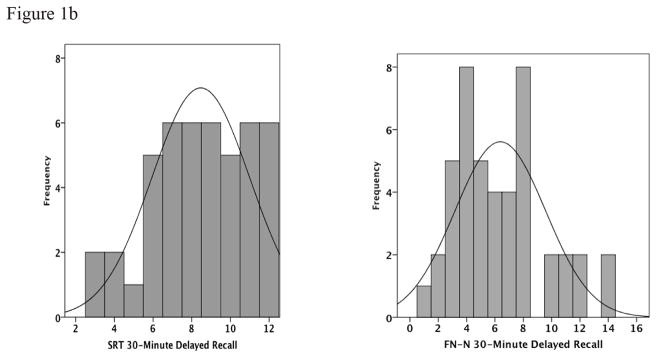
The distribution of scores on the FNAME was examined in relationship to performance on the SRT to determine the range of performance. We found that the FNAME did not exhibit the same ceiling effect in normal controls as other traditional memory measures (see Figure 1a and 1b). Furthermore, successful performance on the FNAME has been associated with increased activity in the brain networks subserving memory in both young and older individuals (Miller, et al., 2008).
Figure 1.
Figure 1a and 1b: Histograms of the frequency of SRT Total Recall and FN-N Total Free Recall as well as SRT 30-Minute Delayed Recall and FN-N 30-Minute Delayed Recall shows that the FN-N distribution of scores has less of a ceiling effect than the SRT scores. (Scales on the x-axis represent the highest score possible on the test)
Development of Composite Factor Scores
To avoid capitalizing on chance due to multiple comparisons, the memory test battery was subjected to a principal components analysis to derive composite factor scores. The initial analysis revealed 5 factors among all the memory tests, explaining 86% of the variance (51%, 17.1%, 7.3%, 6.1% and 4.9% respectively). The Kaiser-Meyer-Oklin value of .83 and Bartlett’s Test of Sphericity (p < .001) supported the factorability of the correlation matrix. However, the scree plot revealed a clear break after the third factor and it was decided to retain only those 3 factors. This resulted in excluding the MC and MC30 of the SRT and FRsrt and FCsrt of the FCSRT. The three-factor solution explained a total of 88% of the variance. Factor 1 contributed to 60.4% of the variance and was associated with the FNAME Name scores. Factor 2 contributed to 20.4% of the variance and was associated with the SRT scores. Factor 3 contributed to 7.3% of the variance and was associated with the FNAME Occupation scores. Table 2 displays the tests that loaded on the 3 factors. The Kaiser-Meyer-Oklin value of .86 and Bartlett’s Test of Sphericity (p < .001) again supported the factorability of the correlation matrix. The component correlation matrix revealed that the FNAME Name Composite (FN-N) was correlated with the SRT-Memory Composite (r = 0.547, p < 0.01) as well as the FNAME Occupation Composite (FN-O) (r = 0.367, p = 0.01). The FN-N and FN-O Composites were also highly correlated with each other (r = 0.693, p < 0.01). In light of these 3 identified factors, we derived regression weighted factor composite scores for each subject. We used these 3 factor composites to explore the relationship of EM performance in CN subjects to Aβ deposition in frontal association and parietal/posterior cingulate cortices that encompass a widely distributed memory network (Buckner, et al., 2005). These regions were chosen because they are associated with early Aβ deposition (Mintun, et al., 2006) and EM in the default network (Buckner, et al., 2005; Sperling, et al., 2009). The average factor composite scores are given in Table 1. The mean (sd) time between PET imaging and testing was 3.4 (5.5) months.
Table 2.
Pattern and structure matrix for PCA with oblim rotation of 3-factor solution from which regression weighted factor scores were derived for each individual.
