Abstract
Background
Mechanisms of allergic transfusion reactions (ATRs) are not well understood. The aim of this study was to distinguish recipient, donor, and product-specific factors associated with ATRs.
Study Design and Methods
We conducted a retrospective cohort study of apheresis platelet (AP) products transfused from 4/2000–3/2010. The concordance rate of ATRs when split AP products were transfused to ≥2 individuals was compared to the overall ATR rate among all AP products. Per person ATR rates also were compared to the overall ATR rate.
Results
We observed 1,616 ATRs among 93,737 transfusions, for an overall incidence of 1.72%(95%CI: 1.64–1.81%). Of the 1,616 ATRs, 630 occurred when split AP products were transfused to ≥2 recipients. Of these 630 AP products, ATRs were observed in ≥2 different recipients of the same AP collection only 6/630 times, for a concordant incidence of 0.95% (95% CI: 0.35–2.06%), which is similar to the overall ATR rate (P=0.17). On an individual level, 30.0% of recipients had ATR rates >5%, and these 30.0% accounted for 62.1% of ATRs. Donors of AP products associated with concordant ATRs donated AP products that had an ATR rate of 5.8% (95% CI 3.1–9.7%), which is higher than the overall ATR rate (P<0.001).
Conclusions
An observed ATR does not predict an ATR in a different recipient of a split AP product. A minority of platelet recipients accounts for the majority of ATRs. Some donors are strongly associated with ATRs. Consequently, recipient and donor factors are implicated in the mechanism of ATRs.
Keywords: Allergy, transfusion reaction, platelet
Introduction
Allergic transfusion reactions (ATR) are common adverse sequelae to all blood products, particularly those with a large plasma component. Estimates of the rate of ATR range from approximately 1–3%1. Mild reactions typically include pruritus and urticaria, and more severe reactions can include angioedema, dyspnea, and shock. Despite the high incidence of ATRs, the mechanisms involved are not understood in the majority of cases. The extent to which ATRs are associated with donor, product, and/or recipient factors is not well defined.
Recipient predispositions to ATRs have been studied. Recipient hypersensitivities resulting from severe IgA2,3, haptoglobin4, and C45 deficiencies have been described, but these deficiencies are too rare to explain the high incidence of ATRs. Transfusion recipients who experience ATRs also appear to be more atopic by laboratory screening than recipients who do not experience an ATR6. Since atopy is so prevalent (~35%)7 and ATRs manifest with symptoms of immediate hypersensitivity, recipient atopy may be a factor that contributes to ATRs.
Donor and blood product factors have been investigated, as well. Atopy in blood donors8,9 and pro-allergic mediators in the plasma of blood products10–12 have been studied. The observations that 1) plasma reduction of platelet products can reduce the incidence of ATRs and 2) platelet and plasma products produce more ATRs than PRBCs would seem to implicate a plasma-based mediator of an ATR13. However, a mediator in plasma may be necessary, but not sufficient, to cause an ATR.
A fundamental question for discerning the mechanisms of ATR is to determine the extent to which donor, product, or recipient factors contribute to the development of an ATR. A unique situation in which to assess the contribution of donor/product factors versus recipient factors occurs when apheresis platelet (AP) products are split and transfused into two or more recipients. When an ATR occurs in one recipient, a second recipient should have a higher likelihood of experiencing an ATR if a donor or product-specific factor is responsible. If a recipient factor is dominant, then the rate of ATR in the split product given to a second person should be equal to the overall ATR rate for all platelet products.
Materials and Methods
Study Population and Database
This retrospective cohort study reviewed all platelet transfusions at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital from April 2000–March 2010. The Johns Hopkins University School of Medicine IRB approved this study.
