Abstract
Research has shown that mechanical loading affects matrix biosynthesis of intervertebral disc (IVD) cells; however the pathway(s) to this effect is currently unknown. Cellular matrix biosynthesis is an energy demanding process. The objective of this study was to investigate the effects of static and dynamic compressive loading on energy metabolism of IVD cells. Porcine annulus fibrosus (AF) and nucleus pulposus (NP) cells seeded in 2% agarose were used in this experiment. Experimental groups included 15% static compression and 0.1 and 1 Hz dynamic compression at 15% strain magnitude for 4 hours. ATP, lactate, glucose and nitric oxide (NO) contents in culture media, and ATP content in cell-agarose construct were measured using biochemical assays. While the total ATP content of AF cells was promoted by static and dynamic loading, only 1 Hz dynamic loading increased total ATP content of NP cells. Increases in lactate production and glucose consumption of AF cells suggest that ATP production via glycolysis is promoted by dynamic compression. ATP release and NO production of AF and NP cells were significantly increased by dynamic loading. Thus, this study clearly illustrates that static and dynamic compressive loading affect IVD cell energy production while cellular responses to mechanical loading were both cell type and compression type dependent.
Keywords: intervertebral disc, ATP, mechanical loading, energy production
Introduction
Back pain is a significant socio-economic burden and the leading cause of pain and disability affecting more than 80% of the total US population during their lifetime [1]. Despite its prevalence, our understanding of the disease process is poor. Degeneration of the intervertebral disc (IVD) has been suggested as a significant contributor to low-back pain (LBP). Nutrient supply, soluble regulators of cell function, genetic influences, ageing, and mechanical loading have been identified as contributory factors to IVD degeneration; however the pathways to this effect are not clearly understood [2,3]. Thus, identifying the degenerative pathways may shed light to development of novel treatment and preventive methods for disc degeneration and LBP.
Extracellular matrix (ECM) synthesis, which is crucial to retaining IVD tissue integrity, is an energy demanding process [4–7]. The avascular nature of IVD tissue may subsequently lead to poor nutrient supply to IVD cells, resulting in decreased cell energy production. Over time, this could cause insufficient maintenance of IVD tissue matrix, thus initiating or promoting disc degeneration. A previous study conducted on a guinea pig model of osteoarthritis showed that depletion of ATP was associated with cartilage degeneration [8]. Thus, given the limited nutrient supply to IVD, cellular energy production may become a critical factor in sustaining the normal function of IVD.
Previous studies have been conducted to evaluate the effects of mechanical loading on IVD tissue. Although direct comparison between these studies is difficult due to wide variations in experiment design, the data collectively demonstrates that mechanical loading affects nutrient transport to IVD tissue [9,10] and IVD cell biosynthesis [11–15]. A previous study conducted by Lee et al. on cartilage suggested that cellular biosynthesis can be affected by mechanical loading due to change in ATP turnover [16]. During daily activities, the spine (or IVD) is subjected to both static (e.g., sitting down and standing) and dynamic (e.g., walking, running, etc) loading at various magnitudes and frequencies when comparing to its supine position. Therefore, the objective of this study was to systematically investigate the effects of static and dynamic compressive mechanical loading on IVD cell energy metabolism.
Materials and Methods
IVD tissues from 4–6 month old pigs were obtained from a local slaughter house and dissected within approximately 2 hr of sacrifice. IVDs from five pigs were used in this experiment. Outer annulus fibrosus (AF) and nucleus pulposus (NP) tissues were extracted from all thoracic and lumbar discs (T1-L5) of each pig and pooled in respective enzyme solutions overnight at 37°C and 5% CO2 in a tissue culture incubator. The enzyme solutions were made of Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen Corp.) supplemented with 10% fetal bovine serum (FBS; Invitrogen Corp.), 1% antibiotic-antimycotic, collagenase type II (AF: 1.5 mg/mL, NP: 0.75 mg/mL; Worthington Biochemical Corp., Lakewood, NJ) and protease (0.6 mg/mL; Sigma Aldrich). Following removal of undigested tissue using a 70 μm filter (BD Biosciences, San Jose, CA), IVD cells were re-suspended in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic. These cells were mixed at a 1:1 ratio to yield 5×106 cells/mL in 2% agarose cylindrical constructs (8 mm in diameter, 2 mm in thickness). Fresh porcine IVD cells were used to prevent phenotypic changes due to passaging and two-dimensional expansion [17,18]. Three dimensional agarose culture was chosen because of its mechanical stability under compression, minimal binding interaction with cells [19–21] and capability to maintain the phenotype of IVD cells [22]. Additionally, IVD cells were isolated from their tissue matrix to eliminate extrinsic effects of mechanical loading (e.g., altered solute diffusivity, induction of convective flow) on nutrient supply [9]. Due to high water content and isotropic properties of 2% agarose, mechanical loading had minimal effects on the transport of small solutes in agarose discs used in this study [23].