| Pattern Coefficients | Structure Coefficients | ||||||
|---|---|---|---|---|---|---|---|
| Tests | Component | Tests | Component | ||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| FRN30 | .991 | .010 | .045 | FRN30 | .966 | .527 | .623 |
| CRN30 | .976 | .022 | .048 | CRN | .965 | .544 | .667 |
| CRN | .921 | .035 | .037 | CRN30 | .956 | .530 | .614 |
| FRN | .899 | .016 | .096 | FRN | .955 | .501 | .693 |
| ILN | .890 | .009 | .022 | ILN | .900 | .478 | .616 |
| LTR | .029 | 1.008 | .008 | LTR | .508 | .989 | .324 |
| LTS | .034 | 1.005 | .041 | LTS | .480 | .972 | .288 |
| CR | .025 | 1.000 | .039 | CR | .487 | .972 | .293 |
| CLTR | .015 | .941 | .019 | TR | .539 | .951 | .381 |
| TR | .001 | .931 | .055 | CLTR | .508 | .942 | .320 |
| DR30 | .029 | .824 | .083 | DR30 | .528 | .869 | .390 |
| DR | .086 | .764 | .004 | DR | .500 | .812 | .328 |
| FRO | .081 | .024 | .962 | CRO | .659 | .422 | .955 |
| CRO30 | .037 | .051 | .946 | CRO30 | .643 | .299 | .953 |
| CRO | .027 | .110 | .935 | ILO | .702 | .342 | .927 |
| FRO30 | .025 | .039 | .885 | FRO30 | .639 | .362 | .915 |
| ILO | .167 | .037 | .828 | FRO | .551 | .269 | .899 |
Factor 1: FRN30= FNAME free recall of names at 30 minutes; CRN30- FNAME cued recall of names at 30 minutes; CRN= FNAME cued recall of names; FRN= FNAME free recall of names; ILN= FNAME initial learning of names; Factor 2: LTR= SRT long-term retrieval; LTS= SRT long-term store; CR= SRT continuous retrieval; CLTR= SRT continuous long-term retrieval; TR= SRT total recall; DR30= SRT delayed recall at 30-minutes; DR= SRT delayed recall at 10-minutes; Factor 3: FRO= FNAME free recall of occupations; CRO30= FNAME cued recall of occupations at 30-minutes; CRO= FNAME cued recall of occupations; FRO30= FNAME free recall of occupations at 30-minutes; ILO= FNAME initial learning of occupations.
Positron Emission Tomography
PiB was prepared as described by Mathis et al (Mathis, et al., 2003) and PiB PET acquisitions were performed as described previously (Gomperts, et al., 2008; Johnson, et al., 2007b; Rentz, et al., 2010). Following a transmission scan, 8.5 – 15 mCi 11C-PiB was injected as a bolus and followed immediately by a 60-minute dynamic acquisition. PiB PET data were reconstructed with ordered set expectation maximization, corrected for attenuation. Each frame was evaluated to verify adequate count statistics and absence of head motion. The Logan graphical analysis method (Logan, et al., 1996; J. C. Price, et al., 2005) with cerebellar cortex as the reference tissue input function was used to evaluate specific PiB retention expressed as the distribution volume ratio (DVR) (Archer, et al., 2006; Fagan, et al., 2006; Johnson, et al., 2007b; Lopresti, et al., 2005). We calculated the DVR (with cerebellar grey reference) in aggregate cortical regions-of-interest (ROI) for the frontal association and precuneus, posterior cingulate, and lateral parietal (PPCLP) cortices, particularly including brain regions responsible for memory. The ROIs were defined according to the automated anatomical labeling (AAL) parcellation scheme (Tzourio-Mazoyer, et al., 2002) applied to data warped to the Montreal Neurological Institute (MNI) standard space using Statistical Parametric Mapping software. The Frontal association cortex ROI consisted of inferior (opercularis, triangularis, orbitalis), superior and middle gyri as well as the supplementary motor area. The PPCLP ROI comprised the posterior cingulate, parietal lobe, supramarginal gyrus, angular gyrus, and precuneus.”(see Figure 3 in Tzourio-Mazoyer, et al., 2002).
Statistical Analysis
We performed multiple regression analyses relating PiB retention (DVR, cerebellar reference) as a continuous variable in cortical regions, co-varying for age and AMNART IQ, as a proxy for CR (Rentz, et al., 2007; Rentz, et al., 2010) and the interaction (cross-product) of CR and amyloid burden to the 3 memory factor scores. A logistic regression was performed to determine if PiB positivity was related to the 3 memory factor scores, covarying for age and AMNART-IQ. As reported previously (Rentz, et al., 2010), we chose a cut-point for amyloid PiB-positive to be a global mean PiB ≥ 1.15 (n = 17). PiB-negative was defined as a global mean PiB < 1.15 (n = 28). We chose 1.15 because (1) it is the cutoff used in other studies published by our group (Gomperts, et al., 2008; Hedden, et al., 2009; Rentz, et al., 2010); (2) because in a previous study combining CN, MCI and AD subjects it represented a cut point that best separated those with from those without focally elevated PiB retention (Gomperts, et al., 2008); and (3) because it represented a lower limit for PiB retention seen in AD patients (Gomperts, et al., 2008; Johnson, et al., 2007).