The study population included oncology patients who were managed by the platelet coordinating staff. All products were single-donor (AP) products. Split AP units were defined as either multiple container apheresis collections or divided AP products that were aliquoted for pediatric transfusion. Platelet transfusions were included in the split AP portion of the study if they met the definition of a split AP product and 1) the split products were transfused into ≥ 2different recipients; and 2) at least one of the recipients of the split products experienced an ATR.
The diagnosis of ATRs was documented at the time of the reaction by transfusion medicine physicians. ATRs were clinically diagnosed by the definition provided in the AABB technical manual1.
Data acquisition
All platelet transfusions and transfusion reactions were documented electronically. A computer search was performed for ATRs in which platelet products were split and the recipient medical record numbers were different. The search identified transfusion reactions with the following terms, which are entered from a standardized transfusion reaction dictionary for computer entry: “allergic: asthma”, “allergic: shock”, allergic: urticaria”, “allergic: pruritus”, “allergic: dyspnea”, allergic: cough”, “allergic: rash”, “allergic: wheezing”, and “angioedema.” Reactions could be coded with multiple symptoms. Split products were identified as having the same root product number, followed by A, B, C, etc., where applicable. Demographic, allergic history, and transfusion information was extracted from the computerized medical record.
Statistics
The primary analysis was to compare the concordance of ATRs in recipients of split AP units to the overall ATR rate. The term “overall ATR rate” is used in this study to denote the ATR rate that includes all AP transfusions, whether or not they were derived from a split AP product. The overall ATR rate for the 10-year study periods included all AP transfusions. All other analyses were conducted using the population that received split AP products. Confidence intervals for ATR rates were computed using a binomial distribution. ATR rates were compared to the overall ATR rate for the 10 year study period using the exact binomial probability test. The proportion of platelet recipients expected to have ATR rates >5% was calculated using the Poisson probability test. Per person ATR rates were calculated as the number of ATRs for a given individual divided by the total number of AP transfusions for that individual. In order to provide a more intuitive comparison among individuals, the per person ATR rate was transformed from the per person ATR rate to the number of ATRs per 100 transfusions by multiplying the per person ATR rate by a factor of 100. Per person ATR rates were compared between children (≤ 18 years) and adults using the Mann-Whitney test. Comparison of reported ATR rates across seasons was performed using the Kruskal-Wallis test, where seasons were defined as the following groups of months: Jan/Feb/Mar; Apr/May/Jun; Jul/Aug/Sep; Oct/Nov/Dec and numbers of ATRs were normalized by dividing the number of ATRs per season by the total number of AP transfusions during those seasons, over the 10 year study period. Analysis was conducted using Stata v. 11.1 (StataCorp, College Station, Tx).
Results
Patient characteristics
A total of 1,064 AP recipients received AP products that were split among ≥ 2 recipients. The age distribution of included subjects was bimodal, with peaks in the first and sixth decades of life. Pediatric patients ≤18 years accounted for 19.0% of patients included. The median age was 51years (IQR 25–64 years). Primary diagnoses were available on 84.6% patients included in the analysis and are listed in Table 1, with the largest percentage of individuals presenting with acute leukemia.
Table 1.