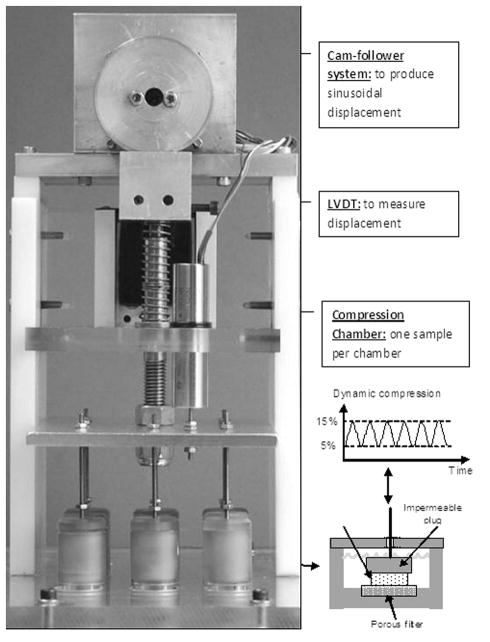
All samples were cultured in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic, for a minimum of 12 hr prior to conducting unconfined uniaxial compression experiments. Due to interference of FBS in certain chemical assays, DMEM without supplements was used in loading experiments. All specimens were washed with serum-free medium three times (5 mins each time) prior to compression experiments. Samples subjected to mechanical loading were placed in a custom-designed bioreactor (Fig. 1). A displacement-control approach was used to apply a compressive strain to samples since the deformational behavior of the cells in 3-dimensional agarose culture under such loading condition has been well characterized in previous studies [19–21]. An eccentric cam-follower system was utilized to apply a sinusoidal displacement to samples at various frequencies (Fig. 1). Each sample was placed in a custom-made chamber with 600 μL of DMEM and compressed between an impermeable plug and a filter (pore size= 20 μm) (Fig. 1). Since the plug inherently reduced surface area for nutrient supply to compressed sample, a similar effect was enforced on control samples by resting a plug on top of each control sample. All experiments were conducted at 37°C and 5% CO2 for 4 hr in a tissue culture incubator. Experiment duration was chosen based on a previous study conducted in our lab where changes in mesenchymal stem cell biosynthesis were observed following 4 hr compression [24]. Experimental groups included a 15% static compressive loading group and three dynamic loading groups at 0.1 Hz and 1 Hz with 15% total strain (5% preloading and 10% sinusoidal strain, Fig. 1). Uncompressed samples were used as a control group. Three cell-agarose constructs of each cell type from the same pig were used for each experimental group and the experiment was repeated with 5 pigs (n=15).
Figure 1.
Schematic of bioreactor.
At the end of each experiment, cell-agarose discs and culture medium were collected. 30 μL of culture medium from each sample was immediately mixed 1:1 with 3 mM EDTA and boiled for 2 min to stabilize ATP in culture media. All specimens were frozen at −80 °C until analysis. At a later point, cell-agarose constructs were dissolved in lysis buffer (15% 1.5 M NaCl, 15% 50 mM EDTA, 1% Triton-X 100 and 10% 100 mM Tris-Cl at pH 7.4) by heating at 65°C for 20 min with intermediate vortexing. These samples were centrifuged at 9000 rpm for 10 min and the supernatant was collected for intracellular ATP and DNA analysis. A live-dead staining conducted in a preliminary experiment exhibited greater than 98% cell viability, confirming that testing conditions were not fatal to cells (Fig. 2).
Figure 2. Viability of (a) AF cells and (b) NP cells after 4-hour 1-Hz dynamic compression (green/red: live/dead cells).