Post-hoc multiple regression analyses were performed to examine the specificity of the relationship between cognitive performance and Aβ burden in frontal and PPCLP regions. The dependent variable was each subtest of the FNAME, the individual cognitive tests for the various domains of attention (Digit Span forward, Trails A), executive functions (Trails B, FAS, Digits Backward), semantic processing (category generation to animals, vegetables and fruit), language (Boston Naming Test) and visuospatial processing (Visual Form Discrimination Test) as well as an executive z-score composite consisting of Trails B, FAS and Digits Backward. The covariates were age, AMNART IQ and the PiB DVR. We also explored in a post-hoc analysis, the relationship of Aβ deposition in frontal and PPCLP regions with APO ε4 carrier status and memory performance, again controlling for age and AMNART IQ. Significance was set a priorias p < 0.05. All data were analyzed using SPSS v18.0.
Results
Subject Characteristics
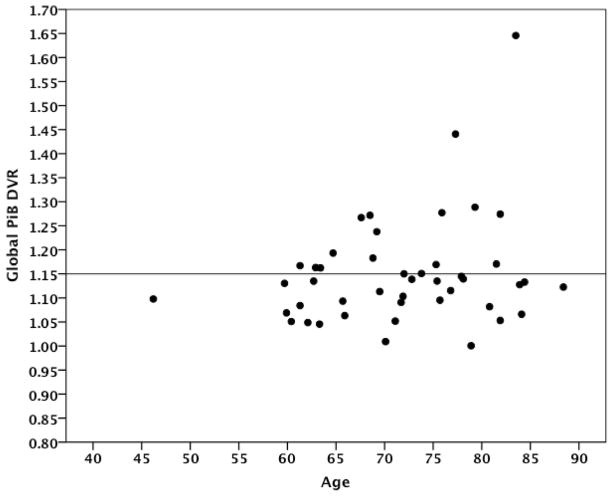
The subject characteristics are provided in Table 1. The sample had an average age of 72 years (46–88 years). Figure 2 displays global amyloid deposition across the age range. There were no significant differences between the numbers of men and women in the sample, with 42% of the sample being men and 58% women. Men were more educated than women (Mann-Whitney U Test, p = .01), but there were no significant differences between men and women on any of the other variables.
Figure 2.
Scatterplot of age and global Aβ deposition. Line at 1.15 indicates the separation between amyloid negative and amyloid positive individuals based on a combined sample of 140 subjects representing the lower limit for PiB retention in AD patients.
Relationship between Amyloid and Memory Performance, Multiple Regression Analysis
We initially explored whether Aβ deposition in cortical regions was associated with memory performance using the 3-factor scores co-varied for age and AMNART IQ. Table 3 provides the results of the multiple regression analyses. We found a significant inverse relationship for the FN-N Composite such that lower performances were associated with Aβ deposition in frontal (R2 = 0.29, β = −2.2, p = 0.02) and PPCLP cortices (R2 = 0.26, β = −2.4, p = 0.05) (see Figure 3a). There were no significant associations found with the SRT Memory Composite (see Figure 3b) or the FN-O Composite (see Figure 3c). No significant relationships were found between FN-N Composite scores, FN-O Composite scores, amyloid deposition and CR.
Table 3.
Multiple regression analyses of the memory factor composite scores and PiB DVR in frontal and PPCLP regions. All models covaried for age and AMNART IQ.
| Factor 1 | Factor 2 | Factor 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FNAME Name Composite | SRT Composite | FNAME Occupation Composite | |||||||
| ROI | a R2 | bβ | p | R2 | β | p | R2 | β | p |
| Frontal | 0.29 | −2.21 | 0.02 | 0.12 | −1.17 | 0.25 | 0.22 | −1.19 | 0.22 |
| PPCLP | 0.26 | −2.44 | 0.05 | 0.14 | −1.99 | 0.14 | 0.21 | −1.44 | 0.26 |
R2 = Full model R-Square
β = unstandardized partial regression coefficient
Figure 3.