Primary diagnoses of evaluated subjects
| Primary Diagnosis | n (%) |
|---|---|
| Acute Leukemia | 363 (34.1) |
| Hematopoietic Stem Cell Transplant | 223 (21.0) |
| Solid tumor | 70 (6.6) |
| Lymphoma | 69 (6.5) |
| Aplastic anemia/Myelodysplastic Syndrome | 56 (5.3) |
| Carcinoma | 52 (4.9) |
| Chronic Leukemia | 24 (2.2) |
| Other | 43 (4.0) |
| Not available | 164 (15.4) |
| Total | 1064 (100) |
Split AP transfusions and ATR rates
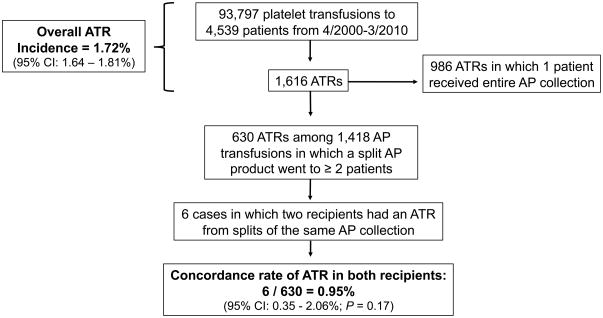
Figure 1 shows the numbers of AP transfusions, ATRs, and split AP products that were included in the study. Overall, during the 10-year study period 4,539 patients received 93,797 AP transfusions, for an average of 20.7 AP transfusions per patient. There were 1,616 ATRs reported among the 93,797transfusions, for an overall incidence of 1.72% (95% CI: 1.64–1.81%). Of the 1,616 ATRs, 630 ATRs occurred in which split AP products were transfused to ≥ 2 recipients, and these AP products were included in the concordance rate analysis. Split AP products associated with these 630 ATRs were given among a total of 1,418 transfusions, for an average of 2.25 splits per AP collection.
Figure 1. Calculation of the overall allergic transfusion reaction (ATR) rate and the concordance rate of ATRs.
The total number of paired transfusions is the number of instances in which a single apheresis platelet (AP) collection was split and transfused into ≥ 2 different recipients. The concordance rate of ATRs is the proportion of these split collections that were associated with an ATR in ≥ 2 recipients.
Among the ATRs reported on split AP products in which at least one unit went to a different patient, only six ATRs were reported in a second recipient. This results in a concordance rate of ATRs in only 0.95% (6/630; 95% CI: 0.35–2.06%), which is lower than the overall ATR rate for all platelet transfusions, although not statistically different from the overall estimate (p=0.17).
Characteristics of concordant ATRs from the same AP collection
As shown in Table 2, there were no distinguishing characteristics of these products in terms of age, equivalent units, volume, or manipulation. No patient listed had a change in temperature > 1°C.
Table 2.
Characteristics of the recipients and apheresis platelet products involved in paired ATRs.
| Concordant ATR | Patient Age (years) | Product Age (days) | Equivalent Units | Volume (mL) | Month of Platelet Donation | Symptoms | Antihistamine Premedication | History of Allergy/Atopy | Per Person ATR Rate to Platelets (%) | Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 39 | 3 | 7 | 300 | April | urticaria, pruritus | No | None | 2/79 (2.5%) | |
| 1B | 12 | 3 | 7 | 300 | urticaria, pruritus, rash | Yes | None | 1/41 (2.4%) | ||
| 2A | 1 | 5 | 1 | 33 | October | dyspnea, wheezing, rash | Yes | None | 1/3 (33.3%) | 2 additional recipients of aliquots, 1 of which was concentrated. Neither caused an ATR. |
| 2B* | 65 | 5 | 6 | 100 | urticaria | Yes | None | 5/157 (3.2%) | ||
| 3A | 59 | 5 | 7 | 202 | October | urticaria | No | Asthma, penicillin (rash) | 1/14 (7.1%) | 1 additional recipient of an aliquot did not have an ATR |
| 3B | 66 | 5 | 7 | 196 | pruritus | No | None | 1/1 (100%) | ||
| 4A | 52 | 5 | 6 | 234 | May | urticaria, pruritus | No | Asthma | 5/71 (7.0%) | |
| 4B | 48 | 4 | 6 | 230 | urticaria, pruritus | Yes | Asthma | 4/36 (11.1%) | ||
| 5A | 66 | 5 | 6 | 292 | May | urticaria, pruritus | Yes | None | 3/21 (13.3%) | |
| 5B* | 63 | 5 | 6 | 100 | urticaria | Yes | None | 5/80 (6.3%) | ||
| 6A | 40 | 4 | 6 | 193 | November | urticaria, pruritus | Yes | None | 3/48 (6.3%) | |
| 6B | 46 | 5 | 6 | 200 | urticaria, pruritus | No | ondansetron (itchy eyes) | 1/19 (5/3%) |
Concentrated platelet unit
It is possible that the likelihood of ≥ 2 patients experiencing an ATR the same AP collection would increase if the patients or products involved had high pre-transfusion probability of an ATR. Patients with high baseline ATR rates would be more likely, by chance alone, to be involved in an ATR with another person with a high baseline ATR rate. Subjects listed in Table 2 who experienced an ATR together from the same AP collection had an overall ATR rate of 5.61% (32 ATRs out of 570 AP transfusions; 95% CI 3.87–7.83%), which is higher than the overall ATR rate of 1.72% (1,616 ATRs out of 93,797 transfusions, P < 0.001). Furthermore, in two cases of concordant ATRs (Cases 2 and 3 in Table 2), there were additional splits of the same product, and there was no ATR in the additional recipients.