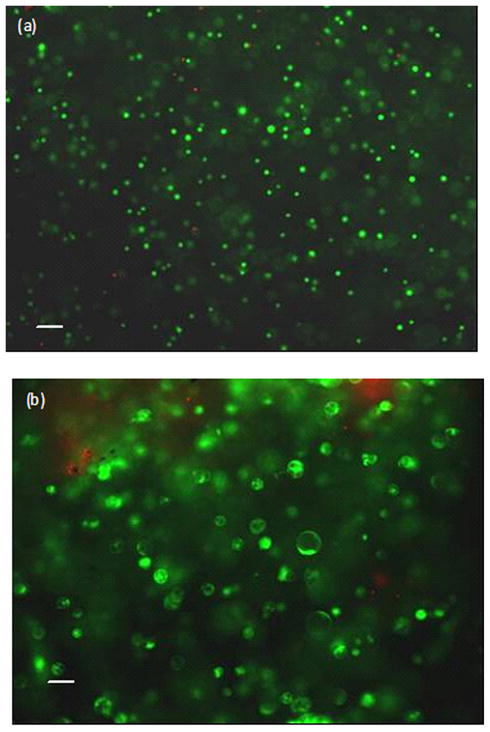
Calcein green and ethidium homodimer were used to detect live and dead cells, respectively. Bar=100μm.
ATP content in culture media and cell-agarose constructs was measured using the Luciferin-luciferase method (Sigma). ATP content in media was used to evaluate ATP released from IVD cells. ATP contents in media and in cell-agarose constructs were combined to account for total ATP content per sample. Lactate content in media was measured using a reaction mix containing 5 mg/mL of β-nicotineamide adenine dinucleotide (Sigma), 0.2 M glycine buffer (Sigma), and 22.25 units/mL of L-lactic dehydrogenase (Sigma) dissolved in distilled water. Sample medium was mixed with the reaction mix 1:1, and the resulting absorbance at 340 nm was measured using a plate reader (model: DTX880, Beckman Coulter, Brea, CA). Lactate was used as a reference for ATP produced via glycolysis. Nitric oxide (NO) content was evaluated by measuring nitrite concentration, a stable by-product of NO, in media using Greiss assay [25]. Previous studies have shown that NO is a potent inhibitor of mitochondrial respiration [26,27]. Standard curves were made from fresh DMEM for all assays performed on culture medium; lysis buffer was used to construct the standard curve for intracellular ATP measurements. Glucose content in media was measured using a Cobas C System (Roche Diagnostics, Indianapolis, IN). Fresh DMEM was used as reference glucose concentration. The change in glucose concentration indicated the total glucose consumed by IVD cells in each sample. Glucose in media was considered the main energy source for ATP production. DNA content in each sample was measured using a QubitTM fluorometer and Quant-iT dsDNA HS Assay Kit (Invitrogen).
Data points from each assay were quantified based on sample volume. These measurements were normalized to DNA content per sample to account for variations in cell number. To evaluate the effects of each loading regimen, values of compressed samples were normalized to respective values of uncompressed samples. To compare dynamic compression with static compression, values of dynamic compression groups were normalized to respective values of static compression. Differential responses of AF and NP cells were evaluated by normalizing values of NP cells to respective values of AF cells under different loading configurations. The measurements from each spine were normalized to respective control sample. The results from five pigs were pooled for statistical analysis. The average values of these ratios and the standard deviations are depicted in the bar graphs (total number of samples per group = 15). Student t-test analysis or one way ANOVA with post-hoc Student-Newman-Keuls test (only for comparison of different frequency groups) was performed using SPSS software (SPSS Inc, Chicago, IL). In all cases p ≤ 0.05 was considered statistically significant.
Results
Static compression of AF cells
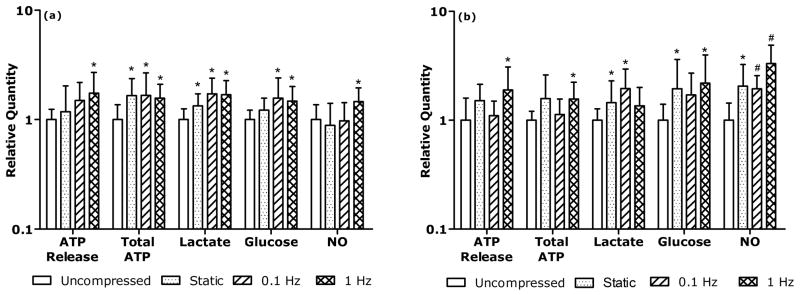
Static compression increased total ATP, lactate production, and glucose consumption of AF cells relative to control conditions (Fig. 3a, p < 0.05). However, ATP release and NO production of AF cells were not significantly altered by static compression.
Figure 3. Significant effects of static and dynamic loading on ATP release, total ATP, lactate production, glucose consumption and NO production of a) AF cells and b) NP cells.