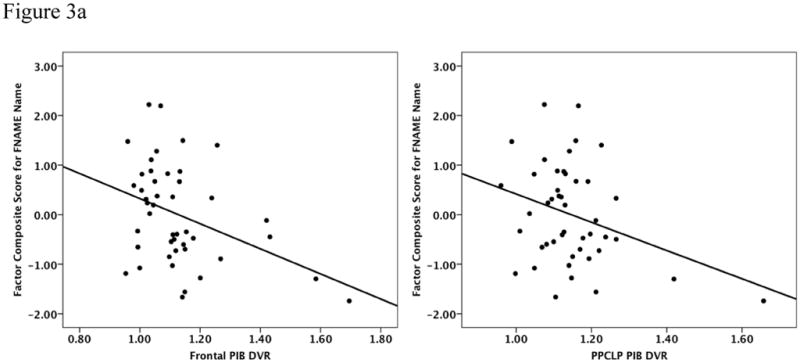
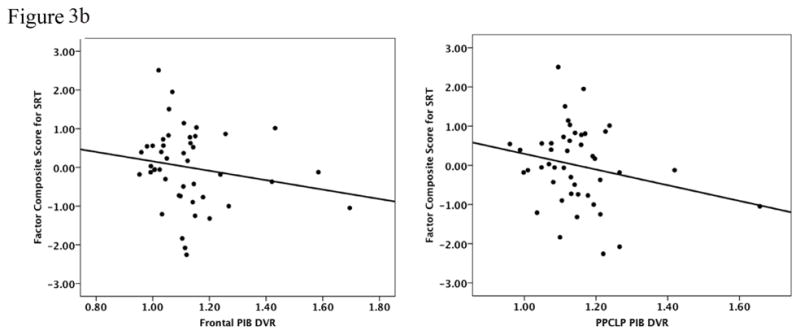
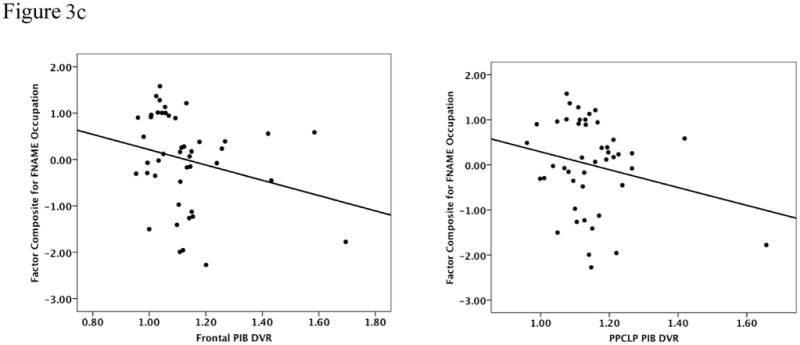
Figure 3a to 3c: Performances on the FN-N Composite (3a) the SRT Memory Composite (3b) and the FN-O Composite (3c) were inversely related to PiB retention in frontal and PPCLP cortices but only the FN-N Composite reached statistical significance. Analyses were co-varied for age and AMNART IQ.
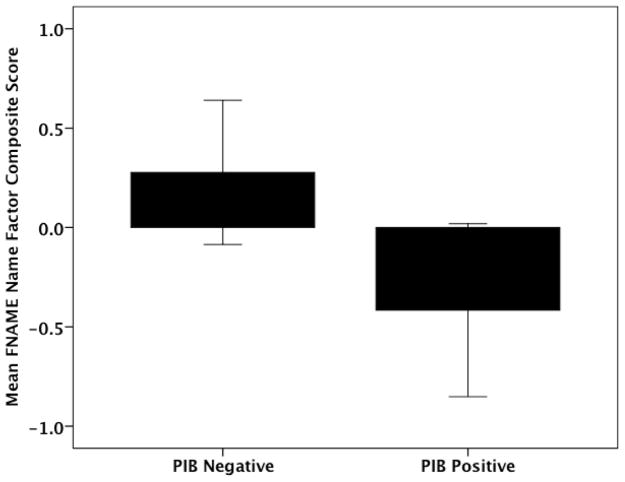
Logistic Regression Analysis
To explore whether PiB positivity was related to memory performance on the 3 factor scores, we performed a logistic regression co-varying for age and AMNART IQ. We found a significant relationship between the PiB-positive classification and lower performance on the FN-N Composite (χ2 = 11.13, β = −1.32, p= 0.04) but there were no significant relationships with the FN-O or SRT Composites (see Figure 4).