On the other hand, donor and product attributes such as pro-allergic plasma IgE or immunogenic proteins may be passively transferred and associated with an ATR, as has been described8,9,14. Indeed, the AP donations that led to concordant ATRs were in the spring and fall months (Table 2), which are peak allergy seasons in Maryland. However, when all AP transfusions to ≥2 recipients over the 10-year study period were analyzed, there was no evidence that ATR rates varied by season (p=0.6). The three months with the highest numbers of ATRs were October (n=71), June (n=62) and April (n=57); the three months with the lowest numbers of ATRs were December (n=40), August (n=43) and February (n=46). These months were still the ones with the most frequent and infrequent numbers of ATRs, respectively, after normalization to the number of total transfusions given in their respective time periods.
ATR rates among individuals
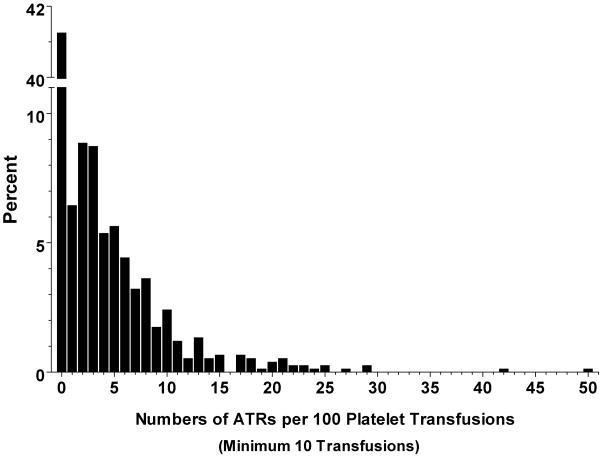
If recipient factors are indeed critical for the development of ATRs, then AP recipients may have varying susceptibilities to ATRs, and some individuals may have ATR rates that are much higher than the overall population average. Figure 2 shows the per person rates of ATRs among individuals with ≥ 10 AP transfusions. Nearly half of all AP recipients (46.6%; 496/1064) never had an ATR after a total of 14,643 transfusions. On the other hand, 30.0% of AP recipients (319/1064) had per person ATR rates of >5%. Given an overall ATR rate of 1.72% (Figure 1), only 1% of the population would be expected to have an ATR rate >5% if ATRs were distributed randomly among AP recipients. The 30.0% who had an ATR rate of >5% accounted for 62.1% of ATRs (660/1062). Adults older than 18 years had a higher proportion of per person ATR rates >5% than children (31.9% vs. 21.8%; P < 0.001).
Figure 2. Per person rates of ATRs among recipients of ≥ 10 platelet transfusions.
Per person ATR rates were calculated as the number of ATRs/number of AP transfusions and normalized to a rate per 100 transfusions. The histogram shows percent of patients with ATR rates categorized into intervals of 1 unit on the x-axis.