The symbols (*:p<0.05; #:p<0.001) indicate statistically significant differences as compared with the uncompressed group.
Static compression of NP cells
Static compression significantly increased glucose consumption and NO production of NP cells (Fig. 3b, p < 0.05). No significant differences were observed in total ATP content, ATP release, and lactate production between the compressed and uncompressed NP groups.
Dynamic compression of AF cells
Dynamic compression at 1 Hz significantly increased ATP released from AF cells (Fig. 3a, p < 0.05). All dynamic frequencies tested promoted total ATP content (Fig. 3a, p < 0.05), lactate production (Fig. 3a, p < 0.05), and glucose consumption (Fig. 3a, p < 0.05) relative to control. NO production was increased by 1 Hz dynamic loading but not by 0.1 Hz (Fig. 3a, p < 0.05). Frequency dependence was not seen among the AF dynamic loading groups except in NO production, where the NO productions of the 1 Hz group were significantly higher than that of the 0.1 Hz group.
Dynamic compression of NP cells
ATP released from NP cells was significantly higher in the 1 Hz group than the control and 0.1 Hz groups (Fig. 3b, p < 0.05). Total ATP of NP cells was significantly higher in the 1 Hz group than the control group (Fig. 3b, p < 0.05). The 0.1 Hz group exhibited a significant increase in lactate production relative to control (Fig. 3b, p < 0.05). Glucose consumption was significantly promoted by 1 Hz dynamic compression relative to control (Fig. 3b, p < 0.05). NO production of NP cells was significantly higher at all tested dynamic frequencies than that of the control group (Fig. 3b, p<0.001). The NO production of the 1 Hz group was also significantly higher than that of the 0.1 Hz group.
Comparison between static and dynamic compression groups
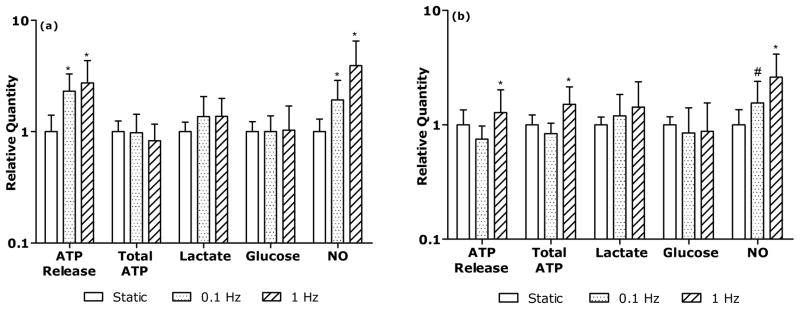
For AF cells, all dynamic compression groups exhibited significantly higher ATP release and NO production relative to static compression group (Fig. 4a). There were no significant differences in total ATP, glucose consumption, and lactate production between the static and dynamic compression groups. For NP cells, total ATP and ATP release at 1 Hz dynamic compression were significantly higher than those of the static compression group (Fig. 4b p<0.05). All dynamic compression groups exhibited significantly higher NO production (Fig. 4b, 0.1 Hz: p<0.001; 1 Hz: p<0.05) than the static compression group. Lactate production of NP cells was not significantly altered by the type of applied compression.
Figure 4. Comparison of ATP release, total ATP, lactate production, glucose consumption, and NO production between the static and dynamic compression for (a) AF cells and (b) NP cells.
The symbols (*:p<0.05; #:p<0.001) indicate statistically significant differences as compared with the static compression group.
Comparison between NP and AF cells
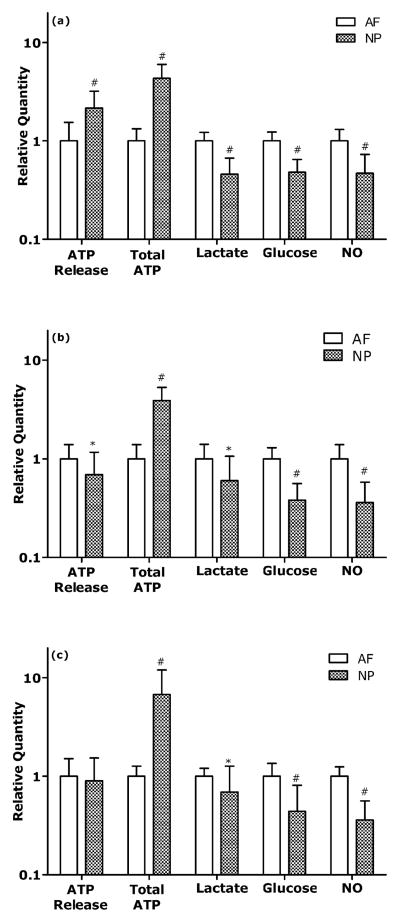
All tested conditions exhibited significantly higher total ATP content (p<0.001) and significantly lower lactate production (Static: p<0.001; 0.1 and 1 Hz: p<0.05), glucose consumption (p<0.001) and NO production (p<0.001) in NP cells (Fig. 5). ATP released from NP cells was higher at static compression (p<0.001) but was lower at 0.1 Hz compression (p<0.05) (Fig. 5). ATP released from IVD cell types did not differ at 1 Hz compression. The measurements of ATP release and total ATP of AF and NP cells are listed in Table 1.