Figure 4.
Logistic regression revealed a significant relationship between PiB positive classification and lower performance on the FN-N Composite.
Post-Hoc Analyses
In post-hoc analyses we explored whether specific aspects of the FNAME were related to amyloid burden in frontal and PPCLP regions. We found that performance on all subtests of the FN-N portion of the FNAME were significant with frontal amyloid deposition including initial learning (ILN, r2 = .38, p = .02) free recall (FRN, r2 = .28, p = .01) and cued recall (CRN, r2 = .28, p = .03), as well as free (FRN30, r2 = .23, p = .04) and cued delayed recall (CRN30, r2 = .20, p = .05). The relationship between performance on these subtests and PPCLP amyloid deposition showed a similar pattern, but was not as significant across all FN-N subtests of the FNAME (ILN, r2 = .38, p = .02; FRN, r2 = .23, p = .05; CRN, r2 = .25, p = .08; FRN30, r2 = .21, p = .08; CRN30, r2 = .16, p = .16). We did not find a significant relationship with amyloid deposition and performance on any of the FN-O subtests of the FNAME. These findings suggest that all aspects of FN-N are similarly challenging and significantly related to amyloid burden.
We also explored whether Aβ burden in frontal and PPCLP regions was related to other cognitive functions. We did not find any significant relationships (p > 0.15) with Aβ burden in frontal or PPCLP regions with tests representing the cognitive domains of attention, executive function, language and visuospatial processing.
Finally, we reran the above regression analyses to determine if there was a relationship between APOE status and memory performance. Thirty-two of the 45 subjects had APOE genotyping available. The remaining 13 individuals did not differ from the 32 subjects in either demographics or performance on the memory factor scores or any of the other cognitive measures. Of these 32 subjects, 8 were ε4 carriers and 24 were non-ε4 carriers. We reran the regression models as described above but added one or no copies of the APOE ε4 allele as a dummy-coded predictor variable. We found that APOE status was not a significant predictor of memory performance on any of the memory composite scores. We also failed to find a significant relationship between APOE carrier status and degree of amyloid deposition in either frontal, PPCLP, precuneus, and global PiB DVR regions (all p > 0.30) in this sample.
Discussion
In this study, we report an association of Aβ burden with performance on a highly challenging test of face-name associative memory, namely (FN-N) of the FNAME, in CN older adults. Similar to other reports (Aizenstein, et al., 2008; Mormino, et al., 2009; Rentz, et al., 2010; Storandt, et al., 2009), we were unable to find this association with less challenging tests of EM, such as face-occupation retrieval (FN-O) or the SRT. The findings of this study suggest that FN-N, in particular, may be a more sensitive probe of Aβ-related memory dysfunction when examining CN older adults.
Forming face-name associations is widely acknowledged to be particularly difficult due to the inherent unrelatedness of a face with a name (Werheid & Clare, 2007). Remembering proper names is the most common memory complaint of older adults (Leirer, Morrow, Sheikh, & Pariante, 1990; Zelinski & Gilewski, 1988). Whereas, forming an association of a face with other biographical information about someone (i.e., occupations, hobbies) is easier (Cohen, 1990; McWeeny, Young, Hay, & Ellis, 1987). Even in paradoxical situations where “Baker” is used as both a name and an occupation, Baker presented as a name was harder to recall than when it was presented as an occupation (McWeeny, et al., 1987). Since there are no contextual properties with which to formulate an associative link, binding a proper name to a unique face can be considered much more effortful, requiring a higher level of cognitive demand, than other less demanding memory tasks that can be associated with previously stored semantic knowledge, such as occupations in the FN-O task, or recalling words that can be associated with well known categories such as on the California Verbal Learning Test or the Free and Cued Selective Reminding Test. It is unknown whether other tests of high cognitive demand would be associated with amyloid deposition. However, based on our analyses, we did not find an association with amyloid deposition and other frontal executive tasks, including Trails B. Consistent with this theory, we found that forming an association between a face and a name was not only more difficult for CN older subjects but also associated with Aβ burden in brain regions related to memory.