ATR rates in products from donors associated with concordant ATRs
We evaluated the possibility that donors of AP products that were associated with concordant ATRs may be associated with a higher ATR rate in recipients of their products during other donation times. Table 3 shows that the six donors who gave products that were associated with concordant ATRs were associated with ATRs in 5.80% of transfusions (13 ATRs out of 224 AP collections transfused; 95% CI 3.12–9.72%), which is higher than the overall ATR rate of 1.72% (1,616 ATRs out of 93,797 transfusions, P < 0.001).
Table 3.
ATRs associated with donors of products that caused concordant ATRs
| Donor of: Concordant ATR # | Platelet Donations (n) | Aphereis Collections associated with an ATR (n) | ATR Frequency Associated with Donor |
|---|---|---|---|
| 1 | 61 | 2 | 3.3% |
| 2 | 20 | 1 | 5.0% |
| 3 | 50 | 4 | 8.0% |
| 4 | 20 | 2 | 10.0% |
| 5 | 55 | 3 | 5.5% |
| 6 | 18 | 1 | 5.6% |
| Overall | 224 | 13 | 5.8% |
Discussion
A first step in determining the mechanisms of ATRs is to differentiate between product and recipient factors. This study was designed to distinguish whether product or recipient factors are more strongly associated with ATRs. We find that product factors appear to be less strongly associated with ATRs than recipient factors. If a product factor were primarily associated with ATRs, then the concordance rate of ATRs among all recipients of a split AP product would be expected to approach 100%. However, the rate of ATRs when an apheresis product is split and transfused to two or more individuals is approximately 1% and statistically similar to the overall ATR rate. The low concordance rate of ATRs among all recipients of split AP products suggested that recipient susceptibility might be important for developing an ATR.
The patients involved in a concordant ATR with another patient collectively had an ATR rate that is more than three times the overall ATR rate. This suggests that finding two ATRs from splits of the same AP product may be due to a chance event determined by an underlying recipient susceptibility to an ATR, and not a particular product characteristic. Furthermore, in two cases of concordant ATRs from a split AP product, there were additional recipients of the same split AP product who did not have an ATR, arguing against a product-specific factor being associated with ATRs in these cases.
Thirty percent of individuals have high rates of ATRs that account for a disproportionate number of ATRs in the study group. Observing that some recipients have much higher rates of ATRs than would be expected by chance suggests that certain platelet recipients harbor an inherent susceptibility to ATRs. Pediatric platelet recipients are more likely to receive split products, as pediatric platelet doses are usually lower than adult doses. Thus, the study population was enriched for pediatric patients. Nevertheless, both adults and pediatric age groups had subsets with high per person ATR rates, even though more adults than children tended to experience repeated ATRs. Possibilities explaining the observation that adults experienced more ATRs than children include differences in reporting ATRs between pediatric and adult units. However, oncology patients represent the largest group receiving AP products, and the same group of oncology platelet coordinators monitors reactions for both pediatric and adult oncology patients. The difference may be due to the difference in types and prevalence of atopic disease in children versus adults. For example, the prevalence of food allergy, eczema, and asthma is higher in children than adults, but the prevalence of allergic disease to environmental allergens increases with age15,16.
In addition to recipient susceptibility, we find evidence that the donors associated with concordant ATRs have donated products that are more frequently associated with ATRs than the overall average, indicating a possible relationship of the donor to the development of an ATR. The rate of ATRs associated with donors of AP products implicated in concordant ATRs (5.8%) is similar to the ATR rate of the recipients who experienced concordant ATRs (5.6%). However, this donor association with ATRs does not appear to be as important as the recipient association because when a single donor AP product was exposed to ≥ 2 recipients, an ATR was recorded in only one recipient in 99% of cases.