Figure 5. Comparison of ATP release, total ATP, lactate production, glucose consumption, and NO production between AF cells and NP cells at a) Static b) 0.1 Hz and c) 1 Hz compression.
The symbols (*:p<0.05; #:p<0.001) indicate statistically significant differences between the AF and NP cells.
Table 1.
ATP release and total ATP of AF and NP cells during 4 hour experiment.
| Control | Static Compression | Dynamic Compression | |||
|---|---|---|---|---|---|
| 0.1 Hz | 1 Hz | ||||
| AF Cells | ATP release | 26.5±6.7 | 31.3±22.8 | 39.9±18.5 | 46.4±25.4 |
| Total ATP | 380±141 | 630±272 | 636±265 | 596±204 | |
| NP cells | ATP release | 27.2±16.0 | 41.0±17.4 | 30.0±10.8 | 51.5±32.2 |
| Total ATP | 1809±382 | 2863±1861 | 2036±796 | 2837±1217 | |
| Unit: pmol/μgDNA | |||||
Discussion
Understanding IVD matrix biosynthesis at physiological and degenerative conditions remains a stepping stone toward developing new treatments for disc degeneration and LBP. Mechanical loading has been shown to modulate IVD matrix biosynthesis [11–15] which requires energy. The human spine is subjected to a wide range of motions during daily activities. Therefore, we examined the energy production of IVD cells under compressive strain at different loading frequencies. To our knowledge, this is the first study to demonstrate the effects of static compressive mechanical loading and multiple compressive dynamic loading frequencies on the energy metabolism of IVD cells.
Aerobic oxidation of glucose in the mitochondria results in the most efficient intracellular ATP production. However, ATP production under anaerobic conditions can also be achieved by oxidation of glucose via glycolysis which consequently results in the production of lactate. Increases in total ATP content, glucose consumption, and lactate production of AF cells under static compression suggest that ATP production of AF cells via glycolysis may be promoted by static compression. Comparison of AF and NP cells in our study suggests that NP cells utilize mitochondrial respiration as their main metabolic pathway (discussed later). As such, increased NO production in NP cells detected under static compression in this study may have an inhibitory effect on the ATP production (via mitochondrial respiration) in NP cells [26,27]. Furthermore, increased glucose consumption and lactate production in NP cells under static compression seen in this study suggests that ATP production in NP cells may be compensated by increased glycolysis under static compression.
Increases in glucose consumption, lactate production and total ATP content in AF cells by dynamic loading at all tested frequencies collectively suggest that glycolysis in AF cells may also be promoted by dynamic compression. Although NO production was increased at 1 Hz loading, its impact on ATP production of AF cells was not observed in the present study. This observation suggests that glycolysis is the main ATP production pathway in AF cells and not mitochondrial respiration [2,28]. However, the effects of dynamic compression on NP cells were significantly varied compared to that of AF cells. Increased NO production detected at all frequencies suggests that ATP production in NP cells via mitochondrial respiration may be inhibited by dynamic loading. Glucose consumption and lactate production of NP cells tended to increase under dynamic compression with statistical significance at 1 Hz for glucose consumption and 0.1 Hz for lactate production. This indicates that glycolysis in NP cells may be promoted by dynamic loading, which may explain the lack of differences observed in ATP production in NP cells despite the increase in NO production. Another possibility is that dynamic compression may promote ATP production in mitochondria of IVD cells (described below).
Current data suggest that dynamic compression has a significantly stronger effect compared to static compression on NO production by and ATP release from IVD cells. Previous studies have shown that extracellular ATP is able to induce NO production in various cell types via activation of P2 nucleotide receptors [29–31]. Thus, extracellular ATP released from the IVD cells under dynamic loading may cause or promote NO production in these cells. Furthermore, this study found that dynamic compression at 1 Hz promoted ATP production of the NP cells without an increase in glucose consumption or lactate production compared to static compression. This finding suggested that more ATP may be produced in the mitochondria of NP cells under dynamic compression than static compression.