Previous fMRI work from multiple groups has suggested that the successful formation and retrieval of face-name pairs required coordinated activity in a distributed memory network (Fletcher & Henson, 2001; Miller, et al., 2008; Rugg, Otten, & Henson, 2002; Sperling, et al., 2003b; Vannini, et al., 2010; Zeineh, Engel, Thompson, & Bookheimer, 2003) This network includes not only the hippocampus and related structures in the medial temporal lobe, but a distributed set of cortical regions, collectively known as the default network (Buckner, et al., 2005; Raichle, et al., 2001). Of particular relevance to our current findings, is the observation that the precuneus/posterior cingulate appears to be a key node in the default network involved in both encoding and retrieval of associative memories (Miller, et al., 2008; Rugg, Otten, & Henson, 2002; Vannini, et al., 2010).
The brain regions particularly vulnerable to early Aβ deposition appear to overlap the anatomic distribution of the default network (Buckner, et al., 2005; Sperling, et al., 2009). Regions within the default network show structural and functional connectivity that converge on the posterior cingulate extending into the precuneus which is strongly interconnected with the hippocampus (Greicius, Srivastava, Reiss, & Menon, 2004). Our previous work, as well as that of other groups, has suggested that Aβ deposition in the default mode network is associated with functional disruption within the default network in cognitively normal older individuals (Hedden, et al., 2009; Sperling, et al., 2009; Vannini, et al., 2011). Thus it is perhaps not surprising that we found an association between reduced performances in FN-N and increased Aβ burden in these brain regions. We did not observe an association between Aβ deposition in medial temporal lobe regions and performance on any cognitive measures. This likely reflects the fact that there is relatively less amyloid pathology in the human hippocampus compared to neocortical regions found in aging or early Alzheimer’s disease.
Another important finding of this study is the association between FN-N and Aβ burden in frontal cortices, in addition to parietal regions. Converging evidence has shown that in addition to medial temporal involvement, cortical regions of the frontal lobe also play an important role in associative memory formation and retrieval (Cabeza, et al., 1997; Fletcher & Henson, 2001) including the forced choice recognition of memory for face-name pairs (Jackson & Schacter, 2004; Vannini, et al., 2010; Werheid & Clare, 2007). When we explored whether specific subtests of the FNAME were related to amyloid deposition in frontal and PPCLP regions, we found that all aspects of FN-N including initial learning, recall and delayed recall were similarly challenging and significantly related to Aβ burden in frontal and PPCLP regions. While it is known that declines in EM and executive functions are predictive of subsequent cognitive decline to AD, (Albert, Moss, Tanzi, & Jones, 2001; Blacker, et al., 2007; Chen, et al., 2000; Grober, et al., 2008), an association of memory and executive dysfunction with Aβ burden in frontal cortex has not been reported in CN older adults. We found that impairments in FN-N were related to Aβ burden in frontal cortices which are also vulnerable to early Aβ deposition (Klunk, et al., 2004).
The findings in this study are consistent with other reports that have suggested that paired associative learning tasks are particularly sensitive in detecting early impairment and preclinical AD, sometimes well before significant deterioration occurred on standard neuropsychological tests (Blackwell, et al., 2004; de Jager, Milwain, & Budge, 2002; Elias, et al., 2000; Fowler, Saling, Conway, Semple, & Louis, 2002; Linn, et al., 1995). In fact, face-name associative memory may be particularly sensitive in dissociating memory impairments related to preclinical AD from those associated with normal aging. Others have also found that face-name associative memory, provides highly sensitive indices of episodic and semantic memory performance in preclinical AD (Clare, Wilson, Carter, Roth, & Hodges, 2002; Parra, et al., 2010; Werheid & Clare, 2007), as was a shape–color associative visual memory task in predicting familial AD (Parra, et al., 2010). It remains to be known whether there are other types of paired associative learning tasks that are equally sensitive to Aβ burden but the development of more challenging tests of memory are needed as future clinical trials move to target individuals in preclinical stages of AD.