There are limitations to this study. Even though product characteristics do not appear to be significantly associated with ATRs, this study was not designed to specifically differentiate between donor and storage-related factors, which maybe associated with ATRs in different ways. While we explored storage time as a factor in concordant ATR, we did not hypothesize that storage time would affect the rate of ATR because this hypothesis has been explored previously and shown not to be a factor20. Nevertheless, it is possible that pro-allergenic mediators may have different concentrations in split AP products, leading to an underestimation of the product as a mediator of ATRs. Apheresis products are split at the time of manufacture, and small differences when the product is split may be amplified during the following days in storage. Additional studies on product-specific factors are needed to assess this possibility.
This study relies on reporting from clinicians for the determination of ATRs, although platelet transfusion coordinators conduct clinical rounds on the oncology inpatients every weekday to assess for adverse reactions. It is possible that reporting bias underestimates the true concordant ATR rate for split apheresis platelet products. However, the overall estimate of the ATR rate is in agreement with data obtained from other surveillance studies21,22.
Two recent trials have demonstrated that premedication with diphenhydramine does not prevent ATRs21,23,24, although antihistamines may mitigate allergic symptoms. The use of antihistamine premedications was prevalent among the six concordant ATRs and did not prevent ATRs in these cases. Importantly, the inability to currently prevent ATRs with premedication underscores the importance of understanding the etiology of ATRs.
We conclude that both donor and recipient factors are associated with incident ATRs. Furthermore, we conclude that storage-related effects, such as pro-allergic mediators that accumulate during storage17,18 or through interaction with plastic19 do not appear to be as strongly associated with ATRs as donor and recipient factors. There are likely both donor and recipient attributes that are needed concurrently to elicit an ATR, and their relative contributions to an ATR developing may change with time and circumstances. Studies investigating the mechanisms of ATRs should take into account recipient and donor factors. Research focusing on the factors that predispose platelet recipients to ATRs is warranted.
Acknowledgments
Support: W.S. received support from the NIH Clinical Scholars in Benign Hematology Program (5K12HL087169).
We thank Sandra Thoman for help in reviewing this manuscript and Parvaneh Baraghi and Farideh Majidi for their assistance with data collection.
Footnotes
Disclaimers: None
Conflicts of interest: None
References
- 1.Roback J. Technical Manual. 16. AABB Press; 2008. [Google Scholar]
- 2.Vassallo RR. Review: IgA anaphylactic transfusion reactions. Part I. Laboratory diagnosis, incidence, and supply of IgA-deficient products. Immunohematology. 2004;20:226–33. [PubMed] [Google Scholar]
- 3.Sandler SG, Eckrich R, Malamut D, Mallory D. Hemagglutination assays for the diagnosis and prevention of IgA anaphylactic transfusion reactions. Blood. 1994;84:2031–5. [PubMed] [Google Scholar]
- 4.Shimada E, Tadokoro K, Watanabe Y, Ikeda K, Niihara H, Maeda I, Isa K, Moriya S, Ashida T, Mitsunaga S, Nakajima K, Juji T. Anaphylactic transfusion reactions in haptoglobin-deficient patients with IgE and IgG haptoglobin antibodies. Transfusion. 2002;42:766–73. doi: 10.1046/j.1537-2995.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 5.Wibaut B, Mannessier L, Horbez C, Coupez B, Courbon B, Mizon P, Goudemand J. Anaphylactic reactions associated with anti-Chido Antibody following platelet transfusions. Vox Sang. 1995;69:150–1. doi: 10.1111/j.1423-0410.1995.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm D, Kluter H, Klouche M, Kirchner H. Impact of allergy screening for blood donors: relationship to nonhemolytic transfusion reactions. Vox Sang. 1995;69:217–21. doi: 10.1111/j.1423-0410.1995.