NP cells exhibited higher total ATP content but lower glucose consumption and lactate production compared to AF cells under all tested experimental conditions, indicating that the NP and AF cells may harbor different pathways of energy metabolism. These findings suggest that intracellular ATP of NP cells may be mainly produced via mitochondrial respiration instead of glycolysis, which previously has been suggested as the main pathway of energy production in mature IVD cells [2,28]. Since AF cells originate from the mesenchyme while NP cells are derived from the notochord [32,33], the difference in energy metabolism between AF and NP cells may be due to inherent differences between the cell types.
ATP is required for the synthesis of macromolecules (e.g., proteoglycan, DNA and RNA) and proteins, and the active transport of molecules which are involved in the majority of cellular activities such as cell proliferation, survival, and metabolism. The current study demonstrates that ATP production of IVD cells is altered by static and dynamic compressive loading, which may subsequently affect cellular activities. This finding is consistent with various studies that have demonstrated a correlation between mechanical loading and cellular biosynthesis [11–15]. The synthesis of proteoglycans is a high ATP-demanding process since ATP not only serves as an energy source for protein synthesis but also serves as building blocks in the formation of UDP-sugars and 3′-phosphoadenosine 5′-phosphosulphate (PAPS) for proteoglycan synthesis [5,7]. This could be the reason that higher intracellular ATP content was found in NP cells, which are active in proteoglycan synthesis. Furthermore, changes in IVD cell energy metabolism due to compression could subsequently affect the distribution of nutrients to IVD cells [2,9] For instance, when mechanical loading increases the glucose consumption of AF cells, it would consequently reduce the transport of glucose into the NP region from the blood supply at the edge of the AF region and as such affect NP cell metabolism.
Our previous theoretical study showed that decreased tissue diffusivity due to static compression could increase lactic acid accumulation in IVDs [9], resulting in low pH. However, that analysis did not include the effects of mechanical loading on metabolic rates of IVD cells. The current study demonstrates that lactate production of IVD cells can be up-regulated intrinsically by compression, suggesting a stronger effect on lactate accumulation and pH than previously assumed. Previous studies have found that pH can be lower than 6.5 in degenerated human discs [34,35]. Low pH environment is detrimental to the viability of IVD cells [36] and reduces proteoglycan synthesis of IVD cells [37,38]. Thus lactate accumulation may play an important role in disc degeneration. Furthermore, low pH (i.e., high proton concentration) can evoke pain sensation through capsaicin (vanilloid) receptor [39]. Although healthy adult discs are avascular and aneural, nerve in-growth and vascularization occur with disc degeneration [3]. It has been shown that patients with back pain often have relatively high lactate levels in IVDs [40,41]. Therefore, the possible increase in lactate accumulation due to mechanical loading may contribute to disc degeneration as well as produce a possible pathway between disc degeneration and the sensation of low back pain.
ATP release in response to mechanical stimulation has been observed in various cell types [42–44], including IVD cells under vibratory loading [44]. Our study also demonstrated that dynamic compression (i.e., 1 Hz) promoted ATP release from IVD cells. The physiological role of ATP released due to mechanical stimulation was not evaluated in this study. However, other studies have shown that extracellular ATP is able to regulate various cellular activities through purinergic pathway [45–47]. In addition, it has been demonstrated that extracellular ATP upregulated expression of glucose transporter and increased cellular glucose uptake in skeletal muscle cells [48] while mechanical loading facilitated the transport of glucose into rat podocytes by increasing the number of glucose transporters on the cell surface [49]. Thus, transport of glucose into IVD cells may be affected by compression, further altering the energy production of IVD cells.
The main limitation of this study is that the agarose gel model of uniaxial compression used in this study may not represent the complex mechanical environment in the IVD. For instance, the IVD cells may be subjected to tensile strain, hydrostatic pressure, shearing force of fluid flow, and high frequency loading (>10 Hz) such as vibration, which were not specifically simulated in this study. In addition, the shape and size of the AF cells residing in the laminar structure of the outer AF region in-vivo could be different from that cultured in agarose gel. This disparity may induce different energy metabolism of AF cells in agarose culture compared to in-vivo conditions since the cytoskeleton is involved in mechanotransduction of IVD cells [50]. Nonetheless, this study provided direct evidences that mechanical loading can affect energy metabolism of IVD cells. Future studies are required to investigate the effects of other mechanical stimuli on energy metabolism of IVD cells and the mechanism behind the data obtained in this study.