This study has several important limitations. The sample size was small and the fact that we did not find an interaction with CR may be due to a lack of power or may suggest that CR plays less of a role in more challenging EM tasks. Another limitation of this report is the lack of APOE genotyping in all subjects, the limited number of APOE ε4 carriers and the absence of any ε4 homozygotes in the study. Previous studies with larger sample sizes, including subjects with family history of AD (Morris, et al., 2010) or recruited specifically to find APOE ε4 carriers (Reiman, et al., 2009) have reported that accelerated memory decline (Caselli, et al., 2009) and fibrillar Aβ as measured with PiB PET imaging was significantly associated with APOE ε4 carrier status in cognitively normal individuals. Future work will be required to determine if APOE has an independent contribution to our observed findings. Finally, the relationship between frontal and PPCLP amyloid and FN-N appears to be driven primarily by a small number of individuals with high amyloid burden. Data from our group, as well as reports from multiple other groups studying amyloid imaging, suggest that the relationship between Aβ burden and cognitive performance, as well as functional and structural imaging variables, is often driven by the subset of individuals with very high levels of PiB retention (Becker, et al., 2011; Hedden, et al., 2009; Sheline, et al., 2010; Sperling, et al., 2009). Although we believe that there is a continuum of Aβ burden, it is likely that these few individuals with very high Aβ deposition represent those at highest risk for subsequent clinical decline, and that these individuals are of particular interest.
In conclusion, we found that performance on a demanding test of face-name retrieval, a common complaint of many normal older individuals, was associated with Aβ burden in frontal and PPCLP cortices, thought to be critical brain regions in a distributed memory network. Challenging tests of associative memory retrieval in clinically normal older individuals may prove to be sensitive markers for detecting the early effects of Aβ deposition.
Acknowledgments
This work was supported by Alzheimer Association grants IIRG-08-90934 (D.R., K.J.) and ZEN-10-174210 (K.J.) and by National Institute on Aging grants P01-AG036694-01 (R.S. and K. J.); P50- AG00513421 (R.S. and K.J.);R01-AG027435 (R.S.); R01- AG027435-S1 (R.S., K.J.), and R01- AG037497 (K.J.) The authors wish to thank the individual research participants without which this work would not be possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Archer HA, Edison P, Brooks DJ, Barnes J, Frost C, Yeatman T, Rossor MN. Amyloid load and cerebral atrophy in Alzheimer's disease: an 11C-PIB positron emission tomography study. Annals of Neurology. 2006;60(1):145–147. doi: 10.1002/ana.20889. [DOI] [PubMed] [Google Scholar]
- Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, … Johnson KA. Amyloid-β associated cortical thinning in clinically normal elderly. Annals of Neurology. 2011 doi: 10.1002/ana.22333. (Article first published online: 17 MAR 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. 66/12/1837 [pii] [DOI] [PubMed] [Google Scholar]
- Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Moss M, Albert M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64(6):862–871. doi: 10.1001/archneur.64.6.862. 64/6/862 [pii] [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17(1–2):42–48. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. 361/3/255 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, Dekosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55:1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Relearning face-name associations in early Alzheimer's disease. Neuropsychology. 2002;16(4):538–547. doi: 10.1037//0894-4105.16.4.538. [DOI] [PubMed] [Google Scholar]
- Cohen GD. Why is it difficult to put names to faces? British Journal of Psychology. 1990;1:249–263. [Google Scholar]
- de Jager CA, Milwain E, Budge M. Early detection of isolated memory deficits in the elderly: the need for more sensitive neuropsychological tests. Psychol Med. 2002;32(3):483–491. doi: 10.1017/s003329170200524x. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer's disease: a 22 year prospective study of the Framingham cohort. Archives of Neurology. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta(42) in humans. Annals of Neurology. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8(1):58–71. [PubMed] [Google Scholar]
- Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Johnson KA. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. Journal of the International Neuropsychological Society. 2008;14(2):266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Merling A, Heimlich T, Lipton RB. Free and cued selective reminding and selective reminding in the elderly. Journal of Clinical and Experimental Neuropsychology. 1997;19(5):643–654. doi: 10.1080/01688639708403750. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29(40):12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. 29/40/12686 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson O, 3rd, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage. 2004;21(1):456–462. doi: 10.1016/j.neuroimage.2003.09.050. S1053811903006001 [pii] [DOI] [PubMed] [Google Scholar]