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 7.Law M, Morris JK, Wald N, Luczynska C, Burney P. Changes in atopy over a quarter of a century, based on cross sectional data at three time periods. BMJ. 2005;330:1187–8. doi: 10.1136/bmj.38435.582975.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Routledge RC, De Kretser DM, Wadsworth LD. Severe anaphylaxis due to passive sensitisation by donor blood. Br Med J. 1976;1:434. doi: 10.1136/bmj.1.6007.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson SG, Nopp A, van Hage M, Olofsson N, Lundahl J, Wehlin L, Soderstrom L, Stiller V, Oman H. Passive IgE-sensitization by blood transfusion. Allergy. 2005;60:1192–9. doi: 10.1111/j.1398-9995.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- 10.Wakamoto S, Fujihara M, Kuzuma K, Sato S, Kato T, Naohara T, Kasai M, Sawada K, Kobayashi R, Kudoh T, Ikebuchi K, Azuma H, Ikeda H. Biologic activity of RANTES in apheresis PLT concentrates and its involvement in nonhemolytic transfusion reactions. Transfusion. 2003;43:1038–46. doi: 10.1046/j.1537-2995.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsuyama N, Hirayama F, Wakamoto S, Yasui K, Furuta RA, Kimura T, Taniue A, Fukumori Y, Fujihara M, Azuma H, Ikeda H, Tani Y, Shibata H. Application of the basophil activation test in the analysis of allergic transfusion reactions. Transfus Med. 2009;19:274–7. doi: 10.1111/j.1365-3148.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa S, Tashiro N, Matsubara T, Furukawa S, Ra C. A comparison of FcepsilonRI-mediated RANTES release from human platelets between allergic patients and healthy individuals. Int Arch Allergy Immunol. 2001;125 (Suppl 1):42–7. doi: 10.1159/000053852. [DOI] [PubMed] [Google Scholar]
- 13.Buck SA, Kickler TS, McGuire M, Braine HG, Ness PM. The utility of platelet washing using an automated procedure for severe platelet allergic reactions. Transfusion. 1987;27:391–3. doi: 10.1046/j.1537-2995.1987.27587320530.x. [DOI] [PubMed] [Google Scholar]
- 14.Arnold DM, Blajchman MA, Ditomasso J, Kulczycki M, Keith PK. Passive transfer of peanut hypersensitivity by fresh frozen plasma. Arch Intern Med. 2007;167:853–4. doi: 10.1001/archinte.167.8.853. [DOI] [PubMed] [Google Scholar]
- 15.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, Massing M, Cohn RD, Zeldin DC. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126:798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbes SJ, Jr, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2007;120:1139–45. doi: 10.1016/j.jaci.2007.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frewin DB, Jonsson JR, Head RJ, Russell WJ, Beal RW. Histamine levels in stored human blood. Transfusion. 1984;24:502–4. doi: 10.1046/j.1537-2995.1984.24685066810.x. [DOI] [PubMed] [Google Scholar]
- 18.Muylle L, Beert JF, Mertens G, Bult H. Histamine synthesis by white cells during storage of platelet concentrates. Vox Sang. 1998;74:193–7. [PubMed] [Google Scholar]
- 19.Salkie ML, Hannon JL. Anti-plasticizer specific IgE is present in the serum of transfused patients. Clin Invest Med. 1995;18:419–23. [PubMed] [Google Scholar]
- 20.Sarkodee-Adoo CB, Kendall JM, Sridhara R, Lee EJ, Schiffer CA. The relationship between the duration of platelet storage and the development of transfusion reactions. Transfusion. 1998;38:229–35. doi: 10.1046/j.1537-2995.1998.38398222865.x. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48:2285–91. doi: 10.1111/j.1537-2995.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 22.Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang SE, Lara PN, Jr, Lee-Ow A, Reed J, Wang LR, Palmer P, Tuscano JM, Richman CM, Beckett L, Wun T. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70:191–4. doi: 10.1002/ajh.10119. [DOI] [PubMed] [Google Scholar]
- 24.Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007;47:1089–96. doi: 10.1111/j.1537-2995.2007.01242.x. [DOI] [PubMed] [Google Scholar]