In summary, this study demonstrated that IVD cell energy production is altered by mechanical loading. Cellular responses to mechanical loading were both cell type and compression type dependent. In future studies, extracellular matrix synthesis will be evaluated under mechanical loading to confirm that change in energy production is translated to changes in cellular biosynthesis.
Acknowledgments
This study was supported by the grants (AR056101 and EB008653) from the NIH. The authors have no conflicts of interest in relation to the presented material.
References
- 1.American Academy of orthopedics surgeons. The burden of musculoskeletal diseases in the united states. The Bone and Joint Decade. 2008:21–53. [Google Scholar]
- 2.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 3.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 4.Baker MS, Feigan J, Lowther DA. The mechanism of chondrocyte hydrogen peroxide damage. Depletion of intracellular ATP due to suppression of glycolysis caused by oxidation of glyceraldehyde-3-phosphate dehydrogenase. J Rheumatol. 1989;16:7–14. [PubMed] [Google Scholar]
- 5.Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- 6.Im MJ, Freshwater MF, Hoopes JE. Enzyme activities in granulation tissue: Energy for collagen synthesis. J Surg Res. 1976;20:121–5. doi: 10.1016/0022-4804(76)90108-6. [DOI] [PubMed] [Google Scholar]
- 7.Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J Cell Sci. 2000;113(Pt 2):193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- 8.Johnson K, Svensson CI, Etten DV, et al. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis Rheum. 2004;50:1216–25. doi: 10.1002/art.20149. [DOI] [PubMed] [Google Scholar]
- 9.Huang CY, Gu WY. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech. 2008;41:1184–96. doi: 10.1016/j.jbiomech.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson AR, Yuan TY, Huang CY, et al. Effect of compression and anisotropy on the diffusion of glucose in annulus fibrosus. Spine (Phila Pa 1976) 2008;33:1–7. doi: 10.1097/BRS.0b013e31815e4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasra M, Merryman WD, Loveless KN, et al. Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. J Orthop Res. 2006;24:1967–73. doi: 10.1002/jor.20253. [DOI] [PubMed] [Google Scholar]
- 12.Korecki CL, Kuo CK, Tuan RS, Iatridis JC. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2009;27:800–6. doi: 10.1002/jor.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohshima H, Urban JP, Bergel DH. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. J Orthop Res. 1995;13:22–9. doi: 10.1002/jor.1100130106. [DOI] [PubMed] [Google Scholar]
- 14.MacLean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329–37. doi: 10.1016/s0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee RB, Wilkins RJ, Razaq S, Urban JP. The effect of mechanical stress on cartilage energy metabolism. Biorheology. 2002;39:133–43. [PubMed] [Google Scholar]
- 17.Chou AI, Bansal A, Miller GJ, Nicoll SB. The effect of serial monolayer passaging on the collagen expression profile of outer and inner anulus fibrosus cells. Spine (Phila Pa 1976) 2006;31:1875–81. doi: 10.1097/01.brs.0000229222.98051.9a. [DOI] [PubMed] [Google Scholar]
- 18.Flagler DJ, Huang CY, Yuan TY, et al. Intracellular Flow Cytometric Measurement of Extracellular Matrix Components in Porcine Intervertebral Disc Cells. Cell Mol Bioeng. 2009;2:264–73. doi: 10.1007/s12195-009-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight MM, Ghori SA, Lee DA, Bader DL. Measurement of the deformation of isolated chondrocytes in agarose subjected to cyclic compression. Med Eng Phys. 1998;20:684–8. doi: 10.1016/s1350-4533(98)00080-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee DA, Bader DL. The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol Anim. 1995;31:828–35. doi: 10.1007/BF02634565. [DOI] [PubMed] [Google Scholar]
- 21.Lee DA, Knight MM, Bolton JF, et al. Chondrocyte deformation within compressed agarose constructs at the cellular and sub-cellular levels. J Biomech. 2000;33:81–95. doi: 10.1016/s0021-9290(99)00160-8. [DOI] [PubMed] [Google Scholar]
- 22.Gruber HE, Fisher EC, Jr, Desai B, et al. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 23.Gu WY, Yao H, Vega AL, Flagler D. Diffusivity of ions in agarose gels and intervertebral disc: effect of porosity. Ann Biomed Eng. 2004;32:1710–7. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 24.Huang CY, Hagar KL, Frost LE, et al. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22:313–23. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- 25.Granger DL, Anstey NM, Miller WC, Weinberg JB. Measuring nitric oxide production in human clinical studies. Methods Enzymol. 1999;301:49–61. doi: 10.1016/s0076-6879(99)01068-x. [DOI] [PubMed] [Google Scholar]