- Johnson KA. Amyloid imaging of Alzheimer's disease using Pittsburgh Compound B. Curr Neurol Neurosci Rep. 2006;6(6):496–503. doi: 10.1007/s11910-006-0052-5. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Greenberg SM. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62(3):229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Moran EK, Becker JA, Blacker D, Fischman AJ, Albert MS. Single photon emission computed tomography perfusion differences in mild cognitive impairment. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78(3):240–247. doi: 10.1136/jnnp.2006.096800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Anderson JM, Fuld P, Kawas C, Brown T, Morgenstern H, Ooi WL. Development of dementing illness in an 80-year-old volunteer cohort. Annals of Neurology. 1989;25:317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Annals of Neurology. 2008;63(1):112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Leirer VO, Morrow DG, Sheikh JI, Pariante GM. Memory skills elders want to improve. Exp Aging Res. 1990;16(3):155–158. doi: 10.1080/07340669008251544. [DOI] [PubMed] [Google Scholar]
- Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, D'Agostino RB. The “preclinical phase” of probable Alzheimer's disease. A 13-year prospective study of the Framingham cohort. Archives of Neurology. 1995;52(5):485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, Price JC. Simplified Quantification of Pittsburgh Compound B Amyloid Imaging PET Studies: A Comparative Analysis. Journal of Nuclear Medicine. 2005;46(12):1959–1972. [PubMed] [Google Scholar]
- Masur DM, Fuld PA, Blau AD, Thal LJ, Levin HS, Aronson MK. Distinguishing normal and demented elderly with the Selective Reminding Test. Journal of Clinical and Experimental Neuropsychology. 1989;11(5):615–630. doi: 10.1080/01688638908400920. [DOI] [PubMed] [Google Scholar]
- Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46(13):2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- McWeeny KH, Young AW, Hay DC, Ellis AW. Putting Names to Faces. British Journal of Psychology. 1987;78(1):143–149. [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating Scale (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. 66/12/1469 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 2010;133(9):2702–2713. doi: 10.1093/brain/awq148. awq148 [pii] [DOI] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130(Pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676 98/2/676. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Huh TJ, Sardinha LM, Moran EK, Becker JA, Daffner KR, Johnson KA. Intelligence quotient-adjusted memory impairment is associated with abnormal single photon emission computed tomography perfusion. J Int Neuropsychol Soc. 2007;13(5):821–831. doi: 10.1017/S1355617707071056. S1355617707071056 [pii] [DOI] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Archives of Neurology. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, Ghoshal N, Williams MM, Grant EA, Marcus DS, Morris JC. Alzheimer disease identification using amyloid imaging and reserve variables: proof of concept. Neurology. 2010;75(1):42–48. doi: 10.1212/WNL.0b013e3181e620f4. 75/1/42 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357(1424):1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. S0006-3223(09)01032-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74(1):44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14(3):129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan A, Phelps CH. Towards defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer Association Workgroup. Alzheimer’s & Dementia. 2011 doi: 10.1016/j.jalz.2011.03.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. S0028-3932(09)00123-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66(12):1476–1481. doi: 10.1001/archneurol.2009.272. 66/12/1476 [pii] 0.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vannini P, O'Brien J, O'Keefe K, Pihlajamaki M, Laviolette P, Sperling RA. What Goes Down Must Come Up: Role of the Posteromedial Cortices in Encoding and Retrieval. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq051. bhq051 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Becker JA, Sullivan C, Putcha D, Rentz D, et al. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.003. S0197-4580(11)00005-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werheid K, Clare L. Are faces special in Alzheimer's disease? Cognitive conceptualisation, neural correlates, and diagnostic relevance of impaired memory for faces and names. Cortex. 2007;43(7):898–906. doi: 10.1016/s0010-9452(08)70689-0. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale. Journal of Psychiatric Research. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305(3):261–266. doi: 10.1001/jama.2010.1995. 305/3/261 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299(5606):577–580. doi: 10.1126/science.1077775 299/5606/577. [pii] [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ. Assessment of memory complaints by rating scales and questionnaires. Psychopharmacol Bull. 1988;24(4):523–529. [PubMed] [Google Scholar]