- 26.Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136–9. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 27.Tomita M, Sato EF, Nishikawa M, et al. Nitric oxide regulates mitochondrial respiration and functions of articular chondrocytes. Arthritis Rheum. 2001;44:96–104. doi: 10.1002/1529-0131(200101)44:1<96::AID-ANR13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976) 2005;30:487–96. doi: 10.1097/01.brs.0000154619.38122.47. [DOI] [PubMed] [Google Scholar]
- 29.Bogle RG, Coade SB, Moncada S, et al. Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells. Biochem Biophys Res Commun. 1991;180:926–32. doi: 10.1016/s0006-291x(05)81154-4. [DOI] [PubMed] [Google Scholar]
- 30.Gendron FP, Chalimoniuk M, Strosznajder J, et al. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem. 2003;87:344–52. doi: 10.1046/j.1471-4159.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 31.Silva G, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension. 2006;47:563–7. doi: 10.1161/01.HYP.0000197954.93874.ef. [DOI] [PubMed] [Google Scholar]
- 32.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–8. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walmsley R. The development and growth of the intervertebral disc. Edinb Med J. 1953;60:341–64. [PMC free article] [PubMed] [Google Scholar]
- 34.Diamant B, Karlsson J, Nachemson A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia. 1968;24:1195–6. doi: 10.1007/BF02146615. [DOI] [PubMed] [Google Scholar]
- 35.Nachemson A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand. 1969;40:23–42. doi: 10.3109/17453676908989482. [DOI] [PubMed] [Google Scholar]
- 36.Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine (Phila Pa 1976) 2001;26:2543–9. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 37.Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine (Phila Pa 1976) 1992;17:1079–82. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12:341–9. doi: 10.1007/s00586-003-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98:6951–6. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine (Phila Pa 1976) 1998;23:1–7. doi: 10.1097/00007632-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 41.Keshari KR, Lotz JC, Link TM, et al. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine (Phila Pa 1976) 2008;33:312–7. doi: 10.1097/BRS.0b013e31816201c3. [DOI] [PubMed] [Google Scholar]
- 42.Graff RD, Lazarowski ER, Banes AJ, Lee GM. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum. 2000;43:1571–9. doi: 10.1002/1529-0131(200007)43:7<1571::AID-ANR22>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 43.Watt WC, Lazarowski ER, Boucher RC. FTR-independent release of ATP: Its implications for the regulation of P2Y2-receptors in airway epithelia. J Biol Chem. 1998;273:14053–8. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki S, Weinhold PS, Graff RD, et al. Annulus cells release ATP in response to vibratory loading in vitro. J Cell Biochem. 2003;90:812–8. doi: 10.1002/jcb.10681. [DOI] [PubMed] [Google Scholar]
- 45.Chowdhury TT, Knight MM. Purinergic pathway suppresses the release of .NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J Cell Physiol. 2006;209:845–53. doi: 10.1002/jcp.20768. [DOI] [PubMed] [Google Scholar]
- 46.Elfervig MK, Graff RD, Lee GM, et al. ATP induces Ca(2+) signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis Cartilage. 2001;9:518–26. doi: 10.1053/joca.2000.0435. [DOI] [PubMed] [Google Scholar]
- 47.Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology. 2004;41:567–75. [PubMed] [Google Scholar]
- 48.Kim MS, Lee J, Ha J, et al. ATP stimulates glucose transport through activation of P2 purinergic receptors in C(2)C(12) skeletal muscle cells. Arch Biochem Biophys. 2002;401:205–14. doi: 10.1016/S0003-9861(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 49.Lewko B, Bryl E, Witkowski JM, et al. Mechanical stress and glucose concentration modulate glucose transport in cultured rat podocytes. Nephrol Dial Transplant. 2005;20:306–11. doi: 10.1093/ndt/gfh612. [DOI] [PubMed] [Google Scholar]
- 50.Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly 2. Dev Dyn. 1999;215:179–89. doi: 10.1002/(SICI)1097-0177(199907)215:3<179::AID-AJA1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]